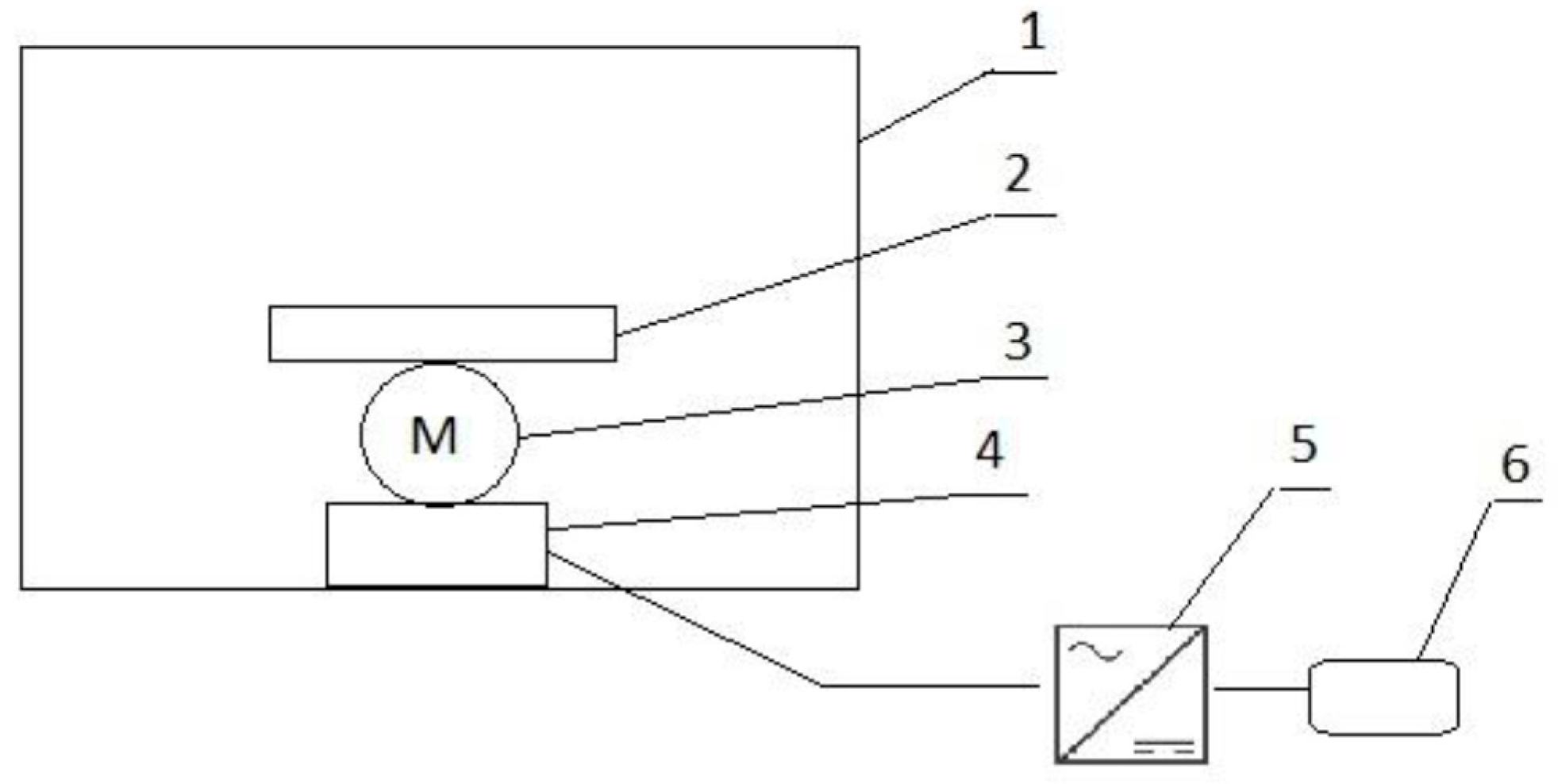
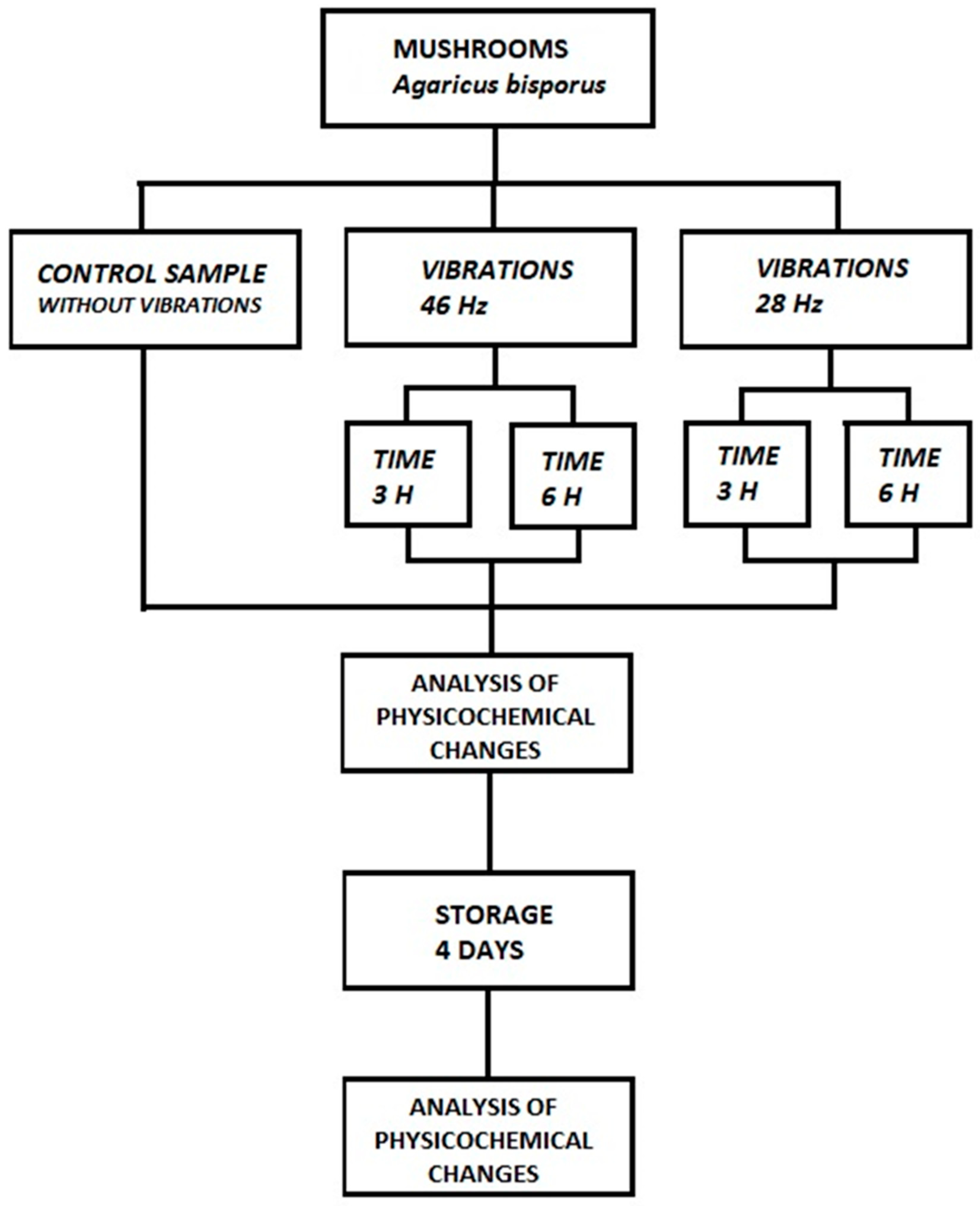
3.1. pH, Total Extract and Dry Matter
Table 1 shows pH values, total extract and dry matter content of mushrooms, depending on the frequencies used, the vibration time and the storage time.
The pH value of mushrooms not subjected to vibration slightly decreased due to their storage for 4 days. The pH value of mushrooms subjected to the vibration of 46 Hz slightly decreased in comparison with mushrooms not subjected to the vibration. Vibrations, regardless of their duration and storage time, resulted in mushrooms achieving a similar pH value (6.42–6.43), except for the mushrooms that were exposed to vibration for 3 h and stored for 4 days. On the other hand, vibrations of 28 Hz, regardless of their duration and storage time, resulted in mushrooms reaching a similar pH value (6.34–6.39).
The measured pH values in the tested mushrooms are in accordance with data from the literature [
31,
32,
33]. The slight decrease in pH value observed during the experiment is probably related to an increase in dry matter and refractometric total extract during transport and storage. Another probable reason for the decrease in pH value is the progressive microbial spoilage of the tested mushrooms.
The measured total extract content in fresh mushrooms is in accordance with the data from the literature [
32]. The content of the total extract of mushrooms not subjected to vibrations increased during storage for 4 days. The total extract content in mushrooms subjected to the vibration at the frequency of 46 Hz increased significantly in comparison with those not subjected to any vibration. It was different for mushrooms stored for 4 days, as the total extract content decreased in comparison with those not treated with vibration. When both frequencies were used, the vibrations did not cause significant changes in the total extract content of mushrooms, regardless of their duration.
The dry matter content of the mushrooms not subjected to vibration increased during their storage for 4 days. The dry matter content of mushrooms subjected to the 46 Hz frequency increased slightly in comparison with those not subjected to any vibration. After 4 days of storage, no significant changes in dry matter values, which ranged from 8.24% to 9.24%, were observed. Further, at the frequency of 28 Hz no significant changes in the dry matter content of mushrooms were observed, regardless of the duration of vibrations.
The recorded levels of dry matter content of mushrooms are in accordance with the data from the literature, which, according to various authors, depending on the variety and yield pattern, range from 7.9% to 8.5% [
34], 11.1% to 12.7% [
35] and 6.8% to 9.1% [
36].
3.2. Antioxidant Activity
Table 2 shows the antioxidant activity of mushrooms subjected to vibrations, depending on the frequencies used, the vibration time and the storage time.
In the first experiment, using the 46 Hz frequency, the antioxidant activity of 127.2 μmol Trolox/g d.m. was recorded in the control sample of mushrooms. As a result of mechanical vibrations, a significant (p ≤ 0.05) increase in this parameter was observed after 3 h, to 230.7 μmol Trolox/g d.m., while after 6 h the increase was insignificant, to 130.7 μmol Trolox/g d.m. After 4 days of storage, an increase in antioxidant activity was observed in all samples, ranging from 33 to 73 units, but these changes were not statistically significant.
In the second experiment, using a frequency of vibrations of 28 Hz in the initial sample of mushrooms (post-harvest control sample), the antioxidant activity was 66.3 μmol Trolox/g d.m. As a result of the application of mechanical vibrations, an increase of this parameter, to 81.29 μmol Trolox/g d.m., was observed over 3 h, while over 6 h of vibrations there was a decrease to 58.9 μmol Trolox/g d.m., however these changes were not statistically significant (p ≤ 0.05). After 4 days of storage, an increase in antioxidant activity of 2–89 units was observed in all samples, which was an increase from 3% to over 100%. The highest, statistically significant increase in antioxidant activity after storage was found in the sample of mushrooms previously subjected to vibrations for 3 h.
Due to the origin of the raw material from two different collective batches, the levels of antioxidant activity in the first and second experiments (vibration frequency 48 and 28 Hz, respectively) differed significantly, but the observed changes in the values of this parameter showed similar trends. In the mushrooms subjected to mechanical vibrations for 3 h, the highest increase in antioxidant activity was observed both in fresh samples and after 4 days of storage. The greater impact of mechanical vibrations lasting 6 h caused an insignificant increase or even decrease in antioxidant activity, which may be associated with progressive mechanical damage to tissues, as a result of which the activity of native oxidoreductive enzymes increases, which leads to the oxidation of bioactive compounds, including polyphenols responsible for antioxidant activity. Moreover, in all experimental variants, the mushrooms after 4 days of storage were characterised by a higher antioxidant activity than those samples tested on the first day of the experiment.
The values of antioxidant activity determined in the mushrooms tested are comparable with those described in the literature. In the study by Skąpska and colleagues [
33], the antioxidant activity of fresh champignons was found to be about 60 μmol Trolox/g d.m., which was almost twice as high as that of oyster mushroom. An increase in antioxidant activity in fresh and sliced champignons during storage (shelf-life) was also observed in the study by Oms-Oliu et al. [
37].
3.3. Polyphenol Content
Table 3 shows the content of polyphenols converted into gallic acid in the mushrooms subjected to vibrations, depending on the frequencies used, the vibration time and the storage time.
In the first experiment conducted with the application of 46 Hz frequency, the initial content of polyphenolic compounds in fresh mushrooms amounted to 796 mg/100 g d.m. As a result of applying 3 h of vibrations, the level of polyphenols increased to 817 mg/100 g d.m., whereas in the variant where the vibrations lasted 6 h, the increase of this parameter was smaller, reaching the level of 791 mg/100 g d.m. The changes of polyphenols content on the first day of the study, directly after applying vibrations, were not significant (p ≤ 0.05). After 4-day storage of the mushrooms, in all samples an increase in polyphenols content by 2–71 units was observed. The highest statistically significant increase in polyphenols content after storage was recorded in the sample previously subjected to vibrations for 6 h, where it reached 864 mg/100 g d.m.
A similar direction of changes in polyphenols content was observed in the second experiment, with the application of vibrations of 28 Hz frequency. The initial level of polyphenols content in fresh mushrooms was 441 mg/100 g d.m., and after the application of vibrations an increase in polyphenols content to the level of 500 and 459 mg/100 g d.m. was observed, after 3 and 6 h of vibrations, respectively. In all experimental variants, as a result of 4-day storage of the mushrooms, increases in polyphenols content to the levels of 607, 555 and 640 mg/100 g d.m. were observed, in the control sample of mushrooms as well as in those which were affected by 3 and 6 h of vibration, respectively. All changes in the value of the analysed parameter were statistically significant (p ≤ 0.05), which was confirmed by ANOVA variance analysis and Fisher’s post-hoc NIR test.
The polyphenol contents determined in the mushrooms tested are consistent with the reference literature’s data. According to various authors, the content of these compounds, expressed in gallic acid equivalents, is on average 575 mg/100 g according to Barros et al. [
38], 618 mg/100 g d.m. [
39], 800–1065 mg/g d.m. [
40] and 855 mg/100 g d.m. [
41]. In the study by Skąpska et al. [
33], in commercial mushrooms the polyphenols content was found to be 12,335 mg/kg d.m. (123.35 mg/100 g d.m.), i.e., significantly less than in the above literature, which may result from the fact that commercially purchased mushrooms were of lower quality than those tested in this study, coming directly from the producer. There are also reports of an increase in the polyphenol content in mushrooms (1.6-fold, on average) during 5-day cold storage of the fruiting bodies [
42]. In the study by Eissa [
32], the changes in the polyphenols content during 15-day cold storage (4 °C) of mushrooms were described. The highest increase in the content of these compounds after 3 days, and then a decrease to a value close to the initial state in fresh produce, was observed. The changes in polyphenols content, and thus in antioxidant activity, may be related to the physiological reaction of the produce to the harvest and to the change in the activity of oxidative enzymes, under the influence of damage during picking and exposure to oxygen [
32].
3.4. Colour Measurement
Table 4 shows the changes in colour parameters of mushrooms subjected to mechanical vibrations of the frequency of 46 Hz, followed by storage.
The lightness parameter (L) was 72.7–86.4. The lowest value was recorded on the first day for mushrooms that had been subjected to vibration for six hours, and the highest for fresh mushrooms, which means that they had the lightest colour. Parameter a* (change of colour in the range from green to red) assumed values from 1.2 (fresh produce, day 1) to 5.1 (vibration time 6 h, day 1), which means that changes occurred in the range of the red colour. Parameter b* (change in colour in the range from blue to yellow) assumed values in the range from 13.3 (fresh material, day 1) to 20.9 (vibration time 6 h, day 4), which means that the changes occurred in the yellow range. The colour difference (ΔE) calculated relative to the control sample (fresh raw material, day 1) for each of the samples was very significant (ΔE > 3), which means that the colour difference would be noticeable to any observer.
Table 5 shows the changes in colour parameters of mushrooms subjected to mechanical vibrations of 28 Hz, followed by storage.
In experiment II, using vibrations of 28 Hz, the lightness parameter (L) assumed values 76.7–87.4. The lowest value was recorded on the fourth day, for mushrooms that had been subjected to vibrations for six hours, and the highest for fresh mushrooms, on the first day, which means that they were characterised by the brightest colour. Parameter a* (change of colour in the range from green to red) assumed values from 0.4 (fresh raw material, day 1) to 4.8 (time of vibrations of 6 h, day 1), which means that the changes occurred in the range of red colour. Parameter b* (change of colour in the range from blue to yellow) assumed values in the range from 11.4 (fresh raw material, day 1) to 20.4 (vibration time 6 h, day 4), which means that the changes occurred in the yellow range. The colour difference (ΔE) calculated relative to the control sample (fresh raw material, day 1) was significant for the mushrooms subjected to vibration for three hours on day 1, amounting to 2.2. For the other samples, ΔE was > 3, which indicates a very significant colour difference.
The analysis of the measured values of colour parameters (
Table 4 and
Table 5) revealed that in both experiments colour changes occurred in an analogous way, and on the basis of the analysis of variance, it was found that the influence of the applied vibration, as well as storage conditions and times, was statistically significant (
p ≤ 0.05). The values of parameters a*, b* and C* increased, while the values of parameters L* and h* decreased with the increase in the application time of mechanical vibrations, as well as as a result of storage. Decreasing values of L* means darkening of the sample, decreasing the proportion of white colour. This was confirmed by the changes in parameters a* and b*, whose increase in value means an increase in the proportion of red and yellow, which reflects the processes of browning of the fruiting bodies during vibration and storage. The saturation of colour C* became more intense, and the tone angle of colour h* shifted from yellowish (h* about 90°) towards reddish brown. The brightness L* in the range of 86–87 for fresh mushrooms was consistent with the data published in the literature. In the sample of fresh mushrooms, the L* lightness ranged from 83 to 87, and did not change after washing in an aqueous solution containing 5% H2O2, 4.5% sodium isoascorbate, 0.2% cysteine hydrochloride and 0.1% EDTA, and after storage for 5 days (2 days at 1 °C and then 3 days at 13 °C) [
43]. However, the application of pre-treatment, involving washing in aqueous solution of sodium pyrosulphite (1000 mg/L) followed by blanching in water, resulted in a decrease in the value of L* to 73–74. The application of washing in aqueous solution of sodium pyrosulphite (1000 mg/L), vacuum soaking with water and then blanching resulted in further darkening of the mushrooms, to L* 68–71. The pre-treatment procedures with the use of antioxidant solutions enabled us to maintain the light colour of the mushrooms during storage in the processing. Fresh mushrooms may be stored at 0–1 °C for up to 7–9 days. In order to maintain the good quality of the mushrooms (shelf-life), a balanced temperature should be maintained during transport and storage, to prevent condensation on the fruiting bodies and packaging [
10].
In order to assess the level of colour change during the experiments, the coefficient ΔE, i.e., the total colour difference, was calculated in relation to the reference sample for which the original material was taken, i.e., fresh mushrooms not subjected to vibration or storage. The calculated values of ΔE were between 3.3 and 15.1, and 2.2 and 14.7, in the first and second experiments, respectively (
Table 4 and
Table 5). This means that all the experimental samples differed significantly from the initial sample, because values ΔE > 2 corresponded to the colour differences perceived by the average observer, and at values ΔE > 5 the observer has the impression of two different colours. The smallest colour differences from the control sample were found in mushrooms subjected to vibrations for 3 h (ΔE from 2.2 to 3.3), while prolonged vibration and storage resulted in noticeable browning of the fruit. The highest coefficient, ΔE = 15.13, was determined for mushrooms after 6 h of shaking at 46 Hz vibration frequency and 4 days of storage.
Table 6 shows the percentage differences in parameter values between initial sample and vibrated mushrooms.
The largest percentage differences in the values of tested parameters between initial samples and vibrated mushrooms occurred in the case of total extract and dry matter content, for mushrooms subjected to vibrations at a frequency of 46 Hz for 6 h, not stored. In the case of antioxidant activity, the largest diffference was in mushrooms vibrated at 28 Hz for 3 h, not stored, and in the case of polyphenol content, the largest difference was in mushrooms vibrated at 28 Hz for 3 h, stored for 4 days.