Effect of Carbon/Nitrogen Ratio, Temperature, and Inoculum Source on Hydrogen Production from Dark Codigestion of Fruit Peels and Sewage Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Preparation
2.2. Inoculum Source
2.3. Batch Bioreactors
2.4. Analytical Techniques
2.5. Data Analysis
3. Results and Discussion
3.1. Characterization of Substrates
3.2. Measuring Biogas
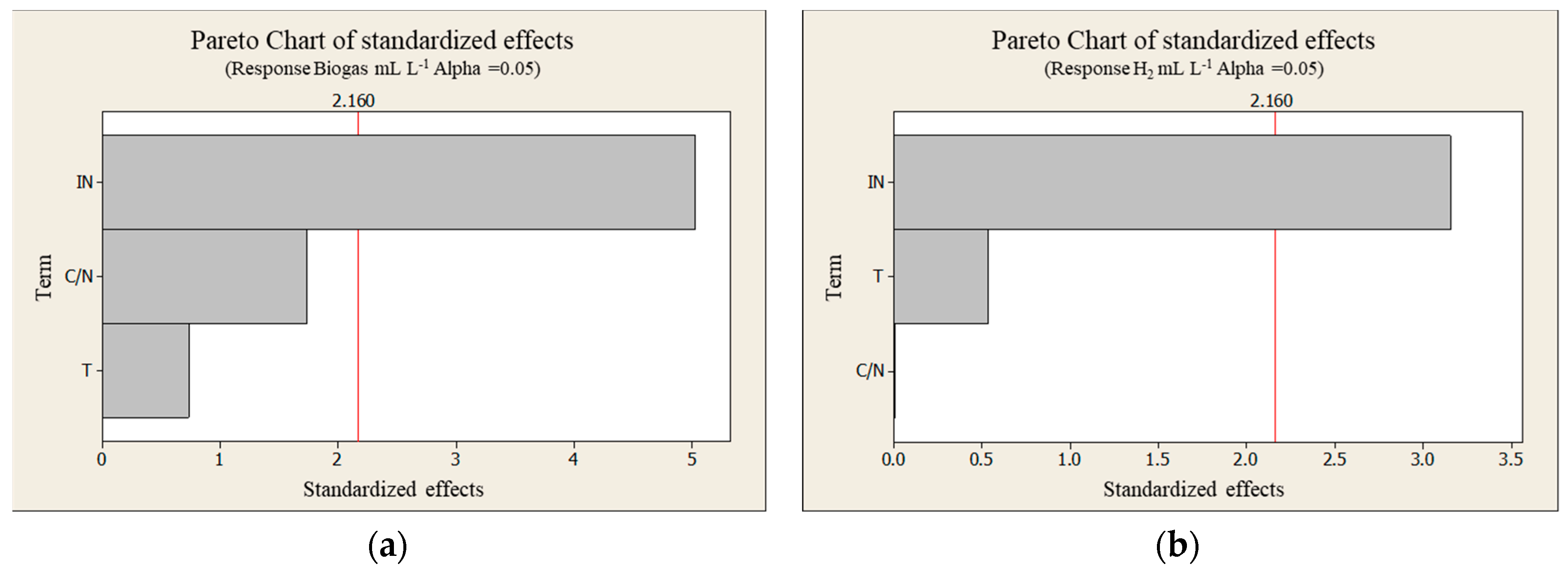
3.3. Biohydrogen Production
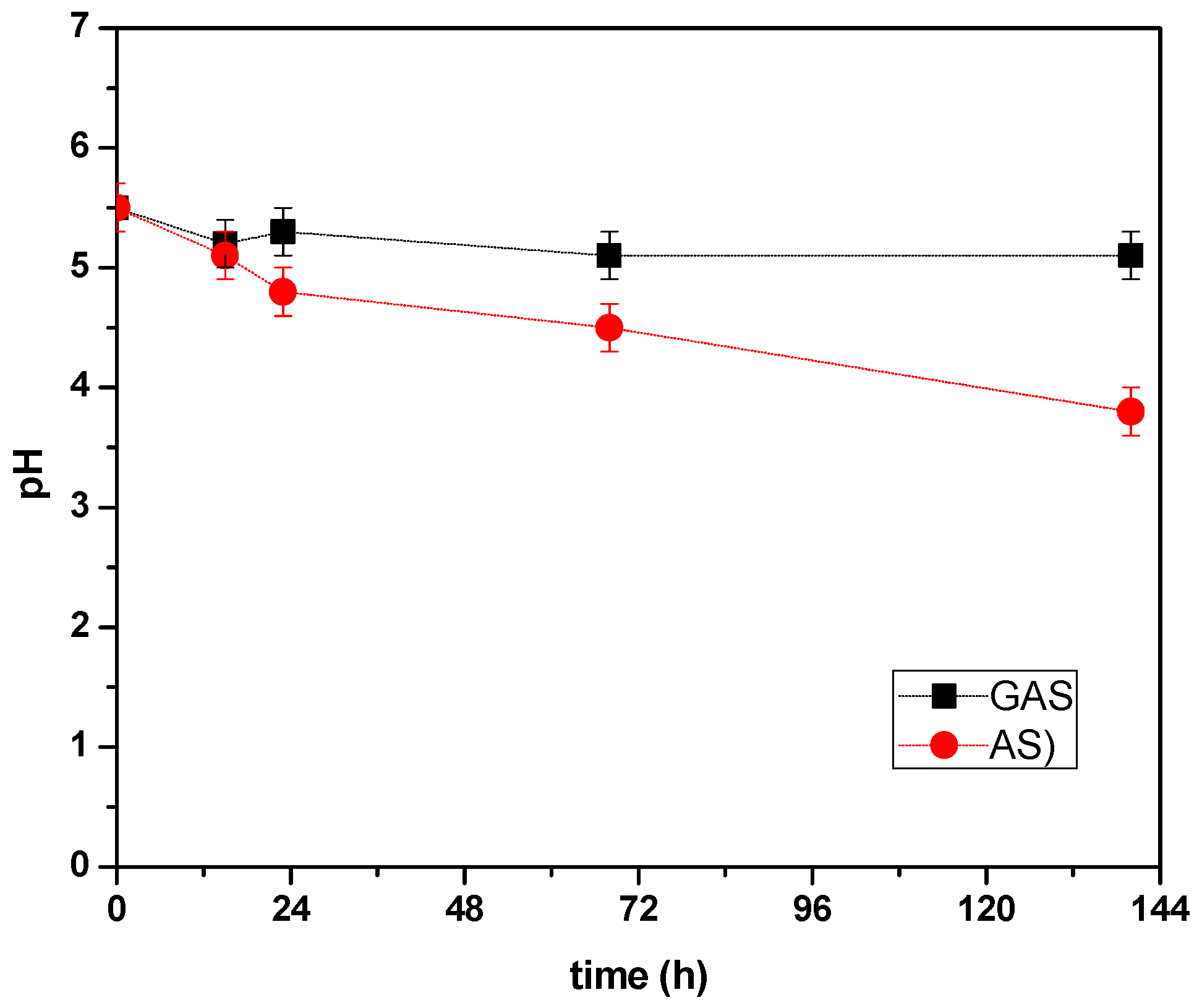
3.4. Monitoring Parameters during DF
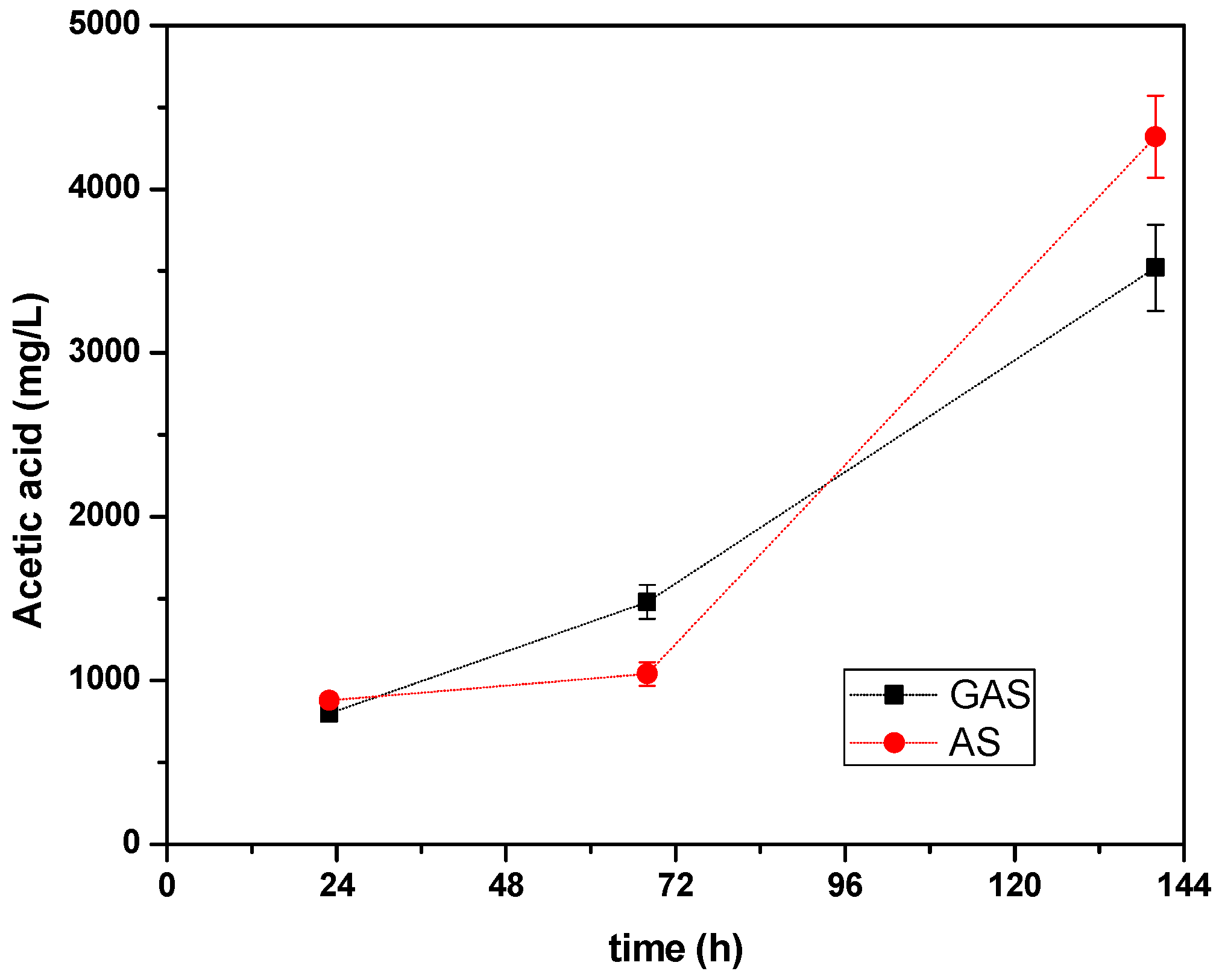
3.5. Production of Soluble Metabolites
4. Practical Application and Future Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Available online: www.eia.gov/forecasts/ieo (accessed on 25 February 2019).
- Ntaikou, I.; Antonopoulou, G.; Lyberatos, G. Biohydrogen production from biomass and wastes via dark fermentation: A review. Waste Biomass Valoriz. 2010, 1, 21–39. [Google Scholar] [CrossRef]
- Gandía, L.M.; Arzamendi, G.; Diéguez, P.M. Chapter 1—Renewable Hydrogen Energy: An Overview. In Renewable Hydrogen Technologies; Gandía, L.M., Arzamendi, G., Diéguez, P.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–17. [Google Scholar]
- Cruz-López, A.; Pozos, A.C.L.; Vázquez, S.; Zanella, R.; Gómez, R. Zn-Ge oxynitride based nano-photocatalyst for hydrogen production under visible light. Mater. Res. Bull. 2016, 83, 603–608. [Google Scholar] [CrossRef]
- Poggi-Varaldo, H.M.; Muñoz-Páez, K.M.; Escamilla-Alvarado, C.; Robledo-Narváez, P.N.; Ponce-Noyola, M.T.; Calva-Calva, G.; Ríos-Leal, E.; Galindez-Mayer, J.; Estrada-Vázquez, C.; Ortega-Clemente, A.; et al. Biohydrogen, biomethane and bioelectricity as crucial components of biorefinery of organic wastes: A review. Waste Manag. Res. 2014, 32, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Zerta, M.; Schmidt, P.; Stiller, C.; Ludwig, H.L. Alternative World Energy Outlook and the Role of hydrogen in a Changing Energy Landscape. In Proceedings of the 2nd World Congress of Young Scientist on Hydrogen Energy Systems, Turin, Italy, 6–8 June 2007. [Google Scholar]
- Mohan, S.V.; Pandey, A. Chapter 1—Biohydrogen Production: An Introduction. In Biohydrogen; Pandey, A., Chang, J.-S., Hallenbeck, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–24. [Google Scholar]
- Kim, S.H.; Han, S.K.; Shin, H.S. Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int. J. Hydrog. Energy 2004, 29, 1607–1616. [Google Scholar] [CrossRef]
- Ghimire, A.; Valentino, S.; Frunzo, L.; Trabli, E.; Escudié, R.; Pirozzi, F.; Lens, N.L.; Esposito, G. Biohydrogen production from food waste by coupling semi-continuous dark-photofermentation and residue post-treatment to anaerobic digestion: A synergy for energy recovery. Int. J. Hydrog. Energy 2015, 40, 16045–16055. [Google Scholar] [CrossRef]
- Ortigueira, J.; Pinto, T.; Gouveia, L.; Moura, P. Production and storage of biohydrogen during sequential batch fermentation of Spirogyra hydrolyzate by Clostridium butyricum. Energy 2015, 88, 528–536. [Google Scholar] [CrossRef]
- Nzihou, A. Toward the valorization of waste and biomass. Waste Biomass Valoriz. 2010, 1, 3–7. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudié, R.; Lens, P.N.L.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Bennemann, J.R. Special Processes for Products Fuel and Energy in the Encyclopedia of Life Support Systems, 1st ed.; Doelle, H.W., Rokem, S., Berovic, M., Eds.; Encyclopedia of Life Support Systems-United Nations Educational, Scientific and Cultural Organization (e-book Academic Collection EOLSS-UNESCO): Singapore, 2009; pp. 338–362. [Google Scholar]
- Akinbomi, J.; Taherzadeh, M. Evaluation of Fermentative Hydrogen Production from Single and Mixed Fruit Wastes. Energies 2015, 8, 4253–4272. [Google Scholar] [CrossRef]
- Alexandropoulou, M.; Antonopoulou, G.; Lyberatos, G. Food Industry Waste’s Exploitation via Anaerobic Digestion and Fermentative Hydrogen Production in an Up-Flow Column Reactor. Waste Biomass Valoriz. 2016, 7, 711–723. [Google Scholar] [CrossRef]
- Valta, K.; Damala, P.; Angeli, E.; Antonopoulou, G.; Malamis, D.; Haralambous, K.J. Current Treatment Technologies of Cheese Whey and Wastewater by Greek Cheese Manufacturing Units and Potential Valorisation Opportunities. Waste Biomass Valoriz. 2017, 8, 1649–1663. [Google Scholar] [CrossRef]
- Gómez-Romero, J.; González-García, A.; Chairez, I.; Torres, L.; García-Peña, E.I. Selective adaptation of an anaerobic microbial community: Biohydrogen production by co-digestion of cheese why and vegetables fruit wastes. Waste Manag. 2015, 43, 144–151. [Google Scholar] [CrossRef]
- De Gioannis, G.; Muntoni, A.; Polettini, A.; Pomi, R. A review of dark fermentative hydrogen production from biodegradable municipal waste fractions. Waste Manag. 2013, 33, 1345–1361. [Google Scholar] [CrossRef] [PubMed]
- Prapinagsorn, W.; Sittijunda, S.; Reungsang, A. Co-digestion of napier grass and its silage with cow dung for bio-hydrogen and methane production by two-stage anaerobic digestion process. Energies 2018, 11, 47. [Google Scholar] [CrossRef]
- Ferreira, A.; Marques, P.; Ribeiro, B.; Assemany, P.; Vieira, H.; Barata, A.; Oliveira, A.C.; Reis, A.; Pinheiro, H.M.; Gouveia, L. Combining biotechnology with circular bioeconomy: From poultry, swine, cattle, brewery, dairy and urban wastewaters to biohydrogen. Environ. Res. 2018, 164, 32–38. [Google Scholar] [CrossRef]
- Basak, B.; Fatima, A.; Jeon, B.-H.; Ganguly, A.; Chatterjee, P.K.; Dey, A. Process kinetic studies of biohydrogen production by co-fermentation of fruit-vegetable wastes and cottage cheese whey. Energy Sustain. Dev. 2018, 47, 39–52. [Google Scholar] [CrossRef]
- García-Depraect, O.; Gómez-Romero, J.; León-Becerril, E.; López-López, A. A novel biohydrogen production process: Co-digestion of vinasse and Nejayote as complex raw substrates using a robust inoculum. Int. J. Hydrog. Energy 2017, 42, 5820–5831. [Google Scholar] [CrossRef]
- Saidi, R.; Liebgott, P.P.; Hamdi, M.; Auria, R.; Bouallagui, H. Enhancement of fermentative hydrogen production by Thermotoga maritima through hyperthermophilic anaerobic co-digestion of fruit-vegetable and fish wastes. Int. J. Hydrog. Energy 2018, 43, 23168–23177. [Google Scholar] [CrossRef]
- Maciel Pinto, M.P.; Mudhoo, A.; Alencar Neves, T.; Donizeti Berni, M.; Forster-Carneiro, T. Co–digestion of coffee residues and sugarcane vinasse for biohythane generation. J. Environ. Chem. Eng. 2018, 6, 146–155. [Google Scholar] [CrossRef]
- Pagliano, G.; Vertorino, V.; PAnico, A.; Romano, I.; Robertiello, A.; Pirozzi, F.; Pepe, O. The effect of bacterial and archaeal populations on anaerobic process fed with mozzarella cheese whey and buttermilk. J. Environ. Manag. 2018, 217, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Liu, J.; Wei, Y. Enhanced biohydrogen production from sewage sludge with alkaline pretreatment. Environ. Sci. Technol. 2004, 38, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
- Argun, H.; Dao, S. Bio-hydrogen production from waste peach pulp by dark fermentation: Effect of inoculum addition. Int. J. Hydrog. Energy 2017, 42, 2569–2574. [Google Scholar] [CrossRef]
- Cappai, G.; De Gioannis, G.; Muntoni, A.; Spiga, D.; Boni, M.R.; Polettini, A.; Pomi, R.; Rossi, A. Biohydrogen production from food waste: Influence of the inoculum-to-Substrate ratio. Sustainability 2018, 10, 4506. [Google Scholar] [CrossRef]
- Pérez-Denicia, E.; Fernández-Luqueño, F.; Vilariñi-Ayala, D.; Montaño-Zetina, L.M.; Maldonado-López, L.A. Renewable energy sources for electricity generation in Mexico: A review. Renew. Sustain. Energy Rev. 2017, 78, 597–613. [Google Scholar] [CrossRef]
- Moya, D.; Aldás, C.; López, G.; Kaparaju, P. Municipal solid waste as a valuable renewable energy resource: A worldwide opportunity of energy recovery by using Waste-to-Energy technologies. Energy Procedia 2017, 134, 286–295. [Google Scholar] [CrossRef]
- Available online: https://www.elfinanciero.com.mx/monterrey/incineraran-basura-para-generar-mas-energia-limpia (accessed on 25 February 2019).
- Bansal, S.K.; Sreekrishnan, T.R.; Singh, R. Effect of heat pretreated consortia on fermentative biohydrogen production from vegetable waste. Natl. Acad. Sci. Lett. 2013, 36, 125–131. [Google Scholar] [CrossRef]
- Buitrón, G.; Carvajal, C. Biohydrogen production from Tequila vinasses in an anaerobic sequencing batch reactor: Effect of initial substrate concentration, temperature and hydraulic retention time. Bioresour. Technol. 2010, 101, 9071–9077. [Google Scholar] [CrossRef]
- Garcia-Peña, I.E.; Canul-Chan, M.; Chairez, I.; Salgado-Manjarez, E.; Aranda-Barradas, J. Biohydrogen production based on the evaluation of kinetic parameters of a mixed microbial culture using glucose and fruit-vegetable waste as feedstocks. Appl. Biochem. Biotechnol. 2013, 171, 279–293. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association. Standard Methods for the Examination of Water and Wastewater, 21th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Budiman, P.M.; Wu, T.Y.; Ramanan, R.N.; Jahim, J. Improvement of Biohydrogen Production through Combined Reuses of Palm Oil Mill Effluent Together with Pulp and Paper Mill Effluent in Photofermentation. Energy Fuels 2015, 29, 5816–5824. [Google Scholar] [CrossRef]
- Dubois, M.; Giles, K.A.; Hamilton, J.; Rebers, P.A.; Simith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, L.A.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Zhang, J.; Sun, H.; Pan, C.; Fan, Y.; Hou, H. Optimization of Process Parameters for Directly Converting Raw Corn Stalk to Biohydrogen by Clostridium sp. FZ11 without Substrate Pretreatment. Energy Fuels 2016, 30, 311–317. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ahmad, D.; Soning, C. Bio-hydrogen generation from mixed fruit peel waste using anaerobic contact filter. Int. J. Hydrog. Energy 2007, 32, 4754–4760. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, S.; Wang, D. Hydrogen-methane production from pulp & paper sludge and food waste by mesophilic-thermophilic anaerobic co-digestion. Int. J. Hydrog. Energy 2013, 38, 15055–15062. [Google Scholar]
- Yossan, S.; O-Thong, S.; Prasertsan, P. Effect of initial pH, nutrients and temperature on hydrogen production from palm oil mill effluent using thermotolerant consortia and corresponding microbial communities. Int. J. Hydrog. Energy 2012, 37, 13806–13814. [Google Scholar] [CrossRef]
- Mishra, P.; Roy, S.; Das, D. Comparative evaluation of the hydrogen production by mixed consortium, synthetic co-culture and pure culture using distillery effluent. Bioresour. Technol. 2015, 198, 593–602. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.H.; Kim, K.Y.; Shin, H.S. Experience of a pilot-scale hydrogen-producing anaerobic sequencing batch reactor (ASBR) treating food waste. Int. J. Hydrog. Energy 2010, 35, 1590–1594. [Google Scholar] [CrossRef]
- Valdez-Vazquez, I.; Torres-Aguirre, G.J.; Molina, C.; Ruiz-Aguilar, G.M.L. Characterization of a Lignocellulolytic Consortium and Methane Production from Untreated Wheat Straw: Dependence on Nitrogen and Phosphorous Content. BioResources 2016, 11, 4237–4251. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ahmad, D.; Ibrahim, M.K.B. Biohydrogen generation from jackfruit peel using anaerobic contact filter. Int. J. Hydrog. Energy 2006, 31, 569–579. [Google Scholar] [CrossRef]
- Hernández-Mendoza, C.E.; Moreno-Andrade, I.; Buitrón, G. Comparison of hydrogen-producing bacterial communities adapted in continuous and discontinuous reactors. Int. J. Hydrog. Energy 2014, 39, 14234–14239. [Google Scholar] [CrossRef]
- Oh, Y.K.; Raj, S.M.; Jung, G.Y.; Park, S. Current status of the metabolic engineering of microorganisms for biohydrogen production. Bioresour. Technol. 2011, 102, 8357–8367. [Google Scholar] [CrossRef] [PubMed]
- Sivagurunathan, P.; Lin, C.Y. Enhanced biohydrogen production from beverage wastewater: Process performance during various hydraulic retention times and their microbial insights. RSC Adv. 2016, 6, 4160–4169. [Google Scholar] [CrossRef]
- Zhang, B.; Li, G.; Cheng, P.; Jim, T.-C.Y.; Hong, M. Landfill Risk Assessment on Groundwater Based on Vulnerability and Pollution Index. Water Resour. Manag. 2016, 30, 1465–1480. [Google Scholar] [CrossRef]
- Bao, M.; Su, H.; Tan, T. Biohydrogen production by dark fermentation of starch using mixed bacterial cultures of Bacillus sp and Brevumdimonas sp. Energy Fuels 2012, 26, 5872–5878. [Google Scholar] [CrossRef]
- Garcia-Peña, E.I.; Parameswaran, P.; Kang, D.W.; Canul-Chan, M.; Krajmalnik-Brown, R. Anaerobic digestion and co-digestion processes of vegetable and fruit residues: Process and microbial ecology. Bioresour. Technol. 2011, 102, 9447–9455. [Google Scholar] [CrossRef] [PubMed]
- Abubackar, H.N.; Keskin, T.; Yazgin, O.; Gunay, B.; Arslan, K.; Azbar, N. Biohydrogen production from autoclaved fruit and vegetable wastes by dry fermentation under thermophilic condition. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef]
| AS | GAS | |
|---|---|---|
| pH | 7.20 | 7.83 |
| TS (%) | 36.6 | 46.6 |
| VS (%) | 28.2 | 35.4 |
| COD (g L−1) | 56.2 | 66.3 |
| TKN (g L−1) | 4.1 | 3.14 |
| Reactor | C/N Ratio | Temperature °C | Inoculum Source |
|---|---|---|---|
| 1 | 20 | 55 | AS |
| 2 | 30 | 37 | AS |
| 3 | 20 | 25 | AS |
| 4 | 40 | 25 | AS |
| 5 | 40 | 55 | GAS |
| 6 | 30 | 37 | GAS |
| 7 | 20 | 55 | GAS |
| 8 | 20 | 55 | GAS |
| 9 | 40 | 25 | GAS |
| 10 | 20 | 25 | AS |
| 11 | 40 | 25 | AS |
| 12 | 20 | 55 | AS |
| 13 | 20 | 25 | GAS |
| 14 | 30 | 37 | GAS |
| 15 | 40 | 55 | AS |
| 16 | 30 | 37 | GAS |
| 17 | 30 | 37 | AS |
| 18 | 30 | 37 | AS |
| Parameter | Melon (Cucumis spp.) | Papaya (Papaya spp.) | Pineapple (Ananas spp.) | Sewage Sludge | |
|---|---|---|---|---|---|
| pH | 5.95 (0.15) | 6.3 (1.4) | 4.18 (0.12) | 7.57 (0.03) | |
| Humidity | % | 92.1 (1.10) | 89.61 (1.3) | 87.45 (1.9) | 92.43 (0.03) |
| TS | % | 7.51 (1.10) | 10.33 (1.29) | 12.55 (1.92) | 6.38 (0.27) |
| VS | % | 6.54 (0.95) | 8.83 (1.19) | 11.5 (1.86) | 3.96 (0.02) |
| TCH | g Glucose 100 g−1 substrate | 10.4 (0.24) | 6 (0.14) | 12.5 (0.3) | - |
| COD | g g−1 sub-strate | 3.6 (0.5) | 6.9 (0.5) | 7.5 (0.5) | 133.7 (0.5) a |
| Proteins | mg g−1substrate | 3.7 (0.18) | 3.5 (0.18) | 1.8 (0.18) | 1180 (60) b |
| Reactor | C/N Ratio | Temperature °C | Inoculum Source | Biogas | H2 | |
|---|---|---|---|---|---|---|
| mL L−1 Reactor | mL L−1 Reactor | mL g−1VS | ||||
| 1 | 20 | 55 | AS | 268 | - | - |
| 2 | 30 | 37 | AS | 1556 | 223 | - |
| 3 | 20 | 25 | AS | 700 | - | - |
| 4 | 40 | 25 | AS | 875 | - | |
| 5 | 40 | 55 | GAS | 1025 | - | - |
| 6 | 30 | 37 | GAS | 2225 | 366 | 21.52 |
| 7 | 20 | 55 | GAS | 787 | 84 | 6.24 |
| 8 | 20 | 55 | GAS | 787 | - | - |
| 9 | 40 | 25 | GAS | 713 | 36 | 3.24 |
| 10 | 20 | 25 | AS | 700 | - | - |
| 11 | 40 | 25 | AS | 567 | - | - |
| 12 | 20 | 55 | AS | 331 | - | - |
| 13 | 20 | 25 | GAS | 700 | 20 | 1.6 |
| 14 | 30 | 37 | GAS | 2215 | 365 | 30.6 |
| 15 | 40 | 55 | AS | 362 | 5 | |
| 16 | 30 | 37 | GAS | 2225 | 367 | 28.67 |
| 17 | 30 | 37 | AS | 1556 | 223 | - |
| 18 | 30 | 37 | AS | 1558 | 212 | - |
| Reactor | Ratio C/N | Temperature °C | Hmax mL L−1 | Rm mL L−1 h−1 | λ h | r2 |
|---|---|---|---|---|---|---|
| R9 | 40 | 25 | 17.5 | 5.0 | 23 | <0.90 |
| R13 | 20 | 25 | 33 | 0.7 | 21 | 1.0 |
| R14 | 30 | 37 | 86.6 | 2.6 | 1.95 | 0.99 |
| R7 | 20 | 55 | 26.9 | 1.9 | 20 | 0.7 |
| R5 | 40 | 55 | n.c. | n.c. | n.c. | n.c. |
| Reactor | R4 | R5 | R6 | R7 | R8 | R9 | R13 | R14 | R16 | |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | I0 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 |
| F | 3.75 | 4.27 | 5.1 | 4.26 | 4.24 | 4.3 | 3.8 | 5.1 | 5.1 | |
| COD g L−1 | I0 | 60.4 | 49.1 | 44.3 | 34.6 | 34.3 | 68.7 | 39.7 | 44.3 | 44.3 |
| F | 30.1 | 27.3 | 21.9 | 14.7 | 18 | 31.7 | 19.1 | 21.7 | 23.6 | |
| TCH mg glucose L−1 | I0 | 2177 | 2156 | 2500 | 755 | 910 | 2312 | 889 | 2500 | 2500 |
| F | 798 | 882 | 1235 | 411 | 540 | 922 | 640 | 1250 | 1200 | |
| Ethyl alcohol %v/v | I0 | 5 | 4.7 | 3.9 | 3 | 3.3 | 4.7 | 3.3 | 5 | 5 |
| F | 4.4 | 5 | 3.4 | 1.3 | 1.9 | 4.5 | 3.7 | 3.5 | 3.5 | |
| SV g L−1 | I0 | 45.7 | 45.5 | 47.3 | 46.9 | 46.3 | 45.3 | 46.1 | 47.3 | 47.3 |
| F | 31.7 | 36.8 | 30.3 | 33.45 | 33.9 | 34.2 | 34.1 | 35.4 | 34.5 | |
| Acetic acid mg L−1 | I0 | 800 | 880 | 680 | 880 | 960 | 960 | 560 | 800 | 800 |
| F | 3000 | 4320 | 3600 | 2120 | 2400 | 1880 | 1400 | 3580 | 3600 |
| Reactor Operation Mode | Type of Substrate or Codigestion | Inoculum/Pretreatment | Conditions | H2 Yield mL H2 gVS−1 | % COD Removal | Reference |
|---|---|---|---|---|---|---|
| Batch | Fruit–Vegetable waste | Anaerobic digester sludge, HST | C/N = Not reported pH = 5.5 * T = 35 °C V = 0.5 L 37 g COD L−1 | 60 | 25 | [34] |
| Batch | Cheese whey—fruit vegetables waste | Anaerobic digester sludge, HST | C/N = 21, pH = 5.5 * T = 37 °C V = 2 L 144.133 g COD L−1 | 449.8 mL H2 g−1 COD | 16 | [17] |
| CSTR | Food waste | Enriched mixed culture, without HST | C/N = 15 T = 39 °C, pH = 6.5 * 4.5 L | 89.8 | Not reported | [52] |
| Batch | Organic Fraction of Municipal Solid Waste–Food Waste | Cow manure, without HST | C/N = Not reported pH = 4.0 T = 30 °C V = 0.125 L 100 g vs. Kg−1 | 14 | Not reported | [53] |
| Batch | Fruit Peels–Sewage Sludge | GAS, HST | C/N = 30 pH = 5.5 T = 37 °C V = 0.1 L 44.3 g COD L−1 | 25 | 50% | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyna-Gómez, L.M.; Molina-Guerrero, C.E.; Alfaro, J.M.; Suárez Vázquez, S.I.; Robledo-Olivo, A.; Cruz-López, A. Effect of Carbon/Nitrogen Ratio, Temperature, and Inoculum Source on Hydrogen Production from Dark Codigestion of Fruit Peels and Sewage Sludge. Sustainability 2019, 11, 2139. https://doi.org/10.3390/su11072139
Reyna-Gómez LM, Molina-Guerrero CE, Alfaro JM, Suárez Vázquez SI, Robledo-Olivo A, Cruz-López A. Effect of Carbon/Nitrogen Ratio, Temperature, and Inoculum Source on Hydrogen Production from Dark Codigestion of Fruit Peels and Sewage Sludge. Sustainability. 2019; 11(7):2139. https://doi.org/10.3390/su11072139
Chicago/Turabian StyleReyna-Gómez, Lirio María, Carlos Eduardo Molina-Guerrero, Juan Manuel Alfaro, Santiago Iván Suárez Vázquez, Armando Robledo-Olivo, and Arquímedes Cruz-López. 2019. "Effect of Carbon/Nitrogen Ratio, Temperature, and Inoculum Source on Hydrogen Production from Dark Codigestion of Fruit Peels and Sewage Sludge" Sustainability 11, no. 7: 2139. https://doi.org/10.3390/su11072139
APA StyleReyna-Gómez, L. M., Molina-Guerrero, C. E., Alfaro, J. M., Suárez Vázquez, S. I., Robledo-Olivo, A., & Cruz-López, A. (2019). Effect of Carbon/Nitrogen Ratio, Temperature, and Inoculum Source on Hydrogen Production from Dark Codigestion of Fruit Peels and Sewage Sludge. Sustainability, 11(7), 2139. https://doi.org/10.3390/su11072139







