Physicochemical Analysis and Essential Oils Extraction of the Gorse (Ulex europaeus) and French Broom (Genista monspessulana), Two Highly Invasive Species in the Colombian Andes
Abstract
1. Introduction
2. Methods
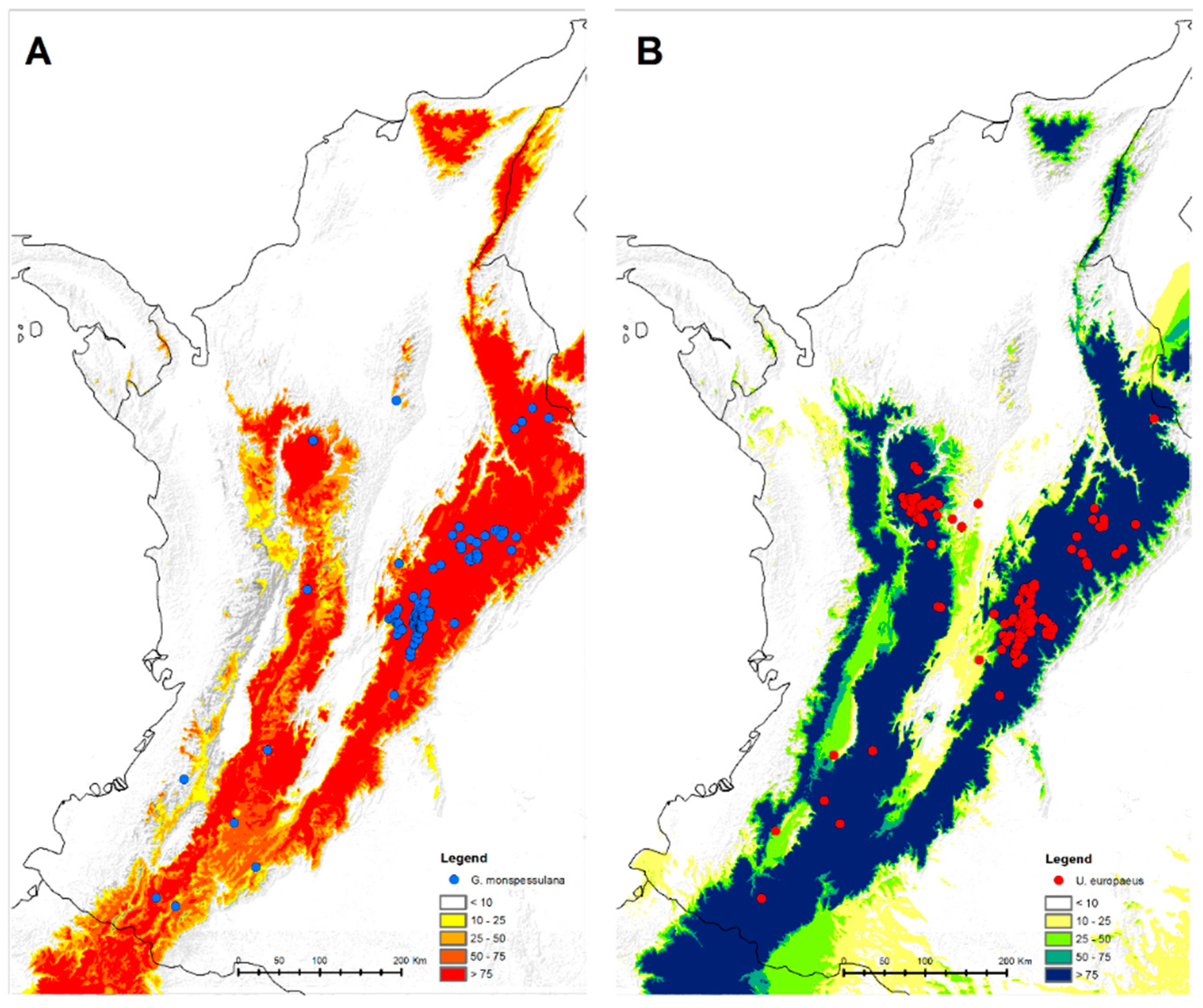
2.1. Data Sampling and Handling Procedure
2.2. Biomass Characterization
2.3. Lignocellulosic Characterization
2.4. Extraction of Essential Oils and Other Chemical Compounds of Interest
2.5. Statistic Analysis
3. Results
3.1. Biomass Characterization
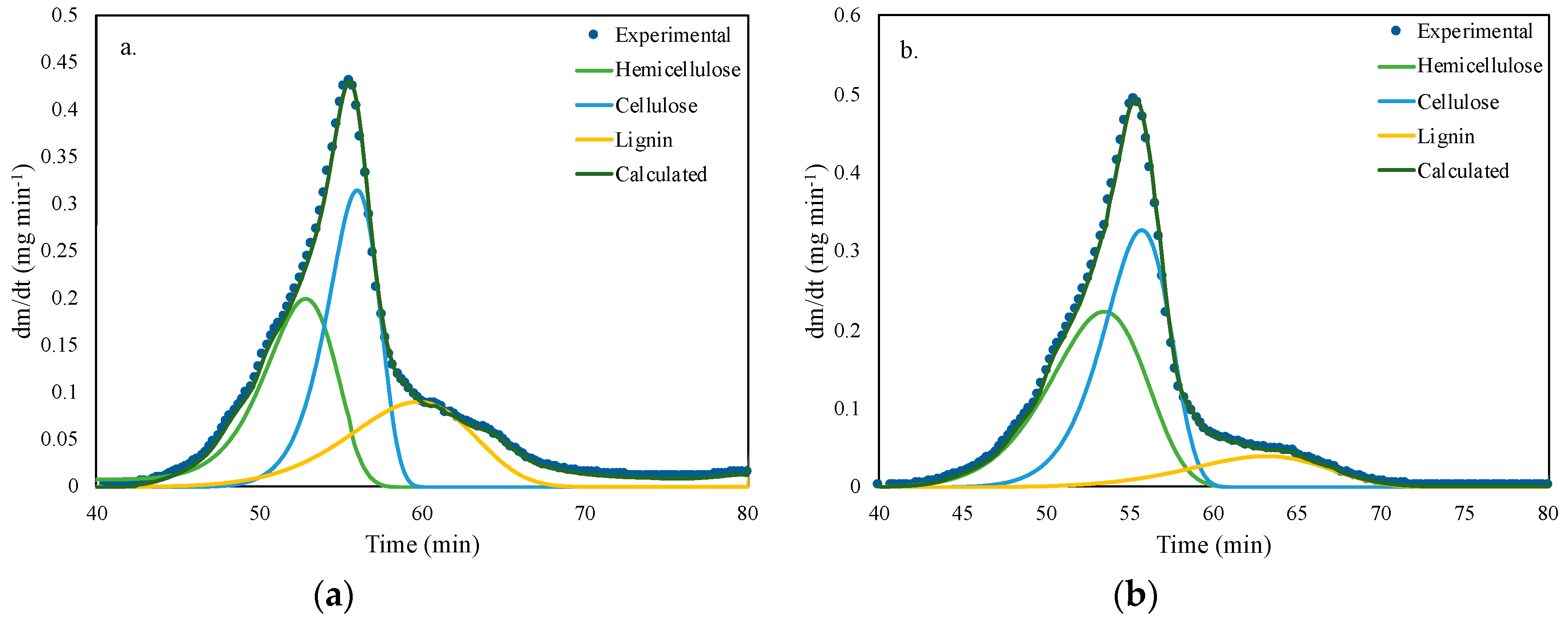
3.2. Thermogravimetric Analysis
3.3. Essential Oils and Other Chemical Compounds
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dragicevic, A.Z. Comparing forest governance models against invasive biological threats. J. Theor. Biol. 2019, 462, 270–282. [Google Scholar] [CrossRef] [PubMed]
- CABI. Genista Monspessulana (Montpellier Broom). Available online: https://www.cabi.org/isc/datasheet/25059#BFC61822-C72D-4D93-960A-66CDDF034DE4 (accessed on 20 September 2019).
- Minambiente. Invasive Exotic Species; Minambiente: Bogotá, Colombia, 2010; Volume 207, p. 7.
- León, O.; Vargas, O. Las especies invasoras: Un reto para la restauración ecológica. In Restauración Ecológica en Zonas Invadidas por Retamo Espinoso y Plantaciones Forestales de Especies Exóticas; Universidad Nacional de Colombia: Bogotá, Colombia, 2009; pp. 19–38. [Google Scholar]
- Baptiste, M.P.; Castaño, N.; Cárdenas, D.; Gutiérrez, F.P.; Gil, D.; Lasso, C. Análisis de Riesgo y Propuesta de Categorización de Especies Introducidas para Colombia; Instituto de Investigación en Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2010; ISBN 978-958-8343-46-4. [Google Scholar]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic Niche Shifts Are Rare Among Terrestrial Plant Invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.; Browbe, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species A Selection from the Global Invasive Species Database, 2nd ed.; The Invasive Species Specialist Group (ISSG) a Specialist Group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN): Auckland, New Zealand, 2004. [Google Scholar]
- Díaz, A.M.; Vargas, O. Chapter 3. Rasgos de historia de vida y ecología de las invasiones de Ulex europaeus. In Restauración Ecológica en Zonas Invadidas por Retamo Espinoso y Plantaciones Forestales de Especies Exóticas; Universidad Nacional de Colombia: Bogotá, Colombia, 2009; pp. 59–67. ISBN 978-958-719-314-5. [Google Scholar]
- Lee, W.G.; Allen, R.B.; Johnson, P.N. Succession and dynamics of gorse (Ulex europaeus L.) communities in the dunedin ecological district South Island, New Zealand. N. Z. J. Bot. 1986, 24, 279–292. [Google Scholar] [CrossRef]
- Ivens, G.W. Some aspects of seed ecology of gorse [Ulex europaeus]. Proc. N. Z. Weed Pest Control Conf. 1978, 31, 53–57. [Google Scholar]
- Alexander, J.M.; D’Antonio, C.M. Seed Bank Dynamics of French Broom in Coastal California Grasslands: Effects of Stand Age and Prescribed Burning on Control and Restoration. Restor. Ecol. 2003, 11, 185–197. [Google Scholar] [CrossRef]
- Popay, A.I.; Adams, C. Emergence of gorse seedlings from a root-raked area. In Proceedings of the Forty Third New Zealand Weed and Pest Control Conference, Dunedin, New Zealand, 14–16 August 1990; pp. 166–169. [Google Scholar]
- Ríos, H.F. Guía Técnica para la Restauración Ecológica de Áreas Afectadas por Especies Vegetales Invasoras en el Distrito Capital: Complejo Invasor Retamo Espinoso (Ulex europaeus L.)—Retamo Liso (Teline Monspessulana (L) C. Koch.); Jardin Botanico Jose Celestino Mutis: Bogotá, Colombia, 2005; ISBN 978-958-96823-9-5. [Google Scholar]
- Vargas, O. Restauración Ecológica del Bosque Altoandino - Estudios Diagnósticos y Experimentales en los Alrededores del Embalse de Chisacá, Localidad de Usme, Bogotá; Universidad Nacional de Colombia: Bogotá, Colombia, 2007; ISBN 978-958-701-848-6. [Google Scholar]
- Pauchard, A.; García, R.A.; Peña, E.; González, C.; Cavieres, L.A.; Bustamante, R.O. Positive feedbacks between plant invasions and fire regimes: Teline monspessulana (L.) K. Koch (Fabaceae) in central Chile. Biol. Invasions 2008, 10, 547–553. [Google Scholar] [CrossRef]
- García, R.A.; Engler, M.L.; Peña, E.; Pollnac, F.W.; Pauchard, A. Fuel characteristics of the invasive shrub Teline monspessulana (L.) K. Koch. Int. J. Wildland Fire 2015, 24, 372–379. [Google Scholar] [CrossRef]
- Gonzalez-Andres, F.; Ortiz, J.-M. Specificity of Rhizobia Nodulating Genista monspessulana and Genista linifolia In Vitro and in Field Situations. Arid Soil Res. Rehabil. 1999, 13, 223–237. [Google Scholar] [CrossRef]
- Cano, I.; Zamudio, N. Chapter 2. Estrategias de articulación y participación comunitaria con los proyectos de restauración ecológica. In Restauración Ecológica del Bosque Altoandino. Estudios Diagnósticos y Experimentales en los Alrededores del Embalse de Chisacá (Localidad de Usme, Bogotá D.C.); Universidad Nacional de Colombia: Bogotá, Colombia, 2007; pp. 104–145. ISBN 978-958-701-848-6. [Google Scholar]
- Alarcón, E.; Lozano de Yunda, A.; Chaparro, H. Caracterización fenotípica de aislamientos rizobianos de Acacia (Acacia sp.) y retamo (Teline monspessulana). Rev. Colomb. Quím. 1997, 26, 21–33. [Google Scholar]
- Smith, J.M.B. An introduction to the biogeography and ecology of broom (Cytisus scoparius) in Australia. Plant Prot. Q. 2000, 15, 140–144. [Google Scholar]
- García, R.A.; Fuentes-Ramírez, A.; Pauchard, A. Effects of two nitrogen-fixing invasive plants species on soil chemical properties in south-central Chile. Gayana Bot. 2012, 69, 189–192. [Google Scholar] [CrossRef][Green Version]
- Parsons, W.T.; Cuthbertson, E.G. Noxious Weeds of Australia, 2nd ed.; CSIRO Publishing: Clayton, Victoria, 2001; ISBN 978-0-643-06514-7. [Google Scholar]
- CABI Ulex Europaeus (Gorse). Available online: https://www.cabi.org/isc/datasheet/55561 (accessed on 22 February 2019).
- Ríos, A. Eliminación de la Especie Invasora Ulex Europaeus L. (Fabaceae) Como Estrategia Experimental de Restauración de la Vegetación del Cerro de Monserrate, Bogotá. Ph.D. Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2001. [Google Scholar]
- Hornoy, B.; Tarayre, M.; Hervé, M.; Gigord, L.; Atlan, A. Invasive Plants and Enemy Release: Evolution of Trait Means and Trait Correlations in Ulex europaeus. PLoS ONE 2011, 6, e26275. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, A.; Cely, J.P.; Etter, A.; Miranda, A.; Fuentes-Ramirez, A.; Acevedo, P.; Salas, C.; Vargas, R. The invasive species Ulex europaeus (Fabaceae) shows high dynamism in a fragmented landscape of south-central Chile. Environ. Monit. Assess 2016, 188, 495. [Google Scholar] [CrossRef] [PubMed]
- López-Hortas, L.; Conde, E.; Falqué, E.; Domínguez, H. Flowers of Ulex europaeus L.—Comparing two extraction techniques (MHG and distillation). Comptes Rendus Chim. 2016, 19, 718–725. [Google Scholar] [CrossRef]
- Udo, N.; Darrot, C.; Atlan, A. From useful to invasive, the status of gorse on Reunion Island. J. Environ. Manag. 2019, 229, 166–173. [Google Scholar] [CrossRef]
- Morin, L.; Gianotti, A.F.; Lauren, D.R. Trichothecene production and pathogenicity of Fusarium tumidum, a candidate bioherbicide for gorse and broom in New Zealand. Mycol. Res. 2000, 104, 993–999. [Google Scholar] [CrossRef]
- López-López, N.; López-Fabal, A. Compost based ecological growing media according EU eco-label requirements. Sci. Hortic. 2016, 212, 1–10. [Google Scholar] [CrossRef]
- Audette, G.F.; Vandonselaar, M.; Delbaere, L.T. The 2.2 A resolution structure of the O(H) blood-group-specific lectin I from Ulex europaeus. J. Mol. Biol. 2000, 304, 423–433. [Google Scholar] [CrossRef]
- Kostova, I.N.; Nikolov, N.M.; Chipilska, L.N. Antimicrobial properties of some hydroxycoumarins and Fraxinus ornus bark extracts. J. Ethnopharmacol. 1993, 39, 205–208. [Google Scholar] [CrossRef]
- Mongrand, S.; Bessoule, J.-J.; Cabantous, F.; Cassagne, C. The C16:3\C18:3 fatty acid balance in photosynthetic tissues from 468 plant species. Phytochemistry 1998, 49, 1049–1064. [Google Scholar] [CrossRef]
- Saldarriaga, J.F.; Patiño, J.L.; Lizarazo, M.J. Kinetic Study of Spiny Retamo (Ulex Eurioaeus, L.) Waste Oxidative Pyrolysis. Chem. Eng. Trans. 2018, 70, 1249–1254. [Google Scholar]
- Ocampo-Zuleta, K.; Solorza-Bejarano, J. Banco de semillas de retamo espinoso Ulex europaeus L. en bordes del matorral invasor en un ecosistema zonal de bosque altoandino, Colombia. Biota Colomb. 2017, 18, 89–98. [Google Scholar] [CrossRef]
- Zabaleta, A. Caracterización horizontal y vertical de los bancos de semillas germinables de Ulex europaeus L. (Fabaceae) en parches de diferentes tamaños en el Embalse de Chisacá Localidad de Usme, Bogotá, D.C. In Restauración Ecológica del Bosque Altoandino. Estudios Diagnósticos y Experimentales en los Alrededores del Embalse de Chisacá (Localidad de Usme, Bogotá D.C.); Universidad Nacional de Colombia: Bogotá, Colombia, 2007; pp. 353–367. ISBN 978-958-701-848-6. [Google Scholar]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. Biomod2: Ensemble Platform for Species Distribution Modeling. R Package Vers. 2013, 2, r560. [Google Scholar]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Saldarriaga, J.F.; Aguado, R.; Pablos, A.; Amutio, M.; Olazar, M.; Bilbao, J. Fast characterization of biomass fuels by thermogravimetric analysis (TGA). Fuel 2015, 140, 744–751. [Google Scholar] [CrossRef]
- Gaspar, F. Extraction of Essential Oils and Cuticular Waxes with Compressed CO2: Effect of Extraction Pressure and Temperature. Ind. Eng. Chem. Res. 2002, 41, 2497–2503. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Saldarriaga, J.F.; Grace, J.; Lim, C.J.; Wang, Z.; Xu, N.; Atxutegi, A.; Aguado, R.; Olazar, M. Bed-to-surface heat transfer in conical spouted beds of biomass–sand mixtures. Powder Technol. 2015, 283, 447–454. [Google Scholar] [CrossRef]
- Saldarriaga, J.F.; Aguado, R.; Atxutegi, A.; Grace, J.; Bilbao, J.; Olazar, M. Correlation for Calculating Heat Transfer Coefficient in Conical Spouted Beds. Ind. Eng. Chem. Res. 2016, 55, 9524–9532. [Google Scholar] [CrossRef]
- Lambert, M.G.; Jung, G.A.; Harpster, H.W.; Lee, J. Forage shrubs in North Island hill country 4. Chemical composition and conclusions. N. Z. J. Agric. Res. 1989, 32, 499–506. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An overview of the organic and inorganic phase composition of biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Aguado, R.; Artetxe, M.; Bilbao, J.; Olazar, M. Kinetic study of lignocellulosic biomass oxidative pyrolysis. Fuel 2012, 95, 305–311. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-Depth Investigation of Biomass Pyrolysis Based on Three Major Components: Hemicellulose, Cellulose and Lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Sebio-Puñal, T.; Naya, S.; López-Beceiro, J.; Tarrío-Saavedra, J.; Artiaga, R. Thermogravimetric analysis of wood, holocellulose, and lignin from five wood species. J. Therm. Anal. Calorim. 2012, 109, 1163–1167. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Boulanouar, B.; Abdelaziz, G.; Aazza, S.; Gago, C.; Miguel, M.G. Antioxidant activities of eight Algerian plant extracts and two essential oils. Ind. Crops Prod. 2013, 46, 85–96. [Google Scholar] [CrossRef]
- Nihei, K.; Shibata, K.; Kubo, I. (+)-2,3-Dehydro-10-oxo-α-isosparteine in Uresiphita reversalis larvae fed on Cytisus monspessulanus leaves. Phytochemistry 2002, 61, 987–990. [Google Scholar] [CrossRef]
- Parra, C. Actividad Antimicrobiana y Caracterización Química del Aceite Esencial de Ulex Europaeus L. (Fabaceae); Universidad Dristrial Francisco José de Caldas: Bogotá, Colombia, 2017. [Google Scholar]
- Máximo, P.; Lourenço, A.; Tei, A.; Wink, M. Chemotaxonomy of Portuguese Ulex: Quinolizidine alkaloids as taxonomical markers. Phytochemistry 2006, 67, 1943–1949. [Google Scholar] [CrossRef]
- Spínola, V.; Llorent-Martínez, E.J.; Gouveia-Figueira, S.; Castilho, P.C. Ulex europaeus: From noxious weed to source of valuable isoflavones and flavanones. Ind. Crops Prod. 2016, 90, 9–27. [Google Scholar] [CrossRef]
- Ligero, P.; de Vega, A.; van der Kolk, J.C.; van Dam, J.E.G. Gorse (Ulex europæus) as a possible source of xylans by hydrothermal treatment. Ind. Crops Prod. 2011, 33, 205–210. [Google Scholar] [CrossRef]
- Li, J.; Hao, H.; Guo, N.; Wang, N.; Hao, Y.; Luan, Y.; Chen, K.; Huang, X. Solubility and thermodynamic properties of maltol in different pure solvents. J. Mol. Liq. 2017, 243, 313–323. [Google Scholar] [CrossRef]
- Gan, T.; Lv, Z.; Liu, N.; Sun, J.; Shi, Z.; Zhao, A. Ultrasensitive Electrochemical Sensor for Maltol in Wines Using Graphene Oxide-Wrapped Amino-Functionalized Carbon Sphere as Sensing Electrode Materials. Electroanalysis 2016, 28, 103–110. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.; Ali Khan, K.; Javed Ansari, M.; Almasaudi, S.B.; Al-Kahtani, S. Effect of gut bacterial isolates from Apis mellifera jemenitica on Paenibacillus larvae infected bee larvae. Saudi J. Biol. Sci. 2018, 25, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, C.; Balloi, A.; Essanaa, J.; Crotti, E.; Gonella, E.; Raddadi, N.; Ricci, I.; Boudabous, A.; Borin, S.; Manino, A.; et al. Gut microbiome dysbiosis and honeybee health. J. Appl. Entomol. 2011, 135, 524–533. [Google Scholar] [CrossRef]
- Skandamis, P.N.; Nychas, G.-J.E. Development and Evaluation of a Model Predicting the Survival of Escherichia coli O157:H7 NCTC 12900 in Homemade Eggplant Salad at Various Temperatures, pHs, and Oregano Essential Oil Concentrations. Appl. Environ. Microbiol. 2000, 66, 1646–1653. [Google Scholar] [CrossRef]
- Osaili, T.M.; Al-Nabulsi, A.A.; Jaradat, Z.; Shaker, R.R.; Alomari, D.Z.; Al-Dabbas, M.M.; Alaboudi, A.R.; Al-Natour, M.Q.; Holley, R.A. Survival and growth of Salmonella Typhimurium, Escherichia coli O157:H7 and Staphylococcus aureus in eggplant dip during storage. Int. J. Food Microbiol. 2015, 198, 37–42. [Google Scholar] [CrossRef]
- Alali, W.Q.; Mann, D.A.; Beuchat, L.R. Viability of Salmonella and Listeria monocytogenes in Delicatessen Salads and Hummus as Affected by Sodium Content and Storage Temperature. J. Food Prot. 2012, 75, 1043–1056. [Google Scholar] [CrossRef]
- Tassou, C.C.; Samaras, F.J.; Arkoudelos, J.S.; Mallidis, C.G. Survival of acid-adapted or non-adapted Salmonella Enteritidis, Listeria monocytogenes and Escherichia coli O157:H7, in traditional Greek salads. Int. J. Food Sci. Technol. 2009, 44, 279–287. [Google Scholar] [CrossRef]
- Al-Holy, M.; Al-Qadiri, H.; Lin, M.; Rasco, B. Inhibition of Listeria innocua in Hummus by a Combination of Nisin and Citric Acid. J. Food Prot. 2006, 69, 1322–1327. [Google Scholar] [CrossRef]
- Al-Rousan, W.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Ajo, R.Y.; Holley, R.A. Use of acetic and citric acids to inhibit Escherichia coli O157:H7, Salmonella Typhimurium and Staphylococcus aureus in tabbouleh salad. Food Microbiol. 2018, 73, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Al-Nabulsi, A.A.; Olaimat, A.N.; Osaili, T.M.; Shaker, R.R.; Zein Elabedeen, N.; Jaradat, Z.W.; Abushelaibi, A.; Holley, R.A. Use of acetic and citric acids to control Salmonella Typhimurium in tahini (sesame paste). Food Microbiol. 2014, 42, 102–108. [Google Scholar] [CrossRef] [PubMed]
| Property | Functional Part Analyzed | |||||||
|---|---|---|---|---|---|---|---|---|
| G-Flower | FB-Flower | G-Leaf | FB-Leaf | G-Seed | FB-Seed | G-Stem | FB-Stem | |
| HHV (MJ/kg) | 18.41 | 19.33 | 19.11 | 21.61 | 20.83 | 20.76 | 19.15 | 19.35 |
| C (wt. %. d.b.) | 42.10 | 42.50 | 45.70 | 45.90 | 45.80 | 46.50 | 44.50 | 44.40 |
| H (wt. %. d.b.) | 5.85 | 6.23 | 6.08 | 6.17 | 6.44 | 6.49 | 5.78 | 5.92 |
| N (wt. %. d.b.) | 2.23 | 2.54 | 2.19 | 3.53 | 5.28 | 4.51 | 1.51 | 1.45 |
| S (wt. %. d.b.) | 0.46 | 0.60 | 0.49 | 0.66 | 0.47 | 0.50 | 0.51 | 0.34 |
| O (wt. %. d.b.) | 49.36 | 48.13 | 45.54 | 43.74 | 42.01 | 42.00 | 47.70 | 47.89 |
| Moisture (wt. %. w.b.) | 0.38 | 0.41 | 0.40 | 0.37 | 0.56 | 0.58 | 0.16 | 0.04 |
| Volatile matter (wt. %. d.b.) | 85.05 | 96.44 | 81.28 | 93.16 | 88.75 | 96.24 | 82.47 | 97.47 |
| Fixed carbon (wt. %. d.b.) | 11.12 | 0.21 | 12.30 | 3.84 | 6.76 | 0.003 | 13.05 | 0.54 |
| Ashes (wt. %. d.b.) | 3.51 | 2.95 | 6.10 | 2.66 | 4.00 | 3.18 | 4.35 | 1.96 |
| Functional Part | Kinetic Parameter | Hemicellulose | Cellulose | Lignin |
|---|---|---|---|---|
| G-flower | Ln k0 (s−1) | 5.42 | 13.26 | 1.30 |
| E (kJ mol−1) | 50.32 | 93.04 | 41.28 | |
| Content (% p/p) | 26.24 | 28.65 | 40.73 | |
| FB-flower | Ln k0 (s−1) | 2.00 | 4.43 | 1.52 |
| E (kJ mol−1) | 35.32 | 50.04 | 51.28 | |
| Content (% p/p) | 29.68 | 29.01 | 37.82 | |
| G-leaf | Ln k0 (s−1) | 9.36 | 6.97 | 13.65 |
| E (kJ mol−1) | 70.32 | 64.04 | 111.28 | |
| Content (% p/p) | 26.99 | 35.50 | 30.86 | |
| FB-leaf | Ln k0 (s−1) | 5.43 | 7.01 | 5.90 |
| E (kJ mol−1) | 50.32 | 63.04 | 69.28 | |
| Content (% p/p) | 26.94 | 33.93 | 35.74 | |
| G-seed | Ln k0 (s−1) | 4.82 | 12.62 | 1.30 |
| E (kJ mol−1) | 50.32 | 93.04 | 41.28 | |
| Content (% p/p) | 27.52 | 30.06 | 37.70 | |
| FB-seed | Ln k0 (s−1) | 4.94 | 12.09 | −0.13 |
| E (kJ mol−1) | 50.32 | 89.04 | 45.28 | |
| Content (% p/p) | 34.47 | 33.79 | 27.96 | |
| G-stem | Ln k0 (s−1) | 11.99 | 20.84 | 5.40 |
| E (kJ mol−1) | 80.32 | 130.94 | 61.28 | |
| Content (% p/p) | 24.95 | 30.50 | 39.78 | |
| FB-stem | Ln k0 (s−1) | 7.35 | 14.87 | 5.28 |
| E (kJ mol−1) | 60.32 | 100.04 | 65.28 | |
| Content (% p/p) | 36.13 | 37.20 | 24.34 |
| Compound | U. europaeus | G. monspessulana |
|---|---|---|
| (R)-(-)-14-Methyl-8-hexadecyn-1-ol | Seed | Flower and stem |
| 1,2-15,16-Diepoxyhexadecane | Leaf and Seed | Flower and stem |
| 1,2-Benzisothiazole, 3-(hexahydro-1H-azepin-1-yl)-, 1,1-dioxide | Flower, leaf and seed | |
| 10,13-Octadecadienoic acid, methyl ester | Seed and stem | |
| 12-Methyl-E,E-2,13-octadecadien-1-ol | Flower and leaf | stem |
| 2,3-Butanediol | Leaf, seed and stem | |
| 2-Methoxy-4-vinylphenol | Leaf and seed | Flower and leaf |
| 2-Methyl-3-(3-methyl-but-2-enyl)-2-(4-methyl-pent-3-enyl)-oxetane | Flower and leaf | |
| 2-Methyl-Z,Z-3,13-octadecadienol | Flower and leaf | |
| 2-Pyrrolidinone | Seed and stem | |
| 3-Buten-2-ol, 2-methyl- | Flower and seed | Flower and leaf |
| 4-(2,6,6-Trimethylcyclohexa-1,3-dienyl)but-3-en-2-one | Flower, leaf and stem | |
| 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | Flower, leaf and seed | |
| 5,6-Dehydrolupanine | Flower, leaf and stem | |
| 9,17-Octadecadienal, (Z)- | Flower and seed | Seed |
| Acetic acid | All organs | Flower, seed and stem |
| Amylene Hydrate | All organs | Flower and seed |
| Anagyrine | Flower | Leaf and stem |
| Aphyline | All organs | |
| Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester | Flower, seed and stem | Flower, seed and stem |
| Bufotenine | All organs | |
| Butyrolactone | Flower and stem | |
| Caulophylline | Seed | All organs |
| Cyclododecane | Flower | Leaf and stem |
| Cyclohexasiloxane, dodecamethyl- | Seed | Leaf and stem |
| Cyclopentadecanone, 2-hydroxy- | Flower and stem | |
| Cyclopropaneoctanal, 2-octyl- | Flower, leaf and seed | Stem |
| Diethylene glycol monobutyl ether | Leaf and Seed | |
| Dihydroactinidiolide | Leaf and stem | |
| Eicosane | Flower, leaf and stem | Stem |
| Ethanol, 1-(2-butoxyethoxy)- | Flower, leaf and stem | Seed and stem |
| Heptadecane | Leaf | Flower and stem |
| Lauric acid | Flower and seed | Flower, leaf and stem |
| Linoleic Acid | Flower, leaf and seed | Flower, seed and stem |
| Maltol | Seed | Flower, seed and stem |
| Methyl linolenate | Flower and leaf | Flower and leaf |
| Methyl palmitate | Flower, seed and stem | Seed and stem |
| m-Xylene | Flower and stem | Seed |
| Octadecane | Flower and stem | |
| Oleamide | Leaf and seed | |
| Oleic Acid | Flower and leaf | Stem |
| Palmitic acid | Flower, leaf and stem | Leaf, seed and stem |
| Phytol | Leaf and Seed | Flower, leaf and stem |
| Psilocin | Leaf and stem | |
| Pyridine, 3-methoxy- | Leaf and Seed | Seed and stem |
| Squalene | Flower and stem | |
| Stearic acid | All organs | All organs |
| Tetracosane | Flower and stem | |
| Tetradecanoic acid | Flower and seed | Flower and stem |
| Triacetin | Seed | Seed and stem |
| Undecane | Flower, seed and stem | |
| Vitamin E | Leaf and stem | |
| α-Isoaphylline | All organs |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio-Castiblanco, D.F.; Peyre, G.; Saldarriaga, J.F. Physicochemical Analysis and Essential Oils Extraction of the Gorse (Ulex europaeus) and French Broom (Genista monspessulana), Two Highly Invasive Species in the Colombian Andes. Sustainability 2020, 12, 57. https://doi.org/10.3390/su12010057
Osorio-Castiblanco DF, Peyre G, Saldarriaga JF. Physicochemical Analysis and Essential Oils Extraction of the Gorse (Ulex europaeus) and French Broom (Genista monspessulana), Two Highly Invasive Species in the Colombian Andes. Sustainability. 2020; 12(1):57. https://doi.org/10.3390/su12010057
Chicago/Turabian StyleOsorio-Castiblanco, Diego F., Gwendolyn Peyre, and Juan F. Saldarriaga. 2020. "Physicochemical Analysis and Essential Oils Extraction of the Gorse (Ulex europaeus) and French Broom (Genista monspessulana), Two Highly Invasive Species in the Colombian Andes" Sustainability 12, no. 1: 57. https://doi.org/10.3390/su12010057
APA StyleOsorio-Castiblanco, D. F., Peyre, G., & Saldarriaga, J. F. (2020). Physicochemical Analysis and Essential Oils Extraction of the Gorse (Ulex europaeus) and French Broom (Genista monspessulana), Two Highly Invasive Species in the Colombian Andes. Sustainability, 12(1), 57. https://doi.org/10.3390/su12010057






