Capability of the Invasive Tree Prosopis glandulosa Torr. to Remediate Soil Treated with Sewage Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Growth Media Analyses
2.3. Plant Traits
2.4. Statistical Analysis
3. Results
3.1. Effects of Sewage Sludge on Growth Media
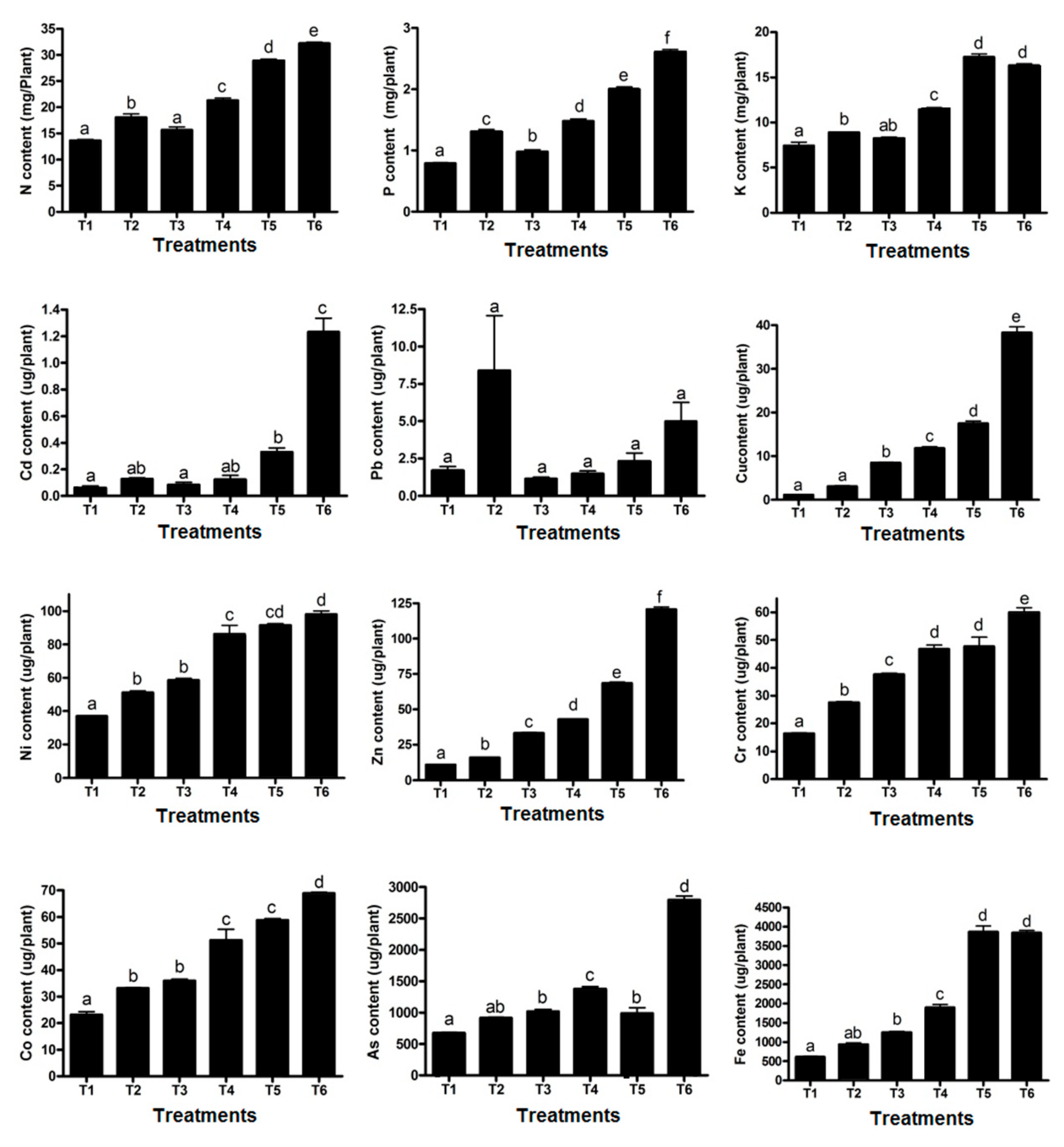
3.2. Effect of Sewage Sludge on Plant Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mosquera-Losada, M.R.; Morán-Zuloaga, D.; Rigueiro-Rodriguez, A. Effects of lime and sewage sludge on soil, pasture production, and tree growth in a six-year-old Populus Canadensis Moench silvopastoral system. J. Plant Nutr. Soil Sci. 2011, 174, 145–153. [Google Scholar] [CrossRef]
- Cerqueira, B.; Vega, F.A.; Silva, L.F.O.; Andrade, L. Effects of vegetation on chemical and mineralogical characteristics of soils developed on a decantation bank from a copper mine. Sci. Total Environ. 2012, 421–422, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J. Land application of sewage sludge in China. Sci. Total Environ. 1997, 197, 149–160. [Google Scholar] [CrossRef]
- Dar, M.I.; Khan, F.A.; Green, I.D.; Naikoo, M.I. The transfer and fate of Pb from sewage sludge amended soil in a multi-trophic food chain: A comparison with the labile elements Cd and Zn. Environ. Sci. Pollut. Res. 2015, 22, 16133–16142. [Google Scholar] [CrossRef]
- Yuswir, N.S.; Praveena, S.M.; Aris, A.Z.; Ismail, S.N.S.; Hashim, Z. Health risk assessment of heavy metal in urban surface soil (Klang District, Malaysia). Bull. Environ. Contam. Toxicol. 2015, 95, 80–89. [Google Scholar] [CrossRef]
- Nissim, W.G.; Cincinelli, A.; Martellini, T.; Alvisi, L.; Palm, E.; Mancuso, S.; Azzarello, E. Phytoremediation of sewage sludge contaminated by trace elements and organic compounds. Environ. Res. 2018, 164, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Pendergrass, A.; Butcher, D.J. Uptake of lead and arsenic in food plants grown in contaminated soil from Barber Orchard, NC. Microchem. J. 2006, 83, 14–16. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Reeves, R.D.; McGrath, S.P. In Situ Decontamination of Heavy Metal Polluted Soils Using Crops of Metal-Accumulating Plants—A Feasibility Study. In Situ Bioreclamation 1991, 600–605. [Google Scholar] [CrossRef]
- Greipsson, S. Phytoremediation. Nat. Educ. Knowl. 2011, 3, 7. [Google Scholar]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals-Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Soliman, W.S.; Sugiyama, S. Phytoremediation and tolerance capacity of moringa to cadmium and its relation to nutrients content. Pollut. Res. 2016, 35, 23–27. [Google Scholar]
- Krzciuk, K.; Galuszka, A. Prospecting for hyperaccumulators of trace elements: A review. Crit. Rev. Biotechnol. 2015, 35, 522–532. [Google Scholar] [CrossRef]
- Shen, Z.G.; Zhao, F.J.; McGrath, S.P. Uptake and transport of zinc in the hyperaccumulator Thlaspi caerulescens and the nonhyperaccumulator Thlaspi Ochroleucum. Plant Cell Environ. 1997, 20, 898–906. [Google Scholar] [CrossRef]
- McGrath, S.P.; Shen, Z.G.; Zhao, F.J. Heavy metal uptake and chemical changes in the rhizosphere of Thiaspi caerulescens and Thlaspi ochroleucum grown in contaminated soils. Plant Soil 1997, 188, 153–159. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Factors Affecting Phytoextraction: A Review. Pedosphere 2016, 26, 148–166. [Google Scholar] [CrossRef]
- Dickinson, N.M. Strategies for sustainable woodland on contaminated soils. Chemosphere 2000, 41, 259–263. [Google Scholar] [CrossRef]
- Meers, E.; Lamsal, S.; Vervaeke, P.; Hopgood, M.; Lust, N.; Tack, F.M.G. Availability of heavy metals for uptake by Salix viminalis on a moderately contaminated dredged sediment disposal site. Environ. Pollut. 2005, 137, 354–364. [Google Scholar] [CrossRef]
- French, C.J.; Dickinson, N.M.; Putwain, P.D. Woody biomass phytoremediation of contaminated brownfield land. Environ. Pollut. 2006, 141, 387–395. [Google Scholar] [CrossRef]
- Pulford, I.D.; Watson, C. Phytoremediation of heavy metal-contaminated land by trees—A review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef]
- Pandey, V.C. Invasive species based efficient green technology for phytoremediation of fly ash deposits. J. Geochem. Explor. 2012, 123, 13–18. [Google Scholar] [CrossRef]
- Abbas, A.M.; Hammad, S.; Soliman, W.S. Influence of copper and lead on germination of three Mimosoideae plant species. Asian J. Agric. Biol. 2017, 5, 320–327. [Google Scholar]
- Yang, R.; Tang, H.J.; Yang, Y.; Chen, X. Invasive and non-invasive plants differ in response to soil heavy metal lead contamination. Bot. Stud. 2007, 48, 453–458. [Google Scholar]
- Mateos-Naranjo, E.; Andrades-Moreno, L.; Redondo-Gómez, S. Comparison of germination, growth, photosynthetic responses and metal uptake between three populations of Spartina densiflora under different soil pollution conditions. Ecotoxicol. Environ. Saf. 2011, 74, 2040–2049. [Google Scholar] [CrossRef] [PubMed]
- Pasiecznik, N.M.; Felker, P.; Harris, P.J.C.; Harsh, L.N.; Cruz, G.; Tewari, J.C.; Cadoret, K.; Maldonado, L.J. The Prosopis Juliflora—Prosopis Pallida Complex: Complex: A Monograph; HDRA: Coventry, UK, 2001; p. 172. [Google Scholar]
- Global Invasive Species Database. 2019. Available online: http://www.iucngisd.org/gisd/100_worst.php (accessed on 10 May 2019).
- Andersson, S. Spread of the Introduced Tree Species Prosopis Juliflora (SW.) DC in the Lake Baringo Area, Kenya; Institutionen for Skoglif Vegetationsekologi, SLU (Swedish Agricultural University): Umea, Sweden, 2005. [Google Scholar]
- Abbas, A.M.; Soliman, W.S.; Mansour, A.; Taher, E.; Hassan, I.N.; Mahmoud, M.; Youssif, M.F.; Mansour, H.; Abdelkareem, M. Predicting the spatial spread of invasive Prosopis juliflora (SW.) D.C. along environmental gradients in Gabel Elba National Park, Egypt. Int. J. Sci. Eng. Res. 2016, 7, 596–599. [Google Scholar] [CrossRef]
- Usha, B.; Venkataraman, G.; Parida, A. Heavy metal and abiotic stress inducible metallothionein isoforms from Prosopis juliflora (SW) DC show differences in binding to heavy metals in vitro. Mol. Genet. Genom. 2009, 281, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Shukla, O.P.; Juwarkar, A.A.; Singh, S.K.; Khan, S.; Rai, U.N. Growth responses and metal accumulation capabilities of woody plants during the phytoremediation of tannery sludge. Waste Manag. 2011, 31, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Varun, M.; D’Souza, R.; Prata, J.; Paul, M.S. Phytoextraction potential of Prosopis juliflora (Sw.) DC. with specific reference to lead and cadmium. Bull. Environm. Contam. Toxicol. 2011, 87, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Khan, S.; Baunthiyal, M.; Sharma, V. Effects of fluoride on germination, early growth and antioxidant enzyme activities of legume plant species Prosopis juliflora. J. Environ. Biol. 2013, 34, 205–209. [Google Scholar] [PubMed]
- Abdalla, M.A.; Elkarim, A.H.A.; Taniguchi, T.; Endo, T.; Yamanaka, N. Phytoremediation of calcareous saline-sodic soils with mesquite (Prosopis glandulosa). Acta Agric. Scand. B-Soil Plant Sci. 2017, 67, 352–361. [Google Scholar] [CrossRef]
- Guo, L.; Cutright, T.J.; Duirk, S. Effect of citric acid, rhizosphere bacteria, and plant age on metal uptake in reeds cultured in acid mine drainage. Water Air Soil Pollut. 2015, 226, 1. [Google Scholar] [CrossRef]
- Ball, J. The Geography and Geology of South-Eastern Egypt; Ministry of Edu. Government Press: Cairo, Egypt, 1912; p. 394.
- Al-Gohary, I.H. Floristic composition of eleven wadis in Gebel Elba, Egypt. Int. J. Agric. Biol. 2008, 10, 151–160. [Google Scholar]
- Schulte, E.E.; Kaufmann, C.; Peter, J.B. The influence of sample size and heating time on soil weight loss-onignition. Commun. Soil Sci. Plant Anal. 1991, 22, 159–168. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and waters table aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Kołodziej, B.; Bielińska, E.J. The use of reed canary grass and giant miscanthus in the phytoremediation of municipal sewage sludge. Environ. Sci. Pollut. Res. 2016, 23, 9505–9517. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Chiroma, T.M.; Ebewele, R.O.; Hymore, F.K. Comparative assessment of heavy metal levels in soil, vegetables and urban grey waste water used for irrigation in Yola and Kano. Int. Refereed J. Eng. Sci. 2014, 3, 1–9. [Google Scholar]
- Smith, S.R. Agricultural recycling of sewage sludge and the environment. CAB Int. 1996. [Google Scholar] [CrossRef]
- Gupta, A.K.; Sinha, S. Phytoextraction capacity of the plants growing on tannery sludge dumping sites. Bioresour. Technol. 2007, 98, 1788–1794. [Google Scholar] [CrossRef]
- Dussault, M.; Bécaert, V.; François, M.; Sauvé, S.; Deschênes, L. Effect of copper on soil functional stability measured by relative soil stability index (RSSI) based on two enzyme activities. Chemosphere 2008, 72, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Lago, D.; Vega, F.A.; Silva, L.F.O.; Andrade, M.L. Soil interaction and fractionation of added cadmium in some Galician soils. Microchem. J. 2013, 110, 681–690. [Google Scholar] [CrossRef]
- Solis-Dominguez, F.A.; Valentin-Vargas, A.; Chorover, J.; Maier, R.M. Effect of arbuscular mycorrhizal fungi on plant biomass and the rhizosphere microbial community structure of mesquite grown in acidic lead/zinc mine tailings. Sci. Total Environ. 2011, 409, 1009–1016. [Google Scholar] [CrossRef]
- Khan, M.U.; Sessitsch, A.; Harris, M.; Fatima, K.; Imran, A.; Arslan, M.; Shabir, G.; Khan, Q.M.; Afzal, M. Cr-resistant rhizo- and endophytic bacteria associated with Prosopis juliflora and their potential as phytoremediation enhancing agents in metal-degraded soils. Front. Plant Sci. 2015, 5, 755. [Google Scholar] [CrossRef]
- Keeran, N.S.; Ganesan, G.; Parida, A.K. A novel heavy metal ATPase peptide from Prosopis juliflora is involved in metal uptake in yeast and tobacco. Transgenic Res. 2017, 26, 247–261. [Google Scholar] [CrossRef]
- Mokgalaka-Matlala, N.S.; Flores-Tavizon, E.; Castillo-Michel, H.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Toxicity of arsenic (III) and (V) on plant growth, element uptake, and total amylolytic activity of mesquite (Prosopis juliflora x P. velutina). Int. J. Phytoremediation 2008, 10, 47–60. [Google Scholar] [CrossRef]
- Yoda, K.Y.; Tsuji, W.; Inoune, T.; Saito, T.; Abd Elbasit, M.A.M.; Eldoma, A.M.; Magzoub, M.K.; Hoshino, B.; Nawata, H.; Yasuda, H. Evaluation of the Effect of a Rain Pulse on the Initial Growth of Prosopis Seedlings. Arid Land Res. Manag. 2015, 29, 210–221. [Google Scholar] [CrossRef]
- Belhaj, D.; Elloumi, N.; Jerbi, B.; Zouari, M.; Abdallah, F.B.; Ayadi, H.; Kallel, M. Effects of sewage sludge fertilizer on heavy metal accumulation and consequent responses of sunflower (Helianthus annuus). Environ. Sci. Pollut. Res. 2016, 23, 1–10. [Google Scholar] [CrossRef]
- Yang, X.E.; Li, T.Q.; Yang, J.C.; He, Z.L.; Lu, L.L.; Meng, F.H. Zinc compartmentation in root, transport into xylem, and absorption into leaf cells in the hyperaccumulating species of Sedum alfredii Hance. Planta 2006, 224, 185–195. [Google Scholar] [CrossRef]
- Hammond, C.M.; Root, R.A.; Maier, R.M.; Chorover, J. Mechanisms of arsenic sequestration by Prosopis juliflora during the phytostabilization of metalliferous mine tailings. Environ. Sci. Technol. 2018, 52, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Gardea-Torresdey, J.L.; Peralta-Videa, J.R.; de la Rosa, G.; Parsons, J.G. Phytoremediation of heavy metals and study of the metal coordination by X-ray absorption spectroscopy. Coordinat. Chem. Rev. 2005, 249, 1797–1810. [Google Scholar] [CrossRef]
- Van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulation of metal and metalloid trace elements: Facts and function. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Burges, A.; Alkorta, I.; Epelde, L.; Garbisu, C. From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int. J. Phytoremediation 2018, 20, 384–397. [Google Scholar] [CrossRef] [PubMed]



| T1 | T2 | T3 | T4 | T5 | T6 | ||
|---|---|---|---|---|---|---|---|
| pH | Before planting | 8.25 a * | 7.85 b * | 7.29 c * | 7.43 c * | 7.12 cd * | 6.86 d |
| After harvesting | 7.98 a | 7.10 b | 6.85 c | 6.70 cd | 6.74 cd | 6.60 d | |
| OM (%) | Before planting | 4.10 d * | 5.00 d * | 8.03 c | 17.60 c * | 18.10 b * | 27.57 a * |
| After harvesting | 5.10 d | 5.30 d | 8.03 c | 10.44 b | 10.50 b | 16.80 a | |
| N (%) | Before planting | 0.19 c * | 0.19 c | 0.29 c | 0.52 b | 0.64 b * | 0.80 a * |
| After harvesting | 0.14 e | 0.20 de | 0.30 d | 0.51 c | 1.09 b | 1.71 a | |
| P (%) | Before planting | 0.13 c | 0.33 ab | 0.25 b | 0.10 c * | 0.30 b * | 0.43 a * |
| After harvesting | 0.11 e | 0.21 de | 0.31 d | 0.83 c | 1.44 b | 1.84 a | |
| K (%) | Before planting | 0.67 a * | 0.68 a * | 0.65 ab * | 0.53 bc * | 0.40 c | 0.24 d * |
| After harvesting | 0.26 b | 0.26 b | 0.24 b | 0.28 b | 0.47 a | 0.51 a | |
| Cd (μg/g) | Before planting | 1.34 a | 1.73 a * | 1.84 a | 1.37 a | 1.43 a | 1.93 a |
| After harvesting | 0.60 a | 0.80 a | 1.23 a | 1.23 a | 1.47 a | 0.93 a | |
| Pb (μg/g) | Before planting | 9.94 a | 15.50 a | 15.08 a | 16.47 a | 11.83 a | 13.53 a |
| After harvesting | 6.93 a | 7.00 a | 7.63 a | 10.37 a | 14.13 a | 8.40 a | |
| Cu (μg/g) | Before planting | 21.57 e * | 25.46 e * | 36.63 d * | 51.87 c * | 64.93 b * | 96.00 a * |
| After harvesting | 4.90 f | 8.63 e | 13.17 d | 24.50 c | 46.47 b | 65.45 a | |
| Ni (μg/g) | Before planting | 115.54 a * | 95.23 ab * | 83.70 b * | 87.93 b * | 70.47 bc * | 54.63 c * |
| After harvesting | 20.07 a | 17.13 a | 11.90 a | 2.70 b | 1.36 b | 1.21 b | |
| Zn (μg/g) | Before planting | 75.82 d * | 93.63 d * | 156.65 d * | 254.83 c * | 511.90 b * | 1530.1 a * |
| After harvesting | 20.13 e | 23.47 e | 35.93 d | 42.93 c | 59.70 b | 83.55 a | |
| Cr (μg/g) | Before planting | 116.15 d * | 127.90 c * | 129.27 c * | 129.87 c * | 141.30 b * | 177.3 a * |
| After harvesting | 79.33 c | 100.87 ab | 106.67 ab | 91.50 bc | 113.63 a | 109.6 ab | |
| Co (μg/g) | Before planting | 55.96 ab * | 59.60 a * | 67.74 a * | 48.70 ab * | 44.97 ab * | 34.23 b * |
| After harvesting | 23.23 a | 10.13 b | 26.20 a | 10.27 b | 8.40 b | 1.80 c | |
| As (μg/g) | Before planting | 0.234 a * | 0.280 a * | 0.334 a * | 0.235 a * | 0.200 a | 0.166 a |
| After harvesting | 0.124 c | 0.039 b | 0.140 c | 0.025 a | 0.188 d | 0.095 cd | |
| Fe (μg/g) | Before planting | 2.084 a * | 2.055 a * | 2.042 a * | 1.643 a * | 1.229 a * | 1.408 a * |
| After harvesting | 0.770 a | 0.432 ab | 0.384 ab | 0.353 b | 0.459 ab | 0.461 ab |
| DF | SS | MS | F. Value | |
|---|---|---|---|---|
| Plant height | 5 | 1771.6 | 354.3 | 16.1 ** |
| Number of leaves | 5 | 623.6 | 124.7 | 11.3 ** |
| Leaf biomass | 5 | 0.423 | 0.085 | 2.59 * |
| Root biomass | 5 | 0.782 | 0.156 | 2.40 n.s. |
| Cd | Pb | Cu | Ni | Zn | Cr | Co | As | Fe | |
|---|---|---|---|---|---|---|---|---|---|
| Bioconcentration Factor (BCF) | |||||||||
| T1 | 0.118 | 0.295 | 0.280 | 2.235 | 0.660 | 0.250 | 1.208 | 0.661 | 0.099 |
| T2 | 0.163 | 0.166 | 0.374 | 3.131 | 0.724 | 0.286 | 3.426 | 2.464 | 0.228 |
| T3 | 0.073 | 0.170 | 0.724 | 5.581 | 1.046 | 0.400 | 1.553 | 1.017 | 0.369 |
| T4 | 0.078 | 0.108 | 0.370 | 25.733 | 0.764 | 0.392 | 3.816 | 4.186 | 0.411 |
| T5 | 0.129 | 0.095 | 0.216 | 38.576 | 0.663 | 0.241 | 4.019 | 0.302 | 0.484 |
| T6 | 0.749 | 0.337 | 0.333 | 46.077 | 0.822 | 0.312 | 21.753 | 1.674 | 0.474 |
| Translocation Factor (TF) | |||||||||
| T1 | 0.586 | 1.393 | 0.251 | 1.133 | 0.580 | 0.271 | 1.219 | 0.588 | 0.141 |
| T2 | 0.729 | 4.192 | 0.051 | 1.157 | 0.701 | 0.484 | 0.993 | 0.622 | 0.254 |
| T3 | 1.816 | 1.761 | 0.216 | 0.747 | 0.469 | 0.378 | 0.776 | 0.593 | 0.574 |
| T4 | 0.485 | 0.945 | 0.371 | 0.998 | 0.596 | 0.608 | 1.613 | 4.098 | 0.371 |
| T5 | 4.829 | 1.373 | 0.295 | 0.914 | 0.344 | 0.625 | 1.055 | 3.584 | 0.330 |
| T6 | 0.301 | 0.141 | 0.137 | 0.651 | 0.179 | 0.461 | 0.936 | 0.889 | 0.176 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, A.M.; Abd-Elmabod, S.K.; El-Ashry, S.M.; Soliman, W.S.; El-Tayeh, N.; Castillo, J.M. Capability of the Invasive Tree Prosopis glandulosa Torr. to Remediate Soil Treated with Sewage Sludge. Sustainability 2019, 11, 2711. https://doi.org/10.3390/su11092711
Abbas AM, Abd-Elmabod SK, El-Ashry SM, Soliman WS, El-Tayeh N, Castillo JM. Capability of the Invasive Tree Prosopis glandulosa Torr. to Remediate Soil Treated with Sewage Sludge. Sustainability. 2019; 11(9):2711. https://doi.org/10.3390/su11092711
Chicago/Turabian StyleAbbas, Ahmed Mahmoud, Sameh K. Abd-Elmabod, Soad M. El-Ashry, Wagdi Saber Soliman, Noha El-Tayeh, and Jesus M. Castillo. 2019. "Capability of the Invasive Tree Prosopis glandulosa Torr. to Remediate Soil Treated with Sewage Sludge" Sustainability 11, no. 9: 2711. https://doi.org/10.3390/su11092711
APA StyleAbbas, A. M., Abd-Elmabod, S. K., El-Ashry, S. M., Soliman, W. S., El-Tayeh, N., & Castillo, J. M. (2019). Capability of the Invasive Tree Prosopis glandulosa Torr. to Remediate Soil Treated with Sewage Sludge. Sustainability, 11(9), 2711. https://doi.org/10.3390/su11092711







