Understanding Phytomicrobiome: A Potential Reservoir for Better Crop Management
Abstract
1. Introduction
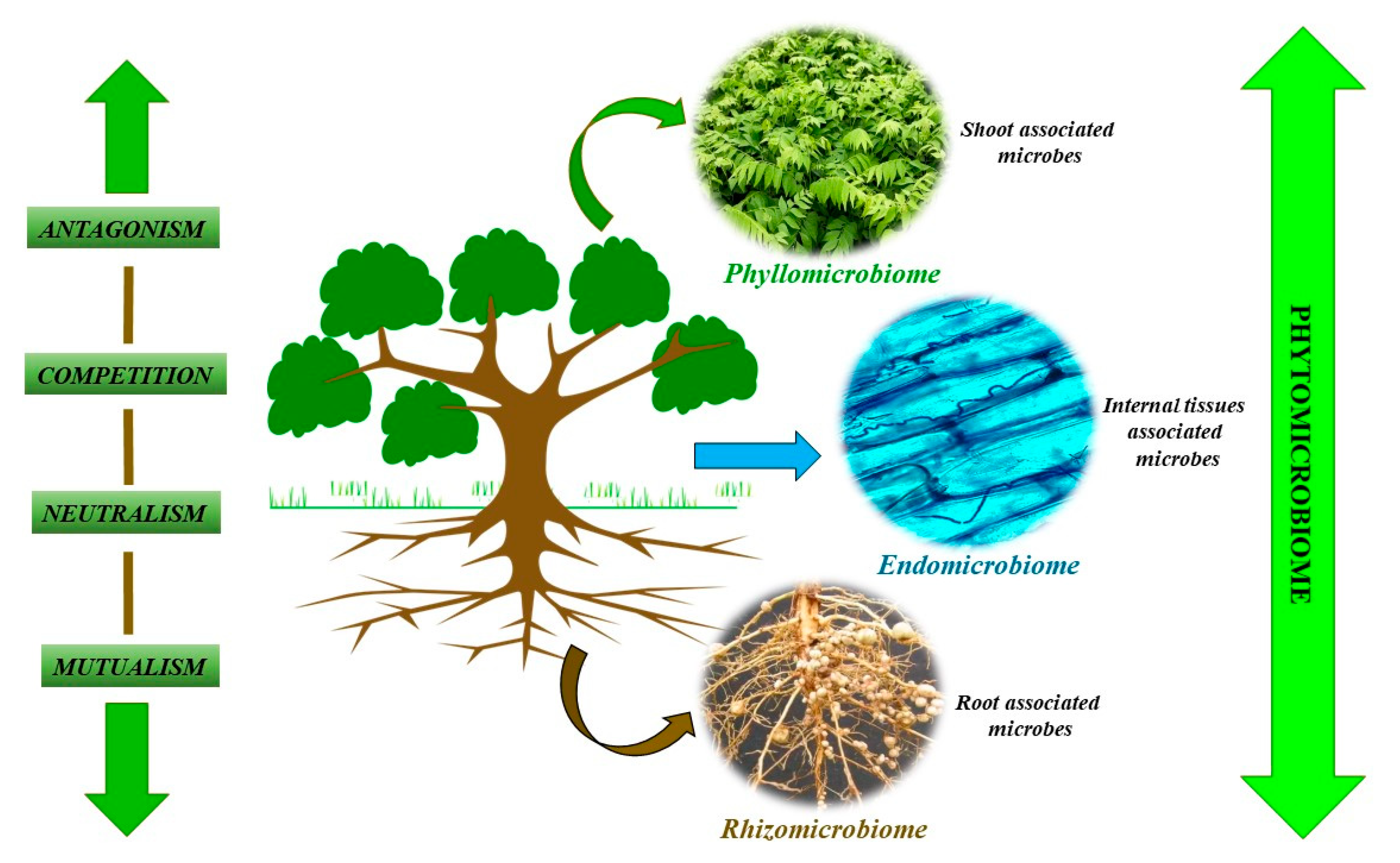
2. Phytomicrobiome: Its Composition and Biomass
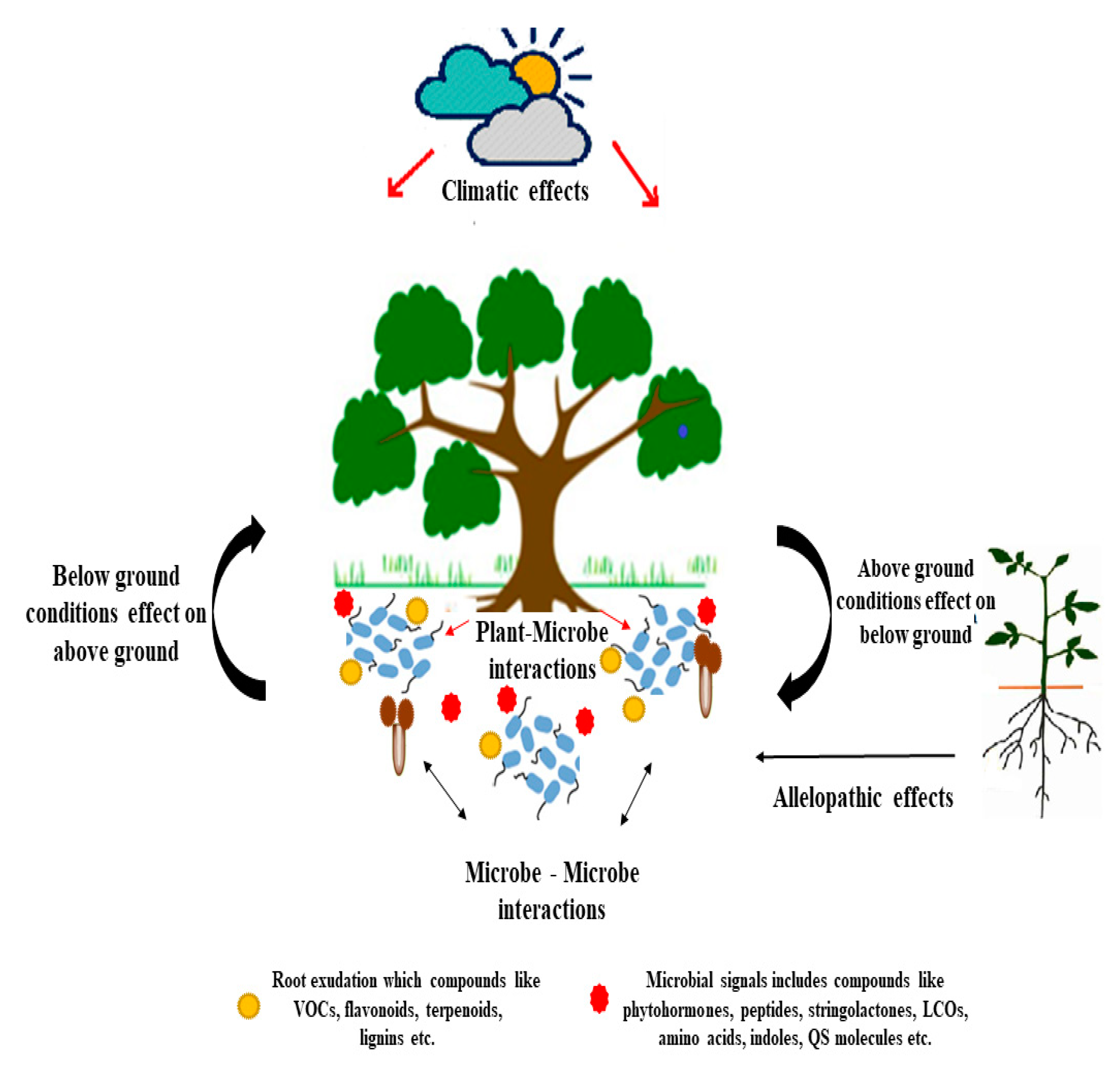
3. Phytomicrobiome Signaling
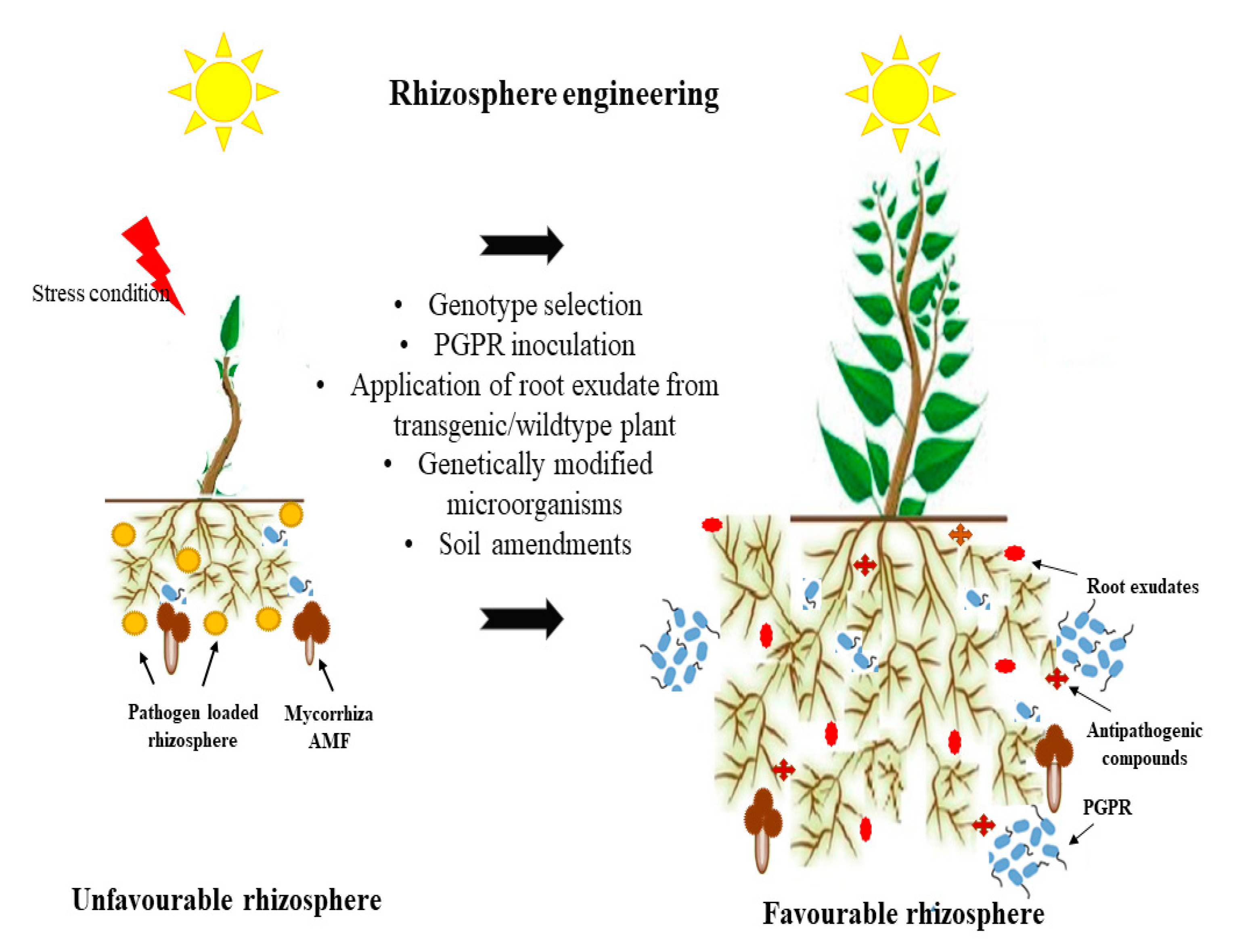
4. Rhizosphere Engineering
5. Phytomicrobiome and Rhizoremediation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, D.L.; Subramanian, S.; Lamont, J.; Bywater-Ekegärd, M. Signaling in the phytomicrobiome: Breadth and potential. Front. Plant Sci. 2015, 6, 709. [Google Scholar] [CrossRef]
- Primo, E.D.; Cossovich, S.; Nievas, F.; Bogino, P.; Humm, E.A.; Hirsch, A.M.; Giordano, W. Exopolysaccharide production in Ensifer meliloti laboratory and native strains and their effects on alfalfa inoculation. Arch. Microbiol. 2019, 202, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Bákonyi, N.; Kisvarga, S.; Barna, D.; Tóth, I.O.; El-Ramady, H.; Abdalla, N.; Kovács, S.; Rozbach, M.; Fehér, C.; Elhawat, N.; et al. Chemical traits of fermented alfalfa brown juice: Its implications on physiological, biochemical, anatomical, and growth parameters of Celosia. Agronomy 2020, 10, 247. [Google Scholar] [CrossRef]
- Khan, A.L.; Asaf, S.; Abed, R.M.; Ning Chai, Y.; Al-Rawahi, A.N.; Mohanta, T.K.; Schachtman, D.; Al-Harrasi, A.; Al-Rawahi, A.N.; Rawahi, A. Rhizosphere microbiome of arid land medicinal plants and extra cellular enzymes contribute to their abundance. Microorganisms 2020, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.; Davidson, J.K.; Smart, C.D. Induction of plant defense gene expression by plant activators and Pseudomonas syringae pv. tomato in greenhouse-grown tomatoes. Phytopathology 2008, 98, 1226–1232. [Google Scholar] [CrossRef]
- Siddiqui, M.F.; Sakinah, M.; Singh, L.; Zularisam, A. Targeting N-acyl-homoserine-lactones to mitigate membrane biofouling based on quorum sensing using a biofouling reducer. J. Biotechnol. 2012, 161, 190–197. [Google Scholar] [CrossRef]
- Audenaert, K.; Pattery, T.; Cornelis, P.; Höfte, M. Induction of systemic resistance to botrytis cinereain tomato by Pseudomonas aeruginosa 7NSK2: Role of salicylic acid, pyochelin, and pyocyanin. Mol. Plant-Microbe Interact. 2002, 15, 1147–1156. [Google Scholar] [CrossRef]
- Yasmin, S.; Hafeez, F.Y.; Mirza, M.S.; Rasul, M.; Arshad, H.M.I.; Zubair, M.; Iqbal, M. Biocontrol of bacterial leaf blight of rice and profiling of secondary metabolites produced by rhizospheric Pseudomonas aeruginosa BRp3. Front. Microbiol. 2017, 8, 1985. [Google Scholar] [CrossRef]
- De Meyer, G.; Höfte, M. Salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 induces resistance to leaf infection by Botrytis cinerea on bean. Phytopathology 1997, 87, 588–593. [Google Scholar] [CrossRef]
- Munhoz, L.D.; Fonteque, J.P.; Santos, I.M.O.; Navarro, M.O.P.; Simionato, A.; Goya, E.T.; Rezende, M.I.; Balbi-Peña, M.L.; De Oliveira, A.G.; Andrade, G. Control of bacterial stem rot on tomato by extracellular bioactive compounds produced by Pseudomonas aeruginosa LV strain. Cogent Food Agric. 2017, 3, 1282592. [Google Scholar] [CrossRef]
- Ma, Z.; Hua, G.K.H.; Ongena, M.; Höfte, M. Role of phenazines and cyclic lipopeptides produced by pseudomonas sp. CMR12a in induced systemic resistance on rice and bean. Environ. Microbiol. Rep. 2016, 8, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Arseneault, T.; Goyer, C.; Filion, M. Phenazine production by Pseudomonas sp. LBUM223 contributes to the biological control of potato common scab. Phytopathology 2013, 103, 995–1000. [Google Scholar] [CrossRef]
- Weller, D.M.; Mavrodi, D.V.; Van Pelt, J.A.; Pieterse, C.M.; Van Loon, L.C.; Bakker, P.A. Induced systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato by 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens. Phytopathology 2012, 102, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Bán, R.; Baglyas, G.; Virányi, F.; Barna, B.; Posta, K.; Kiss, J.; Körösi, K. The chemical inducer, BTH (benzothiadiazole) and root colonization by mycorrhizal fungi (Glomus spp.) trigger resistance against white rot (Sclerotinia sclerotiorum) in sunflower. Acta Biol. Hung. 2017, 68, 50–59. [Google Scholar] [CrossRef]
- Martin, F.; Kohler, A.; Murat, C.; Veneault-Fourrey, C.; Hibbett, D.S. Unearthing the roots of ectomycorrhizal symbioses. Nat. Rev. Microbiol. 2016, 14, 760–773. [Google Scholar] [CrossRef]
- León-Martínez, D.G.; Vielle-Calzada, J.-P.; Olalde-Portugal, V. Expression of phenazine biosynthetic genes during the arbuscular mycorrhizal symbiosis of Glomus intraradices. Braz. J. Microbiol. 2012, 43, 716–738. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.-I.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef]
- Nuccio, E.E.; Starr, E.; Karaoz, U.; Brodie, E.L.; Zhou, J.; Tringe, S.G.; Malmstrom, R.R.; Woyke, T.; Banfield, J.F.; Firestone, M.K.; et al. Niche differentiation is spatially and temporally regulated in the rhizosphere. ISME J. 2020, 14, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Copeland, J.K.; Yuan, L.; Layeghifard, M.; Wang, P.W.; Guttman, D.S. Seasonal community succession of the phyllosphere microbiome. Mol. Plant-Microbe Interact. 2015, 28, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, R.A.; Lorca, G.L.; Meyer, J.; Gonzalez, C.F.; Teplitski, M. Defining the core citrus leaf- and root-associated microbiota: Factors associated with community structure and implications for managing Huanglongbing (Citrus greening) disease. Appl. Environ. Microbiol. 2017, 83, e00210-17. [Google Scholar] [CrossRef]
- Khosravi, H.; Dolatabad, H.K. Identification and molecular characterization of Azotobacter chroococcum and Azotobacter salinestris using ARDRA, REP, ERIC, and BOX. Mol. Biol. Rep. 2019, 47, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Tkacz, A.; Pini, F.; Turner, T.R.; Bestion, E.; Simmonds, J.; Howell, P.; Greenland, A.; Cheema, J.; Emms, D.M.; Uauy, C.; et al. Agricultural selection of wheat has been shaped by plant-microbe interactions. Front. Microbiol. 2020, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.R.; Ramakrishnan, K.; Walshaw, J.; Heavens, D.; Alston, M.; Swarbreck, D.; Osbourn, A.; Grant, A.; Poole, P.S. Comparative metatranscriptomics reveals kingdom level changes in the rhizosphere microbiome of plants. ISME J. 2013, 7, 2248–2258. [Google Scholar] [CrossRef]
- Brisson, V.L.; Schmidt, J.E.; Northen, T.R.; Vogel, J.P.; Gaudin, A.C.M. Impacts of maize domestication and breeding on rhizosphere microbial community recruitment from a nutrient depleted agricultural soil. Sci. Rep. 2019, 9, 15611–15614. [Google Scholar] [CrossRef]
- Da Silva, D.A.F.; Cotta, S.R.; Vollú, R.E.; Jurelevicius, D.; Marques, J.M.; Marriel, I.E.; Seldin, L. Endophytic microbial community in two transgenic maize genotypes and in their near-isogenic non-transgenic maize genotype. BMC Microbiol. 2014, 14, 332. [Google Scholar] [CrossRef] [PubMed]
- Schlaeppi, K.; Dombrowski, N.; Oter, R.G.; Van Themaat, E.V.L.; Schulze-Lefert, P. Quantitative divergence of the bacterial root microbiota in Arabidopsis thaliana relatives. Proc. Natl. Acad. Sci. USA 2013, 111, 585–592. [Google Scholar] [CrossRef]
- Redford, A.J.; Bowers, R.M.; Knight, R.; Linhart, Y.; Fierer, N. The ecology of the phyllosphere: Geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ. Microbiol. 2010, 12, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Maignien, L.; Deforce, E.A.; Chafee, M.E.; Eren, A.M.; Simmons, S.L. Ecological succession and stochastic variation in the assembly of Arabidopsis thaliana phyllosphere communities. MBio 2014, 5, e00682-13. [Google Scholar] [CrossRef]
- Lebeis, S.L. The potential for give and take in plant–microbiome relationships. Front. Plant Sci. 2014, 5, 287. [Google Scholar] [CrossRef]
- Aswathy, S.K.; Sridar, R.; Sivakumar, U. Mitigation of drought in rice by a phyllosphere bacterium Bacillus altitudinis FD48. Afr. J. Microbiol. Res. 2017, 11, 1614–1625. [Google Scholar] [CrossRef]
- He, Z.; Piceno, Y.; Deng, Y.; Xu, M.; Lu, Z.; DeSantis, T.; Andersen, G.; Hobbie, S.E.; Reich, P.B.; Zhou, J. The phylogenic composition and structure of soil microbial communities shifts in response to elevated carbon dioxide. Int. Soc. Microb. Ecol. 2012, 6, 259–272. [Google Scholar]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Marasco, R.; Mosqueira, M.J.; Fusi, M.; Ramond, J.-B.; Merlino, G.; Booth, J.M.; Maggs-Kölling, G.; Cowan, D.A.; Daffonchio, D. Rhizosheath microbial community assembly of sympatric desert speargrasses is independent of the plant host. Microbiome 2018, 6, 215. [Google Scholar] [CrossRef] [PubMed]
- Yurgel, S.; Douglas, G.M.; DuSault, A.; Percival, D.; Langille, M.G.I. Dissecting community structure in wild blueberry root and soil microbiome. Front. Microbiol. 2018, 9, 1187. [Google Scholar] [CrossRef] [PubMed]
- Shakya, M.; Gottel, N.; Castro, H.F.; Yang, Z.K.; Gunter, L.; Labbé, J.; Muchero, W.; Bonito, G.; Vilgalys, R.; Tuskan, G.A.; et al. A multifactor analysis of fungal and bacterial community structure in the root microbiome of mature Populus deltoides trees. PLoS ONE 2013, 8, e76382. [Google Scholar] [CrossRef]
- Schreiter, S.; Ding, G.-C.; Heuer, H.; Neumann, G.; Sandmann, M.; Grosch, R.; Kropf, S.; Smalla, K. Effect of the soil type on the microbiome in the rhizosphere of field-grown lettuce. Front. Microbiol. 2014, 5, 144. [Google Scholar] [CrossRef]
- Dohrmann, A.B.; Küting, M.; Jünemann, S.; Jaenicke, S.; Schlüter, A.; Tebbe, C.C. Importance of rare taxa for bacterial diversity in the rhizosphere of Bt- and conventional maize varieties. ISME J. 2012, 7, 37–49. [Google Scholar] [CrossRef]
- Williams, T.R.; Marco, M.L. Phyllosphere microbiota composition and microbial community transplantation on lettuce plants grown indoors. MBio 2014, 5, 01564. [Google Scholar] [CrossRef]
- Dees, M.W.; Lysøe, E.; Nordskog, B.; Brurberg, M.B. Bacterial communities associated with surfaces of leafy greens: Shift in composition and decrease in richness over time. Appl. Environ. Microbiol. 2015, 81, 1530–1539. [Google Scholar] [CrossRef]
- Lopez-Velasco, G.; Welbaum, G.; Boyer, R.; Mane, S.; Ponder, M. Changes in spinach phylloepiphytic bacteria communities following minimal processing and refrigerated storage described using pyrosequencing of 16S rRNA amplicons. J. Appl. Microbiol. 2011, 110, 1203–1214. [Google Scholar] [CrossRef]
- Agler, M.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.-T.; Weigel, D.; Kemen, E. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef] [PubMed]
- Yergeau, E.; Bell, T.H.; Champagne, J.; Maynard, C.; Tardif, S.; Tremblay, J.; Greer, C.W. Transplanting soil microbiomes leads to lasting effects on willow growth, but not on the rhizosphere microbiome. Front. Microbiol. 2015, 6, 921. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martínez, M.; López-García, A.; Domínguez, M.T.; Navarro-Fernández, C.M.; Kjøller, R.; Tibbett, M.; Marañón, T. Ectomycorrhizal fungal communities and their functional traits mediate plant–soil interactions in trace element contaminated soils. Front. Plant Sci. 2018, 9, 1682. [Google Scholar] [CrossRef] [PubMed]
- Syranidou, E.; Thijs, S.; Avramidou, M.; Weyens, N.; Venieri, D.; Pintelon, I.; Vangronsveld, J.; Kalogerakis, N. Responses of the endophytic bacterial communities of Juncus acutus to pollution with metals, emerging organic pollutants and to bioaugmentation with indigenous strains. Front. Plant Sci. 2018, 9, 1526. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, K.; Basheer, C.; Bajic, V.B.; Swarup, S. Enhancement of plant-microbe interactions using a rhizosphere metabolomics-driven approach and its application in the removal of polychlorinated biphenyls1. Plant Physiol. 2003, 132, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Desbrosses, G.J.; Stougaard, J. Root nodulation: A paradigm for how plant-microbe symbiosis influences host developmental pathways. Cell Host Microbe 2011, 10, 348–358. [Google Scholar] [CrossRef]
- Bhatt, P.; Joshi, D.; Kumar, N.; Kumar, N. Recent Trends to Study the Functional Analysis of Mycorrhizosphere. In Mycorrhizosphere and Pedogenesis; Varma, A., Choudhary, D., Eds.; Springer: Singapore, 2019; pp. 181–190. [Google Scholar]
- Oldroyd, G.E.D. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Gough, C.; Cullimore, J. Lipo-chitooligosaccharide signaling in endosymbiotic plant-microbe interactions. Mol. Plant-Microbe Interact. 2011, 24, 867–878. [Google Scholar] [CrossRef]
- Frantzeskasis, L.; Pietro, A.D.; Rep, M.; Schirawski, J.; Wu, C.H.; Panstruga, R. Rapid evolution in plant–microbe interactions—A molecular genomics perspective. New Phytol. 2020, 225, 1134–1142. [Google Scholar] [CrossRef]
- Antolín-Llovera, M.; Ried, M.K.; Binder, A.; Parniske, M. Receptor kinase signaling pathways in plant-microbe interactions. Annu. Rev. Phytopathol. 2012, 50, 451–473. [Google Scholar] [CrossRef]
- Zamioudis, C.; Mastranesti, P.; Dhonukshe, P.; Blilou, I.; Pieterse, C.M. Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant Physiol. 2013, 162, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Boriss, R. Phytostimulation and Biocontrol by the Plant-Associated Bacillus Amyloliquefaciens FZB42: An Update. In Phyto-Microbiome in Stress Regulation. Environmental and Microbial Biotechnology; Kumar, M., Kumar, V., Prasad, R., Eds.; Springer: Singapore, 2020. [Google Scholar]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2013, 8, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, E.; Rey, T.; Schornack, S. Cross-interference of plant development and plant–microbe interactions. Curr. Opin. Plant Biol. 2014, 20, 118–126. [Google Scholar] [CrossRef]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Nod factor [Nod Bj V (C18:1, MeFuc)] and lumichrome enhance photosynthesis and growth of corn and soybean. J. Plant Physiol. 2008, 165, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Khan, W.; Smith, D.L. Changes in soybean global gene expression after application of lipo-chitooligosaccharide from Bradyrhizobium japonicum under sub-optimal temperature. PLoS ONE 2012, 7, e31571. [Google Scholar] [CrossRef]
- Oláh, B.; Brière, C.; Bécard, G.; Dénarié, J.; Gough, C. Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway. Plant J. 2005, 44, 195–207. [Google Scholar] [CrossRef]
- Chen, M.; Wei, H.; Cao, J.; Liu, R.; Wang, Y.; Zheng, C. Expression of Bacillus subtilis proBA genes and reduction of feedback inhibition of proline synthesis increases proline production and confers osmotolerance in transgenic Arabidopsis. J. Biochem. Mol. Biol. 2007, 40, 396–403. [Google Scholar] [CrossRef]
- Dyachok, J.; Wiweger, M.; Kenne, L.; von Arnold, S. Endogenous nod factor-like signal molecules promote early somatic embryo development in Norway spruce. Plant Physiol. 2002, 128, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Rodriguez-Moreno, L.; Mansurkhodzaev, A.; Wang, P.; Berg, W.V.D.; Gasciolli, V.; Cottaz, S.; Fort, S.; Thomma, B.P.H.J.; Bono, J.; et al. A lysin motif effector subverts chitin-triggered immunity to facilitate arbuscular mycorrhizal symbiosis. New Phytol. 2019, 225, 448–460. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Murray, J.D.; Poole, P.S.; Downie, J.A. The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef] [PubMed]
- De-La-Peña, C.; Loyola-Vargas, V.M. Biotic interactions in the rhizosphere: A diverse cooperative enterprise for plant productivity. Plant Physiol. 2014, 166, 701–719. [Google Scholar] [CrossRef] [PubMed]
- Engelmoer, D.J.P.; Behm, J.E.; Kiers, E.T. Intense competition between arbuscular mycorrhizal mutualists in anin vitroroot microbiome negatively affects total fungal abundance. Mol. Ecol. 2013, 23, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.J.S.; Micheli, F. RRGPredictor, a set-theory-based tool for predicting pathogen-associated molecular pattern receptors (PRRs) and resistance (R) proteins from plants. Genomics 2020, 112, 2666–2676. [Google Scholar] [CrossRef]
- Hartmann, A.; Rothballer, M.; Hense, B.A.; Schröder, P. Bacterial quorum sensing compounds are important modulators of microbe-plant interactions. Front. Plant Sci. 2014, 5, 131. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, V.; Kitto, S.L.; Caplan, J.L.; Hsueh, Y.H.; Kearns, D.B.; Wu, Y.S.; Bais, H. Microbe-associated molecular patterns-triggered root responses mediate beneficial rhizobacterial recruitment in Arabidopsis. Plant Physiol. 2012, 160, 1642–1661. [Google Scholar] [CrossRef]
- Barea, J.M. Future challenges and perspectives for applying microbial biotechnology in sustainable agriculture based on a better understanding of plant-microbe interactions. J. Soil Sci. Plant Nutr. 2015, 15, 261–282. [Google Scholar]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Ortiz-Castro, R.; Martínez-Trujillo, M.; López-Bucio, J. N-acyl-L-homoserine lactones: A class of bacterial quorum-sensing signals alter post-embryonic root development in Arabidopsis thaliana. Plant Cell Environ. 2008, 31, 1497–1509. [Google Scholar] [CrossRef]
- Rudrappa, T.; Czymmek, K.J.; Paré, P.W.; Bais, H. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 2008, 148, 1547–1556. [Google Scholar] [CrossRef]
- Tena, G.; Boudsocq, M.; Sheen, J. Protein kinase signaling networks in plant innate immunity. Curr. Opin. Plant Biol. 2011, 14, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Mosquito, S.; Meng, X.; Devescovi, G.; Bertani, I.; Geller, A.M.; Levy, A.; Myers, M.P.; Bez, C.; Covaceuszach, S.; Venturi, V. LuxR solos in the plant endophyte Kosakonia sp. strain KO348. Appl. Environ. Microbiol. 2020, 86, e00622-20. [Google Scholar] [CrossRef] [PubMed]
- Tarkka, M.; Schrey, S.; Hampp, R. Plant associated soil microorganisms. In Molecular Mechanisms of Plant and Microbe Coexistence; Nautiyal, C., Dion, P., Eds.; Springer: Heidelberg, Germany, 2008; pp. 3–51. [Google Scholar]
- Bais, H.P.; Park, S.W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How plants communicate using the underground information superhighway. Trends Plant Sci. 2004, 9, 26–32. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Bakker, M.G.; Sugiyama, A.; Manter, D.K.; Vivanco, J.M. Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS ONE 2013, 8, e55731. [Google Scholar] [CrossRef]
- Bengough, A.G.; Mullins, C.E. Mechanical impedance to root growth: A review of experimental techniques and root growth responses. J. Soil Sci. 1990, 41, 341–358. [Google Scholar] [CrossRef]
- Fitter, A.H.; Lynch, J.M. The Rhizosphere. J. Appl. Ecol. 1991, 28, 748. [Google Scholar] [CrossRef]
- Hiltner, L. Uber neure erfahrungen und probleme auf dem Gebiet der Boden-bakteriologie und unter besondere Berucksichtigung der grundungung und Bracke. Arb. Dtsch. Landwirtsch. Ges. 1904, 98, 59–78. [Google Scholar]
- Knudson, L. The secretion of invertase by plant roots. Am. J. Bot. 1920, 7, 371–379. [Google Scholar] [CrossRef]
- Lyon, T.L.; Wilson, J.K. Liberation of Organic Matter by Roots of Growing Plants; New York Agricultural Experimental Station Bulletin: New York, NY, USA, 1921. [Google Scholar]
- Martin, M.H.; Marschner, H. The Mineral Nutrition of Higher Plants. J. Ecol. 1988, 76, 1250. [Google Scholar] [CrossRef]
- Whipps, J.M. Carbon economy. In The Rhizosphere; Lynch, J.M., Ed.; JohnWiley & Sons: Essex, UK, 1990. [Google Scholar]
- Uren, N. Types, Amounts, and Possible Functions of Compounds Released into the Rhizosphere by Soil-Grown Plants. In The Rhizosphere; CRC Press: Boca Raton, FL, USA, 2000; pp. 35–56. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Bais, H.P.; Broeckling, C.D.; Vivanco, J.M. Root Exudates Modulate Plant—Microbe Interactions in the Rhizosphere. In Secondary Metabolites in Soil Ecology. Soil Biology; Karlovsky, P., Ed.; Springer: Heidelberg, Germany, 2008; Volume 14, pp. 241–252. [Google Scholar]
- Bhatt, K.; Maheshwari, D.K. Decoding multifarious role of cow dung bacteria in mobilization of zinc fractions along with growth promotion of C. annuum L. Sci. Rep. 2019, 9, 14232. [Google Scholar] [CrossRef] [PubMed]
- Prithiviraj, B.; Paschke, M.W.; Vivanco, J.M.; Goodman, R.M. Root Communication: The Role of Root Exudates. In Encyclopedia of Plant and Crop Science; Routledge: New York, NY, USA, 2004; pp. 1–5. [Google Scholar]
- Segonzac, C.; Zipfel, C. Activation of plant pattern-recognition receptors by bacteria. Curr. Opin. Microbiol. 2011, 14, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Nailwal, P.; Tapan, K.; Negi, L.; Panwar, A. Isolation and characterization of rhizobial isolates from the rhizospheric soil of an endangered plant Meizotropis pellita. Asian Microbiol. Biotech. Envion. Sci. 2014, 16, 301–306. [Google Scholar]
- Berlec, A. Novel techniques and findings in the study of plant microbiota: Search for plant probiotics. Plant Sci. 2012, 193, 96–102. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Sheng, M.; Tang, M. Effects of Rhizophagus irregularis on Photosynthesis and Antioxidative Enzymatic System in Robinia pseudoacacia L. under Drought Stress. Front. Plant Sci. 2017, 8, 576. [Google Scholar] [CrossRef]
- Elmore, J.M.; Liu, J.; Smith, B.; Phinney, B.; Coaker, G. Quantitative proteomics reveals dynamic changes in the plasma membrane during arabidopsis immune signaling. Mol. Cell. Proteom. 2012, 11, 1796–1813. [Google Scholar] [CrossRef]
- Nguyen, T.H.N.; Brechenmacher, L.; Aldrich, J.T.; Clauss, T.R.; Gritsenko, M.A.; Hixton, K.K.; Libault, M.; Tanaka, K.; Yang, F.; Yao, Q.; et al. Quantitative phosphoproeomic analysis of soybean root hairs inoculated with Bradyrhizobium japonicum. Mol. Cell. Proteom. 2012, 11, 1140–1155. [Google Scholar] [CrossRef]
- Rose, C.M.; Venkateshwaran, M.; Volkening, J.D.; Grimsrud, P.A.; Maeda, J.; Bailey, D.J.; Park, K.; Howes-Podoll, M.; Os, D.; Yeun, L.H.; et al. Rapid phosphoproteomic and transcriptomic changes in the rhizobia-legume symbiosis. Mol. Cell. Proteom. 2012, 11, 724–744. [Google Scholar] [CrossRef]
- Watrous, J.; Roach, P.; Alexandrov, T.; Heath, B.S.; Yang, J.Y.; Kersten, R.D.; Van Der Voort, M.; Pogliano, K.; Gross, H.; Raaijmakers, J.M.; et al. Mass spectral molecular networking of living microbial colonies. Proc. Natl. Acad. Sci. USA 2012, 109, E1743–E1752. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Z.; Wang, W.J.; Liu, G.F.; Shtykova, E.V.; Xu, J.H.; Li, L.F.; Su, X.D.; Dong, Y. The crystal structure of the MPN domain from the COP9 signalosome subunit CSN6. FEBS Lett. 2012, 586, 1147–1153. [Google Scholar] [CrossRef]
- Rahi, P. Phytomicrobiome: A reservoir for sustainable agriculture. In Mining of Microbial Wealth and Metagenomics; Kalia, V., Shouche, Y., Purohit, H., Rahi, P., Eds.; Springer: Singapore, 2017. [Google Scholar]
- Kudjordjie, E.N.; Sapkota, R.; Steffensen, S.K.; Fomsgaard, I.S.; Nicolaisen, M. Maize synthesized benzoxazinoids affect the host associated microbiome. Microbiome 2019, 7, 59. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Shen, C.; Li, H.; Qiu, L. 1-Aminocyclopropane-1-Carboxylate: A novel and strong chemoattractant for the plant beneficial rhizobacterium Pseudomonas putida UW4. Mol. Plant-Microbe Interact. 2019, 32, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Jochum, M.D.; McWilliams, K.L.; Pierson, E.A.; Jo, Y.K. Host-mediated microbiome engineering (HMME) of drought tolerance in the wheat rhizosphere. PLoS ONE 2019, 14, e0225933. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.N.; Van Bui, L.; Hoang, M.T.T. Pseudomonas PS01 isolated from maize rhizosphere alters root system architecture and promotes plant growth. Microorganisms 2020, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Guichard, M.; Allain, J.M.; Bianchi, M.W.; Frachisse, J.M. Root Hair Sizer: An algorithm for high throughput recovery of different root hair and root developmental parameters. Plant Methods 2019, 15, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, Y.; Wang, Y.; Guo, Y.; Long, H.; Deng, G.; Chen, Q.; Xuan, P. Molecular markers and cytogenetics to characterize a wheat-Dasypyrum villosum 3V (3D) substitution line conferring resistance to stripe rust. PLoS ONE 2018, 13, e0202033. [Google Scholar] [CrossRef]
- Fasoula, V.A. Prognostic breeding: A new paradigm for crop improvement. Plant Breed. Rev. 2013, 37, 297–347. [Google Scholar]
- López-Bucio, J.; De La Vega, O.M.; Guevara-García, A.; Herrera-Estrella, L. Enhanced phosphorus uptake in transgenic tobacco plants that overproduce citrate. Nat. Biotechnol. 2000, 18, 450–453. [Google Scholar] [CrossRef]
- Tesfaye, M.; Temple, S.J.; Allan, D.L.; Vance, C.P.; Samac, D.A. Overexpression of malate dehydrogenase in transgenic alfalfa enhances organic acid synthesis and confers tolerance to aluminum. Plant Physiol. 2001, 127, 1836–1844. [Google Scholar] [CrossRef]
- Yang, H.; Knapp, J.; Koirala, P.; Rajagopal, D.; Peer, W.A.; Silbart, L.K.; Murphy, A.; Gaxiola, R.A. Enhanced phosphorus nutrition in monocots and dicots over-expressing a phosphorus-responsive type IH+-pyrophosphatase. Plant Biotech. J. 2007, 5, 735–745. [Google Scholar] [CrossRef]
- Deng, W.; Luo, K.; Li, Z.; Yang, Y.; Hu, N.; Wu, Y. Overexpression of Citrus junos mitochondrial citrate synthase gene in Nicotiana benthamiana confers aluminum tolerance. Planta 2009, 230, 355–365. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L.; Fernández, I.G.; Colucci, G.; Harman, G.; Pintor-Toro, J.; Filippone, E.; Muccifora, S.; Lawrence, C.B.; Zoina, A.; et al. Genes from mycoparasitic fungi as a source for improving plant resistance to fungal pathogens. Proc. Natl. Acad. Sci. USA 1998, 95, 7860–7865. [Google Scholar] [CrossRef] [PubMed]
- Volpi, C.; Janni, M.; Lionetti, V.; Bellincampi, D.; Favaron, F.; D’Ovidio, R. The ectopic expression of a pectin methyl esterase inhibitor increases pectin methyl esterification and limits fungal diseases in wheat. Mol. Plant-Microbe Interact. 2011, 24, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Pogorelko, G.; Lionetti, V.; Fursova, O.; Sundaram, R.M.; Qi, M.; Whitham, S.A.; Bogdanove, A.J.; Bellincampi, D.; Zabotina, O.A. Arabidopsis and brachypodium dis-tachyon transgenic plants expressing Aspergillus nidulans acetylesterases have decreased degree of polysaccharide acetylation and increased resistance to pathogens. Plant Physiol. 2013, 162, 9–23. [Google Scholar] [CrossRef]
- Azad, A.K.; Amin, L.; Sidik, N.M. Gene technology for papaya ringspot virus disease management. Sci. World J. 2014, 2014, 768038. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Knill, E.; Glick, B.R.; Défago, G. Effect of transferring 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase genes into Pseudomonas fluorescens strain CHA0 and its gacA derivative CHA96 on their growth-promoting and disease-suppressive capacities. Can. J. Microbiol. 2000, 46, 898–907. [Google Scholar] [CrossRef]
- Sundheim, L.; Poplawsky, A.R.; Ellingboe, A.H. Molecular cloning of two chitinase genes from Serratia marcescens and their expression in Pseudomonas species. Physiol. Mol. Plant Pathol. 1988, 33, 483–491. [Google Scholar] [CrossRef]
- Alsanius, B.W.; Hultberg, M.; Englund, J.E. Effect of lacZY marking of the 2, 4-diaceyl phloroglucinol producing Pseudomonas fluorescens strain 5–2/4 on its physiological performance and root colonization ability. Microbial. Res. 2002, 157, 39–45. [Google Scholar] [CrossRef]
- Dong, Y.H.; Xu, J.L.; Li, X.Z.; Zhang, L.H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 2000, 97, 3526–3531. [Google Scholar] [CrossRef]
- Savka, M.A.; Dessaux, Y.; Oger, P.M.; Rossbach, S. Engineering bacterial competitiveness and persistence in the phytosphere. Mol. Plant-Microbe Interact. 2002, 15, 866–874. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, Y.S.; Baek, K.H.; Jung, H.; Ha, S.H.; Choi, Y.D.; Kim, M.; Reuzeau, C.; Kim, J.K. Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol. 2010, 153, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Pitino, M.; Duan, Y.; Stover, E. Reduced susceptibility to Xanthomonas citriin transgenic citrus expressing the FLS2 receptor from Nicotiana benthamiana. Mol. Plant-Microbe Interact. 2016, 29, 132–142. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; van der Sluis, L.; Bakker, P.A.H.M.; Schippers, B.; Koster, M.; Weisbeek, P.J. Utilization of heterologous siderophores and rhizosphere competence of fluorescent Pseudomonas spp. Can. J. Microbiol. 1995, 41, 126–135. [Google Scholar] [CrossRef]
- Delgadillo, J.; Lafuente, A.; Doukkali, B.; Redondo-Gómez, S.; Mateos-Naranjo, E.; Caviedes, M.A.; Pajuelo, E.; Rodríguez-Llorente, I.D. Improving legume nodulation and Cu rhizostabilization using a genetically modified rhizobia. Environ. Technol. 2014, 36, 1237–1245. [Google Scholar] [CrossRef]
- Barac, T.; Taghavi, S.; Borremans, B.; Provoost, A.; Oeyen, L.; Colpaert, J.V.; Vangronsveld, J.; van der Lelie, N. Engineered endophytic bacteria improve phytoremediation of water-soluble, volatile, organic pollutants. Nat. Biotechnol. 2004, 22, 583–588. [Google Scholar] [CrossRef]
- Chen, J.M.C.; Yang, Y.B.Y.; Schultz, B.; McIver, A. Foliar application of lipo-chitooligosaccharides (Nod factors) to tomato (Lycopersicon esculentum) enhances flowering and fruit production. Can. J. Plant Sci. 2007, 87, 365–372. [Google Scholar]
- Nautiyal, C.S.; Srivastava, S.; Chauhan, P.S.; Seem, K.; Mishra, A.; Sopory, S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013, 66, 1–9. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.; Seo, S.G.; Lee, C.; Woo, S.Y.; Kim, S.H. Gene expression profile affected by volatiles of new plant growth promoting rhizobacteria, Bacillus subtilis strain JS, in tobacco. Genes Genom. 2015, 37, 387–397. [Google Scholar] [CrossRef]
- Oliver, R.P.; Henricot, B.; Segers, G. Cladosporium fulvum, Cause of Leaf Mould of Tomato. In Fungal Pathology; Kronstad, J.W., Ed.; Springer: Dordrecht, The Netherlands, 2000; pp. 65–91. [Google Scholar]
- Yuttavanichakul, W.; Lawongsa, P.; Wongkaew, S.; Teaumroong, N.; Boonkerd, N.; Nomura, N.; Tittabutr, P. Improvement of peanut rhizobial inoculant by incorporation of plant growth promoting rhizobacteria (PGPR) as biocontrol against the seed borne fungus, Aspergillus niger. Biol. Control. 2012, 63, 87–97. [Google Scholar] [CrossRef]
- Bhatt, P.; Pal, K.; Bhandari, G.; Barh, A. Modelling of the methyl halide biodegradation in bacteria and its effect on environmental systems. Pestic. Biochem. Physiol. 2019, 158, 88–100. [Google Scholar] [CrossRef]
- Bhatt, P.; Gangola, S.; Chaudhary, P.; Khati, P.; Kumar, G.; Sharma, A.; Srivastava, A. Pesticide induced up-regulation of esterase and aldehyde dehydrogenase in indigenous Bacillus spp. Bioremediation J. 2019, 23, 42–52. [Google Scholar] [CrossRef]
- Bhatt, P.; Huang, Y.; Zhan, H.; Chen, S. Insight into microbial applications for the biodegradation of pyrethroid insecticides. Front. Microbiol. 2019, 10, 1778. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Bhatt, K.; Huang, Y.; Lin, Z.; Chen, S. Esterase is a powerful tool for the biodegradation of pyrethroid insecticides. Chemosphere 2020, 244, 125507. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Huang, Y.; Zhang, W.; Sharma, A.; Chen, S. Enhanced cypermethrin degradation kinetics and metabolic pathway in Bacillus thuringiensis strain SG4. Microorganisms 2020, 8, 223. [Google Scholar] [CrossRef]
- Bhatt, P.; Huang, Y.; Rene, E.R.; Kumar, A.J.; Chen, S. Mechanism of allethrin biodegradation by a newly isolated Sphingomonas trueperi strain CW3 from wastewater sludge. Bioresour. Technol. 2020, 305, 123074. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhang, W.; Pang, S.; Huang, Y.; Mishra, S.; Bhatt, P.; Chen, S. Current approaches to and future perspectives on methomyl degradation in contaminated soil/water environments. Molecules 2020, 25, 738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lin, Z.; Pang, S.; Bhatt, P.; Chen, S. Insights into the biodegradation of lindane (γ-hexachlorocyclohexane) using a microbial system. Front. Microbiol. 2020, 11, 522. [Google Scholar] [CrossRef]
- Mishra, S.; Zhang, W.; Lin, Z.; Pang, S.; Huang, Y.; Bhatt, P.; Chen, S. Carbofuran toxicity and its microbial degradation in contaminated environments. Chemosphere 2020, 259, 127419. [Google Scholar] [CrossRef]
- Zhan, H.; Huang, Y.; Lin, Z.; Bhatt, P.; Chen, S. New insights into the microbial degradation and catalytic mechanism of synthetic pyrethroids. Environ. Res. 2020, 182, 109138. [Google Scholar] [CrossRef]
- Negi, G.; Pankaj, S.A.; Sharma, A. In situ biodegradation of endosulfan, imidacloprid, and carbendazim using indigenous bacterial cultures of agriculture fields of Uttarakhand, India. Int. J. Biol. Food Vat. Agric. Eng. 2014, 8, 953–961. [Google Scholar]
- Bhatt, K.; Maheshwari, D.K. Zinc solubilizing bacteria (Bacillus megaterium) with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. 3 Biotech 2020, 10, 36. [Google Scholar] [CrossRef]
- Singh, H.; Verma, A.; Kumar, M.; Sharma, R.; Gupta, R.; Kaur, M. Phytoremediation: A green technology to clean up the sites with low and moderate level of heavy metals. Austin Biochem. 2017, 2, 1012. [Google Scholar]
- Jalil, S.U.; Ansari, M.I. Plant microbiome and its functional mechanism in response to environmental stress. Int. J. Green Pharm. 2018, 12, 81–92. [Google Scholar]
- Martínez, M.T.; San-José, M.D.C.; Arrillaga, I.; Cano, V.; Morcillo, M.; Cernadas, M.J.; Corredoira, E. Holm oak somatic embryogenesis: Current status and future perspectives. Front. Plant Sci. 2019, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lin, Z.; Zhang, W.; Pang, S.; Bhatt, P.; Rene, E.R.; Kumar, A.J.; Chen, S. New insights into the microbial degradation of D-cyphenothrin in contaminated water/soil environments. Microorganims 2020, 8, 473. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, Y.; Zhan, H.; Bhatt, P.; Chen, S. An overview of strobilurin fungicide degradation: Current status and future perspective. Front. Microbiol. 2020, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Feng, Y.; Fan, X.; Chen, S. Recent advances in glyphosate biodegradation. Appl. Microbiol. Biotechnol. 2018, 10, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chang, C.; Deng, Y.; An, S.; Dong, Y.H.; Zhou, J.; Hu, M.; Zhong, G.; Zhang, L.H. Fenpropathrin biodegradation pathway in Bacillus sp. DG-02 and its potential for bioremediation of pyrethroid-contaminated soils. J. Agric. Food Chem. 2014, 62, 2147–2157. [Google Scholar] [CrossRef]
- Pang, S.; Lin, Z.; Zhang, W.; Mishra, S.; Bhatt, P.; Chen, S. Insights into the microbial degradation and biochemical mechanisms of neonicotinoids. Front. Microbiol. 2020, 11, 868. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, W.; Pang, S.; Lin, Z.; Zhang, Y.; Huang, Y.; Bhatt, P.; Chen, S. Kinetics and new mechanism of azoxystrobin biodegradation by an Ochrobactrum anthropi strain SH14. Microorganisms 2020, 8, 625. [Google Scholar] [CrossRef]
- Huang, Y.; Zhan, H.; Bhatt, P.; Chen, S. Paraquat degradation from contaminated environments: Current achievements and perspectives. Front. Microbiol. 2019, 10, 1754. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Deng, Y.; Chang, C.; Lee, J.; Cheng, Y.; Cui, Z.; Zhou, J.; He, F.; Hu, M.; Zhang, L.H. Pathway and kinetics of cyhalothrin biodegradation by Bacillus thuringiensis strain ZS-19. Sci. Rep. 2015, 5, 8784. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Zhang, W.; Lin, Z.; Pang, S.; Huang, Y.; Chen, S. Biodegradation of allethrin by a novel fungus Fusarium proliferatum strain CF2, isolated from contaminated soils. Microorganisms 2020, 8, 593. [Google Scholar] [CrossRef] [PubMed]

| Plant System | Microorganism | Interaction Signals | Techniques Used | References |
|---|---|---|---|---|
| Tomato | Pseudomonas syringae pv. tomato | Benzothiadiazole | RT-PCR | [5] |
| Mung bean (Vigna radiata) | Agrobacterium Tumefaciens | N-Acyl-homoserine-lactones (AHLs) | RT-PCR Fluorescence Microscopy | [6] |
| Tomato | Pseudomonas aeruginosa | Pyochelin, its precursor salicylic acid & pyocyanin | Thin Layer Chromatography (TLC) | [7] |
| Rice | Pseudomonas aeruginosa | 1-Hydroxy-phenazine, pyocyanin, lahorenoic acid, pyochellin, rhamnolipids | Mass spectrometric analysis | [8] |
| Bean | Pseudomonas aeruginosa | Pyoverdin, pyochelin, and salicylic acid | TLC and colorimetry | [9] |
| Tomato | Pseudomonas aeruginosa | Phenazine | TLC and HPLC analysis | [10] |
| Rice | Pseudomonas sp. CMR12 | Orfamides and sessilins (cyclic lipopeptides); phenazine | UPLC-MS | [11] |
| Bean | Pseudomonas sp. CMR12 | Orfamides and sessilins (cyclic lipopeptides); phenazine | UPLC-MS | [11] |
| Potato | Pseudomonas sp. LBUM223 | Phenazine | qPCR and RT-qPCR analysis | [12] |
| Arabidopsis thaliana | Pseudomonas fluorescens | Polyketide antibiotic 2,4-diacetylphloroglucinol | Different assays | [13] |
| Sunflower | Glomus sp. | Benzothiadiazole | - | [14] |
| Arabidopsis sp. | Laccaria bicolor | Benzothiadiazole | Different assays | [15] |
| Sorghum sp. and Lolium sp. | Glomus intraradices | Phenazine | RT-PCR | [16] |
| Lotus japonicus | Gigaspora margarita | Strigolactone | Spectroscopic analysis | [17] |
| Orobanche weed | AM fungi | sesquiterpene lactones | Spectroscopy | [17] |
| Phytomicrobiome | Plant System | Method of Study | Community Structure | References |
|---|---|---|---|---|
| Rhizomicrobiome | Spear grass | Illumina sequencing and metaphylogenomic analysis | Bacterial (Actinobacteria and Alphaproteobacteria) and fungal (Curvularia, Aspergillus, and Thielavia) communities | [33] |
| Root and soil microbiome | Wild blueberry | Illumina sequencing | Fungal (Glomeromycota, Mucoromycotina, and Chytridiomycota) and protist communities along with bacterial communities (Aprospirales, Actinomycetales, Rhizobiales, and Xanthomonadales) | [34] |
| Root microbiome | Populus deltoides | 454 pyrosequencing | 35 bacterial and 4 fungal taxa in the rhizosphere and 1 bacterial and 1 fungal in the endosphere | [35] |
| Rhizosphere | Lettuce (Lactuca sativa) | Pyrosequencing | Bacterial communities (Sphingomonas, Rhizobium, Pseudomonas, and Variovorax) | [36] |
| Rhizosphere | Maize | Pyrosequencing | Bacterial diversity (Proteobacteria and Actinobacteria) | [37] |
| Phyllosphere | Lettuce plants (Lactuca sativa) | Pyrosequencing | Bacterial communities (Enterobacteriaceae and Moraxellaceae families) | [38] |
| Phyllosphere | Rocket salad (Diplotaxis tenuifolia) and lettuce (Lactuca sativa) | Illumina sequencing | Bacterial colonization of leaves (Proteobacteria, Actinobacteria, Firmicutes, and Bacteroidetes) | [39] |
| Phyllosphere | Spinach | Pyrosequencing | Bacteria communities (Proteobacteria and Firmicutes) | [40] |
| Phyllosphere | Arabidopsis thaliana | Illumina sequencing | Bacteria (Caulobacter sp., Flavobacterium), fungi (Albugo sp., Dioszegia sp., Udeniomyces sp.) and oomycetes symbionts | [41] |
| Rhizosphere | Willows (Salix purpurea “Fish Creek”) | Illumina sequencing | Bacterial (Nitrososphaerales, Methanobacteriales, E2 group, Methanosarcinales, and Methanomicrobiales) and fungal (Sordariomycetes, Dothideomycetes, Chytridiomycetes, and Zygomycota) communities | [42] |
| Endosphere | Holm oak trees (Quercus ilex subsp. ballota) | ITS region | Fungal communities (Hebeloma cavipes and Thelephora terrestris) | [43] |
| Endosphere | Transgenic maize | DGGE analysis | Endophytic communities (bacteria, archaea, and fungi) | [44] |
| Phyllosphere | Bean, soybean, and canola | Illumina sequencing | Bacterial communities (Firmicutes, Bacteroidetes, Thermi, and Chloroflexi) | [18] |
| Phyllosphere | Arabidopsis thaliana | 454 sequencing | Bacterial communities (Acinetobacter, Variovorax, Pseudomonas, unidentified Sphingobacteriaceae, Rhodococcus, Ochrobactrum, and Chryseobacterium) | [28] |
| Different Chemical Components of Plant Root Exudate | Microbial Diversity | |
|---|---|---|
| Carbohydrates | Arabinose, glucose, galactose, fructose, sucrose, pentose, rhamnose, raffinose, ribose, xylose and mannitol | Bacterial species like Actinobacteria, Proteobacteria and Firmicutes; Fungal species like Pythium |
| Amino acids | All 20 proteinogenic amino acids, l-hydroxyproline, homoserine, mugineic acid, aminobutyric acid | Bacterial species like Proteobacteria, Acidobacteria, Actinobacteria; Fungal families like Gigasporaceae, Acaulosporaceae |
| Organic acids | Acetic acid, succinic acid, l-aspartic acid, malic acid, l-glutamic acid, salicylic acid, shikimic acid, isocitric acid, chorismic acid, sinapic acid, caffeic acid, p-hydroxybenzoic acid, gallic acid, tartaric acid, ferulic acid, protocatacheuic acid, p-coumaric acid, mugineic acid, oxalic acid, citric acid, piscidic acid | Bacterial species like Actinobacteria, Gemmatimonadetes, and Chloroflexi and fungal species like Rhizophagus intraradices, Funneliformis mosseae |
| Flavonols | Naringenin, kaempferol, quercitin, myricetin, naringin, rutin, genistein, strigolactone and their substitutes with sugars | Bacterial species like Actinobacteria, Proteobacteria and fungal species like Funneliformis mosseae, and Rhizophagus irregularis |
| Lignins | Catechol, benzoic acid, nicotinic acid, phloroglucinol, cinnamic acid, gallic acid, ferulic acid, syringic acid, sinapoyl aldehyde, chlorogenic acid, coumaric acid, vanillin, sinapyl alcohol, quinic acid, pyroglutamic acid | Bacterial species like Bacillus, Flavisolibacter, Actinobacteria, and fungal species like Rhizoctonia solani, and Scletorina sclerotium. |
| Coumarins | Umbelliferone | Bacterial species like Lysobacter, Phormidium, Proteobacteria and fungal families like Ascomycetes, Scutellosporaceae |
| Aurones | Benzyl aurones synapates, sinapoyl choline | Bacterial species like Acidobacteria, Actinobacteria and fungal families like Basidiomycetes, Acaulosporaceae |
| Glucosinolates | Cyclobrassinone, desuphoguconapin, desulphoprogoitrin, desulphonapoleiferin, desulphoglucoalyssin | Bacterial species like Actinobacteria, Proteobacteria and fungal species like Alternaria solani, Rhizophagus intraradices, Funneliformis mosseae. |
| Anthocyanins | Cyanidin, delphinidin, pelargonidin and their substitutes with sugar molecules | Bacterial species like Acidobacteria, Actinobacteria and fungal species like Fusarium equiseti, Rhizophagus intraradices, Rhizophagus irregularis |
| Indole compounds | Indole-3-acetic acid, brassitin, sinalexin, brassilexin, methyl indole carboxylate, camalexin glucoside | Bacterial species like Kaistobacter, Flavisolibacter, Actinobacteria and fungal species like Rhizoctonia solani |
| Fatty acids | Linoleic acid, oleic acid, palmitic acid, stearic acid | Bacterial species like Actinobacteria, Lysobacter, Balneimonas and fungal species like Funneliformis mosseae, Fusarium equiseti, Alternaria solani. |
| Sterols | Campestrol, sitosterol, stigmasterol | Bacterial species like Flavisolibacter, Balneimonas and fungal species like Rhizoctonia solani, Rhizophagus irregularis |
| Allomones | Jugulone, sorgoleone, 5,7,4′-trihydroxy-3′, 5′-dimethoxyflavone, DIMBOA, DIBOA | Bacterial species like Gemmatimonadetes, Chloroflexi and fungal species like Alternaria solani, Verticillium sp. |
| Proteins and enzymes | PR proteins, lectins, proteases, acid phosphatases, peroxidases, hydrolases, lipase | Bacterial species like Balneimonas, Lysobacter, Actinobacteria and fungal families like Zygomycetes, Gigasporaceae |
| Engineered Plant/Microbes | Gene | Host | Effect | References |
|---|---|---|---|---|
| Tobacco roots | Citrate synthase gene | Pseudomonas aeruginosa | Phosphorous acquisition, Al tolerance | [107] |
| Medicago sativa | Malate dehydrogenase | Medicago sativa | Enhanced the organic anion efflux | [108] |
| Arabidopsis thaliana | Pyrophosphates gene | Arabidopsis thaliana | Enhanced the tolerant capacity of Al | [109] |
| Nicotiana benthamiana | Citrate synthase gene | Yuzu tree | Enhanced the tolerant capacity of Al | [110] |
| Transgenic tobacco, potato | Trichoderma harzianum endochitinase | - | Enhanced the tolerate capacity from fungal pathogen (Alternaria alternate, Botrytis cinerea) | [111] |
| Durum wheat transgenic lines | Pectin methyl esterase inhibitor gene | Golden kiwi tree | Enhanced the tolerate capacity from fungal pathogen (Fusarium graminearum, Bipolaris sorokiniana) | [112] |
| Arabidopsis, purple false brome | Aspergillus nidulans acetyl esterases | - | Enhanced the tolerate capacity from fungal pathogen (Botrytis cinere, Bipolaris sorokiniana) | [113] |
| Papaya | Papaya ring spot coat protein gene | Papaya | Virus resistant plants | [114] |
| Cucumber, Canola | Pseudomonas fluorescens (CHA 0) transformed with ACC deaminase gene acdS from P. putida UW4 | - | Improved root architect and plant protection | [115] |
| Pseudomonas strain | Chi A gene | Serratia macrcescens | Enhanced protection from fungal pathogen | [116] |
| P. fluorescens 5–2/4 | DAPG biosynthesis operon | Pseudomonas fluorescens Q2–87 | Protection from plant pathogen P. ultimum | [117] |
| Potato | Bacterial lactonase gene aii A | Bacillus sp. | Protection from plant pathogen Pectobacterium | [118] |
| Lotus corniulatus | Opines biosynthesis gene | Agrobacterium tumefacience | Phytoremediation | [119] |
| Rice | OsNac10 gene | Rice | Enhanced drought tolerance and increased grain yield | [120] |
| Citrus sweet orange | Pattern recognition receptor FLS 2 | Tobacco (Nicotiana benthamiana) | Increased canker resistant and defence | [121] |
| Radish | Heterologous gene encoding siderophore responsible for iron uptake | Pseudomonas fluorescens | Enhanced the competitiveness in soil | [122] |
| Ensifer medicae strain | copAB genes | Pseudomonas fluorescens | Improved the tolerance of plant in copper contaminated soil and enhanced nodulation | [123] |
| Yellow lupin | pTOM toluene-degradation plasmid | Burkholderia cepacia G4 | Participated in phytoremidiation | [124] |
| Arabidopsis | proBA gene | Bacillus subtilis | Salt tolerant | [125] |
| Rice, maize | SN13, SQR9 | Bacillus amyloliquefaciens | Salt tolerant | [126] |
| Tobacco | VOCs related gene | Bacillus subtilis | Enhancement of plant growth | [127] |
| Cultivar Tomato | Cf-4 (Fungal gene) | Wild tomato | Resistance to the fungal tomato pathogen Cladosporium fulvum | [128] |
| Aspergillus niger | α-Galactosidase | Aspergillus niger | Increased the protein secretion 9 times | [129] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatt, P.; Verma, A.; Verma, S.; Anwar, M.S.; Prasher, P.; Mudila, H.; Chen, S. Understanding Phytomicrobiome: A Potential Reservoir for Better Crop Management. Sustainability 2020, 12, 5446. https://doi.org/10.3390/su12135446
Bhatt P, Verma A, Verma S, Anwar MS, Prasher P, Mudila H, Chen S. Understanding Phytomicrobiome: A Potential Reservoir for Better Crop Management. Sustainability. 2020; 12(13):5446. https://doi.org/10.3390/su12135446
Chicago/Turabian StyleBhatt, Pankaj, Amit Verma, Shulbhi Verma, Md. Shahbaz Anwar, Parteek Prasher, Harish Mudila, and Shaohua Chen. 2020. "Understanding Phytomicrobiome: A Potential Reservoir for Better Crop Management" Sustainability 12, no. 13: 5446. https://doi.org/10.3390/su12135446
APA StyleBhatt, P., Verma, A., Verma, S., Anwar, M. S., Prasher, P., Mudila, H., & Chen, S. (2020). Understanding Phytomicrobiome: A Potential Reservoir for Better Crop Management. Sustainability, 12(13), 5446. https://doi.org/10.3390/su12135446







