Conversion of Secondary Forests into Chestnut Forests Affects Soil Nutrients in Anji County, China
Abstract
1. Introduction
2. Materials and Methods
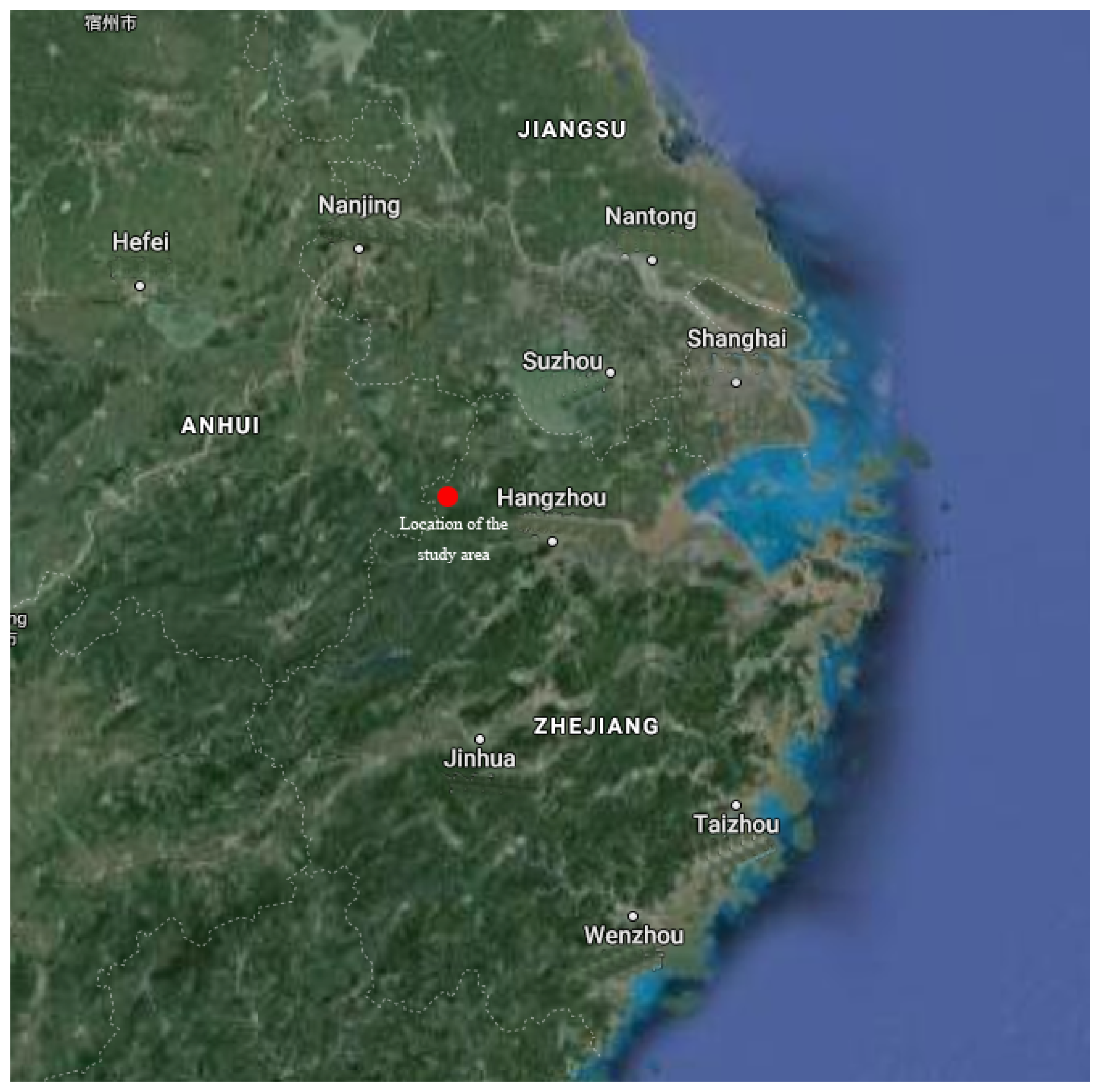
2.1. Description of Site
2.2. Details of the Secondary and Chestnut Forests
2.3. Soil Sample Collection and Determination of Nutrient Contents
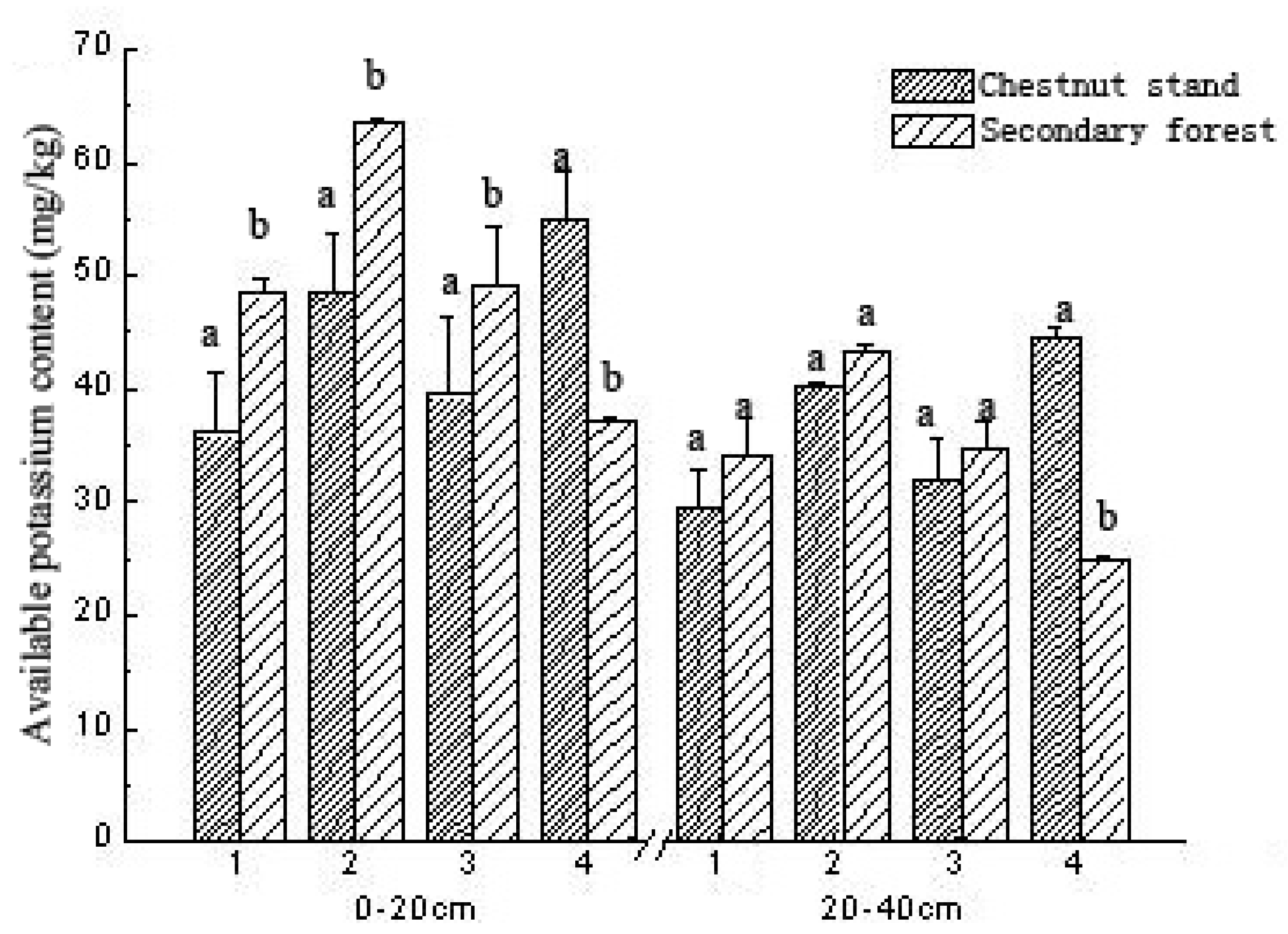
3. Results
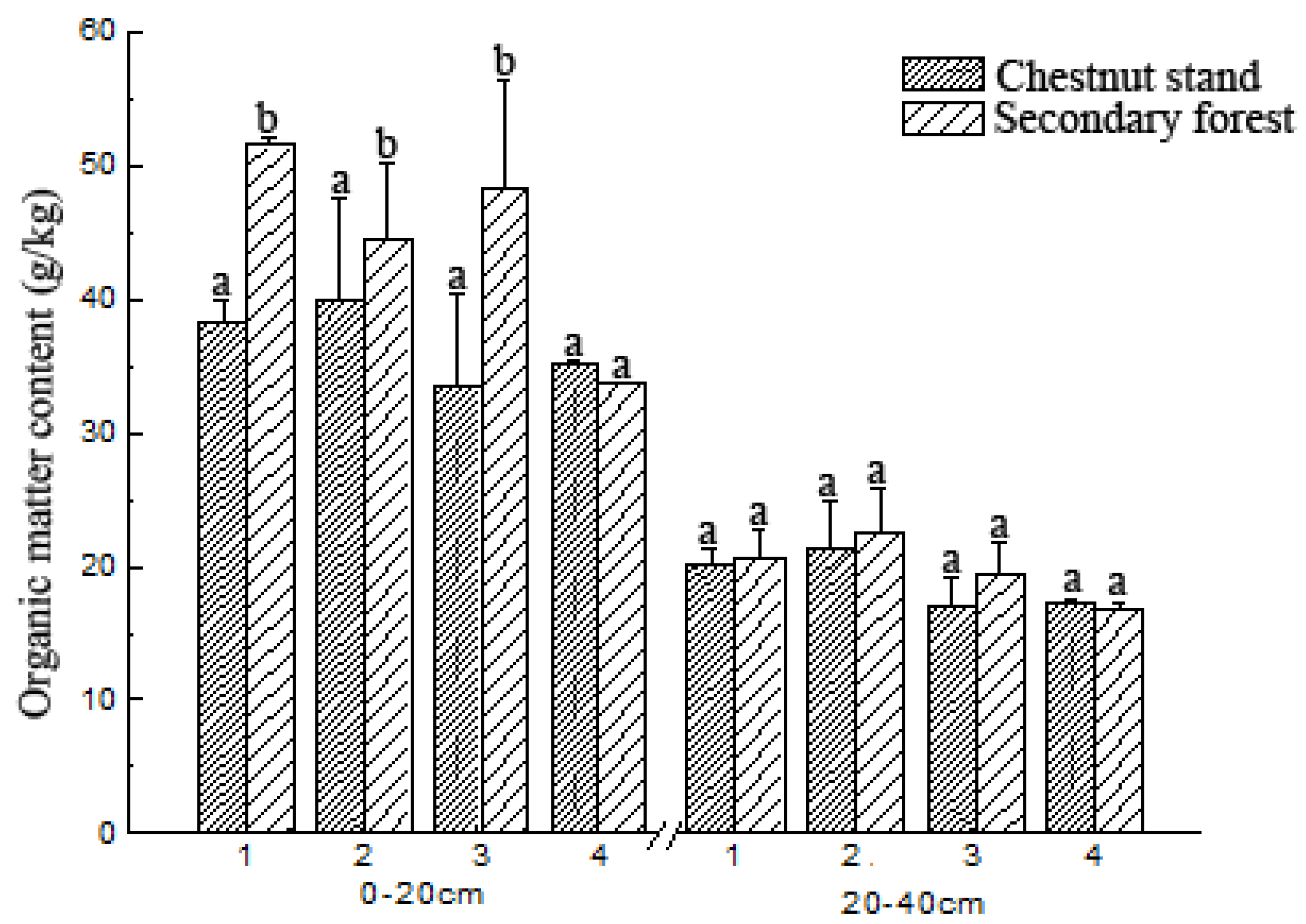
3.1. Soil Organic Matter Content Change
3.2. Comparison of Soil TN Content
3.3. Comparison of Soil Hydrolyzable Nitrogen Content
3.4. Comparison of Soil TP Content
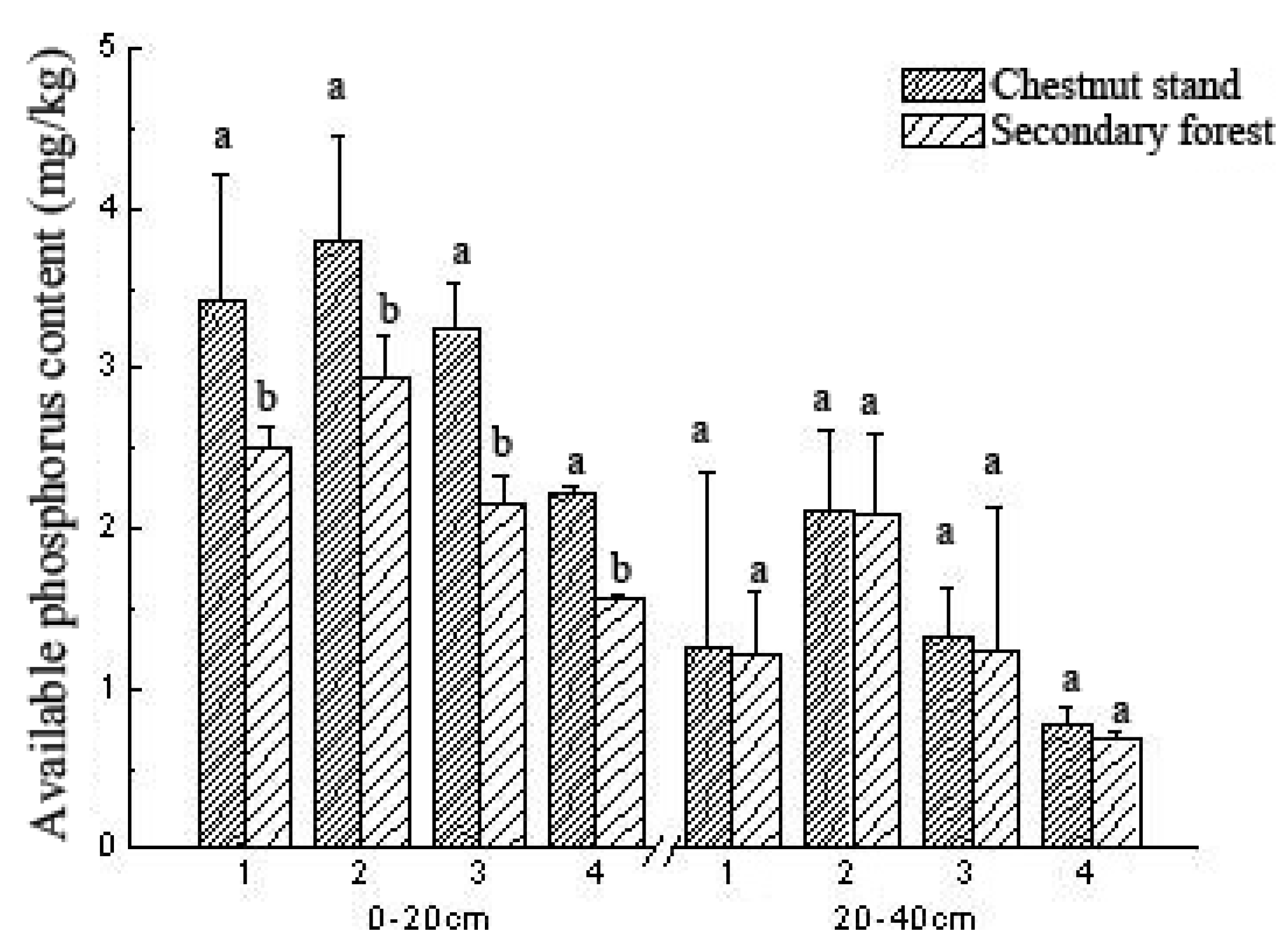
3.5. Comparison of Soil AP Content
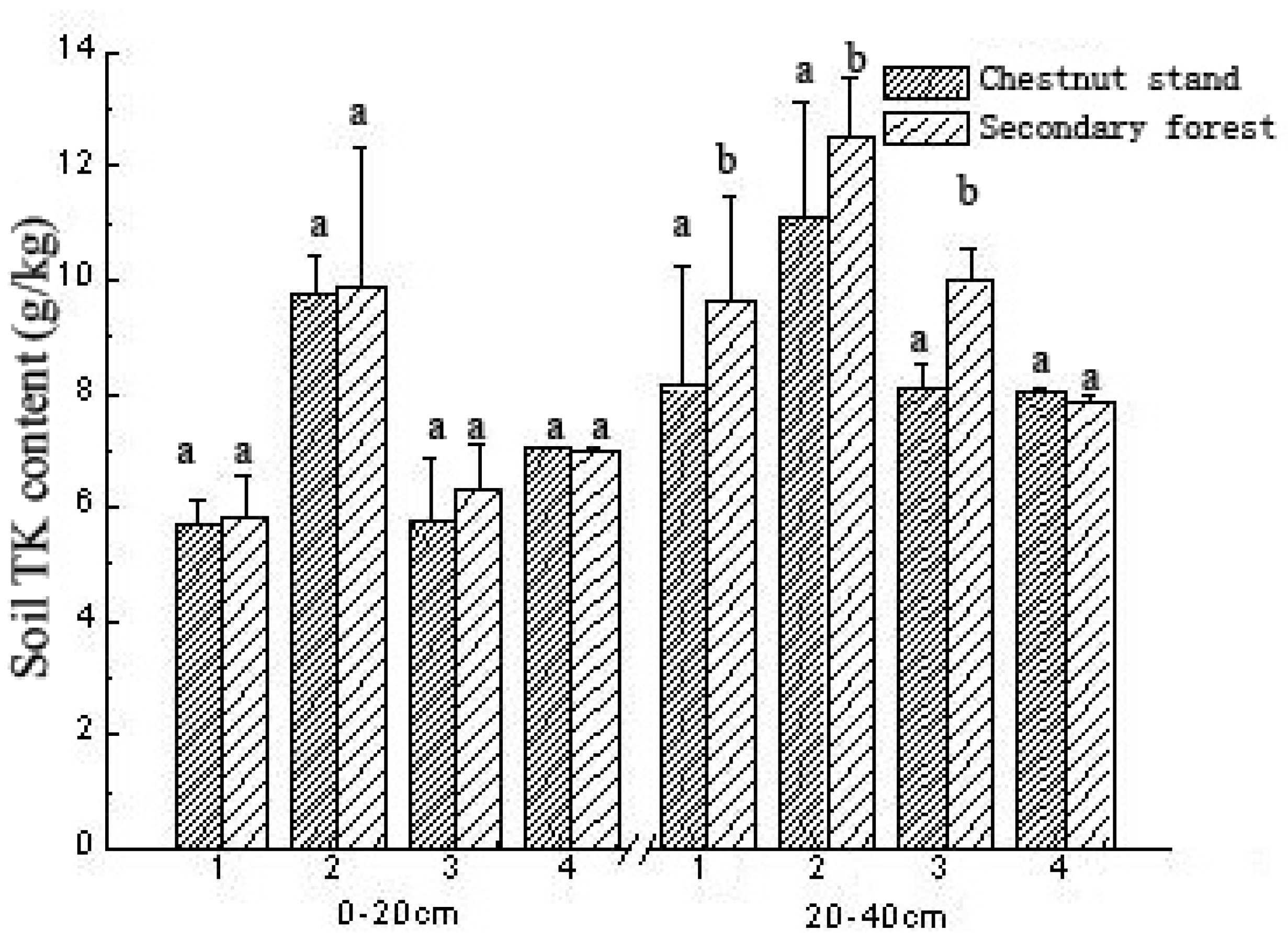
3.6. Comparison of Soil TK Content
3.7. Comparison of Soil AK Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Creed, I.F.; van Noordwijk, M. (Eds.) Forest and Water on a Changing Planet: Vulnerability, Adaptation and Governance Opportunities. A Global Assessment Report; IUFRO World Series Volume 38; IUFRO: Vienna, Austria, 2018. [Google Scholar]
- Rosen, M.R.; Lapham, W.W. Introduction to the US Geological Survey National Water-Quality Assessment (NAWQA) of ground-water quality trends and comparison to other national programs. J. Environ. Qual. 2008, 37, 190–198. [Google Scholar]
- Neary, D.G.; Ice, G.G.; Jackson, C.R. Linkages between forest soils and water quality and quantity. For. Ecol. Manag. 2009, 258, 2269–2281. [Google Scholar] [CrossRef]
- Eriksson, L.O.; Löfgren, S.; Öhman, K. Implications for forest management of the EU Water Framework Directive’s stream water quality requirements-A modeling approach. For. Policy Econ. 2011, 13, 284–291. [Google Scholar] [CrossRef]
- Wang, R.J.; Gao, P.; Li, C.; Dong, X.D.; Jiang, Y.K.; Wang, J.X. Characteristics of Subsurface Runoff and Phosphorus Loss in QuercusAcutissimaand Robinia Pseudoacacia Forest in the Grain to Green Program. J. Soil Water Conserv. 2019, 33, 9–13. [Google Scholar]
- Wang, Q.B.; Cao, Y.N.; Zhang, J.F.; Chen, G.C.; Li, Z.B.; Wu, H.; Wang, L. Characteristics of nitrogen transportation in Castanea mollissima forest around the Fushi Reservoir watershed in north Zhejiang Province, China. Chin. J. Appl. Ecol. 2017, 28, 545–553. [Google Scholar]
- Sabine, Z.; Sabine, G.; Claus-Felix, T.; Karin, H.; Wilhelm, C. Effects of Reduced Tillage on Crop Yield, Plant Available Nutrients and Soil Organic Matter in a 12-Year Long-Term Trial under Organic Management. Sustainability 2013, 5, 3876–3894. [Google Scholar]
- Li, J.X.; Cao, F.F.; Wu, D.; Fu, X.; Tian, Y.; Wu, G. Determining Soil Nutrients Reference Condition in Alpine Region Grassland, China: A Case Study of Hulun Buir Grassland. Sustainability 2018, 10, 4666. [Google Scholar] [CrossRef]
- Shin, C.S.; Sharif, O.; Lee, H.Y. Evaluating the Effect of Bacterial Inoculation and Fertilization on the Soil Nutrient Status of Coal Mine Soil by Growing Soybean (Glycine max) and Shrub Lespedeza (Lespedeza bicolor). Sustainability 2018, 10, 4793. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhang, J.Q.; Wang, C. Impact on Soil Nutrient of Populus tomentosa and Robinia pseudoacaia Plantations in Loess Plateau. J. Northwest For. Univ. 2011, 26, 12–17. [Google Scholar]
- Liu, Y.J.; Li, Y.; Ma, K.; He, W.S. Effects of potato, silkworm bean and buckwheat on soil were studied. Jiangsu Agric. Sci. 2018, 46, 79–83. [Google Scholar]
- Hua, D.W.; Li, J. Evaluation of Soil Nutrients in Tea Region of Shangnan County. Mod. Agric. Sci. Technol. 2018, 20, 184–185,194. [Google Scholar]
- Liu, J.M.; Yao, Y.; Liu, Z.L.; Lu, Y.D. Study on Nutrient Characteristics of Soil in Natural Corylus Forest Stands of Different Stocking Densities. For. Eng. 2018, 34, 1–5. [Google Scholar]
- Oladele, S.O.; Adeyemo, A.J.; Awodun, M.A. Influence of rice husk biochar and inorganic fertilizer on soil nutrients availability and rain-fed rice yield in two contrasting soils. Geoderma 2019, 339, 1–11. [Google Scholar] [CrossRef]
- Fu, B.J. Blue Skies for China. Science 2008, 321, 611. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cai, Z.C.; Yan, X.Y.; Zhu, Z.L. A great challenge to solve nitrogen pollution from intensive agriculture. J. Plant Nutr. Fertil. 2014, 20, 1–6. [Google Scholar]
- Jeppesen, E.; Ndergaard, M.; Jensen, J.P.; Havens, K.E.; Anneville, O.; Carvalho, L. Lake responses to reduced nutrient loading-an analysis of contemporary long-term data from 35 case studies. Freshw. Biol. 2005, 50, 1747–1771. [Google Scholar] [CrossRef]
- Bunting, L.; Leavitt, P.R.; Gibson, C.E.; Mcgee, E.J.; Hall, V.A. Degradation of water quality in lough neagh, Northern Ireland, by diffuse nitrogen flux from a phosphorus-rich catchment. Limnol. Oceanogr. 2007, 52, 354–369. [Google Scholar] [CrossRef]
- Guo, L. Doing battle with the green monster of Taihu Lake. Science 2007, 317, 1166. [Google Scholar] [CrossRef]
- Stone, R. China aims to turn tide against toxic lake pollution. Science 2011, 333, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y. Indirect impact of bluegillr, lepomis macrochirusr, on the development of cyanobacterial blooms in lake biwa, Japan. J. Freshw. Ecol. 2011, 26, 323–335. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Wang, Y.; Sheng, H.; Dong, F.; Zou, R.; Zhao, L.; Guo, H.C.; Zhu, X.; He, B. Quantitative evaluation of lake eutrophication responses under alternative water diversion scenarios: A water quality modeling based statistical analysis approach. Sci. Total Environ. 2014, 468–469, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.N.; Paterson, M.J.; Hecky, R.E.; Schindler, D.W.; Venkiteswaran, J.J.; Findlay, D.L. Biological nitrogen fixation prevents the response of a eutrophic lake to reduced loading of nitrogen: Evidence from a 46-year whole-lake experiment. Ecosystems 2018, 21, 1088–1100. [Google Scholar] [CrossRef]
- Matisoff, G.; Watson, S.B.; Guo, J.; Duewiger, A.; Steely, R. Sediment and nutrient distribution and resuspension in lake winnipeg. Sci. Total Environ. 2017, 575, 173–186. [Google Scholar] [CrossRef]
- Paytan, A.; Roberts, K.; Watson, S.; Peek, S.; Chuang, P.C.; Defforey, D.; Carol, K. Internal loading of phosphate in lake erie central basin. Sci. Total Environ. 2017, 579, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, Y.; Liang, Z.; Wu, S.; Guo, H. Internal cycling, not external loading, decides the nutrient limitation in eutrophic lake: A dynamic model with temporal bayesian hierarchical inference. Water Res. 2017, 116, 231–240. [Google Scholar] [CrossRef]
- Wang, S.S.; Gao, Y.N.; Li, Q. Long-term and inter-monthly dynamics of aquatic vegetation and its relation with environmental factors in Taihu Lake, China. Sci. Total Environ. 2019, 651, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.B.; Zhang, J.F.; Liu, G.Y.; Chen, G.C.; Pan, C.X.; Wu, H. Study on the Control of TN and TP for Agricultural Non-point Source Pollution by Ponds-Wetlands Combined System—An Example of Shenji River Watershed in Anji County. Chin. Agric. Sci. Bull. 2014, 30, 197–204. [Google Scholar]
- Lu, R.K. Soil Agro-Chemical Analysis Methods; China Agro-Science Press: Beijing, China, 1999. [Google Scholar]
- Li, Z.B.; Zhang, J.F.; Chen, G.C.; Wang, Q.B.; Pan, C.X.; Wu, H.; Wang, L. Research on Characteristics of Soil Erosion Pollutants in Chestnut (Castanea mollissima) Stands Around Fushi Reservoir in Anji County. J. Soil Water Conserv. 2013, 27, 90–94. [Google Scholar]
- Li, H.P.; Geng, Y.Q.; Wu, Y.J.; Zhang, X.J.; Duan, F.J.; Li, Y.C.; Ning, P. Study on Forest Soil Nutrient in the Middle Part of Zhongtiao Mountain. For. Sci. Technol. 2018, 9, 8–12. [Google Scholar]
- Wang, C.Y.; Yan, X.; Gu, M.H. The interaction effects of vegetation and soil nutrients on vegetation succession in abandoned farmland on the Loess Plateau. Acta Prataculturae Sin. 2018, 27, 26–35. [Google Scholar]
- Zhou, X.B.; Tao, Y.; Wu, L.; Li, Y.G.; Zhang, Y.M. Soil stoichiometry in different land-use categories in desert-oasis ecotones of the southern Taklimakan Desert. Acta Ecol. Sin. 2019, 39, 1–3. [Google Scholar]
- Xiao, W.Y.; Chen, H.Y.H.; Kumar, P.; Chen, C.; Guan, Q.W. Multiple interactions between tree composition and diversity and microbial diversity underly litter decomposition. Geoderma 2019, 341, 161–171. [Google Scholar] [CrossRef]
- Meng, M.J.; Lin, J.; Guo, X.P.; Liu, X.; Wu, J.S.; Zhao, Y.P.; Zhang, J.C. Impacts of forest conversion on soil bacterial community composition and diversity in subtropical forests. Catena 2019, 175, 167–173. [Google Scholar] [CrossRef]
- Wan, P.; Zhang, G.Q.; Zhao, Z.H.; Hu, Y.B.; Liu, W.Z.; Hui, G.Y. Short-term effects of different forest management methods on soil microbial communities of a natural Quercus aliena var. acuteserrata forest in Xiaolongshan, China. Forests 2019, 10, 161. [Google Scholar] [CrossRef]
- Jasso-Flores, I.; Galicia, L.; Garcia-Oliva, F.; Martinez-Yrizar, A. Are vegetation-soil systems drivers of ecosystem carbon contents along an elevational gradient in a highland temperate forest? Can. J. For. Res. 2019, 49, 296–304. [Google Scholar] [CrossRef]
- Fan, X.H.; Ge, H.Y.; Zhang, C.C.; Deng, W.P.; Chen, F.S.; Bu, W.S. Variations in soil fertility of typical non-commercial forest types in Jiangxi Province of eastern China. J. Beijing For. Univ. 2018, 11, 84–92. [Google Scholar]
- Yao, J.Y.; Qi, J.Q.; Zhang, L.H.; Wang, M.Z.; Li, T.T.; Zhang, X.Y.; Hao, J.F. Effects of anthropogenic disturbance on species diversity and soil physicochemical properties of Symplocos sumuntia secondary forest in Bifengxia. Chin. J. Ecol. 2018, 37, 2942–2950. [Google Scholar]



| Sample Plot Number | Slope (°) | Vegetation Cover in Chinese Chestnut (%) | Vegetation Cover in Secondary Forest Land (%) | Plants in Chinese Chestnut Forest | Plants in Secondary Forests |
|---|---|---|---|---|---|
| 1 | 13 | 80 | 100 | Castanea mollissima BL., Hedyotis chrysotricha (Palib.) Merr., Smilax glabra Roxb., Setaria viridis (L.) Beauv | Cunninghamia lanceolata (Lamb.) Hook (dominant tree species), Diospyros kaki Thunb, Cinnamomum camphora (L.) Presl., Castanopsis sclerophylla (Lindl.) Schott., Litsea cubeba (Lour.) Pers.; Ilex chinensis Sims |
| 2 | 17 | 80 | 100 | Castanopsis sclerophylla (Lindl.) Schott. (dominant tree species), Cinnamomum camphora (L.) Presl.; Ilex chinensis Sims | |
| 3 | 10 | 90 | 98 | Castanopsis sclerophylla (Lindl.) Schott. (dominant tree species), Cunninghamia lanceolata (Lamb.) Hook | |
| 4 | 6 | 85 | 90 | Brachystachyum densiflorum (Rendle) Keng (dominant tree species), Lycium chinense Mill. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Li, Z.; Wang, R.; Zhang, J.; Pan, C.; Cai, Z. Conversion of Secondary Forests into Chestnut Forests Affects Soil Nutrients in Anji County, China. Sustainability 2019, 11, 2373. https://doi.org/10.3390/su11082373
Sun S, Li Z, Wang R, Zhang J, Pan C, Cai Z. Conversion of Secondary Forests into Chestnut Forests Affects Soil Nutrients in Anji County, China. Sustainability. 2019; 11(8):2373. https://doi.org/10.3390/su11082373
Chicago/Turabian StyleSun, Shiyong, Zebo Li, Rongjia Wang, Jianfeng Zhang, Chunxia Pan, and Zeyu Cai. 2019. "Conversion of Secondary Forests into Chestnut Forests Affects Soil Nutrients in Anji County, China" Sustainability 11, no. 8: 2373. https://doi.org/10.3390/su11082373
APA StyleSun, S., Li, Z., Wang, R., Zhang, J., Pan, C., & Cai, Z. (2019). Conversion of Secondary Forests into Chestnut Forests Affects Soil Nutrients in Anji County, China. Sustainability, 11(8), 2373. https://doi.org/10.3390/su11082373





