A Review of Southeast Asian Oil Palm and Its CO2 Fluxes
Abstract
1. Introduction
2. Oil Palm Characteristics and Its Environment
2.1. The Oil Palm Crop
2.2. Climate
2.3. Soil Classification and Characteristics
3. The Oil Palm and Climate Change Factors
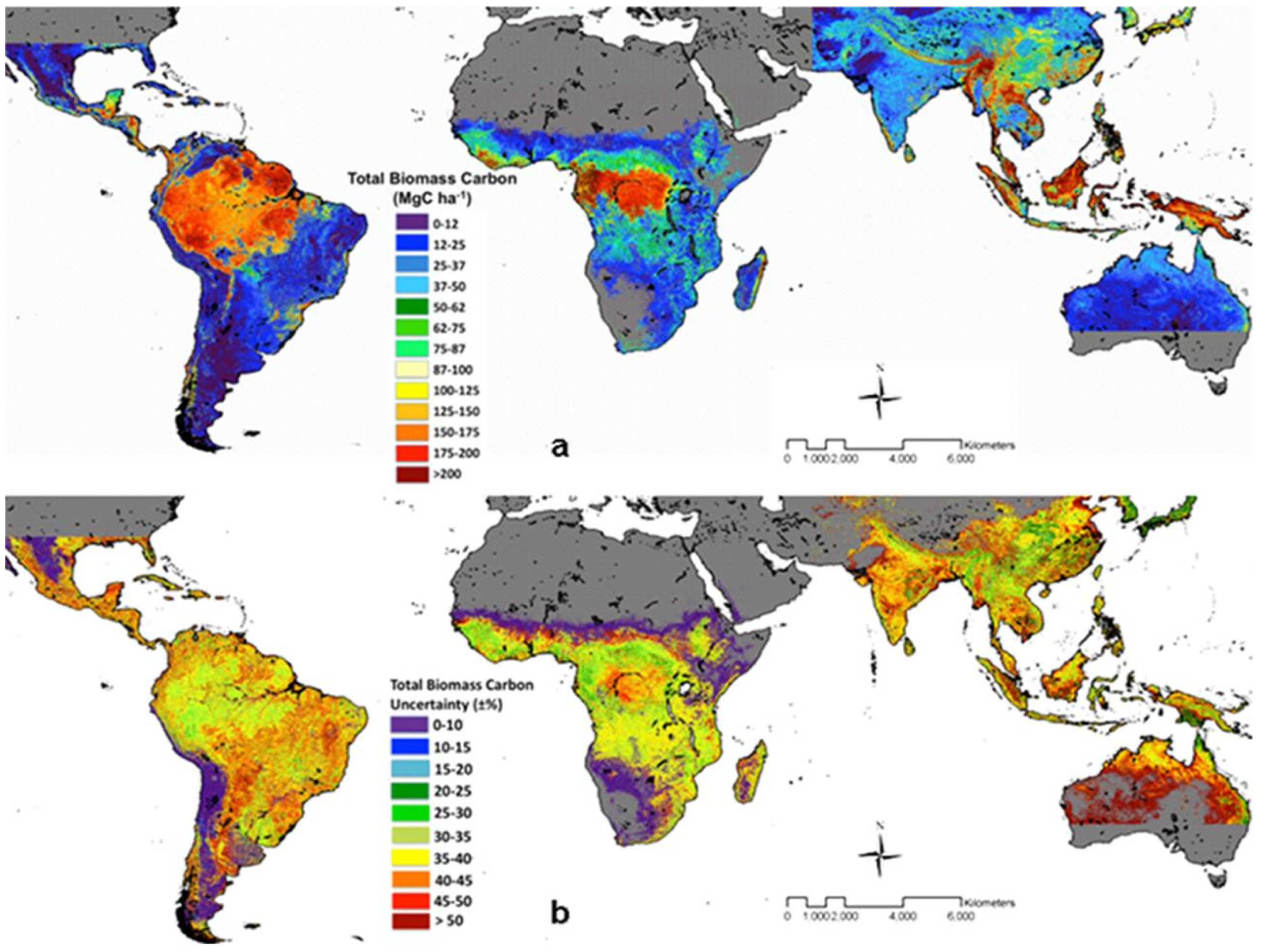
3.1. Deforestation
3.2. Peatland Areas
3.3. Biomass Burning
4. Oil Palm and Gas Emissions
4.1. Emissions of CO2
4.2. Comparison Studies on Oil Palm CO2 with Other Crops
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Ong-Abdullah, M.; Low, E.T.L.; Manaf, M.A.A.; Rosli, R.; Nookiah, R.; Ooi, L.C.L.; Ooi, S.E.; Chan, K.L.; Halim, M.A.; et al. Oil palm genome sequence reveals divergence of interfertile species in Old and New worlds. Nature 2013, 500, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Wahid, M.B.; Abdullah, S.N.A.; Henson, I.E. Oil palm—Achievements and potential. Plant Prod. Sci. 2005, 8, 288–297. [Google Scholar] [CrossRef]
- Ibragimov, A.; Sidique, S.F.; Tey, Y.S. Productivity for sustainable growth in Malaysian oil palm production: A system dynamics modeling approach. J. Clean. Prod. 2019, 213, 1051–1062. [Google Scholar] [CrossRef]
- Pasiecznik, N. Elaeis guineensis (African oil palm); CAB International: Wallingford, UK, 2013. [Google Scholar]
- Wilcove, D.S.; Koh, L.P. Addressing the threats to biodiversity from oil-palm agriculture. Biodivers. Conserv. 2010, 19, 999–1007. [Google Scholar] [CrossRef]
- Fitzherbert, E.B.; Struebig, M.J.; Morel, A.; Danielsen, F.; Brühl, C.A.; Donald, P.F.; Phalan, B. How will oil palm expansion affect biodiversity? Trends Ecol. Evol. 2008, 23, 538–545. [Google Scholar] [CrossRef]
- Edem, D. Palm oil: Biochemical, physiological, nutritional, hematological and toxicological aspects: A review. Plant Foods Hum. Nutr. 2002, 57, 319–341. [Google Scholar] [CrossRef]
- Toulec, T.; Lhota, S.; Soumarová, H.; Putera, A.K.S.; Kustiawan, W. Shrimp farms, fire or palm oil? Changing causes of proboscis monkey habitat loss. Glob. Ecol. Conserv. 2020, 21, e00863. [Google Scholar] [CrossRef]
- Margono, B.A.; Potapov, P.V.; Turubanova, S.; Stolle, F.; Hansen, M.C. Primary forest cover loss in Indonesia over 2000–2012. Nat. Clim. Chang. 2014, 4, 730–735. [Google Scholar] [CrossRef]
- Gaveau, D.L.A.; Sloan, S.; Molidena, E.; Yaen, H.; Sheil, D.; Abram, N.K.; Ancrenaz, M.; Nasi, R.; Quinones, M.; Wielaard, N.; et al. Four Decades of Forest Persistence, Clearance and Logging on Borneo. PLoS ONE 2014, 9, e101654. [Google Scholar] [CrossRef]
- Padfield, R.; Hansen, S.; Davies, Z.G.; Ehrensperger, A.; Slade, E.M.; Evers, S.; Papargyropoulou, E.; Bessou, C.; Abdullah, N.; Page, S.; et al. Co-producing a Research Agenda for Sustainable Palm Oil. Front. For. Glob. Chang. 2019, 2, 13. [Google Scholar] [CrossRef]
- Luskin, M.S.; Potts, M.D. Microclimate and habitat heterogeneity through the oil palm lifecycle. Basic Appl. Ecol. 2011, 12, 540–551. [Google Scholar] [CrossRef]
- Fayle, T.M.; Turner, E.C.; Snaddon, J.L.; Chey, V.K.; Chung, A.Y.C.; Eggleton, P.; Foster, W.A. Oil palm expansion into rain forest greatly reduces ant biodiversity in canopy, epiphytes and leaf-litter. Basic Appl. Ecol. 2010, 11, 337–345. [Google Scholar] [CrossRef]
- Koh, L.P.; Miettinen, J.; Liew, S.C.; Ghazoul, J. Remotely sensed evidence of tropical peatland conversion to oil palm. Proc. Natl. Acad. Sci. USA 2011, 108, 5127–5132. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K.M.; Curran, L.M.; Ratnasari, D.; Pittman, A.M.; Soares-Filho, B.S.; Asner, G.P.; Trigg, S.N.; Gaveau, D.A.; Lawrence, D.; Rodrigues, H.O. Committed carbon emissions, deforestation, and community land conversion from oil palm plantation expansion in West Kalimantan, Indonesia. Proc. Natl. Acad. Sci. USA 2012, 109, 7559–7564. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.H.; Abood, S.; Ghazoul, J.; Barus, B.; Obidzinski, K.; Koh, L.P. Environmental impacts of large-scale oil palm enterprises exceed that of smallholdings in Indonesia. Conserv. Lett. 2014, 7, 25–33. [Google Scholar] [CrossRef]
- Miettinen, J.; Hooijer, A.; Vernimmen, R.; Liew, S.C.; Page, S.E. From carbon sink to carbon source: Extensive peat oxidation in insular Southeast Asia since 1990. Environ. Res. Lett. 2017, 12, 024014. [Google Scholar] [CrossRef]
- Jaenicke, J.; Rieley, J.O.; Mott, C.; Kimman, P.; Siegert, F. Determination of the amount of carbon stored in Indonesian peatlands. Geoderma 2008, 147, 151–158. [Google Scholar] [CrossRef]
- Yule, C.M. Loss of biodiversity and ecosystem functioning in Indo-Malayan peat swamp forests. Biodivers. Conserv. 2010, 19, 393–409. [Google Scholar] [CrossRef]
- Murdiyarso, D.; Lilleskov, E.; Kolka, R. Tropical peatlands under siege: The need for evidence-based policies and strategies. Mitig. Adapt. Strateg. Glob. Chang. 2019, 24, 493–505. [Google Scholar] [CrossRef]
- Austin, K.G.; Kasibhatla, P.S.; Urban, D.L.; Stolle, F.; Vincent, J. Reconciling oil palm expansion and climate change mitigation in Kalimantan, Indonesia. PLoS ONE 2015, 10, e0127963. [Google Scholar] [CrossRef]
- Hergoualc’H, K.; Verchot, L.V. Stocks and fluxes of carbon associated with land use change in Southeast Asian tropical peatlands: A review. Glob. Biogeochem. Cycles 2011, 25, 789–807. [Google Scholar] [CrossRef]
- Rees, A. The apical organization and phyllotaxis of the oil palm. Ann. Bot. 1964, 28, 57–69. [Google Scholar] [CrossRef]
- Johnson, D. Tropical palms. non-wood forest products 10/Rev. 1. In Rome: Food and Agriculture; Organization of the United Nations (FAO): Rome, Italy, 2011. [Google Scholar]
- Woittiez, L.S.; van Wijk, M.T.; Slingerland, M.; van Noordwijk, M.; Giller, K.E. Yield gaps in oil palm: A quantitative review of contributing factors. Eur. J. Agron. 2017, 83, 57–77. [Google Scholar] [CrossRef]
- Adam, H.; Collin, M.; Richaud, F.; Beulé, T.; Cros, D.; Omoré, A.; Nodichao, L.; Nouy, B.; Tregear, J.W. Environmental regulation of sex determination in oil palm: Current knowledge and insights from other species. Ann. Bot. 2011, 108, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Sumathi, S.; Chai, S.; Mohamed, A. Utilization of oil palm as a source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 2008, 12, 2404–2421. [Google Scholar] [CrossRef]
- Langeveld, H.; Dixon, J.; van Keulen, H. Oil palm biodiesel in the Far East. In Biofuel Cropping Systems; Routledge: London, UK, 2014; pp. 178–193. [Google Scholar]
- Zwart, R. Opportunities and challenges in the development of a viable Malaysian palm oil biomass industry. J. Oil Palm Environ. Health (JOPEH) 2013, 4, 41–46. [Google Scholar]
- Henson, I.E. A Brief History of the Oil Palm. In Palm Oil; Lai, O.-M., Tan, C.-P., Akoh, C.C., Eds.; AOCS Press: Champaign, IL, USA, 2012; pp. 1–29. [Google Scholar]
- Duke, J. Elaeis guineensis Jacq; Purdue University: West Lafayette, IN, USA, 1983. [Google Scholar]
- Ollagnier, M.; Ochs, R.; Martin, G. The manuring of the oil palm in the world. Manuring Oil Palm World. 1970, 36, 3–63. [Google Scholar]
- Chuah, T.; Wan Azlina, A.; Robiah, Y.; Omar, R. Biomass as the renewable energy sources in Malaysia: An overview. Int. J. Green Energy 2006, 3, 323–346. [Google Scholar] [CrossRef]
- Yusoff, S. Renewable energy from palm oil–innovation on effective utilization of waste. J. Clean. Prod. 2006, 14, 87–93. [Google Scholar] [CrossRef]
- Goh, K. Climatic requirements of the oil palm for high yields. In Managing Oil Palm for High Yields: Agronomic Principles; Goh, K.J., Ed.; Malaysian Society of Soil Science/Param Agric: Surveys, Malaysia, 2000; pp. 1–17. [Google Scholar]
- Liew, W.L.; Kassim, M.A.; Muda, K.; Loh, S.K.; Affam, A.C. Conventional methods and emerging wastewater polishing technologies for palm oil mill effluent treatment: A review. J. Environ. Manag. 2015, 149, 222–235. [Google Scholar] [CrossRef]
- Ong, H.T. System approach to the climatology of oil palm. I. Identification of rainfall and dry spell aspects. Oleagineux 1982, 37, 93–101. [Google Scholar]
- Corley, R.H.V. Oil palm yield components and yield cycles. Malays. Int. Agric. Oil Palm Conf. Paper 2012, 9, 116–129. [Google Scholar]
- Oettli, P.; Behera, S.K.; Yamagata, T. Climate Based Predictability of Oil Palm Tree Yield in Malaysia. Sci. Rep. 2018, 8, 2271. [Google Scholar] [CrossRef] [PubMed]
- Kamil, N.N.; Omar, S.F. The Impact of El Nino and La Nina on Malaysian Oil Palm Industry. Oil Palm Bulettin 2017, 74, 1–6. [Google Scholar]
- Paterson, R.R.M.; Kumar, L.; Taylor, S.; Lima, N. Future climate effects on suitability for growth of oil palms in Malaysia and Indonesia. Sci. Rep. 2015, 5, 14457. [Google Scholar] [CrossRef]
- Shanmuganathan, S.; Narayanan, A.; Mohamed, M.; Ibrahim, R.; Khalid, H. A Hybrid Approach to Modelling the Climate Change Effects on Malaysia’s Oil Palm Yield at the Regional Scale. In Recent Advances on Soft Computing and Data Mining. Advances in Intelligent Systems and Computing; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Keong, Y.K.; Keng, W.M. Statistical Modeling of Weather-based Yield Forecasting for Young Mature Oil Palm. APCBEE Procedia 2012, 4, 58–65. [Google Scholar] [CrossRef]
- Stevenson, S.L. Significant changes to ENSO strength and impacts in the twenty-first century: Results from CMIP5. Geophys. Res. Lett. 2012, 39, L17703. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; US Government Printing Office: Washington, DC, USA, 1975. [Google Scholar]
- Olivin, J. Étude pour la localisation d’un bloc industriel de palmiers à huile. Oléagineux 1968, 23, 499–504. [Google Scholar]
- Olivin, J. Study for the siting of a commercial oil palm plantation. Oleagineux (France) 1986, 41, 175–182. [Google Scholar]
- Paramananthan, S. Soil requirements of oil palm for high yields. In Managing Oil Palm for High Yields: Agronomic Principles; Malaysian Society of Soil Science and Param Agricultural: Kuala Lumpur, Malaysia, 2000; pp. 18–38. [Google Scholar]
- Corley, R.H.V.; Tinker, P.B.H. The Oil Palm; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Gobin, A.; Campling, P.; Deckers, J.A.; Poesen, J.; Feyen, J. Soil erosion assessment at the Udi-Nsukka Cuesta (southeastern Nigeria). Land Degrad. Dev. 1999, 10, 141–160. [Google Scholar] [CrossRef]
- Hamdan, J.; Burnham, C.; Ruhana, B. Degradation effect of slope terracing on soil quality for Elaeis guineensis Jacq.(oil palm) cultivation. Land Degrad. Dev. 2000, 11, 181–193. [Google Scholar] [CrossRef]
- Paramananthan, S. Managing marginal soils for sustainable growth of oil palms in the tropics. J. Oil Palm Environ. Health (JOPEH) 2013, 4, 1–16. [Google Scholar] [CrossRef]
- Schrier-Uijl, A.P.; Silvius, M.; Parish, F.; Lim, K.H.; Rosediana, S.; Anshari, G. Environmental and Social Impacts of Oil Palm Cultivation on Tropical Peat—A Scientific Review; RSPO (Roundtable on Sustainable Palm Oil): Kuala Lumpur, Malaysia, 2013. [Google Scholar]
- van Der Werf, G.R.; Randerson, J.T.; Giglio, L.; Collatz, G.J.; Mu, M.; Kasibhatla, P.S.; Morton, D.C.; Defries, R.S.; Jin, Y.; van Leeuwen, T.T. Global fire emissions and the contribution of deforestation, savanna, forest, agricultural, and peat fires (1997–2009). Atmos. Chem. Phys. 2010, 10, 11707–11735. [Google Scholar] [CrossRef]
- NASA. Carbon Stored in Earth’s Tropical Forest. Available online: https://www.nasa.gov/topics/earth/features/earth20110531-i.html (accessed on 25 December 2019).
- Jaafar, W.S.W.M.; Maulud, K.N.A.; Kamarulzaman, A.M.M.; Raihan, A.; Sah, S.M.; Ahmad, A.; Saad, S.N.M.; Azmi, A.T.M.; Syukri, N.K.A.J.; Khan, W.R. The Influence of Deforestation on Land Surface Temperature—A Case Study of Perak and Kedah, Malaysia. Forests 2020, 11, 670. [Google Scholar] [CrossRef]
- Kamlun, K.U.; Goh, M.H.; Teo, S.; Tsuyuki, S.; Phua, M.-H. Monitoring of deforestation and fragmentation in Sarawak, Malaysia between 1990 and 2009 using landsat and SPOT images. J. For. Sci. 2012, 28, 152–157. [Google Scholar] [CrossRef]
- Hooijer, A.; Silvius, M.; Wosten, H.; Page, S. PEAT-CO2, Assessment of CO2 Emissions from Drained Peatlands in SE Asia; Delft Hydraulics Report: Delft, The Netherlands, 2006. [Google Scholar]
- van Beukering, P.; Schaafsma, M.; Davis, O.; Oskolokaite, I. The Economic alue of Petland Resources within the Central Kalimantan Peatland Project in Indonesia: Perceptions of Local Communities; Institute for Environmental Studies: Amsterdam, The Netehrlands, 2008. [Google Scholar]
- Wetland International. Assessment on Peatlands, Biodiversity and Climate Change; Wetland International: Kuala Lumpur, Malaysia, 2007. [Google Scholar]
- Tyson, A.; Varkkey, H.; Choiruzzad, S.A.B. Deconstructing the Palm Oil Industry Narrative in Indonesia: Evidence from Riau Province. Contemp. Southeast Asia 2018, 40, 422–448. [Google Scholar] [CrossRef]
- Wolf, H.A. Deforestation in Cambodia and Malaysia: The case for an international legal solution. Pac. Rim Law Policy J. 1996, 5, 431–432. [Google Scholar]
- Busch, J.; Ferretti-Gallon, K.; Engelmann, J.; Wright, M.; Austin, K.G.; Stolle, F.; Turubanova, S.; Potapov, P.V.; Margono, B.; Hansen, M.C.; et al. Reductions in emissions from deforestation from Indonesia’s moratorium on new oil palm, timber, and logging concessions. Proc. Natl. Acad. Sci. USA 2015, 112, 1328–1333. [Google Scholar] [CrossRef]
- Chua, K.B.; Chua, B.H.; Wang, C.W. Anthropogenic deforestration, El Nino and the emergence of Nipah virus in Malaysia. Malays. J. Pathol. 2002, 24, 15–21. [Google Scholar]
- Yong, C. Deforestration Drivers and Human Rights in Malaysia: A National Overview and Two Sub-Regional Case Studies; Forest Peoples Programme: Moreton-in-Marsh, UK, 2014. [Google Scholar]
- IPCC. Climate Change and Water; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2009. [Google Scholar]
- Gaveau, D.L.A.; Salim, M.A.; Hergoualc’h, K.; Locatelli, B.; Sloan, S.; Wooster, M.; Marlier, M.E.; Molidena, E.; Yaen, H.; DeFries, R.; et al. Major atmospheric emissions from peat fires in Southeast Asia during non-drought years: Evidence from the 2013 Sumatran fires. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Sloan, S.; Campbell, M.J.; Alamgir, M.; Lechner, A.M.; Engert, J.; Laurance, W.F. Trans-national conservation and infrastructure development in the Heart of Borneo. PLoS ONE 2019, 14, e0221947. [Google Scholar] [CrossRef]
- Page, S.E.; Rieley, J.O.; Banks, C.J. Global and regional importance of the tropical peatland carbon pool. Glob. Chang. Biol. 2011, 17, 798–818. [Google Scholar] [CrossRef]
- Hooijer, A.; Page, S.; Canadell, J.G.; Silvius, M.; Kwadijk, J.; Wösten, H.; Jauhiainen, J. Current and future CO2 emissions from drained peatlands in Southeast Asia. Biogeosciences 2010, 7, 1505–1514. [Google Scholar] [CrossRef]
- Yoshino, K.; Ishida, T.; Nagano, T.; Setiawan, Y. Landcover pattern analysis of tropical peat swamp lands in Southeast Asia. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2010, 38, 941–946. [Google Scholar]
- Huijnen, V.; Wooster, M.J.; Kaiser, J.W.; Gaveau, D.L.A.; Flemming, J.; Parrington, M.; Inness, A.; Murdiyarso, D.; Main, B.; Van Weele, M. Fire carbon emissions over maritime southeast Asia in 2015 largest since 1997. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Kløve, B.; Sveistrup, T.E.; Hauge, A. Leaching of nutrients and emission of greenhouse gases from peatland cultivation at Bodin, Northern Norway. Geoderma 2010, 154, 219–232. [Google Scholar] [CrossRef]
- Kusumaningtyas, S.D.A.; Aldrian, E. Impact of the June 2013 Riau province Sumatera smoke haze event on regional air pollution. Environ. Res. Lett. 2016, 11, 075007. [Google Scholar] [CrossRef]
- Miettinen, J.; Shi, C.; Liew, S.C. Two decades of destruction in Southeast Asia’s peat swamp forests. Front. Ecol. Environ. 2011, 10, 124–128. [Google Scholar] [CrossRef]
- Othman, M.; Latif, M.T. Dust and gas emissions from small-scale peat combustion. Aerosol Air Qual. Res. 2013, 13, 1045–1105. [Google Scholar] [CrossRef]
- Ram, K.; Sarin, M.M.; Sudheer, A.K.; Rengarajan, R. Carbonaceous and secondary inorganic aerosols during wintertime fog and haze over urban sites in the Indo-Gangetic plain. Aerosol Air Qual. Res. 2012, 12, 359–370. [Google Scholar] [CrossRef]
- Yokelson, R.J.; Griffith, D.W.T.; Ward, D.E. Open-path fourier transform infrared studies of large-scale laboratory biomass fires. J. Geophys. Res. 1996, 101, 21067–21080. [Google Scholar] [CrossRef]
- Langmann, B.; Duncan, B.; Textor, C.; Trentmann, J.; Vanderwerf, G. Vegetation fire emissions and their impact on air pollution and climate. Atmos. Environ. 2009, 43, 107–116. [Google Scholar] [CrossRef]
- Mead, M.I.; Castruccio, S.; Nadzir, M.S.M.; Dominick, D.; Thota, A.; Crippa, P. Impact of the 2015 wildfires on Malaysian air quality and exposure: A comparative study of observed and modeled data. Environ. Res. Lett. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Hyer, E.J.; Chew, B.N. Aerosol transport model evaluation of an extreme smoke episode in Southeast Asia. Atmos. Environ. 2010, 44, 1422–1427. [Google Scholar] [CrossRef]
- Varkkey, H.; Poh Onn, L. The Haze Problem in Southeast Asia: Palm Oil and Patronage. J. Southeast Asian Econ. 2017, 34, 573–575. [Google Scholar] [CrossRef]
- Pegas, P.N.; Nunes, T.; Alves, C.A.; Silva, J.R.; Vieira, S.L.A.; Caseiro, A.; Pio, C.A. Indoor and outdoor characterisation of organic and inorganic compounds in city centre and suburban elementary schools of Aveiro, Portugal. Atmos. Environ. 2012, 55, 80–89. [Google Scholar] [CrossRef]
- Seiler, W.; Crutzen, J.P. Estimates of the gross and net fluxes of carbon between the biospere and the atmosphere from biomass burning. Clim. Change 1980, 2, 207–247. [Google Scholar] [CrossRef]
- Song, Y.; Chang, D.; Liu, B.; Miao, W.; Zhu, L.; Zhang, Y. A new emission inventory for nonagricultural open fires in Asia from 2000 to 2009. Environ. Res. Lett. 2010, 5, 1–6. [Google Scholar] [CrossRef]
- Shi, Y.; Sasai, T.; Yamaguchi, Y. Spatio-temporal evaluation of carbon emissions from biomass burning in Southeast Asia during the period 2001–2010. Ecol. Model. 2014, 272, 98–115. [Google Scholar] [CrossRef]
- Henson, I.E. Notes on oil palm productivity. IV. Carbon dioxide gradients and fluxes and evapotranspiration, above and below the canopy. J. Oil Palm Res. 1999, 11, 33–40. [Google Scholar]
- Nadzir, M.S.M.; Ooi, C.G.; Harris, N.R.P.; Ashworth, K.; Bolas, C.; Bran, S.H.; Macatangay, R.; Jaafar, W.S.W.M.; Yusup, Y.; Latif, M.T.; et al. Driving factors for isoprene-surface ozone chemistry over an oil palm plantation in Malaysia. Environ. Int. 2020, submitted. [Google Scholar]
- Holzinger, R.; Sanhueza, E.; von Kuhlmann, R.; Kleiss, B.; Donoso, L.; Crutzen, P.J. Diurnal cycles and seasonal variation of isoprene and its oxidation products in the tropical savanna atmosphere. Glob. Biogeochem. Cycles 2002, 16, 1074. [Google Scholar] [CrossRef]
- Biesenthal, T.A.; Bottenheim, J.W.; Shepson, P.B.; Brickell, P.C. The chemistry of biogenic hydrocarbons at a rural site in eastern Canada. J. Geophys. Res. Atmos. 1998, 103, 25487–25498. [Google Scholar] [CrossRef]
- Goldan, P.D.; Kuster, W.C.; Fehsenfeld, F.C.; Montzka, S.A. Hydrocarbon measurements in the southeastern United States: The Rural Oxidants in the Southern Environment (ROSE) Program 1990. J. Geophys. Res. Atmos. 1995, 100, 25945–25963. [Google Scholar] [CrossRef]
- Montzka, S.A.; Trainer, M.; Goldan, P.D.; Kuster, W.C.; Fehsenfeld, F.C. Isoprene and its oxidation products, methyl vinyl ketone and methacrolein, in the rural troposphere. J. Geophys. Res. Atmos. 1993, 98, 1101–1111. [Google Scholar] [CrossRef]
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. 2006, 6, 3181–3210. [Google Scholar] [CrossRef]
- Wennberg, P.O.; Bates, K.H.; Crounse, J.D.; Dodson, L.G.; McVay, R.C.; Mertens, L.A.; Nguyen, T.B.; Praske, E.; Schwantes, R.H.; Smarte, M.D.; et al. Gas-Phase Reactions of Isoprene and Its Major Oxidation Products. Chem. Rev. 2018, 118, 3337–3390. [Google Scholar] [CrossRef]
- Ahamad, F.; Latif, M.T.; Tang, R.; Juneng, L.; Dominick, D.; Juahir, H. Variation of surface ozone exceedance around Klang Valley, Malaysia. Atmos. Res. 2014, 139, 116–127. [Google Scholar] [CrossRef]
- Latif, M.T.; Huey, L.S.; Juneng, L. Variations of surface ozone concentration across the Klang Valley, Malaysia. Atmos. Environ. 2012, 61, 434–445. [Google Scholar] [CrossRef]
- Nadzir, M.S.M.; Ashfold, M.J.; Khan, M.F.; Robinson, A.D.; Bolas, C.; Latif, M.T.; Wallis, B.M.; Mead, M.I.; Hamid, H.H.A.; Harris, N.R.P.; et al. Spatial-temporal variations in surface ozone over Ushuaia and the Antarctic region: Observations from in situ measurements, satellite data, and global models. Environ. Sci. Pollut. Res. Int. 2018, 25, 2194–2210. [Google Scholar] [CrossRef]
- Khatun, R.; Reza, M.I.H.; Moniruzzaman, M.; Yaakob, Z. Sustainable oil palm industry: The possibilities. Renew. Sustain. Energy Rev. 2017, 76, 608–619. [Google Scholar] [CrossRef]
- Wijedasa, L.S.; Jauhiainen, J.; Könönen, M.; Lampela, M.; Vasander, H.; Leblanc, M.-C.; Evers, S.; Smith, T.E.L.; Yule, C.M.; Varkkey, H.; et al. Denial of long-term issues with agriculture on tropical peatlands will have devastating consequences. Glob. Chang. Biol. 2017, 23, 977–982. [Google Scholar] [CrossRef]
- Evers, S.; Yule, C.M.; Padfield, R.; O’Reilly, P.; Varkkey, H. Keep wetlands wet: The myth of sustainable development of tropical peatlands – implications for policies and management. Glob. Chang. Biol. 2017, 23, 534–549. [Google Scholar] [CrossRef]
- Carter, C.; Finley, W.; Fry, J.; Jackson, D.; Willis, L. Palm oil markets and future supply. Eur. J. Lipid Sci. Technol. 2007, 109, 307–314. [Google Scholar] [CrossRef]
- Hooijer, A.; Page, S.; Jauhiainen, J.; Lee, W.A.; Lu, X.X.; Idris, A.; Anshari, G. Subsidence and carbon loss in drained tropical peatlands. Biogeosciences 2012, 9, 1053–1071. [Google Scholar] [CrossRef]
- Dariah, A.; Marwanto, S.; Agus, F. Root- and peat-based CO2 emissions from oil palm plantations. Mitig. Adapt. Strateg. Glob. Chang. 2014, 19, 831–843. [Google Scholar] [CrossRef]
- Matysek, M.; Evers, S.; Samuel, M.K.; Sjogersten, S. High heterotrophic CO2 emissions from a Malaysian oil palm plantations during dry-season. Wetl. Ecol. Manag. 2017, 26, 415–424. [Google Scholar] [CrossRef]
- Melling, L.; Chaddy, A.; Goh, K.J.; Hatano, R. Soil CO2 Fluxes from Different Ages of Oil Palm in Tropical Peatland of Sarawak, Malaysia as Influenced by Environmental and Soil Properties. Acta Hortic. 2013, 982, 25–35. [Google Scholar] [CrossRef]
- Marwanto, S.; Agus, F. Is CO2 flux from oil palm plantations on peatland controlled by soil moisture and/or soil and air temperatures? Mitig. Adapt. Strateg. Glob. Chang. 2013, 19, 809–819. [Google Scholar] [CrossRef]
- Husnain, H.; Wigena, I.G.P.; Dariah, A.; Marwanto, S.; Setyanto, P.; Agus, F. CO2 emissions from tropical drained peat in Sumatra, Indonesia. Mitig. Adapt. Strateg. Glob. Chang. 2014, 19, 845–862. [Google Scholar] [CrossRef]
- Marwanto, S.; Sabiham, S.; Funakawa, S. Importance of CO2 production in subsoil layers of drained tropical peatland under mature oil palm plantation. Soil Tillage Res. 2019, 186, 206–213. [Google Scholar] [CrossRef]
- Melling, L.; Hatano, R.; Goh, K.J. Soil CO2 flux from three ecosystems in tropical peatland of Sarawak, Malaysia. Tellus B Chem. Phys. Meteorol. 2005, 57, 1–11. [Google Scholar] [CrossRef]
- Melling, L.; Goh, K.J.; Beauvais, C.; Hatano, R. Carbon Flow and Budget in Young Mature Oil Palm Agroecosystem on Deep Tropical Peat. Planter 2008, 84, 21. [Google Scholar]
- Agus, F.; Wahyunti, A.D.; Setyanto, P.; Subiksa, I.G.M.; Runtunuwu, E.; Susanti, E.; Supriatna, W. Carbon Budget and Management Strategies for Conserving Carbon in Peatland: Case Study in Kubu Raya and Pontianak Districts, West Kalimantan, Indonesia. In Proceeding of International Workshop on Evaluation and Sustainable Management of Soil Carbon Sequestration in Asian Countries; Indonesian Soil Research Institute: Bogor, Indonesia, 2010. [Google Scholar]
- Manning, F.C.; Kho, L.K.; Hill, T.C.; Cornulier, T.; Teh, Y.A. Carbon Emissions From Oil Palm Plantations on Peat Soil. Front. For. Glob. Chang. 2019, 2, 37. [Google Scholar] [CrossRef]
- Fowler, D.; Nemitz, E.; Misztal, P.; Di Marco, C.; Skiba, U.; Ryder, J.; Helfter, C.; Cape, J.N.; Owen, S.; Dorsey, J.; et al. Effects of land use on surface-atmosphere exchanges of trace gases and energy in Borneo: Comparing fluxes over oil palm plantations and a rainforest. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2011, 366, 3196–3209. [Google Scholar] [CrossRef]
- Meijaard, E.; Sherman, J.; Ancrenaz, M.; Wich, S.A.; Santika, T.; Voigt, M. Orangutan populations are certainly not increasing in the wild. Curr. Biol. 2018, 28, R1241–R1242. [Google Scholar] [CrossRef]
- Sharvini, S.R.; Noor, Z.Z.; Chong, C.S.; Stringer, L.C.; Glew, D. Energy generation from palm oil mill effluent: A life cycle assessment of two biogas technologies. Energy 2020, 191, 116513. [Google Scholar] [CrossRef]
- Xiao, D.; Ye, Y.; Xiao, S.; Zhang, W.; He, X.; Wang, K. Effects of tillage on CO2 fluxes in a typical karst calcareous soil. Geoderma 2019, 337, 191–201. [Google Scholar] [CrossRef]
- Pareja-Sánchez, E.; Cantero-Martínez, C.; Álvaro-Fuentes, J.; Plaza-Bonilla, D. Tillage and nitrogen fertilization in irrigated maize: Key practices to reduce soil CO2 and CH4 emissions. Soil Tillage Res. 2019, 191, 29–36. [Google Scholar] [CrossRef]
- Wilson, H.M.; Al-Kaisi, M.M. Crop rotation and nitrogen fertilization effect on soil CO2 emissions in central Iowa. Appl. Soil Ecol. 2008, 39, 264–270. [Google Scholar] [CrossRef]
- West, T.O.; Marland, G. Net carbon flux from agriculture: Carbon emissions, carbon sequestration, crop yield, and land-use change. Biogeochemistry 2003, 63, 73–83. [Google Scholar] [CrossRef]
- Bernoux, M.; Volkoff, B.; Carvalho, M.d.C.S.; Cerri, C.C. CO2 emissions from liming of agricultural soils in Brazil. Glob. Biogeochem. Cycles 2003, 17, 1049. [Google Scholar] [CrossRef]
- Ball, B.C.; Scott, A.; Parker, J.P. Field N2O, CO2 and CH4 fluxes in relation to tillage, compaction and soil quality in Scotland. Soil Tillage Res. 1999, 53, 29–39. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Goldberg, S.D.; Xu, J.-C.; Harrison, R.D. Spatial and seasonal variation in soil respiration along a slope in a rubber plantation and a natural forest in Xishuangbanna, Southwest China. J. Mt. Sci. 2018, 15, 695–707. [Google Scholar] [CrossRef]
- Sánchez, M.L.; Ozores, M.I.; López, M.J.; Colle, R.; De Torre, B.; García, M.A.; Pérez, I. Soil CO2 fluxes beneath barley on the central Spanish plateau. Agric. For. Meteorol. 2003, 118, 85–95. [Google Scholar] [CrossRef]
| Climatic Element | Highly Suitable | Suitable | Moderately Suitable | Currently Unsuitable | Permanently Unsuitable |
|---|---|---|---|---|---|
| Annual rainfall (mm year−1) | 2000–2500 | 2500–3000 1700–2000 | 3000–4000 1400–1700 | 4000–5000 1100–1400 | >5000 <1100 |
| Duration of dry season (month) | 0 | 1 | 2–4 | 5–6 | >6 |
| Mean annual temperature | 26–29 | 29–32 23–26 | 32–34 20–23 | 34–36 17–20 | >36 <20 |
| Daily solar radiation (MJ m−2) | 16–17 | 17–19 14–16 | 19–21 11–14 | 21–23 8–11 | >23 <8 |
| Wind (m s−1) | <10 | 10–15 | 15–25 | 25–40 | >40 |
| Country | Area | Year | Emissions (t C ha−1 year−1) | Reference |
|---|---|---|---|---|
| Indonesia | Jambi and Riau | 2007–2010 | 95 | Hooijer et al. [102] |
| Jambi | 2010–2011 | 13 | Marwanto and Agus [106] | |
| Jambi | 2011–2012 | 10 | Dariah et al. [103] | |
| Riau | 2011–2012 | 18 | Husnain et al. [107] | |
| Riau | 2016–2017 | 12 | Marwanto et al. [108] | |
| Malaysia | Selangor | 2000 | 79 | Matysek et al. [104] |
| Sarawak | 2002–2003 | 17 | Melling et al. [109] | |
| Sarawak | 2002–2003 | 41 | Melling et al. [110] | |
| Sarawak | 2003 | 7 | Matysek et al. [104] Matysek et al. [104] | |
| Selangor | 2006 | 65 |
| Country | Area | Year | Emissions (t C ha−1 year−1) | Reference |
|---|---|---|---|---|
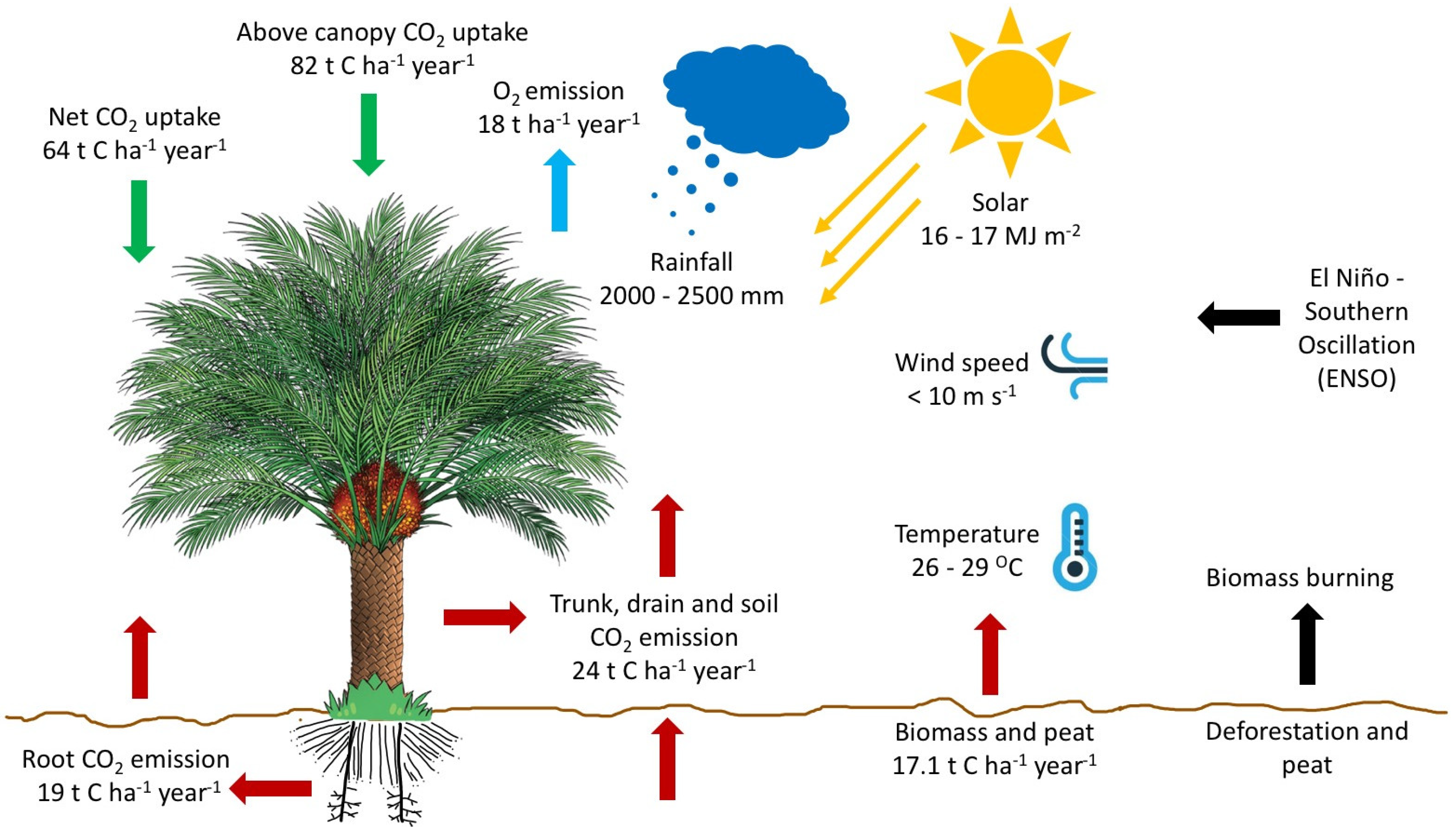
| Indonesia | Aceh Barat | 2008 | 19 (Root) | Agus et al. [111] |
| Malaysia | Sabah | 2008 | 82 (Above canopy-uptake) | Fowler et al. [113] |
| Sarawak | 2015–2017 | 24 (Trunk, drain, soil) | Manning et al. [112] |
| Plant | Type | Time Measured (Location) | Emissions (t C ha−1 year−1) | Reference |
|---|---|---|---|---|
| Barley | Soil | November 1998 to October 2000, over non-irrigated barley (Central Spain) | 0.63 | Sánchez et al. [123] |
| Corn | Soil | Based on agricultural inputs detailed by Frye and Blevins (1997) and Ismail et al. (1994), Blevins et al. (1983). (Kentucky, USA) | 0.4 | West and Marland [119] |
| Rubber | Soil | January and March 2016 (China) | 5.7 | Zhao et al. [122] |
| Intact Forest | 9.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uning, R.; Latif, M.T.; Othman, M.; Juneng, L.; Mohd Hanif, N.; Nadzir, M.S.M.; Abdul Maulud, K.N.; Jaafar, W.S.W.M.; Said, N.F.S.; Ahamad, F.; et al. A Review of Southeast Asian Oil Palm and Its CO2 Fluxes. Sustainability 2020, 12, 5077. https://doi.org/10.3390/su12125077
Uning R, Latif MT, Othman M, Juneng L, Mohd Hanif N, Nadzir MSM, Abdul Maulud KN, Jaafar WSWM, Said NFS, Ahamad F, et al. A Review of Southeast Asian Oil Palm and Its CO2 Fluxes. Sustainability. 2020; 12(12):5077. https://doi.org/10.3390/su12125077
Chicago/Turabian StyleUning, Royston, Mohd Talib Latif, Murnira Othman, Liew Juneng, Norfazrin Mohd Hanif, Mohd Shahrul Mohd Nadzir, Khairul Nizam Abdul Maulud, Wan Shafrina Wan Mohd Jaafar, Nor Fitrah Syazwani Said, Fatimah Ahamad, and et al. 2020. "A Review of Southeast Asian Oil Palm and Its CO2 Fluxes" Sustainability 12, no. 12: 5077. https://doi.org/10.3390/su12125077
APA StyleUning, R., Latif, M. T., Othman, M., Juneng, L., Mohd Hanif, N., Nadzir, M. S. M., Abdul Maulud, K. N., Jaafar, W. S. W. M., Said, N. F. S., Ahamad, F., & Takriff, M. S. (2020). A Review of Southeast Asian Oil Palm and Its CO2 Fluxes. Sustainability, 12(12), 5077. https://doi.org/10.3390/su12125077








