Abstract
Manure is a substantial source of ammonia volatilization into the atmosphere before and after soil application. The purpose of the study was to investigate the effects of temperature and alkalization treatments on the release of ammonia and ammonia recovery (AR) from cow manure and to characterize the chemical properties of the resultant effluents. In a closed glass reactor, 100 g of fresh cow manure was mixed with 100 mL of deionized water and the mixture was treated with various volume of KOH to increase the manure pH to 7, 9, and 12. Ammonia was distilled from the mixture at temperatures of 75, 85, 95, and 100 °C for a maximum of 5 h. Ammonia was received as diluted boric and sulfuric acids. Results indicated that the highest ammonia recovery was 86.3% and 90.2%, which were achieved at a pH of 12 and temperatures of 100 and 95 °C, respectively. The recovered ammonia in boric acid was higher than in sulfuric acid, except at a pH of 12 and temperatures of 95 and 100 °C. The effluents, after ammonia was removed, showed that the variation in pH ranged between 6.30 and 9.38. The electrical conductivity ranged between 4.5 and 9. (dS m−1) and total potassium ranged between 9.4 and 57.2 mg kg−1.
1. Introduction
Substantial amounts of liquid and solid manures are produced as a by-product of dairy feeding worldwide [1]. Unfortunately, only a small portion of this is further processed into compost or organic fertilizers [2]. The rest of the manure is either left on bare soils or used in agriculture as fresh manure, which poses severe environmental concerns [3]. The volatilization, seepage, and leaching of various compounds from manure result in air and water pollution, especially during the handling and storage of manure [4]. The main sources of ammonia emissions from the animal feeding industry, as described by the US-EPA, are cattle (54%), poultry (33%), and hogs or pigs (12%) [5]. Nearly half of the emissions are from manure operations, which include manure applied to pasture (15%), manure management (7%), and manure applied to soil (3%) [3]. Barrett [6] reported that agricultural activities are responsible for 90% of the atmospheric emissions in Western Europe. Moreover, the emissions in Africa were 0.79 Gt in 2010 and 0.87 Gt in 2014 [7]. The generation of ammonia emissions occurs due to nitrogen in the feces and urine of cattle, where the breakdown of manure protein produces ammonia as well as uric acid during manure storage and decomposition [8]. When ammonia is produced with water present, it becomes ammonium and stays in the liquid form under specific conditions: at a low pH, 99% of ammonia remains as ammonium. However, at a high pH some of the ammonium converts to ammonia [9]. Animal manure has 0.04–0.88% (w/w) ammonia [10]. Animal manure increases air pollution through volatilization of free ammonia [11]. Anaerobic manure digestion is rapidly applied to liquid cow manure to stabilize the organic matter [12].
Therefore, there is an urgent need for improved environmental technology that reduces the release of gases from livestock facilities to the environment. Many studies have suggested that recycling and reusing the manure is a viable option in dealing with ammonia emissions [13]. Dairy manure is generally considered to be a rich ammonium source; therefore, it can potentially be utilized to recover ammonia and produce an ammonium-based liquid fertilizer [14]. Extensive research has studied the removal of ammonium during municipal and industrial wastewater treatment [15]. However, little has been done on the ammonium recovery from dairy manure wastes used for agricultural purposes. Most of the nitrogen removal processes that have been developed for municipal and industrial wastewaters have been applied to animal wastes too, such as biological nitrogen removal [16], ammonia stripping [17], ion exchange [18], and struvite crystallization [19]. Among the nitrogen removal processes above, ammonia stripping is widely used due to its simplicity and lower costs [20]. Ammonia stripping is a simple desorption process used to remove the ammonia from the waste material. It is often easier and less expensive to remove nitrogen from wastewater in the form of ammonia rather than converting ammonia to nitrate-nitrogen before being removed [21].
In conventional ammonia stripping, an alkali is added to the manure or wastewater to raise the pH [20], it converts ammonium ions to ammonia gas according to Equation (1):
NH4+ + OH−→H2O + NH3.
Ammonia recovery from manure is based on the disassociation of ammonium and the equilibrium of ammonia among liquid and gas (Equations (2) and (3)) [22]. The efficiency of ammonia recovery is governed by the liquid free ammonia content, which is increased by the temperature and pH as show in Equation (4) [23]. The value of Ka can be obtained from Equation (5) [24].
where Ka is the ammonium disassociation constant, H is the Henry’s constant, dimensionless, [H+] is the H+ concentration in mole L−1, and T is temperature in K.
NH4+ ↔ NH3 + H+ Ka = 10−9.25 at 20 °C,
NH3(aq)↔ NH3 (gas) H = 0.0006 at 20 °C,
Apart from the alkali reaction, many ammonia recovery procedures that are currently in use at a large scale utilize chemical or physical methods to enhance the nitrogen recovery process [25]. These methods include many techniques, such as nano filtration, reverse osmosis, membrane distillation, air stripping, steam stripping, chemical precipitation, and ion exchange [26]. Chemical additions and micro- and ultra filtration are sometimes also performed to enhance the nitrogen recovery [27]. In a study by Gustin et al. [28] that compared the effects of pH, temperature, and air flow on nitrogen ammonia recovery from anaerobic wastewater, the results suggested that a high pH had the most significant impact on stripping, causing a change in the ammonia to ammonium ratio to favor of ammonia accumulation. The second important factor was the amount of air passing through the stripping bench plant, which promoted the transition of ammonia from its liquid phase to its gas phase [29]. The temperature effect on wastewater ammonia was studied and results revealed that, when the temperature greater than 70 °C, maximum ammonia removal (92.2%) was achieved [28]. In another study by Garcia-Gonzalez [30] that estimated the effect of aeration on the recovery of ammonia from swine manure using gas permeable membranes, the results showed that aeration increased the pH above 8.5, allowing for a quick transformation of NH4+ into gaseous ammonia (NH3) and the efficient recovery of ammonia by permeation through the submerged membrane. The overall NH4+ recovery obtained with aeration was 98% and ammonia emissions losses were less than 1.5%. These results suggest that pH and temperature can significantly affect the recovery of ammonia from manure in the stripping process. Therefore, the objectives of this research are to investigate the impacts of alkalization and temperature treatments on ammonia volatilization and recovery from dairy manure and to determine the chemical properties of the solid and effluent by-products.
2. Materials and Methods
2.1. Cow Manure Collection and Characterization
Fresh cow manure was collected from Al Safi-Danone dairy farm situated in Al-Kharj, Saudi Arabia. A composite sample of fresh manure was packed in polythene bags and transported to the laboratory in an ice-box container to minimize ammonium–nitrogen loss. The collected cow manure was stored at −20 °C in a freezer. The moisture content of the samples was determined in subsamples at 70 °C in an oven. Water-extractable ammonium was obtained using the method described by Curtin et al. [31] and dissolved ammonia-nitrogen was analyzed using micro-Kjeldahl (UDK 132 Automatic distillation system 230V, Italy) according to the method described by Estefan et al. [32]. The total nitrogen content was determined using the same procedure after a 0.2 g of the dried at 70 °C cow manure was digested with 10 mL of concentrated sulfuric acid. The total carbon content was determined after the oxidation of 0.2 g dry manure in a mixture of K2Cr2O7 and concentrated sulfuric acid, following the method of Schumacher et al. [33]. For the determination of total phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), zinc (Zn), copper (Cu), iron (Fe), and manganese (Mn), a 0.2 g of dried manure was treated with 10 mL of concentrated nitric acid and digested according to the procedure of Creed et al. [34] using microwave digestion (MARS, CEM Corporation, USA). The total concentrations were determined for the digestate using inductively coupled plasma-optical emission spectrophotometer (ICP-OES, PerkinElmer Optima 4300 DV, USA). The pH was determined in a 1:5 water extract (v/w) using a pH meter (Orion star A211), and the electrical conductivity (EC) expressed in deciSiemens/ meter (dS m−1) was measured in the filtrated extracts using an EC meter (YSI, USA). The approximate chemical composition of the cow manure and cow manure effluent was determined by scanning electron microscopy using an SEM (JSM-6380-LA, JEOL, Japan). The surface functional groups of the produced material (organic amendments) were characterized using Fourier Transform Infrared spectroscopy (FTIR, Nicolet 6700).
2.2. Adjustment of the Initial Manure Paste with KOH
A titration experiment was conducted before the ammonia removal trial to determine and simulate the alkali volumes needed to obtain a specific initial pH. Briefly, 25 g of fresh manure was mixed with 25 mL of deionized water in a 250 mL Erlenmeyer flask placed on a magnetic stirrer. A 0.1 N KOH solution was added to the mixture in drops until the pH reached 13. The pH of the mixture was instantly recorded using the attached pH meter (Cole Parmer). The pH readings from the titration were fitted to a sigmoidal curve, as described by Nelson and Su [35]. The use of the sigmoidal formula allowed for the prediction of manure pH under KOH addition. A nonlinear regression module, generated with Sigma Plot 12, was used to simulate the observed data and calculate the curve parameters. The four-parameter sigmoidal function can be described as follows:
where, pHmin is the starting pH value, a is the maximum pH value reached, A is the amount of alkali (positive) added, Amid is the A value of the curve’s inflection point, and b defines the shape of the curve.
2.3. Ammonia Stripping
The ammonia stripping was performed in a thermal stripping assembly, consisting of a round flask bottle placed on a 300W electric mantle set at the desired temperature. The flask was closed tightly with an L–shaped glass tube, inserted with plastic rubber to prevent leakage of the generated ammonia from the system. The glass tube was further connected to a heat-resistant plastic tube that was inserted into a conical flask containing 50 mL of 4% boric acid (w/w) to capture the stripped ammonia in the form of ammonium borate. A suspension of cow manure and deionized water was prepared by adding 100 g of the fresh cow manure into 100 mL of water. The pH of the suspension was adjusted to 7, 9 and 12 using the required volumes of 15 N KOH as determined from the pH calibration trial. The suspension was heated to different stripping temperature, i.e., 75, 85, 95 and 100 °C for 5 h. The boric acid was collected after the stripping and titrated with a 0.01 N sulfuric acid solution, using a methyl orange mixture as an indicator, to estimate the amount of ammonia absorbed by the boric acid during the stripping process. After the completion of the stripping process, the treated effluents (digestate) were dried at 70 °C, ground, sieved using1 mm sieves, and stored at 4 °C in closed glass bottles for further analyses.
2.4. Cow Manure Effluents Analysis
The pH and EC of the effluent were measured in a 1:10 (v/w) ratio. The total nitrogen was extracted using sulfuric acid and measured using micro-Kjeldahl (UDK 132 automatic distillation system 230V, Italy). The total carbon concentration was oxidized using K2Cr2O7 following the method described in [33]. Total P, K, Ca, Mg, Na, Zn, Cu, Fe and Mn were extracted and analyzed as mentioned above. EDX and FTIR were performed using the same procedure as mentioned in cow manure analysis.
2.5. Dynamics of Ammonia Stripping
The temporal changes in ammonia stripping were studied in the thermal stripping assembly at different time intervals. The manure suspension in a1:1 ratio (w/v) with deionized water was prepared. The pH was adjusted with the addition of0, 0.5, and 2.5 mL of 15N KOH to obtain different pH levels of 7, 9, and 12, respectively. The stripping was performed at different temperatures, i.e., 75, 85, 95, and 100 °C. Sulfuric acid was used to capture the stripped ammonia instead of boric acid. The stripping solution was changed after 0.5, 1, 2, 3, 4 and 5 h. At the end of experiment, sulfuric acid was back-titrated against 0.25N NaOH to estimate the concentration of the adsorbed ammonia. The filtrate temperature in the stripping was flask monitored with a thermometer. Following the recovery of ammonia, treated effluents were collected, dried at 70 °C, and ground with an electric mixer. The treated effluents were then analyzed for total C, N, P, K, Ca, Mg, and other micronutrients (Fe, Cu, Zn, and Mn) following the same procedure that was used for the fresh manure analysis.
2.6. Statistical Analysis
In the ammonia recovery trial, two-way ANOVA was performed to test the effects of temperature and pH on ammonia recovery. While for the ammonia dynamics, a three-way ANOVA was performed to test the effects of temperature, pH, and stripping time on ammonia recovery.
3. Results and Discussion
3.1. Adjustment of the Initial Manure Paste with KOH
In order to obtain the selected manure initial pH, the effect of 0.1N KOH on the manure pH was investigated. The relation between the added alkali and the pH rise followed a sigmoidal function with three parameters (p < 0.0001 and R2 = 0.9967). Increasing the pH of the cow manure to 12 required 40% more alkali than for the increase to a pH of 9. The pH increased rapidly with the addition of KOH at the beginning of titration, while it slowed down subsequently, and stabilized afterward. It was noted that the pH rapidly increased to 10.5 with the addition of just 0.2 meq g−1 of KOH, the pH increased by almost 2, from 10.5 to 12.3, with the addition of 0.4 meq g−1 of KOH, while the addition of 0.4–0.9 meq g−1 of KOH exhibited minimal increase in the pH. The Best fit was achieved using the sigmoidal curve as shown in Figure 1. The linear fit adequately described the starting range of the base additions, while increasing the KOH led to the distinct sigmoidal shape with an inflation point around pH 12 that then plateaued until the end, suggesting that a lower pH can reduce the amount and cost of alkali needed for ammonia stripping. One study [14] used NaOH to raise cow manure pH and stated that raising the cow manure pH to a pH of 9 in order to produce 1 kg (NH4) of 2SO4 required 0.28 × 10−7 kg NaOH for digested manure and 1.43 × 10−7 kg for undigested manure. Also, the results of El-Bourawi et al. [36] mentioned that KOH was used to increase wastewater pH to achieve high ammonia recovery.
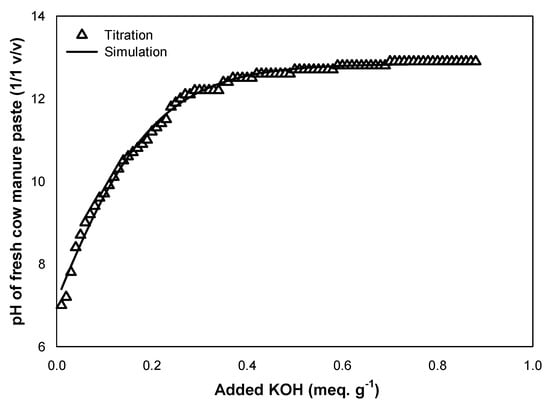
Figure 1.
Theeffect of the addition of KOH (meq g−1) on the pH of fresh cow manure paste (1/1 v/v).
3.2. Effect of Temperature on Ammonia Recovery
Initial pH and distillation temperatures showed a significant impact on ammonia recovery (AR) as shown in Table 1. Data showed that the highest concentrations of ammonia were recovered at an increased temperature and pH. The two-way ANOVA statistical analysis showed that the stripping temperature and pH were highly significant factors that determined the efficiency of ammonia recovery (p = 0.000). The maximum ammonia recovery (90.2%) was obtained at a distillation temperature of 95 °C and an initial pH of 12, while the lowest recovery value (20.2%) was observed at 75 °C and a pH of 7. At a low pH (7 and 9), increasing the distillation temperature from 75 to 100 °C resulted in approximately two-fold increase in the recovered ammonia, from 20% to 53% when pH = 7, and from 46% to 72% when pH = 9. A similar, but less pronounced, trend was observed in using a higher initial pH (pH = 12), where the AR increased by only 7% when distillation temperature was increased from 75 to 100 °C. The statistical analysis showed that a 75 °C distillation temperature significantly reduced AR in all the treatments, while increasing the distillation temperature from 95 to 100 °C did not show a statistically significant increase in AR. These results agree with those of Ding et al. [37], who found that increasing the feed temperature resulted in increased ammonia removal from solutions. He attributed this increase to the overall increase in the liquid mass transfer coefficient (kL) and the gas transfer coefficient (kG). Similar results were reported by Tao et al. [14], who studied how AR from digested and undigested dairy manure was affected by temperature, pH, and dissolved solids content. They found that 102 °C was the most favorable distillation temperature for ammonia stripping. The results are also in agreement with Duong et al. [38] who studied thermal ammonia removal from an aqueous solution when assisted by vacuum membrane and found that higher feed temperatures increased the ammonia removal rate up to 83%.

Table 1.
The effect of pH and distillation temperature on ammonia recovery from fresh cow manure. Small numbers on top indicate significantly level.
3.3. Effect of Alkalinization on Ammonia Recovery
In all cases, the increase in initial pH led to a highly significant increase in AR. At low distillation temperatures of 75 and 85 °C, AR increased dramatically from 20 to 78% and from 27 to 86% due to the increase in the initial pH from 7 to 12, respectively. The increase was less pronounced at higher distillation temperatures of 95 and 100 °C, where the AR increased from 43 to 90% and from 53 to 84%, when the initial pH was increased from 7 to 12, respectively. This increase in AR was due to the conversion of ammonium ions to ammonia gas. Adding an alkali shifted the equilibrium chemical reaction towards the production of more ammonia particles (Equation (1)). Our finding was in agreement with that obtained by El-Bourawi et al. [36], where, when vacuum membrane distillation was used to remove ammonia, pH was a [36] crucial factor in ammonia removal. They concluded that ammonia removal is difficult and inefficient with no pH adjustment. When Ding et al. [37] studied ammonia removal from water using a membrane, their results showed that, without the addition of NaOH, a lower ammonia removal was achieved when compared to the high efficiency of ammonia removal at pH 11, as shown in Table 1.
3.4. Effect of Temperature and Initial Alkalinity on the Dynamics of Ammonia Recovery
Although the volatilization of ammonium from an aqueous solutions has been investigated intensively [39,40], few have investigated the recovery of ammonia from organic amendments. Therefore, there is a need to understand the dynamics of AR from organic amendments. The time that the material resides in the distillation reactor is a critical step for the monetization and the scale-up of AR to an industrial scale. A linear relationship between distillation time and recovered ammonia was observed at a distillation temperature of 75 °C, while at higher temperatures (85, 95, and 100 °C) the relationship followed a logarithmic increase (exponential rise), with an abrupt increase during the first sixty minutes. As shown in Figure 2, the highest ammonia recovery, i.e., 96%, was achieved at 100 °C after five hours of distillation time at a pH of 12, while the lowest recovery was observed at a distillation temperature of 75 °C and a pH of 7. The temperature and pH affected the recovery of ammonia significantly in all cases (p < 0.05).
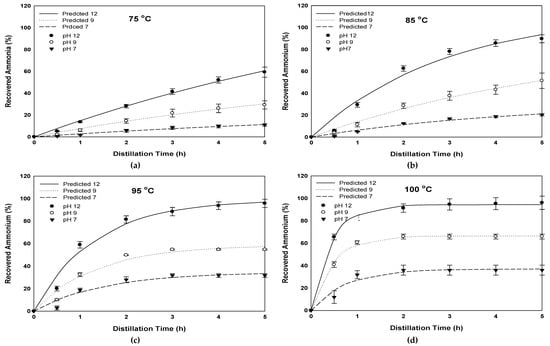
Figure 2.
Thedynamics of the recovered ammonia during the distillation of fresh dairy cow manure under different initial pH and four distillation temperatures: (a) 75°C, (b) 85°C, (c) 95°C, and (d) 100 °C.
Vapor-liquid equilibrium describes the distribution of a compound between its vapor and liquid phases. The vapor pressure of a compound in contact with a liquid is largely dependent on temperature. The variation of the overall mass transfer coefficient is an exponential function of temperature as described by Henry’s law and the Van’t Hoff equation. Thus, in this research, the exponential rise function was used to simulate AR as follow:
where y is the recovered ammonium (percent of the total ammonium), x is the time of distillation in min, a is the time required to reach equilibrium (tipping point), and b is a constant that determines the shape of the curve. The exponential rise function significantly fitted the AR data in all cases (p < 0.0001), as shown in Table 2.
y = a*(1 − exp(−b*x)),

Table 2.
Non-linear regression-dynamic fitting of ammonia recovery for cow manure at different pH and temperature levels.
Hence, parameters (a) and (b) could be used to predict the required preconditions for AR. It was concluded that the temperature determined the rate for reaching maximum recovery (curve plateau,) while pH determined the value of maximum recovery. At lower temperatures, higher values of a were obtained, indicating a longer time required to reach the equilibrium. At the higher temperatures of 95 and 100 °C, the AR curve reached equilibrium in 3 and 2 h, respectively. While at 75 and 85 °C, the recovery was more gradual and the AR curves did not reach the tipping point. This result indicated that a higher temperature is required to achieve efficient AR in a shorter period, while relatively lower temperatures could give similar results after a longer duration.
Results obtained by Yoon et al. [41] revealed that ammonia removal was affected by equilibrium and mass transfer, and showed that the effect of pH and temperature were considered when the model equation was applied. Moreover, the ammonia free fraction was increased exponentially until a pH of 11 was reached. Also, results by Vrečko and Hvala [42] studied the ammonia removal from wastewater and described ammonia stripping through exponential function under aerobic reactors.
3.5. The Efficiency of Ammonia Recovery with Different Acids
The aim of this research was to investigate potential ammonia recovery from manure to produce liquid, ammonium-based fertilizer. Hence, the recovery of ammonia by sulfuric acid is a very convenient option, since ammonium sulfate is well-established as a commercial liquid fertilizer. Boric acid was used to judge the efficiency of sulfuric acid in AR after two hours of distillation. Boric acid has been used traditionally to measure nitrogen through the recovery of ammonia in a Kjeldahl apparatus [43]. The efficiency of boric and sulfuric acids in AR from fresh cow manure was calculated as a percent of the initial ammonium in the raw materials, Figure 3. Overall, boric acid was more efficient at AR compared to sulfuric acid. However, sulfuric acid was more efficient than boric acid when pH was 12 at all temperature treatments, with the exception of 75 °C. The effect of temperature at a lower pH was greater that its effect at a high pH (12). In general, there was a significant difference between boric acid and sulfuric acid in AR as response to pH change. Swartz et al. [44] showed that sulfuric acid increased NH3 uptake in anacidic solution and they attributed this to the reaction of ammonia with H+ at the gas-liquid interface. Boric acid is considered to be a high-ammonia absorbing solution [45]. Results by Manuzon et al. [46] showed that using a concentrated acidic solution (0.2 N or higher) increased ammonia capture efficiency up to 98%. The high affinity of sulfuric acid for absorbing ammonia may be explained by Ndegwa et al. [47], who mentioned that one mole of sulfuric acid could completely trap two moles of ammonia.
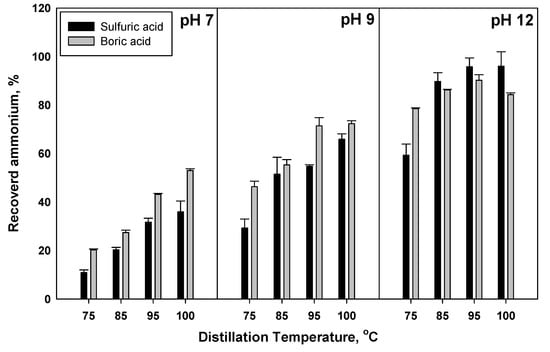
Figure 3.
The efficiency of boric and sulfuric acids in capturing ammonia from fresh cow manure.
3.6. Characterization of the Produced Effluents
The total carbon content and the C:N ratio of the manure effluents increased with the increase in distillation temperature, as shown in Figure 4. The highest carbon content was noted at 100 °C and pH 9 conditions, where total carbon content was 25% more than that of the least carbon content (75 °C with pH 12).
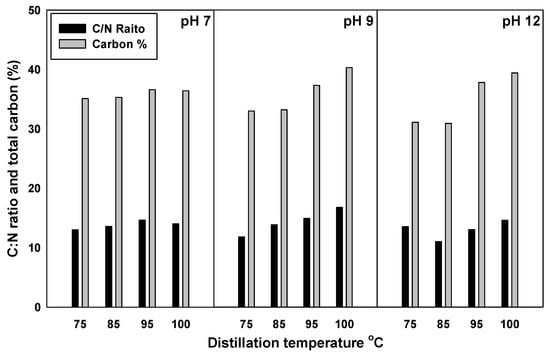
Figure 4.
Thetotal carbon content and C:N ratio as affected by different distillation temperatures and pH levels.
The highest C:N ratio was 16.8 which was obtained at 100 °C and pH 9, while the lowest ratio was 11.04, which was observed at 85 °C with pH 12. Huang et al. [48] studied the chemical structures and characteristics of animal manures and showed a value of 10.7 for the C:N ratio of cow manure. These results showed a high value for the produced cow manure effluent, which could be used as a rich carbon source for soil additions, similar to the study implications suggested by Jiang et al. [49]. Total potassium concentration increased in response to the addition of KOH as Table 3 showed, and the changing of the manure pH during ammonia stripping, while no changes were noted for different temperatures and distillation times. The EC of the ammonia stripping effluent apparently increased at pH 12, while no changes were observed at lower pH levels (7 and 9). The starting pH of the raw manure effluent was 6.3, which slightly increased at pH 12 for ammonia stripping at all the temperatures. Surprisingly, the pH of the effluent ranged from 8.6 to 9.4 at apH of 12, suggesting that the higher buffering capacity of manure reduced the effects of KOH addition. The concentrations of Ca, Na, Zn, Cu, Fe, and Mn were not affected with the addition of KOH to manure.

Table 3.
ThepH, salinity, and elemental composition of the effluent produced from thermal ammonia stripping of fresh cow manure.
3.7. The EDX Spectra along with the Elemental Composition Spectra of the Raw Cow Manure and Cow Manure Effluents
The spectra of EDX, along with elemental composition, were assessed for the highest temperatures (95 and 100 °C) at different pH levels (7, 9, and 12). The carbon content increased with increases in the temperature at all pH levels. It was noted that the carbon content increased from 38.6 to 40.1%, 38.9 to 40.2%, and from 37.7 to 39.4% at pH levels of 7, 9, and 12, respectively, with an increase in temperature from 95 to 100 °C. Also, the results showed that the nitrogen contents decreased from 24.9 to 24.1%, 27.7 to 21.0%, and from 27.6 to 23.5% at pH levels of 7, 9, and 12, respectively, with a change in temperature from 95 to 100 °C. The potassium contents also increased from 1.33 to 1.63% and from 2.3 to 3.4% at a pH of 9 and 12, respectively, with a change in temperature from 95 to 100 °C. It was also noted that the phosphorus contents increased from 0.3 to 0.4%, 0.16 to 0.28%, and from 0.19 to 0.33% at pH levels of 7, 9, and 12, respectively, with a change in temperature from 95 to 100 °C (Figure 5). The other elemental compositions fluctuated as the temperature and pH of manure changed. The higher contents of K in effluents that were found at temperatures of 95 and 100 °C and at pH 9 and 12 when compared to the original material could be due to the addition of KOH during the heating process.
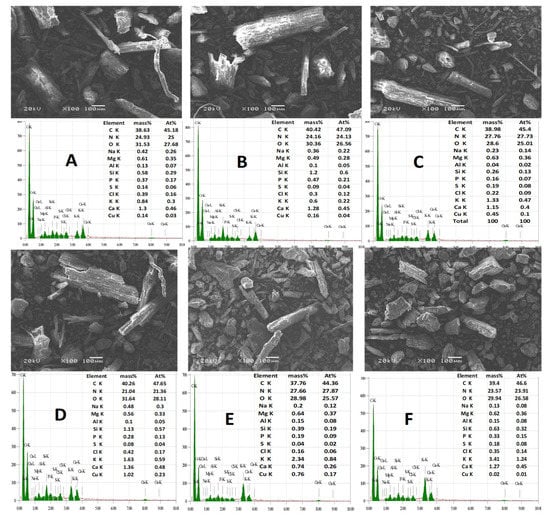
Figure 5.
EDX spectra and elemental composition (weight basis) of the raw cow manure and cow manure effluents produced at (A) pH 7 and 95 °C, (B) pH 7 and 100 °C, (C) pH 9 and 95 °C, (D) pH 9 and 100 °C, (E) pH 12 and 95 °C, and (F) pH 12 and 100 °C.
3.8. FTIR Spectra of the Raw Cow Manure and the Cow Manure Effluents
The FTIR spectra of the fresh cow manure and the striping effluents at low and high temperatures and at different pH levels are presented in Figure 6. FTIR was used to explore the changes in the functional groups and the characteristic bands of cow manure due to the changes in temperature and pH level. Different bands, with different intensities and absorbance, were visible on the spectra. A band appearing at 3400 cm−1 was termed V1 and represented the H-bonded associated OH [12], which could be due to moisture or KOH addition. The influential bands, termed V2 and V3, located around 2800 cm−1 were due to the presence of C–H (aliphatic C–H) [50]. The bands V4 and V5, appearing between 1700–1300 cm−1, were produced by the absorbance of C-bending alkane, C = C stretch of alkene, N–O stretch of nitro, and C = O stretch of carbonyl [51]. Finally, the presence of a sharp peak (V6) at 1000 cm−1 was due to the aromatic ethers and polysaccharides (C–H). The intensity of the –OH peak at 3400 cm−1 increased with the temperature change from 95 to 100 °C, indicating the presence of hydroxyl groups at elevated stripping temperatures [52]. The strength for aliphatic (V4) at 1429 cm−1 decreased with the increase in cow manure temperature, which suggested the decomposition of organic fatty hydrocarbons [53].
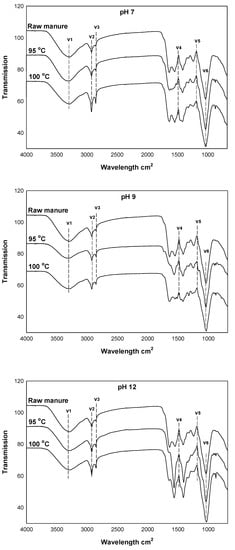
Figure 6.
FTIR spectra of the raw cow manure and the cow manure effluents as affected by two different temperatures and three different pH levels.
3.9. Implications for Water Management
The application of fresh livestock manures as a soil amendment has been in practice for a long time. The manure serves as a substantial source of major nutrients in crop production and improves soil health. However, mismanagement and imbalanced applications of manures may result in various environment problems. The surface runoff of nutrients, such as nitrogen, from the manure may cause significant pollution of nearby water bodies, mainly due to rainfall events. Hence, the runoff losses of nitrogen from the field may potentially contribute to surface water pollution [54]. The elevated nitrogen levels result in the development of eutrophication by producing toxic algal blooms, which affect the human, animal, and aquatic life [55]. Therefore, it is very crucial to reduce nitrogen runoff after manure application. In the current study, we recovered the available nitrogen in the form of ammonia through the stripping process, which reduced the free ammonia levels in the manure effluents. Hence, the application of this effluent would reduce the chances of nitrogen leaching and runoff to nearby water bodies, resulting in an efficient management strategy for surface water pollution. Moreover, the cow manure effluents could enhance the nutrient and water holding capacity of the soil.
4. Conclusions
The effects of temperature and alkalization treatments on ammonia recovery from cow manure were investigated in this study. The manure effluents after ammonia recovery were chemically characterized. The recovery of ammonia was higher in boric acid when compared with sulfuric acid, except at an initial pH of 12 and temperatures of 95 and 100 °C. The combination of 95 °C and pH 12 was found to be the optimum condition for recovering the highest amount of ammonia from fresh cow manure. Therefore, the temperature and initial pH must be controlled for higher efficiency in the ammonia stripping process. The manure effluents, after ammonia recovery, exhibited a pH in range of 6.3 to 9.38, an electrical conductivity of 4.5–9.4 dS m−1, and a total potassium range of 9.4 to 57.2 mg kg−1. Hence, it can be concluded that the recovery of ammonia can be maximized by optimizing the initial pH and temperature of the ammonia stripping process. Moreover, converting cow manure to effluent via ammonia stripping could reduce the environmental risks associated with ammonium nitrogen volatilization and could produce eco-friendly soil amendments.
Author Contributions
For research Ahmed Mohammed-Nour participated in formulating the research hypothesis, research methodology, data analysis, statistical analysis, and manuscript writing. Ahmed H. El-Naggar contributed to the research hypothesis, experimental design, experimental methods, and supervised data analysis and statistical analysis and supervised the manuscript writing. Mohamed Al-Sewailem: contributed to the research hypothesis, methodologies and procedures selections, supervised and revision of methods, data analysis, contributed to manuscript writing and review.
Acknowledgments
The authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for supporting the work through College of Food and Agriculture Sciences Research Center.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Nitrogen Inputs to Agricultural Soils from Livestock Manure, New Statistics, 2018 ed.; Economic and Social Devolopment Department: Rome, Italy, 2018. [Google Scholar]
- Chang, R.; Yao, Y.; Cao, W.; Wang, J.; Wang, X.; Chen, Q. Effects of composting and carbon based materials on carbon and nitrogen loss in the arable land utilization of cow manure and corn stalks. J. Environ. Manag. 2019, 233, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Powell, J.M.; McCrory, D.F.; Jackson-Smith, D.B.; Saam, H. Manure collection and distribution on Wisconsin dairy farms. J. Environ. Qual. 2005, 34, 2036–2044. [Google Scholar] [CrossRef]
- Xu, R.; Tian, H.; Pan, S.; Prior, S.A.; Feng, Y.; Batchelor, W.D.; Chen, J.; Yang, J. Global ammonia emissions from synthetic nitrogen fertilizer applications in agricultural systems: Empirical and process-based estimates and uncertainty. Glob. Chang. Biol. 2019, 25, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Barrett, K. Oceanic ammonia emissions in Europe and their transboundary fluxes. Atmos. Environ. 1998, 32, 381–391. [Google Scholar] [CrossRef]
- Tongwane, M.I.; Moeletsi, M.E. A review of greenhouse gas emissions from the agriculture sector in Africa. Agric. Syst. 2018, 166, 124–134. [Google Scholar] [CrossRef]
- Sun, F.; Aguerre, M.; Wattiaux, M. Starch and dextrose at 2 levels of rumen-degradable protein in iso-nitrogenous diets: Effects on lactation performance, ruminal measurements, methane emission, digestibility, and nitrogen balance of dairy cows. J. Dairy Sci. 2019, 102, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Manuzon, R.; Hadlocon, L.J. Ammonia Emission from Animal Feeding Operations and Its Impacts. In Agriculture and Natural Resources; Ohio State University Extension: Columbus, OH, USA, 2014. [Google Scholar]
- ASABE. Manure Production and Characteristics; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2010. [Google Scholar]
- Koolen, C.D.; Rothenberg, G. Air Pollution in Europe. ChemSusChem 2019, 12, 164–172. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Operating Anaerobic Digester Projects; U.S. Environmental Protection Agency: Washington, DC, USA, 2014. [Google Scholar]
- Risse, L.; Cabrera, M.; Franzluebbers, A.; Gaskin, J.; Gilley, J.E.; Killorn, R.; Radcliffe, D.; Tollner, W.; Zhang, H. Land Application of Manure for Beneficial Reuse; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2006. [Google Scholar]
- Tao, W.; Ukwuani, A.T. Coupling thermal stripping and acid absorption for ammonia recovery from dairy manure: Ammonia volatilization kinetics and effects of temperature, pH and dissolved solids content. Chem. Eng. J. 2015, 280, 188–196. [Google Scholar] [CrossRef]
- Madakka, M.; Jayaraju, N.; Rajesh, N.; Chandra, M.R.G.S. Development in the Treatment of Municipal and Industrial Wastewater by Microorganism. In Recent Developments in Applied Microbiology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 263–273. [Google Scholar]
- Delgado Vela, J.; Stadler, L.B.; Martin, K.J.; Raskin, L.; Bott, C.B.; Love, N.G. Prospects for biological nitrogen removal from anaerobic effluents during mainstream wastewater treatment. Environ. Sci. Technol. Lett. 2015, 2, 234–244. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, Y.-W.; Jahng, D. Ammonia stripping for enhanced biomethanization of piggery wastewater. J. Hazard. Mater. 2012, 199, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, T.; Weatherley, L. Ammonia removal from wastewater by ion exchange in the presence of organic contaminants. Water Res. 2003, 37, 1723–1728. [Google Scholar] [CrossRef]
- Liu, Y.; Kwag, J.-H.; Kim, J.-H.; Ra, C. Recovery of nitrogen and phosphorus by struvite crystallization from swine wastewater. Desalination 2011, 277, 364–369. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Xiang, S.; Huang, Z.; Ruan, R.; Liu, Y. Nutrient removal from digested swine wastewater by combining ammonia stripping with struvite precipitation. Environ. Sci. Pollut. Res. 2019, 26, 6725–6734. [Google Scholar] [CrossRef]
- Minocha, V.K.; Rao, A.P. Ammonia removal and recovery from urea fertilizer plant waste. Environ. Technol. 1988, 9, 655–664. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Harza, B.M.W. Water Treatment: Principles and Design; Wiley: New York, NY, UAS, 2005. [Google Scholar]
- Tchobanoglus, G.; Burton, F.; Stensel, H.D. Wastewater engineering: Treatment and reuse. Am. Water Works Assoc. J. 2003, 95, 201. [Google Scholar]
- Emerson, K.; Russo, R.C.; Lund, R.E.; Thurston, R.V. Aqueous ammonia equilibrium calculations: Effect of pH and temperature. J. Fish. Board Can. 1975, 32, 2379–2383. [Google Scholar] [CrossRef]
- Escudero, A.; Blanco, F.; Lacalle, A.; Pinto, M. Struvite precipitation for ammonium removal from anaerobically treated effluents. J. Environ. Chem. Eng. 2015, 3, 413–419. [Google Scholar] [CrossRef]
- Buonomenna, M.G. Membrane processes for a sustainable industrial growth. RSC Adv. 2013, 3, 5694–5740. [Google Scholar] [CrossRef]
- Kunz, A.; Mukhtar, S. Hydrophobic membrane technology for ammonia extraction from wastewaters. Eng. Agríc. 2016, 36, 377–386. [Google Scholar] [CrossRef]
- Guštin, S.; Marinšek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Prot. 2011, 89, 61–66. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Zhou, T.; Li, Z.; Xiang, S.; Xu, F.; Ruan, R.; Liu, Y. Evaluation of ammonia recovery from swine wastewater via a innovative spraying technology. Bioresour. Technol. 2019, 272, 235–240. [Google Scholar] [CrossRef] [PubMed]
- García-González, M.; Vanotti, M.; Szogi, A. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of aeration. J. Environ. Manag. 2015, 152, 19–26. [Google Scholar] [CrossRef]
- Curtin, D.; Wright, C.; Beare, M.; McCallum, F. Hot water-extractable nitrogen as an indicator of soil nitrogen availability. Soil Sci. Soc. Am. J. 2006, 70, 1512–1521. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant, and Water Analysis. A Manual for the West Asia and North Africa Region; International Center for Agricultural Research in the Dry Areas (ICARDA): Beirut, Lebanon, 2013; pp. 170–176. [Google Scholar]
- Schumacher, B.A. Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments; U.S. Environmental Protection Agency: Washington, DC, USA, 2002. [Google Scholar]
- Creed, J.; Brockhoff, C.; Martin, T. US-EPA Method 200.8: Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma-Mass Spectrometry; Environmental Monitoring Systems Laboratory Office of Research and Development: Cincinnati, OH, USA, 1994. [Google Scholar]
- Nelson, P.N.; Su, N. Soil pH buffering capacity: A descriptive function and its application to some acidic tropical soils. Soil Res. 2010, 48, 201–207. [Google Scholar] [CrossRef]
- El-Bourawi, M.; Khayet, M.; Ma, R.; Ding, Z.; Li, Z.; Zhang, X. Application of vacuum membrane distillation for ammonia removal. J. Membr. Sci. 2007, 301, 200–209. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, L.; Li, Z.; Ma, R.; Yang, Z. Experimental study of ammonia removal from water by membrane distillation (MD): The comparison of three configurations. J. Membr. Sci. 2006, 286, 93–103. [Google Scholar] [CrossRef]
- Batstone, D.; Hülsen, T.; Mehta, C.; Keller, J. Platforms for energy and nutrient recovery from domestic wastewater: A review. Chemosphere 2015, 140, 2–11. [Google Scholar] [CrossRef]
- Manto, M.J.; Xie, P.; Keller, M.A.; Liano, W.E.; Pu, T.; Wang, C. Recovery of ammonium from aqueous solutions using ZSM-5. Chemosphere 2018, 198, 501–509. [Google Scholar] [CrossRef]
- Duong, T.; Xie, Z.; Ng, D.; Hoang, M. Ammonia removal from aqueous solution by membrane distillation. Water Environ. J. 2013, 27, 425–434. [Google Scholar] [CrossRef]
- Yoon, H.; Lim, J.-H.; Chung, H.-K. Ammonia removal model based on the equilibrium and mass transfer principles. Bull. Korean Chem. Soc. 2008, 29, 555–561. [Google Scholar]
- Vrečko, D.; Hvala, N. Model-Based Control of the Ammonia Nitrogen Removal Process in a Wastewater Treatment Plant. In Case Studies in Control; Springer: London, UK, 2013. [Google Scholar]
- Ma, T.; Zuazaga, G. Micro-Kjeldahl determination of nitrogen. A new indicator and an improved rapid method. Ind. Eng. Chem. Anal. Ed. 1942, 14, 280–282. [Google Scholar] [CrossRef]
- Swartz, E.; Shi, Q.; Davidovits, P.; Jayne, J.; Worsnop, D.; Kolb, C. Uptake of gas-phase ammonia. 2. Uptake by sulfuric acid surfaces. J. Phys. Chem. A 1999, 103, 8824–8833. [Google Scholar] [CrossRef]
- Singh Lodhi, B.; Lal, N. Ammonia Recovery from Dyes and Pigment Manufacturing Industrial Waste Water in Physio-Chemical Treatment Subsequent with Air Stripping at High pH. Academia 2017, 4, 201–213. [Google Scholar]
- Manuzon, R.B.; Zhao, L.; Keener, H.M.; Darr, M.J. A prototype acid spray scrubber for absorbing ammonia emissions from exhaust fans of animal buildings. Trans. ASABE 2007, 50, 1395–1407. [Google Scholar] [CrossRef]
- Ndegwa, P.M.; Vaddella, V.K.; Hristov, A.; Joo, H. Measuring concentrations of ammonia in ambient air or exhaust air stream using acid traps. J. Environ. Qual. 2009, 38, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yu, Z.; Gao, H.; Yan, X.; Chang, J.; Wang, C.; Hu, J.; Zhang, L. Chemical structures and characteristics of animal manures and composts during composting and assessment of maturity indices. PLoS ONE 2017, 12, e0178110. [Google Scholar] [CrossRef]
- Jiang, A.; Zhang, T.; Zhao, Q.; Frear, C.; Chen, S. Integrated Ammonia Recovery Technology in Conjunction with Dairy Anaerobic Digestion, CFF Final Report-AD Component. 2012. Available online: wp2.cahnrs.wsu.edu.s3.amazonaws.com/wp-content/uploads/sites/32/2013/02/CSANR2010-001.Ch08.pdf (accessed on 23 April 2019).
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Mašek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Xue, D.; Pang, F.; Meng, F.; Wang, Z.; Wu, W. Decision-tree-model identification of nitrate pollution activities in groundwater: A combination of a dual isotope approach and chemical ions. J. Contam. Hydrol. 2015, 180, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Ahmad, M.; Usman, A.R.; Al-Faraj, A.S.; Abduljabbar, A.S.; Al-Wabel, M.I. Biochar composites with nano zerovalent iron and eggshell powder for nitrate removal from aqueous solution with coexisting chloride ions. Environ. Sci. Pollut. Res. 2018, 25, 25757–25771. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).