Abstract
The present study aims to investigate the treatment efficiency of soil flushing using waste lemon extract for samples collected from contaminated farmland, in which the copper concentration was measured as 2487 ± 139 mg/kg. The flushing solution, containing 9.9 g/L citric acid, was prepared from the waste lemon extraction process. The soil-flushing treatment using a solution containing commercial citric acids of 10 g/L was also conducted for comparison. Additionally, the collected soil was mixed with crushed waste lemons and the mixture was subjected to a composting process for subsequent stabilization study. After 120-min batch experiments, the desorbed copper concentration for waste lemon-extract experiment was 36.9 mg/L, which was higher than that (28.6 mg/L) for commercial citric solution experiment. The reduction in soil copper concentration (1504 mg/kg) treated by waste lemon-extract flushing was more than that treated by commercial citric solution (1256 mg/kg) at the comparable citric acid concentration. More metals were removed by waste lemon-extract flushing. This is because the waste lemon-extract solution contains additional co-dissolved organic substances with a longer flushing time, which allows more exchange reactions between adsorbed metals and flushing solution. For the treatment with waste lemon extract, the soil pH values were 4.56, 5.70 and 6.29 before, after flushing and after compost treatment, respectively. The observed variation in soil pH also showed that waste lemon extract might be a better flushing agent, while flushing with commercial citric solution decreased the pH in the soil environment. The plant copper availability dropped from 677 mg/kg to 156 mg/kg after waste lemon-extract flushing and stabilization with composted waste lemon. Therefore, the use of waste lemon extract for soil flushing not only removed toxic metals from the soil but also prevented the occurrence of soil acidification, an often-observed phenomenon using an acidic solution in conventional soil flushing. After soil flushing, the application of composted waste lemon could stabilize the toxic metals and increase the pH to a range suitable for plant growth.
1. Introduction
Soil pollution by toxic metals, which is mainly caused by inappropriate utilization, treatment, or disposal of metal-containing substances [1,2,3,4,5], is one of the most concerning issues around the world [6,7]. The adverse impacts of toxic metals on the general population have been shown to result mostly from oral ingestion [8,9]. To mitigate such impacts, the conventional concepts of pollution control, such as source reduction and endpoint treatment, have been applied extensively. For toxic metal remediation, soil plowing and excavation are often-employed and economically-feasible methods. However, these methods can only redistribute or relocate the toxic metals without removing the contaminant physically. To remediate the toxic metal contamination effectively, soil flushing is often applied firstly, followed by a subsequent treatment to remove the toxic metal from the eluent solution. To enhance the removal efficiency, amendment to the flushing solution’s constituents has been considered and applied [10,11].
In the study by Zhang et al. [12], batch experiments were performed to assess the efficiency of soil washing on a contaminated soil under various operating conditions. Results showed that pre-washing with ethylenediaminetetraacetic acid (EDTA) enhanced the chemical immobilization of Cu and Cr, while an opposite effect was observed for Pb and Zn, particularly when Ca(OH)2 was added as the immobilizing agent. Gusiatin and Klimiuk [13] investigated the influence of multiple saponin washing for copper, cadmium, and zinc removal in the soils of loamy sand, loam, and silty clay. The best removal efficiency was found in the loamy sand (82–90%), and loam (67–88%), while the least was in the silty clay (39–62%). In the loamy sand and loam, the metals had higher mobility factors (44–61% Cu, 60–76% Cd, and 68–84% Zn) compared to that of the silty clay (9% Cu, 28% Cd, and 36% Zn). Moon et al. [14] conducted a study of bench-scale soil washing to remove Zn from contaminated soils. Various washing solutions including hydrochloric acid (HCl), nitric acid (HNO3), sodium hydroxide (NaOH), oxalic acid (HOOCCOOH•2H2O), sulfuric acid (H2SO4), phosphoric acid (H3PO4), and tartaric acid (C4H6O6) were used. The concentrations of the washing solutions ranged from 0.1 M to 2 M with a liquid to solid ratio of 10. The results showed that the soil-washing efficiencies showed a decreasing trend for the removal of Zn as follows: HCl > HNO3 > H2SO4 > H3PO4 > C4H6O6 > HOOCCOOH•2H2O > NaOH. Pociecha and Lestan [15] conducted a laboratory scale feasibility study showing that EDTA can be recovered up to 50% from a flushing solution after extraction of Pb (5330 mg/kg), Zn (3400 mg/kg), Cd (35 mg/kg), and As (279 mg/kg) contaminated soil. The EDTA recovery could be up to 100% in the washing solution after 100%, 97%, 98%, and 100% of initial Pb, Zn, Cd, and As were removed in an electrolytic cell using a graphite anode, respectively. Ng et al. [16] explored the application of a two-stage electrokinetic washing system to the remediation of lead (Pb) contaminated soils, in which the system not only provided additional driving force for Pb flushing but also reduced the volume of washing solution. The effects of NaNO3, HNO3, citric acid, and EDTA as the washing solutions in a two-stage electrokinetic washing system were evaluated as well. The results showed that a two-stage electrokinetic washing process enhanced the Pb removal efficiency by 2.52–9.08% and 4.98–20.45% in comparison to a normal electrokinetic process and normal washing process, respectively. Although exhibiting the highest removal efficiency, HNO3 was not suitable for soil washing because of the pH fluctuation in the cathode chamber, corrosion of graphite anode, and excessive power consumption. In contrast, the citric acid not only provided a high Pb removal efficiency with low power consumption but also maintained a low soil-to-solution ratio, stable pH, and electrode integrity. In the study by Zhang et al. [17], selected chelating agents were added in the soil-flushing solutions for toxic metal removal. The results showed that phenyldiaminetetraacetic acid (PDTA) significantly enhanced the Cu removal from the tested soil samples. The major responsible mechanisms of Cu removal were complexation-promoted Cu dissolution and the increased dissolution of soil organic matters (SOMs). PDTA demonstrated a high selectivity for Cu(II) over other cations (i.e., Ca(II), Mg(II), Fe(III), Mn(II), and Al(III)), especially at lower liquid-to-soil ratios under PDTA deficiency. The unwanted dissolution of soil minerals during the washing process can degrade soil texture and interfere with future land use. PDTA-enhanced soil washing increased the exchangeable fractions of Cu, Zn, and Pb, and decreased their ambient residuals, compared to their levels in unwashed soil.
In the United States, acid washing was regarded as one of the popular techniques for toxic metal removal, in which hydrochloric acid was often employed as the chelating agents [18,19,20]. However, previous studies indicated that soil flushing containing hydrochloric acid might result in several disadvantages including the (1) deterioration of soil properties, structure, fertility, and the microorganism within, and (2) further required treatment for the resulting wastewater and soil [21,22,23]. Alternatively, citric acid has been proposed as a replacement of hydrochloric acid. It has been demonstrated that citric acid can effectively remove the toxic metal from the treated soil [24], although a few studies have also pointed out that citric acid may cause the soil acidification problem [25]. Along with this context, the present study evaluated the (1) use of waste lemon extract as the eluent solution for soil flushing, and (2) the addition of lemon compost to alleviate soil acidification and to improve plant availability. No study using waste lemon extract in soil flushing has been reported before.
2. Materials and Methods
2.1. Waste Lemon-Extract Preparation
The waste lemons were collected from the hand-shake drink shops in the metropolitan area of Taipei, which were mixed on a 1:1 w/w ratio with deionized water in a beaker. After the deionized (DI) water was boiled (i.e., at the temperature of 100 °C), the waste lemons were immersed in the boiling water for 3 min. The boiled lemon solution was stored at room temperature for 30 min to cool down, and filtered with a tea bag. The filtrate liquid (i.e., waste lemon extract) was stored in a glass bottle for subsequent use.
2.2. Compost Preparation
The 2000 g waste lemon was crushed using the Electrolux EBR-3416 blender and placed in a compost bin with 250 g clean soil evenly distributed at the bottom, followed by a coverage of 250 g clean soil at the top. After the temperature rose to 40 °C, the mixed compost was stirred with ventilation until the soil aroma emerged. The composting process took 4 weeks. The compost was air-dried and grounded to pass through a 20-mesh sieve, and the ratio of carbon to nitrogen was measured in triplicate to confirm the compost maturity before further use.
2.3. Analysis of Soil Properties
The soil samples were collected from a contaminated farmland using a random sampling strategy following the protocol (NIEA S102.63B) proposed by Taiwan Environmental Protection Administration (EPA), in which the copper concentration was measured as high as 2487 mg/kg. Using a spade, 100 kg soil from ten sampling locations (10 kg from each) was collected, then sieved through a 2-mm screen to remove large objects such as branches, twigs, and rocks. The soil was mixed completely to assure the homogeneity and stored at room temperature for later use.
Soil pH was measured using a pH meter after the soil was mixed with water at the 1:1 (w/v) soil to water ratio [26]. Soil electrical conductivity (EC) measurement was conducted on the soil solution at 1:5 (w/v) soil-to-water ratio using an EC meter. Soil texture was analyzed following the pipette method, measuring the particle-size distribution and comparing it with the soil-texture classification diagram [27]. Soil organic-matter content (OMC) was analyzed using the Walkley–Black wet oxidation method [28]. Soil samples were sieved by a 0.15-mm mesh screen and digested with aqua regia (concentrated HNO3--concentrated HCl at 1:3 v/v ratio) solutions, followed by the analytical measurement for Cd, Cu, Zn, Ni, Pb, and Cr using inductive coupled plasma optical emission spectrometry (ICP-OES), adopting the protocol suggested by the International Organization for Standardization [29].
2.4. Waste Lemon-Extract Analysis
The pH of the waste lemon extract was measured using a pH meter. For citric acid content, the method proposed by Lu and Chen [30] was adopted. The tested sample was mixed with DI water and a few drops of phenolphthalein. The resulting sample was titrated with 0.1 N NaOH until no further color changed and the citric acid content was calculated. For total flavonoids content, the method by Kreft et al. [31] was used. The rutin, as the equivalent of total flavonoid, was extracted with 5 mL methanol/water (67:33) at room temperature, by shaking for 40 min. High-performance liquid chromatography (HPLC) with C-18 column and UV/Vis detector at 380 nm was employed for analytical determination using the mobile phase consisting of two previously-prepared solutions, A: acetonitrile and methanol (1:2), and B: 0.75% aqueous H3PO4. The mobile-phase gradient setting was 100% B solution at the beginning, and the samples were run on a linear gradient to 60% A and 40% B in 20 min, followed by a linear gradient to 100% A and 0% B for another 20 min, and finally 10 min equilibration (100% B).
2.5. Compost Maturity Analysis
The compost was inspected by naked eyes and found to have the color of dark black or dark brown. It was considered matured when its texture was fluffy, with fragrance. The functional group identification of the compost was conducted using FTIR spectroscopy [32]. The C, N, and H compositional percentage was measured by elemental analyzer. Dissolved organic matter (at 1:20 w/v) extracted from the compost solution was used in the phytotoxicity test and seed germination for the Brassica chinensis L. seed. Twenty-five Brassica chinensis L. seeds and 5 mL germination solution were placed on the sterilized petri dishes with paper filters. Deionized water incubation was conducted as the control group and each treatment was triplicated. The petri dishes were kept in the darkness for 3 days at 25 °C. The germination index (GI) was calculated according to the following formula [33]:
For the root-length determination, the root was stretched fully and its length was measured using a ruler.
2.6. Soil-Column Flushing Experiment
Two 800 g soil samples, which were air-dried with a 10-mesh screen (pore size 2 mm), were placed into soil columns (60 cm high × 5 cm width × 5 cm length, with the height of the tested soil sample reaching 30 cm, please refer to Supplementary Materials for illustration), saturated with 400 mL DI water, and flushed with 8000 mL (1) commercial citric solution and (2) waste lemon extract solution, each followed by an additional 8000 mL DI water as the residual cleanup operation in the soil matrix. The total volume of 16 L was used in the soil-flushing treatment. The pore volume of 800 g soil was calculated to be 289.5 mL. The flushing time assessment started at the moment when an outflow was observed from the tested soil column. The timer stopped when no outflow was observed. The tested soil column was mounted on a wooden stand, with a beaker at the bottom outlet for filtrate collection after gravitational flushing. During the flushing process, the pH was measured and toxic metal concentrations of each liter in the flushed liquid were analyzed using ICP-OES.
2.7. Batch Metal Removal Experiment
Two 10 g soil samples, which followed the same pretreatment procedure outlined in Section 2.6, were placed into two beakers. One sample was saturated with 100 commercial citric solution while the other was saturated with waste lemon extract. An aliquot of 1 mL was collected from each sample at the contact times of 5, 15, 30, 60, and 120 min. Each sample was diluted tenfold with deionized water, followed by analytical quantification using ICP-OES.
2.8. Stabilization Experiment
The stabilization experiment is to obtain the fractionation results of the investigated soil. The analysis was conducted according to the protocol described in Table 1 [34,35,36,37]. In short, 1 g soil sample, which was air-dried with a 10-mesh screen (pore size 2 mm), was saturated with 10 mL of DI water, and equilibrated on an oscillator at the speed of 150 rpm. The sample was centrifuged at 4000 rpm for 10 min. The supernatant was screened with a 0.22 µm filter, and the obtained solution was analyzed for metal concentrations in triplicate. The equilibrating, oscillating, centrifuging, screening, and analyzing procedures were repeated using the soil sample in the previous step, but with different saturation solution, equilibrating time, and temperature. The six-step experimental procedure was conducted to obtain various fractionation results of the investigated soil.

Table 1.
Sequential extraction of toxic metal in soil.
2.9. Soil Quality
As indicated by Xu et al. [38], the pH, EC, OMC, texture, Cd, Cu, Zn, Ni, Pb, and Cr availability are important factors by which to evaluate plant-growing soil quality. For the plant availability calculation, soil which has been sieved by a 0.149-mm screen was added into 0.1 N HCl and analyzed the extract for Cd, Cu, Zn, Ni, Pb, and Cr by ICP-OES. This study verified the soil quality using seed tests of twenty-five cultivated Brassica chinensis L. seeds which were placed in the 9-cm petri dishes in the darkness for 3 days at 25 °C, each containing 15 g dried soil and 15 mL deionized water. The number of seeds germinated and the length of the germinated shoots were measured after 3 days.
3. Results and Discussion
3.1. Soil and Waste Lemon Properties
The properties of the tested soil are shown in Table 2. The texture is loamy sand, and its pH is 4.56 with conductivity of 54.1 μS/cm, with organic matter content of 55.6 g/kg. The copper concentration was 2487 mg/kg. In the waste lemon, the citric acid content, pH, and total flavonoid content (TFC) of the waste lemon extract and 10 g/L commercial citric solution were measured. The citric acid content and pH of waste lemon extract were about 9.9 g/L and 3.4, respectively. The pH of commercial citric solution is 2.4, lower than that of the waste lemon extract. As the preliminary analysis indicated the existence of rutin in the waste lemon extract, the TFC was measured as 46 mg rutin/L.

Table 2.
Physical and chemical properties of the soil used in this study (mean ± standard deviation, n = 3).
3.2. Compost Maturity
The compost was odorous and had the color of dark brown. Its carbon-to-nitrogen ratio was 10.09, suitable for soil application since it was considered mature from its appearance, odor, and C/N ratio [39]. The pH of the compost was 8.29, which may neutralize the acidic condition in the soil during application. By comparing these two spectra in Figure 1, the general shape of the FTIR spectra were similar but different in signal intensity, showing the soil modification through the composting process. A small protruding crest at wave number 1650 cm−1 indicated that the compost might contain aromatics and alkenes [40]. A small protruding crest at wave number 1420 cm−1 indicated that the compost might contain lignin [41]. Both Marina et al. [42] and Xu et al. [43] showed that the lignin could adsorb toxic metals. A high protruding peak at the wave number 1030 cm−1 suggested that the compost might contain polysaccharides [41], and Choma et al. [44] indicated that polysaccharides were able to adsorb toxic metals.
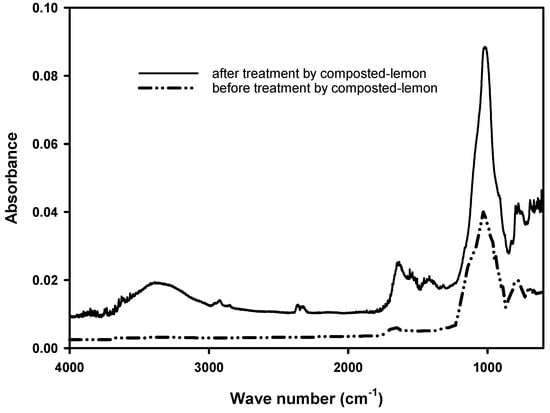
Figure 1.
FTIR spectra of soil sample before and after treatment by composted lemon.
3.3. Waste Lemon-Extract Flushing
In the present study, the tested soil, containing a variety toxic metals, was flushed with waste lemon extract and commercial citric solution, and the toxic metal concentrations in the effluents were compared. As shown in Figure 2, the highest concentration peaks of cadmium, zinc, and copper were at the first 2 L of effluent, and the high concentration peaks of nickel, lead, and chromium were at 7–8 L. This phenomenon was explained by the toxic metal fractionation percentage that the ratios of organic-bound metals to the residuals of cadmium, zinc, and copper in soil are lower than those of nickel, lead, and chromium as shown in Figure S2 (please refer to the Supplementary Materials for Figure S2). Kabata [45] indicated that the organic-bound toxic metals are not easily chelated or ion-exchanged, and even more difficult to migrate.

Figure 2.
The effluent toxic metal concentrations of soil flushing (mean, n = 3).
The reduction in soil copper concentration (1504 mg/kg) treated by waste lemon-extract flushing was more than that treated by commercial citric solution (1256 mg/kg) at the comparable citric acid concentration. The copper removal efficiencies for treatments by waste lemon-extract and commercial citric solution were 67.3% and 65.6%, respectively. These efficiencies increased to 76.9% and 67.7%, respectively, if the soil after flushing was subjected to compost treatment (discussion is presented in the forthcoming section). The effect of waste lemon extract on toxic metal flushing is better than that of commercial citric solution. For the eluting time for the first 8 L flushing (citric-related solution only), the treatment with the commercial citric solution required 90–120 min, while that with waste lemon extract took up 300–360 min. Apparently, the flushing with waste lemon extract demonstrated a better treatment efficiency and a longer contact time. To further explore the influence of contact time on the metal-removal efficiency, a series of batch experiments with various contact time were conducted, and the results are shown in Table 3. The commercial citric solution showed a better metal-removing ability when the contact time was less than 5 min. On the contrary, a better treatment efficiency was observed for waste lemon extract when the contact time was more than 15 min. In this study, the commercial citric solution has a shorter contact time than that of waste lemon extract, so that the flushing efficiency of commercial citric solution for toxic metal removal was lower compared to that of waste lemon extract. Citrus fruits have been identified to contain more than 60 kinds of flavonoids. Lemon is one of the citrus fruits and mainly contains four different kinds of flavonoids: flavones, flavanones, flavonols, and anthocyanins [46]. Jabeen et al. [47] showed that flavonoids (Fls) have the ability to chelate metals (M) to form M-Fl. In short, the waste lemon extract contains not only citric acid but also many flavonoids, which can chelate with toxic metals, so the waste lemon extract can flush out more toxic metals.

Table 3.
Desorbed toxic metal concentrations at different residence time (CCS: Commercial Citric Solution, WLE: Waste Lemon Extract).
3.4. Changes of Soil Fractionation after Flushing and Stabilization
In Figure 3, the exchangeable copper in the stabilized soil was apparently reduced because the soil fraction with weak bonding ability had been removed after flushing. Stabilization enabled the toxic metal to be fixed in the mineral structure or adsorbed onto the clay. The metal capturing ability of the soil after flushing decreased since the acid took away some adsorbing sites, resulting in a decrease in the residual toxic-metal concentration. The direct stabilization might transform the water-soluble, exchangeable, organic-bound carbonate and Fe/Mn oxides forms of toxic metals into residual forms, but the total concentration did not change markedly because stabilization may only immobilize the toxic metals and make them difficult to release. After flushing and compost treatments, many forms of Cd were at the concentration below the detection limits. Therefore, the Cd concentration illustrations are not shown in Figure 3 after deliberate consideration.
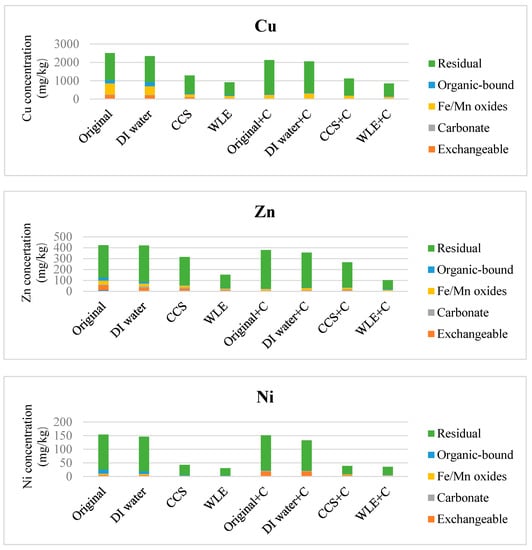
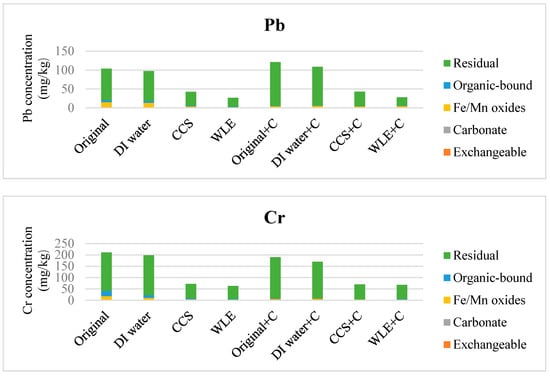
Figure 3.
Effects of soil flushing and stabilization on chemical fractions of Cu, Zn, Ni, Pb, and Cr (mean, n = 3) (CCS: Commercial Citric Solution, WLE: Waste Lemon Extract, C: Compost).
3.5. Soil Quality Improvement
Xu et al. [38] proposed soil texture, pH, EC, OMC, and metal availability as soil-quality persistence indicators. As shown in Table 4, the pH of the investigated soil was acidic, and it was reduced to 4.48 after flushing by commercial citric solution. However, the pH increased to 5.70 after flushing using waste lemon extract. During the soil flushing process, the aqueous citric acid might act as the chelating agent to capture the metal ions adsorbed on the surface of soil particles. The hydrogen ions might take up the soil active sites that used to be occupied by toxic metals, resulting in a decrease in hydrogen ion concentration [48]. At similar citric content, the pH of the waste lemon extract was higher than the commercial citric solution, and the organic constituents in the waste lemon extract reacted with nitrogenous substances in the soil, causing an increase in pH. Stabilization after soil flushing using waste lemon extract increased the pH to 6.29.

Table 4.
Quality of the soil after flushing and stabilization (mean ± standard deviation, n = 3) (CCS: Commercial Citric Solution, WLE: Waste Lemon Extract, C: Compost).
Generally, the soil texture changed after the flushing in the present study. As compared to the original sample, the SOM, EC, sand%, and silt% decreased at different degrees after flushing treatment while clay% increased. Such observation may be due to the fact that the silt and clay might move downwards in the commercial citric solution (CCS) and waste lemon extract (WLE) flushing process, causing the redistribution of soil constituents. After the composting treatment, nutrients were introduced to enhance the microbial activity, causing further variation in soil texture. Although the soil texture variation occurred, the soil samples before and after the experiments were classified as “loamy sand” or “sand”, due to the high and low contents of sand and clay, respectively, according to the soil classification ternary diagram.
The organic matter content of the investigated soil was measured as 55.6 g/kg, which was considered as high organic matter content. After being flushed by commercial citric solution and waste lemon extract, the organic matter is reduced to the range of general cultivated soil. Since stabilization should increase the soil organic matter content, the compost application dose should be controlled carefully if the organic matter content of the original soil is already high. After flushing either with waste lemon extract or natural citric acid, the soil electrical conductivity decreased. The electrical conductivity increased after stabilization, but the soil was still considered in the classification to be without salt damage effect. Moreover, the increase in conductivity makes the soil more nutritious. The investigated soil was loamy sand, and its structure does not change after flushing. Meanwhile, the soil stabilized by compost should increase sand content, but stabilization after flushing by waste lemon compost acid does not cause a significant change in soil texture.
Xu et al. [38] suggested the soil-quality persistence index for the availability assessment of cadmium, lead, copper, and zinc (Table 5). By calculating the upper and lower limits considering half of one standard deviation (i.e., ± half of one standard deviation), the Cu plant availability for CCS flushing ranged from 230.33–236.08 mg/kg and those for WLE flushing was between 213.35 and 229.25 mg/kg, which have the occurring probability of 38.2% (based on the probability distribution of the standard normal distribution). For CCS flushing, the probability for Cu plant availability higher than 230.33 mg/kg is 69.1% (i.e., only considering one tail-end probability). Similarly, the probability for Cu plant availability lower than 229.25 mg/kg is 69.1% for WLE flushing. Therefore, the soil copper availability for plant after flushing by waste lemon extract is lower than that by commercial citric solution at the probability of 69.1%. Subsequent stabilization after flushing can further reduce the soil copper concentration, and the risk of copper on the soil organisms can be reduced markedly. For other toxic metals such as zinc, nickel, and lead, the plant availabilities were reduced by combining waste lemon extract flushing and stabilization. However, adding chromium to the compost for stabilization should increase the plant intake efficiency, and this is because the composted lemon contained a small amount of chromium, which increased the chromium concentration slightly after stabilization.

Table 5.
Toxic metal plant availability after flushing and stabilization (mg/kg) (mean ± standard deviation, n = 3) (CCS: Commercial Citric Solution, WLE: Waste Lemon Extract, C: Compost).
Studies have shown that GI can be regarded as an integrated indicator of phytotoxicity [49], and the GI reached 122.2% after 3-day cultivation. The California Compost Quality Council (CCQC) [50] suggested that the maturity should be considered sufficient when GI was greater than 80%, while Bernal et al. [51] and Lv et al. [52] pointed out that a GI greater than 110% implied the elimination of inhibiting substances for germination during the composting process. Experimental photos of the Brassica chinensis L. germination rate are shown in Table 6. Table 7 shows the root-length observations of the Brassica chinensis L. germination. Among the roots observed for different treatments after 3 days, the root after WLE + C treatment was longer than the one without any treatment. Although the root flushed by waste lemon extract was longer than that flushed by commercial citric solution after 3 days of cultivation on average, the difference may not be significant considering the standard deviations under the 95% confidence level. These observations also implied that metal removal followed by compost would improve the soil environment for plant growth, possibly due to the apparent decrease in metal plant availability.

Table 6.
Initial and 3-day germination photos of compost (n = 3).

Table 7.
Brassica chinensis L. root length (cm) measurement after 1-, 2- and 3-day germination (CCS: Commercial Citric Solution, WLE: Waste Lemon Extract, C: Compost).
4. Conclusions
In this study, the effect of soil flushing using waste lemon extract on copper removal of a contaminated agricultural farmland was investigated, and the effect of composted lemon on soil stabilization was explored. The results showed that soil flushing using waste lemon extract for copper removal has a probability of 69.1% to be more effective than using commercial citric solution because waste lemon extract contained other substances that might also chelate toxic metals. The extra substances in waste lemon extract might increase the retention time, which allowed more time for metal removal. Additionally, the waste lemon extract can not only flush out copper but also cadmium, zinc, nickel, lead, and chromium simultaneously. The soil stabilized by composted lemon could significantly reduce the plant availability of copper and raise its pH to the range in which plants can absorb most of the desired nutrient elements.
Supplementary Materials
The following are available online at https://www.mdpi.com/2071-1050/12/14/5751/s1, Figure S1: Illustration of experimental soil column setup, Figure S2: Percentage of toxic metal fractionation in soil (mean, n = 3).
Author Contributions
Conceptualization, T.-K.C. and C.F.; methodology, T.-K.C. and P.-W.Z.; software, P.-W.Z.; validation, P.-W.Z. and Y.-Z.H.; formal analysis, P.-W.Z. and Y.-Z.H.; investigation, P.-W.Z. and C.F.; resources, T.-K.C. and C.F.; data curation, T.-K.C. and C.F.; writing—original draft preparation, P.-W.Z., Y.-Z.H. and C.F.; writing—review and editing, C.F.; visualization, C.F.; supervision, T.-K.C. and C.F.; project administration, T.-K.C. and C.F.; funding acquisition, T.-K.C. and C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest. The current work is purely a spin-off from another project that was explored out of pure curiosity.
References
- Zhang, T.; Liu, J.M.; Huang, X.F.; Xia, B.; Su, C.Y.; Luo, G.F.; Xu, Y.W.; Wu, Y.X.; Mao, Z.W.; Qiu, R.L. Chelant extraction of heavy metals from contaminated soils using new selective EDTA derivatives. J. Hazard. Mater. 2013, 262, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Wu, J.; Lu, S.; Wang, Y.; Jiao, X.; Song, L. Soil and soil environmental quality monitoring in China: A review. Environ. Int. 2014, 69, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Mahmood, Q.; Peng, D.; Fu, W.; Chen, T.; Wang, Y.; Li, S.; Chen, J.; Liu, D. The spatial distribution pattern of heavy metals and risk assessment of moso bamboo forest soil around lead–zinc mine in Southeastern China. Soil Tillage Res. 2015, 153, 120–130. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, Y.; Zhu, Y.G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2014, 49, 750–759. [Google Scholar] [CrossRef]
- Zhong, B.; Chen, J.; Shafi, M.; Guo, J.; Wang, Y.; Wu, J.; Ye, Z.; He, L.; Liu, D. Effect of lead (Pb) on antioxidation system and accumulation ability of Moso bamboo (Phyllostachys pubescens). Ecotoxicol. Environ. Saf. 2017, 138, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S. Heavy metal pollution in China: Origin, pattern and control. Environ. Sci. Pollut. Res. 2003, 10, 192–198. [Google Scholar] [CrossRef]
- Nriagu, J.O. A silent epidemic of environmental metal poisoning? Environ. Pollut. 1988, 50, 139–161. [Google Scholar] [CrossRef]
- Moon, C.; Zhang, Z.; Shimbo, S.; Watanabe, T.; Moon, D.; Lee, C.; Lee, B.; Ahn, K.; Lee, S.; Ikeda, M. Dietary Intake of Cadmium and Lead among the General Population in Korea. Environ. Res. 1995, 71, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Zhang, Z.W.; Qu, J.B.; Xu, G.F.; Song, L.H.; Wang, J.J.; Shimbo, S.; Nakatsuka, H.; Higashikawa, K.; Ikeda, M. Urban-rural comparison on cadmium exposure among general populations in Shandong Province, China. Sci. Total Environ. 1998, 217, 1–8. [Google Scholar] [CrossRef]
- Isoyama, M.; Wada, S.-I. Remediation of Pb-contaminated soils by washing with hydrochloric acid and subsequent immobilization with calcite and allophanic soil. J. Hazard. Mater. 2007, 143, 636–642. [Google Scholar] [CrossRef]
- Di Palma, L.; Mecozzi, R. Heavy metals mobilization from harbour sediments using EDTA and citric acid as chelating agents. J. Hazard. Mater. 2007, 147, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tong, L.; Yuan, Y.; Liu, Z.; Huang, H.; Tan, F.; Qiu, R. Influence of soil washing with a chelator on subsequent chemical immobilization of heavy metal in a contaminated soil. J. Hazard. Mat. 2010, 178, 578–587. [Google Scholar] [CrossRef]
- Gusiatin, Z.M.; Klimiuk, E. Metal (Cu, Cd and Zn) removal and stabilization during multiple soil washing by saponin. Chemosphere 2012, 86, 383–391. [Google Scholar] [CrossRef]
- Moon, D.H.; Lee, J.R.; Wazne, M.; Park, J.H. Assessment of soil washing for Zn contaminated soils using various washing solutions. J. Ind. Eng. Chem. 2012, 18, 822–825. [Google Scholar] [CrossRef]
- Pociecha, M.; Lestan, D. Recycling of EDTA solution after soil washing of Pb, Zn, Cd and As contaminated soil. Chemosphere 2012, 86, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.S.; Cupta, B.S.; Hashim, M.A. Performance evaluationof two-stage electrokinetic washing as soil remediation method for lead removal using different wash solutions. Electromich. Acta. 2014, 147, 9–18. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, H.; Yang, X.H.; Xia, B.; Liu, J.M.; Su, C.Y.; Qiu, R.L. Influence of the selective EDTA derivative phenyldiaminetetraacetic acid on the speciation and extraction of heavy metals from a contaminated soil. Chemosphere 2014, 109, 1–6. [Google Scholar] [CrossRef]
- Fedje, K.K.; Li, Y.; Strömvall, A. Remediation of metal polluted hotspot areas through enhanced soil washing–Evaluation of leaching methods. J. Environ. Manag. 2013, 125, 489–496. [Google Scholar] [CrossRef]
- Rao, C.R.M.; Sahuquillo, A.; López-Sánchez, J.F. A Review of the Different Methods Applied in Environmental Geochemistry For Single and Sequential Extraction of Trace Elements in Soils and Related Materials. Water Air. Soil Pollut. 2007, 189, 291–333. [Google Scholar] [CrossRef]
- Udovic, M.; Lestan, D. EDTA Leaching of Cu Contaminated Soils Using Ozone/UV for Treatment and Reuse of Washing Solution in a Closed Loop. Water Air Soil Pollut. 2006, 181, 319–327. [Google Scholar] [CrossRef]
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-LaFLèche, M. Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Ko, I.; Chang, Y.; Lee, C.; Kim, K.W. Assessment of pilot-scale acid washing of soil contaminated with As, Zn and Ni using the BCR three-step sequential extraction. J. Hazard. Mater. 2005, 127, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tampouris, S.; Papassiopi, N.; Paspaliaris, I. Removal of contaminant metals from fine grained soils, using agglomeration, chloride solutions and pile leaching techniques. J. Hazard. Mater. 2001, 84, 297–319. [Google Scholar] [CrossRef]
- Bassi, R.; Prasher, S.O.; Simpson, B.K. Extraction of metals from a contaminated sand soil using citric acid. Env. Prog. 2000, 19, 275–282. [Google Scholar] [CrossRef]
- Li, Y.J.; Hu, P.J.; Zhao, J.; Dong, C.X. Remediation of cadmium- and lead-contaminated agricultural soil by composite washing with chlorides and citric acid. Environ. Sci. Pollut. Res. 2015, 22, 5563–5571. [Google Scholar] [CrossRef] [PubMed]
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis, Part 2, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph No. 9; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis, Part 1, 2nd ed.; Klute, A., Ed.; Agronomy Monograph No. 9; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1986; pp. 383–412. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E.; Sparks, L.M.; Page, A.; Helmke, P.; Loeppert, R. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis, Part 2, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph No. 9; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982; pp. 539–577. [Google Scholar]
- International Organization for Standardization (ISO). Soil Quality: Extraction of Trace Elements Soluble in Aqua-Regia; ISO 11466; ISO: Geneva, Switzerland, 1995. [Google Scholar]
- Luo, X.N.; Chen, C.Y. Analytical Chemistry Experiment, 3rd ed.; New Wun Ching Development Publishing Co., Ltd.: New Taipei City, Taiwan, 2014. (In Chinese) [Google Scholar]
- Al, I.K.E.; Fabjan, N.; Yasumoto, K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006, 98, 508–512. [Google Scholar] [CrossRef]
- Taiwan Industrial Development Bureau, Ministry of Economic Affairs. Composting Technology and Equipment Manual and Case Compilation; Taiwan Green Productivity Foundation: Taipei, Taiwan, 2005.
- Zucconi, F.; Monaco, A.D.E.; Forte, M.; De, B.M. Biological evaluation of compost maturity. Biocycle 1981, 22, 27–29. [Google Scholar]
- Huang, Z.Y.; Li, J.; Cao, Y.L.; Cai, C.; Zhang, Z. Behaviors of exogenous Pb in P-based amended soil investigated with isotopic labeling method coupled with Tessier approach. Geoderma 2016, 264, 126–131. [Google Scholar] [CrossRef]
- Liu, L.; Chen, H.; Cai, P.; Liang, W.; Huang, Q. Immobilization and phytotoxicity of Cd in contaminated soil amended with chicken manure compost. J. Hazard. Mater. 2009, 163, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Ure, A.M. Methods of analysis for heavy metals in soils. In Heavy Metals in Soils; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1995; pp. 58–102. [Google Scholar]
- Xu, Z.Y.; Chen, Z.X.; Cai, C.Q. Selected Indicators and Conceptual Framework for Assessment Methods of Soil Quality in Arable Soils of Taiwan. Soil Environ. 1999, 2, 77–88. (In Chinese) [Google Scholar]
- Riffaldi, R.; Levi-Minzi, R.; Pera, A.; De Bertoidi, M. Evaluation of Compost Maturity by Means of Chemical and Microbial Analyses. Waste Manag. Res. 1986, 4, 387–396. [Google Scholar] [CrossRef]
- Amir, S.; Jouraiphy, A.; Meddich, A.; El Gharous, M.; Winterton, P.; Hafidi, M. Structural study of humic acids during composting of activated sludge-green waste: Elemental analysis, FTIR and 13C NMR. J. Hazard. Mater. 2010, 177, 524–529. [Google Scholar] [CrossRef]
- Vaca-Paulín, R.; Esteller, M.; De La Fuente, J.A.L.; Zavaleta-Mancera, H. Effect of sewage sludge or compost on the sorption and distribution of copper and cadmium in soil. Waste Manag. 2006, 26, 71–81. [Google Scholar] [CrossRef]
- Šćiban, M.; Klašnja, M.; Antov, M.G. Study of the biosorption of different heavy metal ions onto Kraft lignin. Ecol. Eng. 2011, 37, 2092–2095. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, T.T.; Rao, Q.Q.; Shui, S.W.; Li, W.-W.; He, H.B.; Yao, R.S. Fabrication of mesoporous lignin-based biosorbent from rice straw and its application for heavy-metal-ion removal. J. Environ. Sci. 2017, 53, 132–140. [Google Scholar] [CrossRef]
- Choma, A.; Złotko, K.; Komaniecka, I.; Waśko, A.; Pleszczyńska, M.; Siwulski, M.; Wiater, A. Chemical characterization of alkali-soluble polysaccharides isolated from a Boletus edulis (Bull.) fruiting body and their potential for heavy metal biosorption. Food Chem. 2018, 266, 329–334. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Metals in Soils of Poland—Occurrence and Behavior. In Trace Substances in Environmental Health XXV; Science Reviews Limited: Northwood, NH, USA, 1991; pp. 53–70. [Google Scholar]
- Matheyambath, A.C.; Padmanabhan, P.; Paliyath, G. Citrus Fruits: Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 136–140. [Google Scholar]
- Jabeen, E.; Janjua, N.K.; Ahmed, S. Removal of metal ions using metal-flavonoid-DNA adduct protocol author links open overlay panel. J. Saudi Chem. Soc. 2019, 23, 118–126. [Google Scholar] [CrossRef]
- Lestan, D.; Luo, C.L.; Li, X.D. The use of chelating agents in the remediation of metal-contaminated soils: A review. Environ. Pollut. 2008, 153, 3–13. [Google Scholar] [CrossRef]
- Tiquia-Arashiro, S.; Tam, N.F.Y. Elimination of phytotoxicity during co-composting of spent pig-manure sawdust litter and pig sludge. Bioresour. Technol. 1998, 65, 43–49. [Google Scholar] [CrossRef]
- California Compost Quality Council (CCQC). Compost Maturity Index; The California Compost Quality Council: Nevada, CA, USA, 2001. [Google Scholar]
- Bernal, M.; Alburquerque, J.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Xing, M.; Yang, J.; Qi, W.; Lu, Y. Chemical and spectroscopic characterization of water extractable organic matter during vermicomposting of cattle dung. Bioresour. Technol. 2013, 132, 320–326. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).