Gas Multiple Flow Mechanisms and Apparent Permeability Evaluation in Shale Reservoirs
Abstract
:1. Introduction
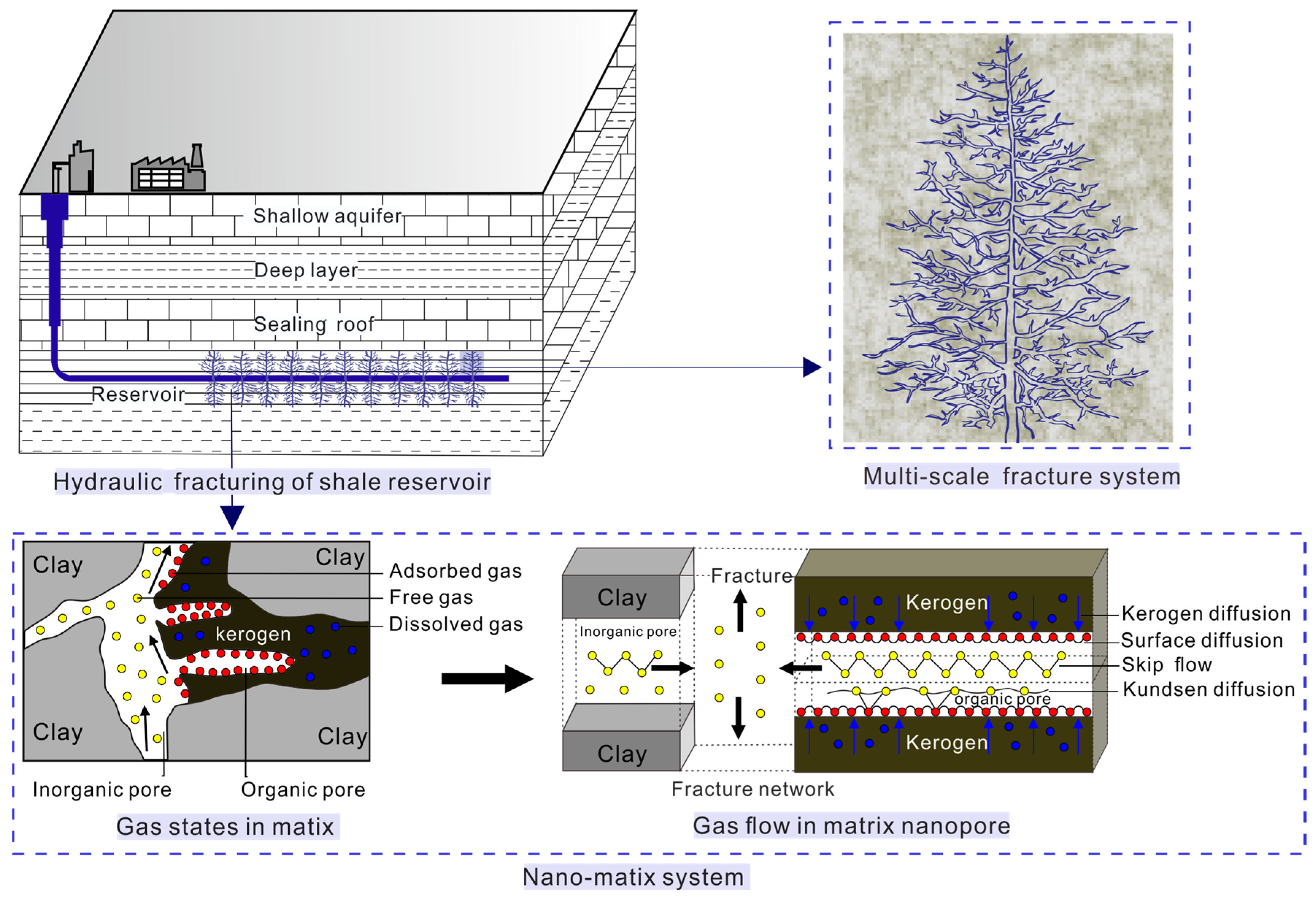
2. Shale Gas Multiple Flow Mechanisms
2.1. Kerogen Diffusion
2.2. Surface Diffusion
2.3. Gas Flow Regimes in Nanopores
3. Model Establishment and Analysis
3.1. Establishment of Apparent Permeability Model
3.2. Permeability Dynamic Changes under Matrix Shrinkage and Stress Sensitivity
4. Methodology
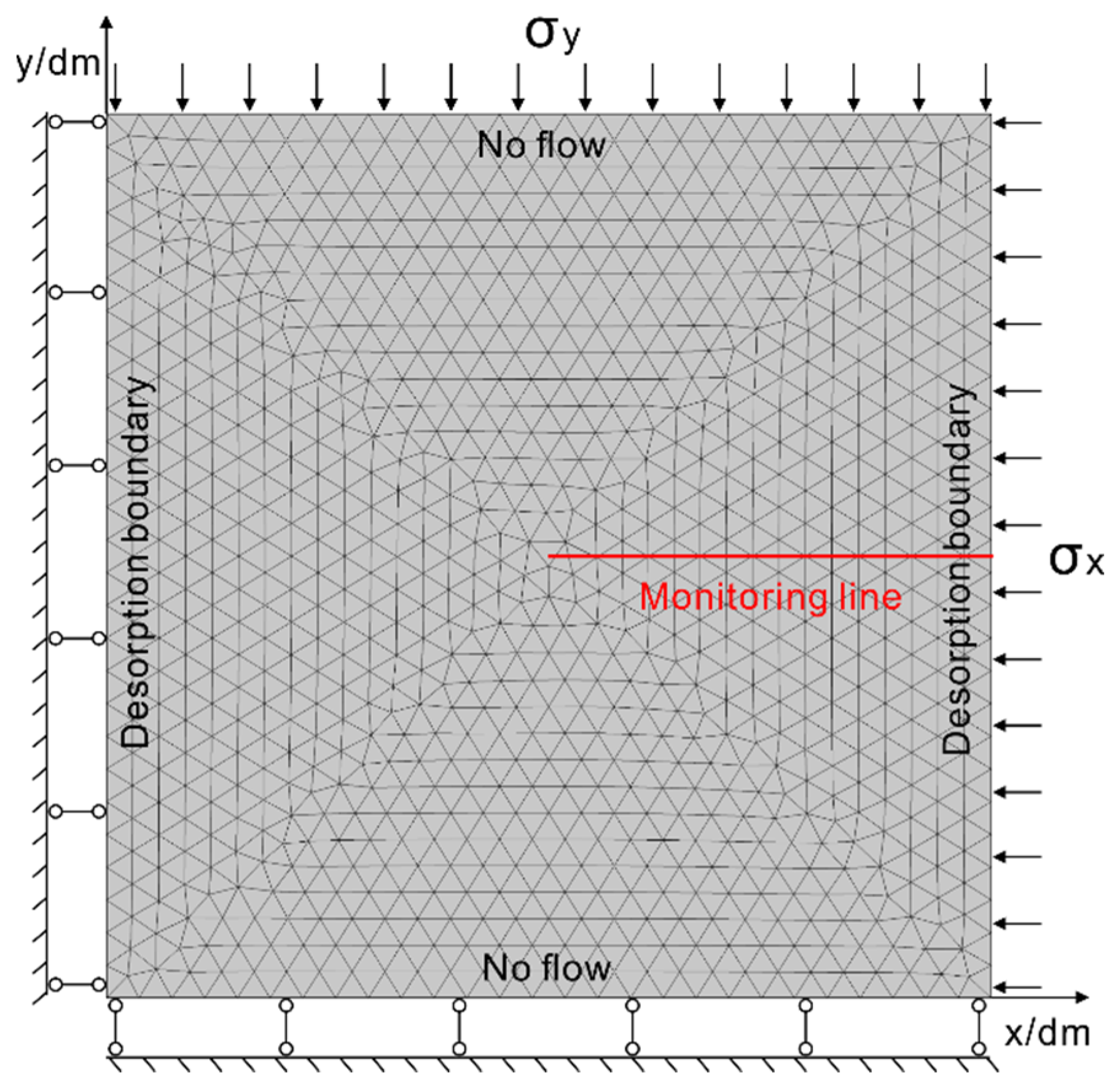
4.1. Numerical Model and Module
4.2. Physical Boundary Conditions and Input Parameters
5. Results and Discussion
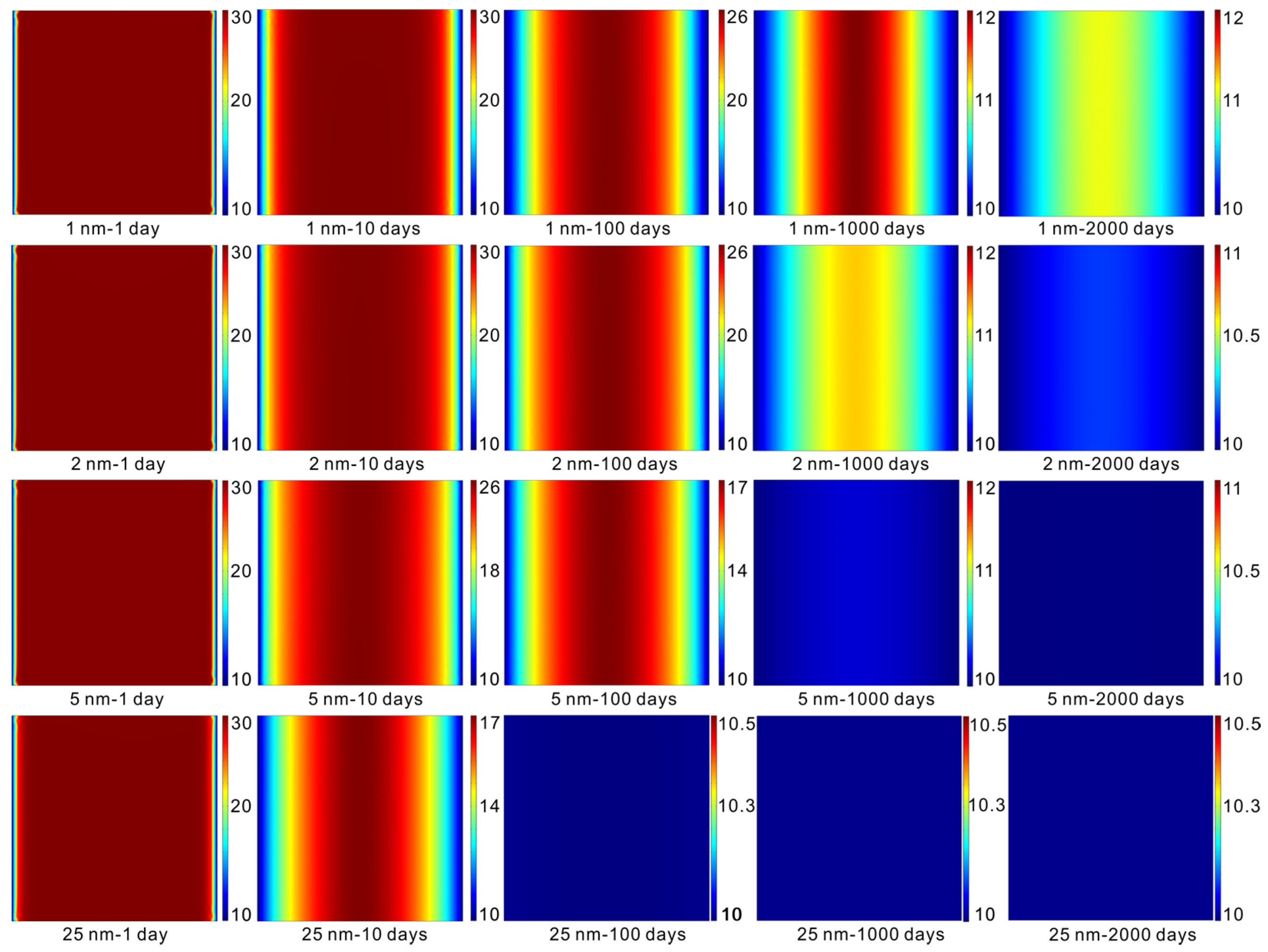
5.1. Apparent Permeability Evaluation during Production
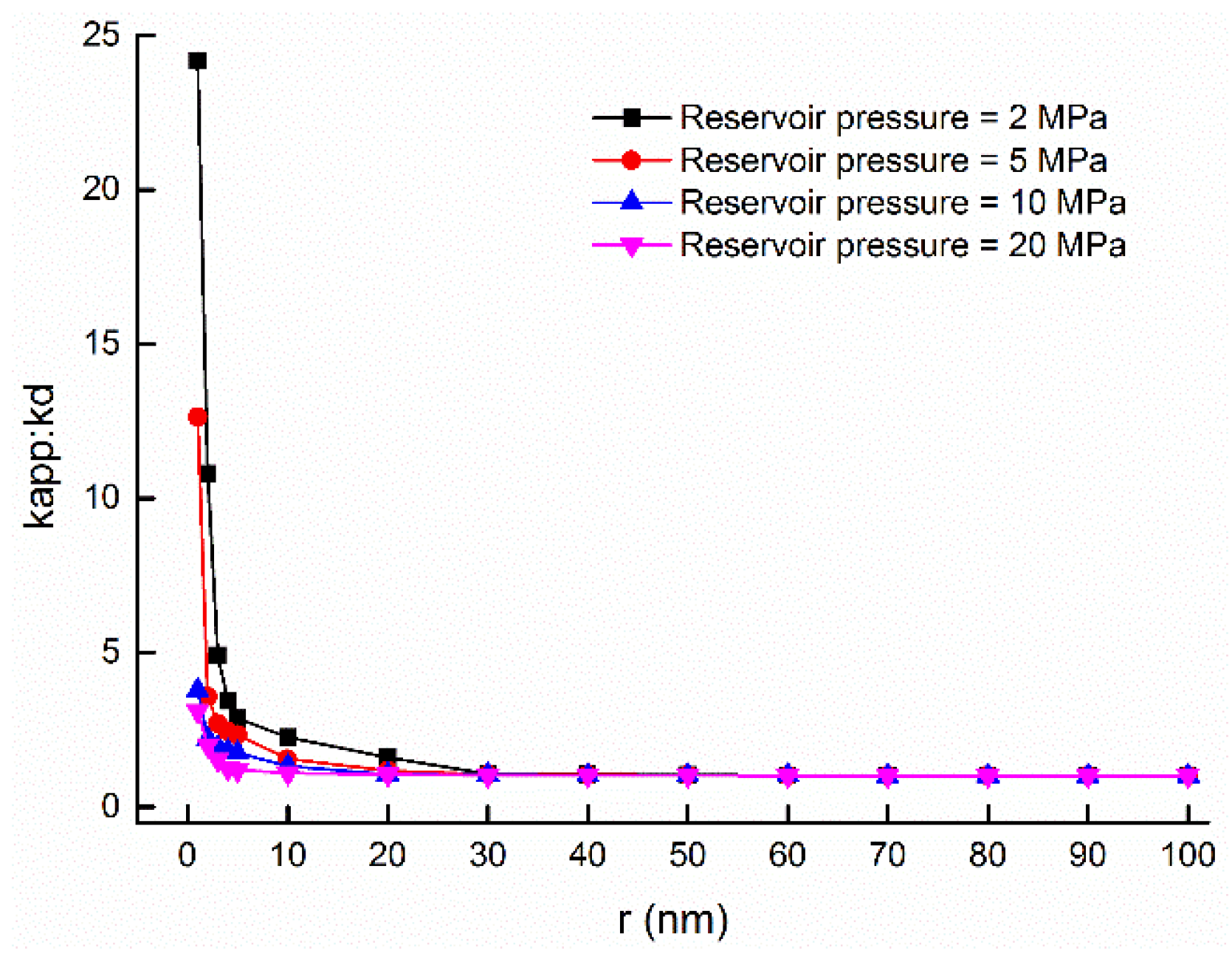
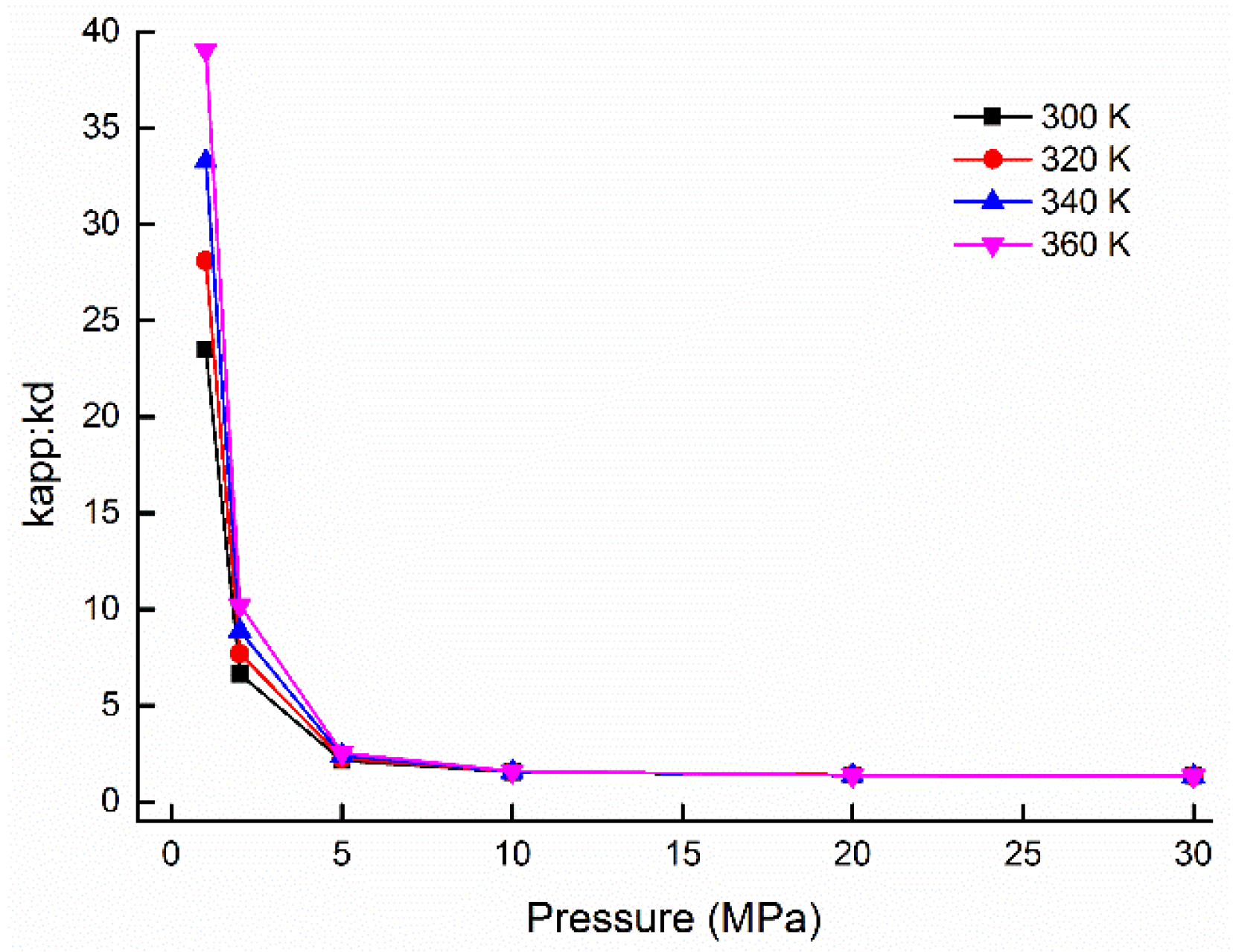
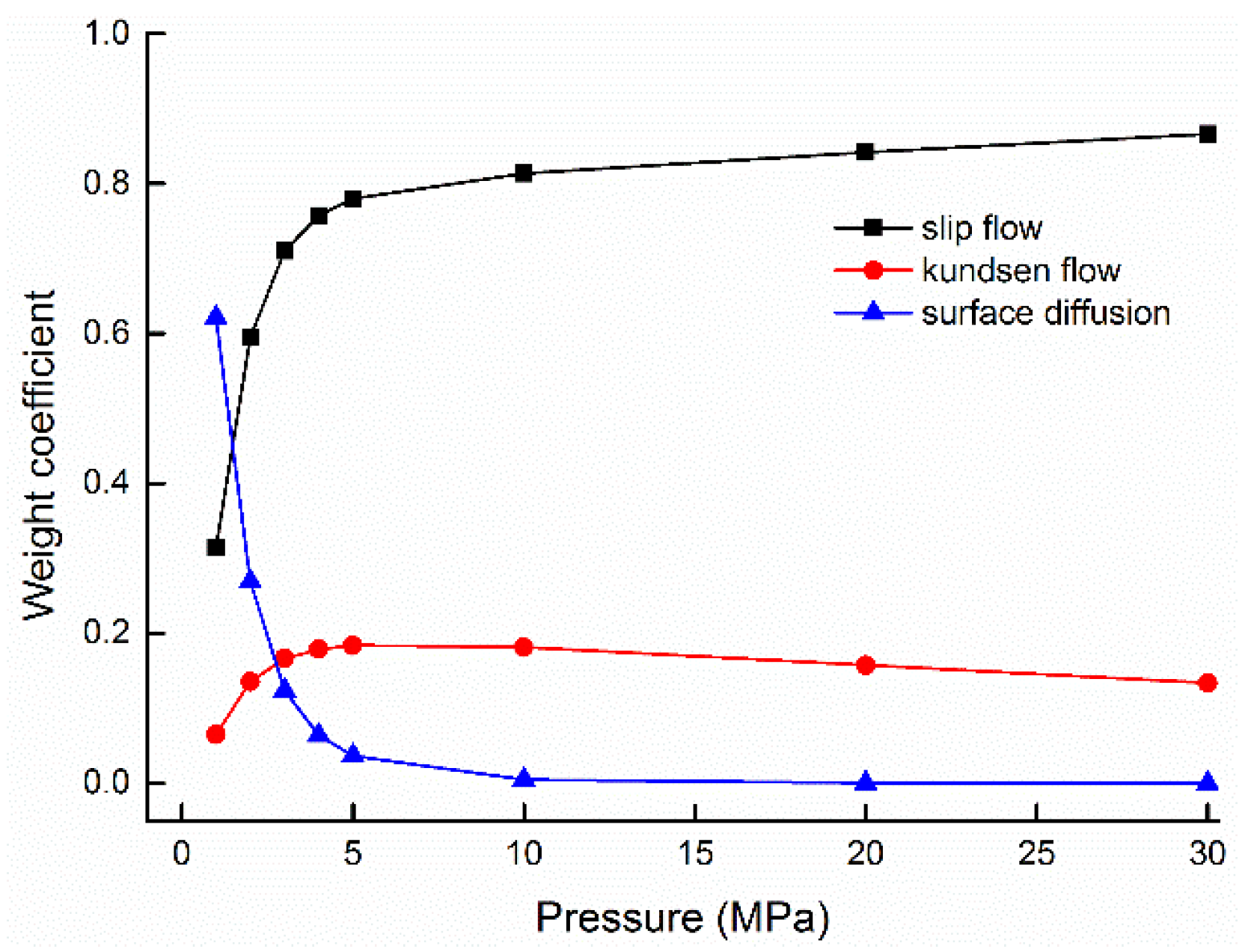
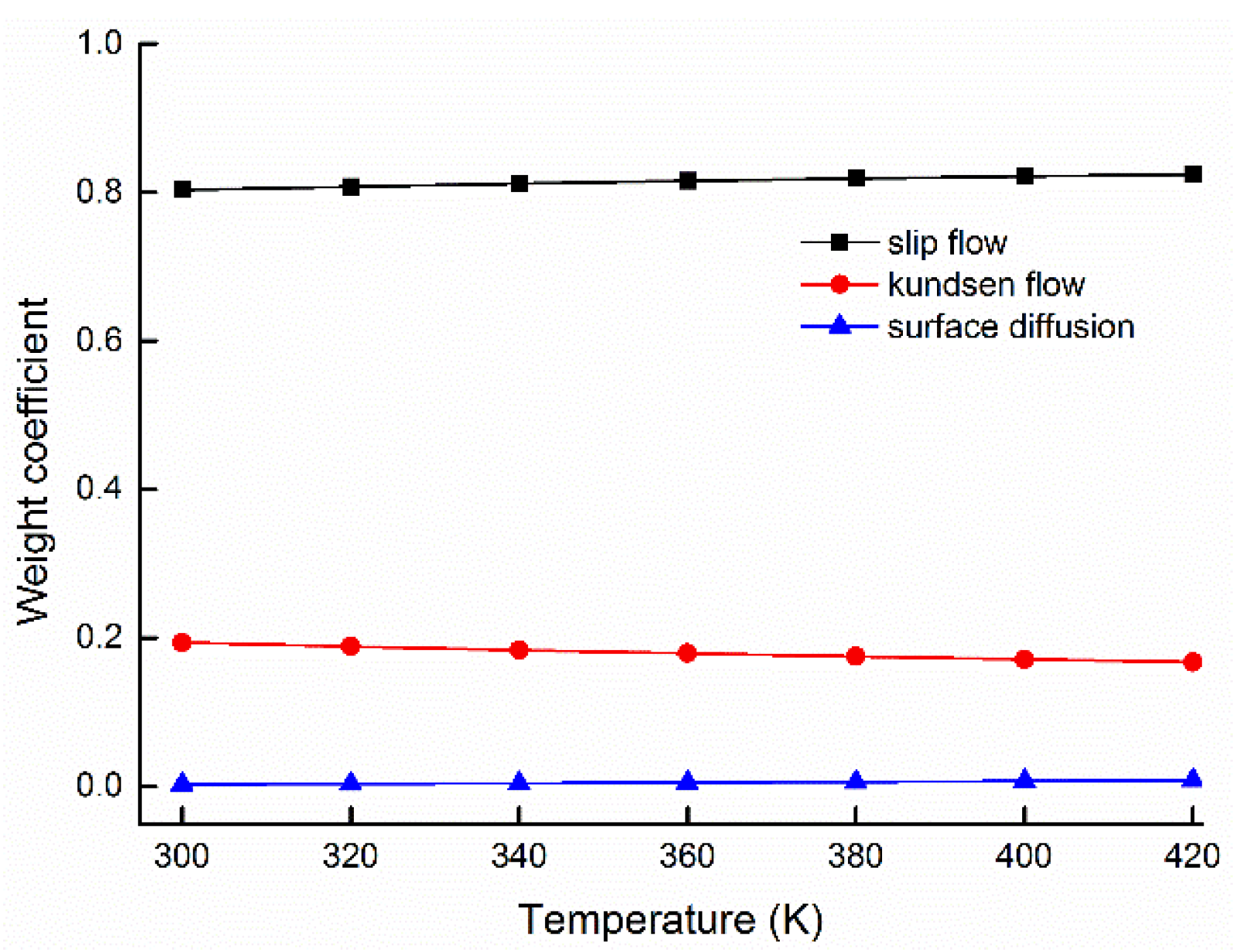
5.2. Weight Ratios of Gas Flow Mechanisms and Its Influenced Factors
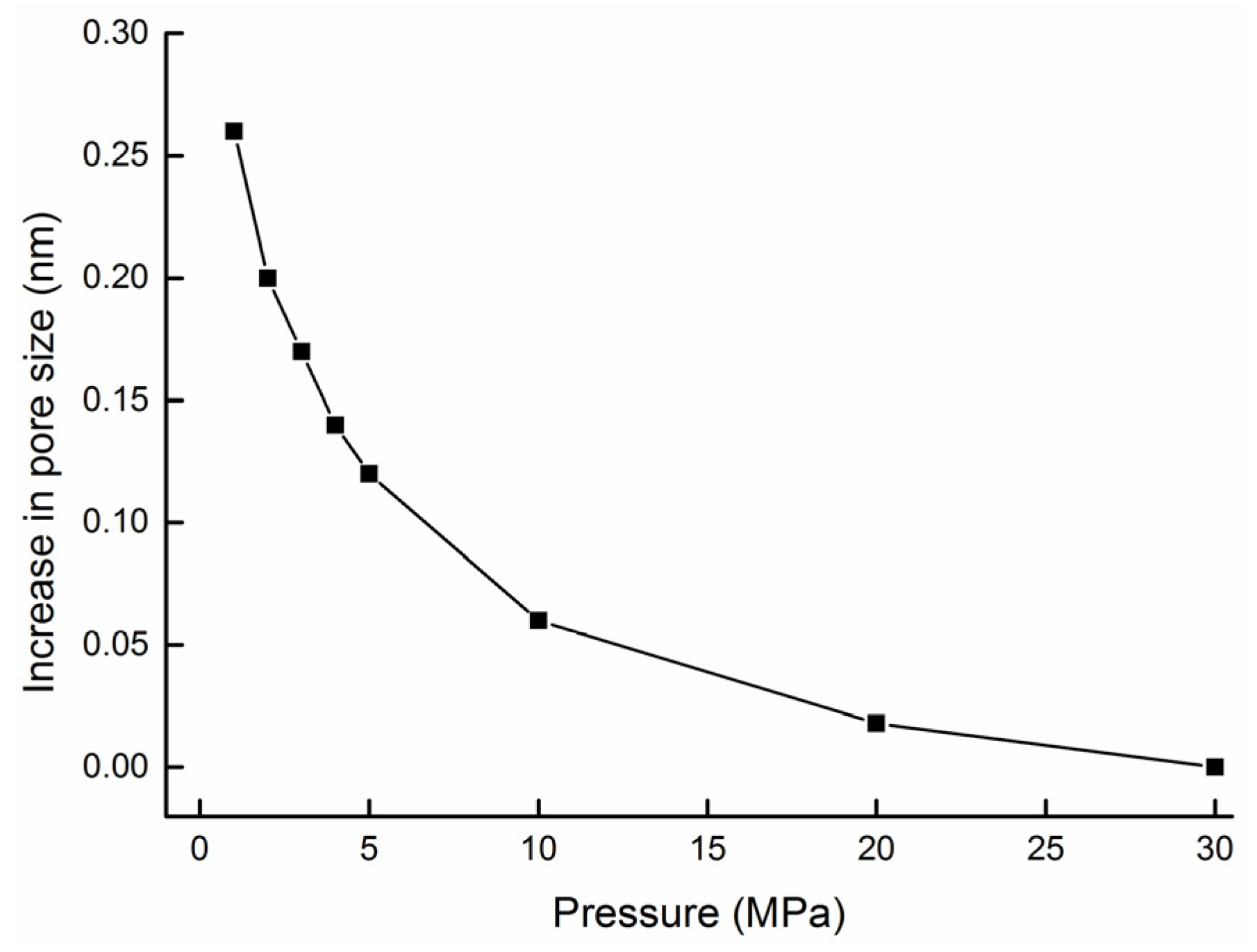
5.3. Permeability Dynamic Change
5.4. Comparions of Apparment Permeability Model to Previous Models
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Curtis, J.B. Fractured shale-gas systems. AAPG Bull. 2002, 86, 1921–1938. [Google Scholar] [CrossRef]
- Liu, H.X.; Li, J.L. The US Shale Gas Revolution and Its Externality on Crude Oil Prices: A Counterfactual Analysis. Sustainability 2018, 10, 697. [Google Scholar] [CrossRef]
- Howarth, R.W.; Santoro, R.; Ingraffea, A. Methane and the greenhouse–gas footprint of natural gas from shale formation. Clim. Chang. 2011, 106, 679–690. [Google Scholar] [CrossRef]
- Rahm, D. Regulating hydraulic fracturing in shale gas plays: The case of Texas. Energy Policy 2011, 39, 2974–2981. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, Y.; Wang, W.; Lu, M.; Sheng, G. Apparent permeability for liquid transport in nanopores of shale reservoirs: Coupling flow enhancement and near wall flow. Int. J. Heat Mass Transf. 2017, 115, 224–234. [Google Scholar] [CrossRef]
- Wang, S.; Javadpour, F.; Feng, Q. Fast mass transport of oil and supercritical carbon dioxide through organic nanopores in shale. Fuel 2016, 181, 741–758. [Google Scholar] [CrossRef]
- Wang, S.; Javadpour, F.; Feng, Q. Molecular dynamics simulations of oil transport through inorganic nanopores in shale. Fuel 2016, 171, 74–86. [Google Scholar] [CrossRef]
- Wu, K.; Li, X.; Guo, C.; Wang, C.; Chen, Z. A unified model for gas transfer in nanopores of shale–gas reservoirs: Coupling pore diffusion and surface diffusion. SPE J. 2016, 21, 1583–1611. [Google Scholar] [CrossRef]
- Javadpour, F.; Farshi, M.M.; Amrein, M. Atomic–force microscopy: A new tool for gas–shale characterization. J. Can. Pet. Technol. 2012, 51, 236–243. [Google Scholar] [CrossRef]
- Nozawa, M.; Tanizawa, K.; Kanaoka, Y. BIB–SEM study of the pore space morphology in early mature Posidonia shale from the Hils area, Germany. Int. J. Coal Geol. 2012, 103, 12–25. [Google Scholar] [CrossRef]
- Mehmani, A.; Prodanović, M.; Javadpour, F. Multiscale, multiphysics network modeling of shale matrix gas flows. Transp. Porous Media 2013, 99, 377–390. [Google Scholar] [CrossRef]
- Javadpour, F. Nanopores and apparent permeability of gas flow in mudrocks (shales and siltstone). J. Can. Pet. Technol. 2009, 48, 16–21. [Google Scholar] [CrossRef]
- Lilley, C.R.; Sader, J.E. Velocity profile in the knudsen layer according to the boltzmann equation. Proc. Math. Phys. Eng. Sci. 2008, 464, 2015–2035. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, D. Impact of adsorption on gas transport in nanopores. Sci. Rep. 2016, 6, 23629. [Google Scholar] [CrossRef]
- Beskok, A.; Karniadakis, G.E. Report: A model for flows in channels, pipes, and ducts at micro and nano scales. Microscale Thermophys. Eng. 1999, 3, 43–77. [Google Scholar] [CrossRef]
- Hornyak, G.L.; Tibbals, H.F.; Dutta, J.; Moore, J.J. Introduction to Nanoscience and Nanotechnology; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Karniadakis, G.; Beskok, A.; Aluru, N. Microflows and Nanoflows: Fundamentals and Simulation; Springer: New York, NY, USA, 2005. [Google Scholar]
- Bird, G.A. Molecular Gas Dynamics and the Direct Simulation of Gas Flows; Clarendon Press: Oxford, UK, 2003. [Google Scholar]
- Guo, Z.; Shi, B.; Zheng, C.G. An extended Navier–Stokes formulation for gas flows in the Knudsen layer near a wall. Europhys. Lett. 2007, 80, 24001. [Google Scholar] [CrossRef]
- Ziarani, A.S.; Aguilera, R. Knudsen’s permeability correction for tight porous media. Transp. Porous Media 2012, 91, 239–260. [Google Scholar] [CrossRef]
- Maxwell, J.C. On stresses in rarified gases arising from inequalities of temperature. Philos. Trans. R. Soc. Lond. 1879, 170, 231–256. [Google Scholar] [CrossRef]
- Knudsen, M. Die Gesetze der Molekularströmung und der inneren Reibungsströmung der Gase durch Röhren. Ann. Phys. 1909, 333, 75–130. [Google Scholar] [CrossRef]
- Pollard, W.G.; Present, R.D. On gaseous self–diffusion in long capillary tubes. Phys. Rev. 1948, 73, 762–774. [Google Scholar] [CrossRef]
- Klinkenberge, L.J. The permeability of porous media to liquids and gases. Drill. Prod. Pract. 1941, 22, 200–213. [Google Scholar] [CrossRef]
- Ertekin, T.; King, G.R.; Schwerer, F.C. Dynamic gas slippage: A unique dual–mechanism approach to the flow of gas in tight formations. SPE Form. Eval. 1986, 1, 43–52. [Google Scholar] [CrossRef]
- Javadpour, F.; Fisher, D.; Unsworth, M. Nanoscale gas flow in shale gas sediments. J. Can. Pet. Technol. 2007, 46, 55–61. [Google Scholar] [CrossRef]
- Civan, F. Effective correlation of apparent gas permeability in tight porous media. Transp. Porous Media 2010, 82, 375–384. [Google Scholar] [CrossRef]
- Civan, F.; Rai, C.S.; Sondergeld, C.H. Shale–gas permeability and diffusivity inferred by improved formulation of relevant retention and transport mechanisms. Transp. Porous Media 2011, 86, 925–944. [Google Scholar] [CrossRef]
- Ren, W.; Li, G.; Tian, S.; Sheng, M.; Geng, L. Adsorption and surface diffusion of supercritical methane in shale. Ind. Eng. Chem. Res. 2017, 56, 3446–3455. [Google Scholar] [CrossRef]
- Wu, K.; Li, X.; Wang, C.; Yu, W.; Chen, Z. Model for surface diffusion of adsorbed gas in nanopores of shale gas reservoirs. Ind. Eng. Chem. Res. 2015, 54, 3225–3236. [Google Scholar] [CrossRef]
- Wu, K.; Chen, Z.; Li, X.; Guo, C.; Wei, M. A model for multiple transport mechanisms through nanopores of shale gas reservoirs with real gas effect–adsorption–mechanic coupling. Int. J. Heat Mass Transf. 2016, 93, 408–426. [Google Scholar] [CrossRef]
- George, J.D.S.; Barakat, M.A. The change in effective stress associated with shrinkage from gas desorption in coal. Int. J. Coal Geol. 2001, 45, 105–113. [Google Scholar] [CrossRef]
- Harpalani, S.; Schraufnagel, R.A. Influence of matrix shrinkage and compressibility on gas production from coalbed methane reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 23–26 September 1990; pp. 338–339. [Google Scholar]
- Levine, J.R. Model study of the influence of matrix shrinkage on absolute permeability of coal bed reservoirs. Geol. Soc. Lond. Spec. Publ. 1996, 109, 197–212. [Google Scholar] [CrossRef]
- An, C.; Fang, Y.; Liu, S.; Alfi, M.; Yan, B.; Wang, Y. Impacts of Matrix Shrinkage and Stress Changes on Permeability and Gas Production of Organic–Rich Shale Reservoirs. In Proceedings of the SPE Reservoir Characterisation and Simulation Conference and Exhibition, Abu Dhabi, UAE, 8–10 May 2017. [Google Scholar]
- Dong, J.J.; Hsu, J.Y.; Wu, W.J.; Shimamoto, T.; Hung, J.H.; Yeh, E.C. Stress–dependence of the permeability and porosity of sandstone and shale from TSDP Hole–A. Int. J. Rock Mech. Min. Sci. 2010, 47, 1141–1157. [Google Scholar] [CrossRef]
- Mckee, C.R.; Bumb, A.C. Stress–dependent permeability and porosity of coal. SPE Form. Eval. 1998, 3, 81–91. [Google Scholar] [CrossRef]
- Fan, D.; Ettehadtavakkol, A.; Chen, X.; Zhang, J.; Lu, J. Analytical model of gas transport in heterogeneous hydraulically-fractured organic-rich shale media. Fuel 2017, 207, 625–640. [Google Scholar] [CrossRef]
- Chong, Z.; Li, X.; Chen, X.; Zhang, J.; Lu, J. Numerical investigation into the effect of natural fracture density on hydraulic fracture network propagation. Energies 2017, 10, 914. [Google Scholar] [CrossRef]
- Bowker, K.A. Barnett shale gas production, Fort Worth basin: issues and discussion. AAPG Bull. 2007, 91, 523–533. [Google Scholar] [CrossRef]
- Wang, H.; Qu, Z.G.; Yin, Y.; Bai, J.Q.; Yu, B. Review of molecular simulation method for gas adsorption/desorption and diffusion in shale matrix. J. Therm. Sci. 2019, 28, 1–16. [Google Scholar] [CrossRef]
- Cunningham, R.E.; Williams, R.J.J. Diffusion in Gases and Porous Media; Springer: New York, NY, USA, 1980. [Google Scholar]
- Krishna, R.; Wesselingh, J. The Maxwell Stefan approach to mass transfer. Chem. Eng. Sci. 1997, 52, 861–911. [Google Scholar] [CrossRef]
- Guo, L.; Peng, X.; Wu, Z. Dynamical characteristics of methane adsorption on monolith nanometer activated carbon. J. Chem. Ind. Eng. 2008, 59, 2726–2732. [Google Scholar] [CrossRef]
- Keliu, W.U.; Xiangfang, L.I.; Chen, Z.X. The mechanism and mathematical model for the adsorbed gas surface diffusion in nanopores of shale gas reservoirs. Sci. China 2015, 45, 525–540. [Google Scholar]
- Whitaker, S. Flow in porous media i: A theoretical derivation of Darcy’s law. Transp. Porous Media 1986, 1, 3–25. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, T.H. Transport in Shale Reservoirs; Elsevier BV: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Brown, G.P.; Dinardo, A.; Cheng, G.K.; Sherwood, T.K. The flow of gases in pipes at low pressures. J. Appl. Phys. 1946, 17, 802–813. [Google Scholar] [CrossRef]
- Wei, M.Y.; Liu, Y.L.; Liu, J.H.; Elsworth, D.; Zhou, F.H. Micro–scale investigation on coupling of gas diffusion and mechanical deformation of shale. J. Pet. Sci. Eng. 2019, 175, 961–970. [Google Scholar] [CrossRef]
- Carman, P.C. Flow of Gases through Porous Media; Butterworth: London, UK, 1956. [Google Scholar]
- Pan, Z.; Ma, Y.; Connell, L.D.; Down, D.I.; Camilleri, M. Measuring anisotropic permeability using a cubic shale sample in a triaxial cell. J. Nat. Gas Sc. Eng. 2015, 26, 336–344. [Google Scholar] [CrossRef]
- Kwon, O.; Kronenberg, A.K.; Gangi, A.F.; Johnson, B. Permeability of wilcox shale and its effective pressure law. J. Geophys. Res. Sol. Earth 2001, 106, 19339–19353. [Google Scholar] [CrossRef]
- Metwally, Y.M.; Sondergeld, C.H. Measuring low permeabilities of gas–sands and shales using a pressure transmission technique. Int. J. Rock Mech. Min. Sci. 2011, 48, 1135–1144. [Google Scholar] [CrossRef]
- Guo, X.S. Enrichment Mechanism and Exploration Technology of Jiaoshiba Block in Fuling Shale Gas Field; Science Press: Beijing, China, 2014. [Google Scholar]
- Zou, C. Unconventional Petroleum Geology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 363–373. [Google Scholar]
- Langmuir, I. Constitution and fundamental properties of solids and liquids: Ii, liquids. J. Am. Chem. Soc. 1917, 3, 1848–1904. [Google Scholar] [CrossRef]
- Bangham, D.H.; Razouk, R.I. The saturation and immersion expansions and the heat of wetting. R. Soc. 1938, 166, 1471–2946. [Google Scholar] [CrossRef]
- Seidle, J.P.; Jeansonne, M.W.; Erickson, D.J. Application of matchstick geometry to stress dependent permeability in coals. In Proceedings of the SPE Rocky Mountain Regional Meeting, Casper, WY, USA, 18–21 May 1992. [Google Scholar]
- Seidle, J.R.; Huitt, L.G. Experimental measurement of coal matrix shrinkage due to gas desorption and implications for cleat permeability increases. In Proceedings of the International Meeting on Petroleum Engineering, Beijing, China, 14–17 November 1995. [Google Scholar]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Zheng, J.; Ju, Y.; Liu, H.H.; Zheng, L.; Wang, M. Numerical prediction of the decline of the shale gas production rate with considering the geomechanical effects based on the two–part hooke’s model. Fuel 2016, 185, 362–369. [Google Scholar] [CrossRef]
- COMSOL Multiphysics 5.4a. Available online: https://cn.comsol.com/ (accessed on 24 February 2019).





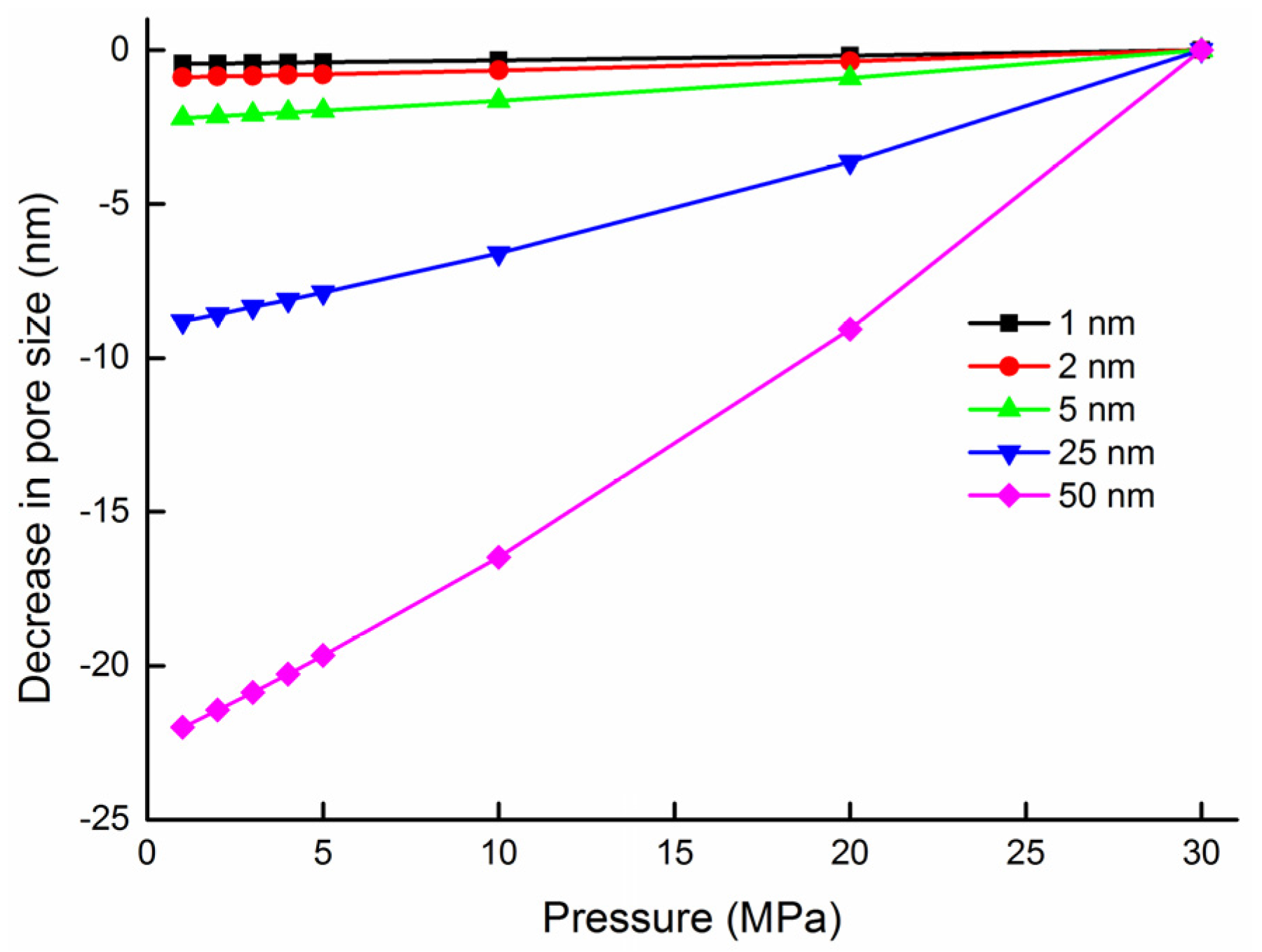
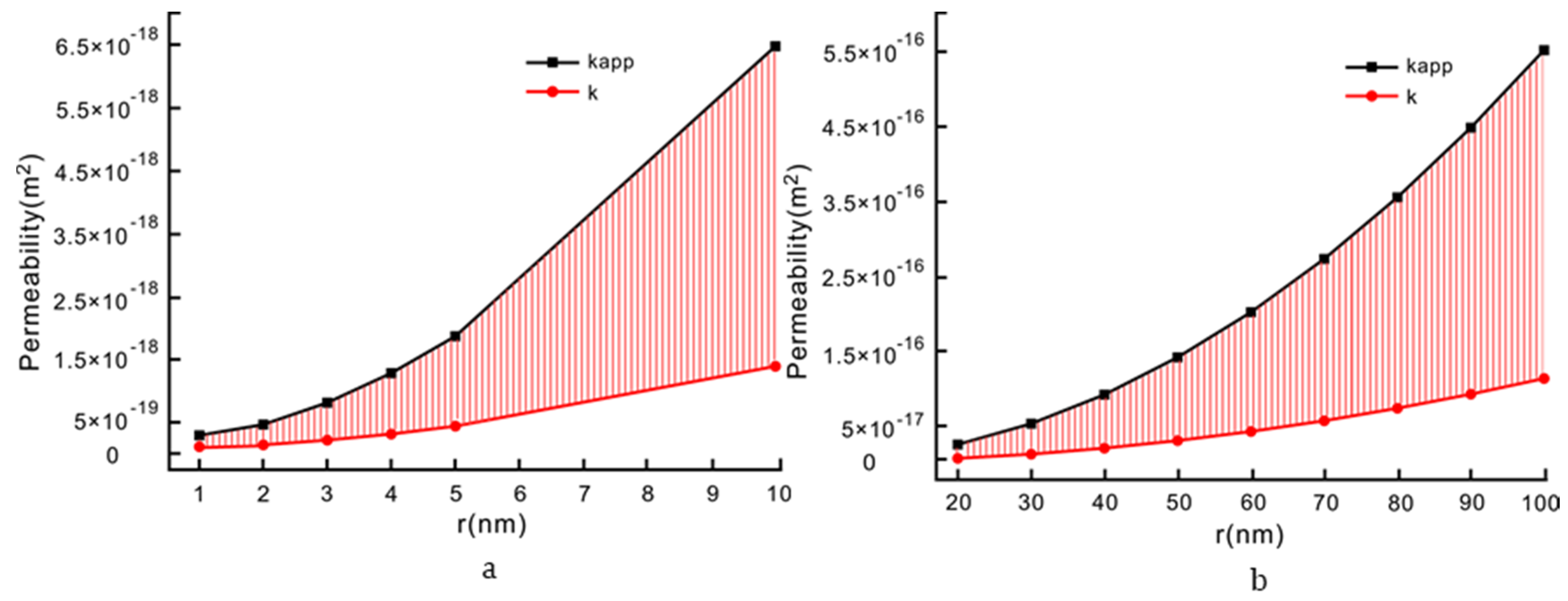

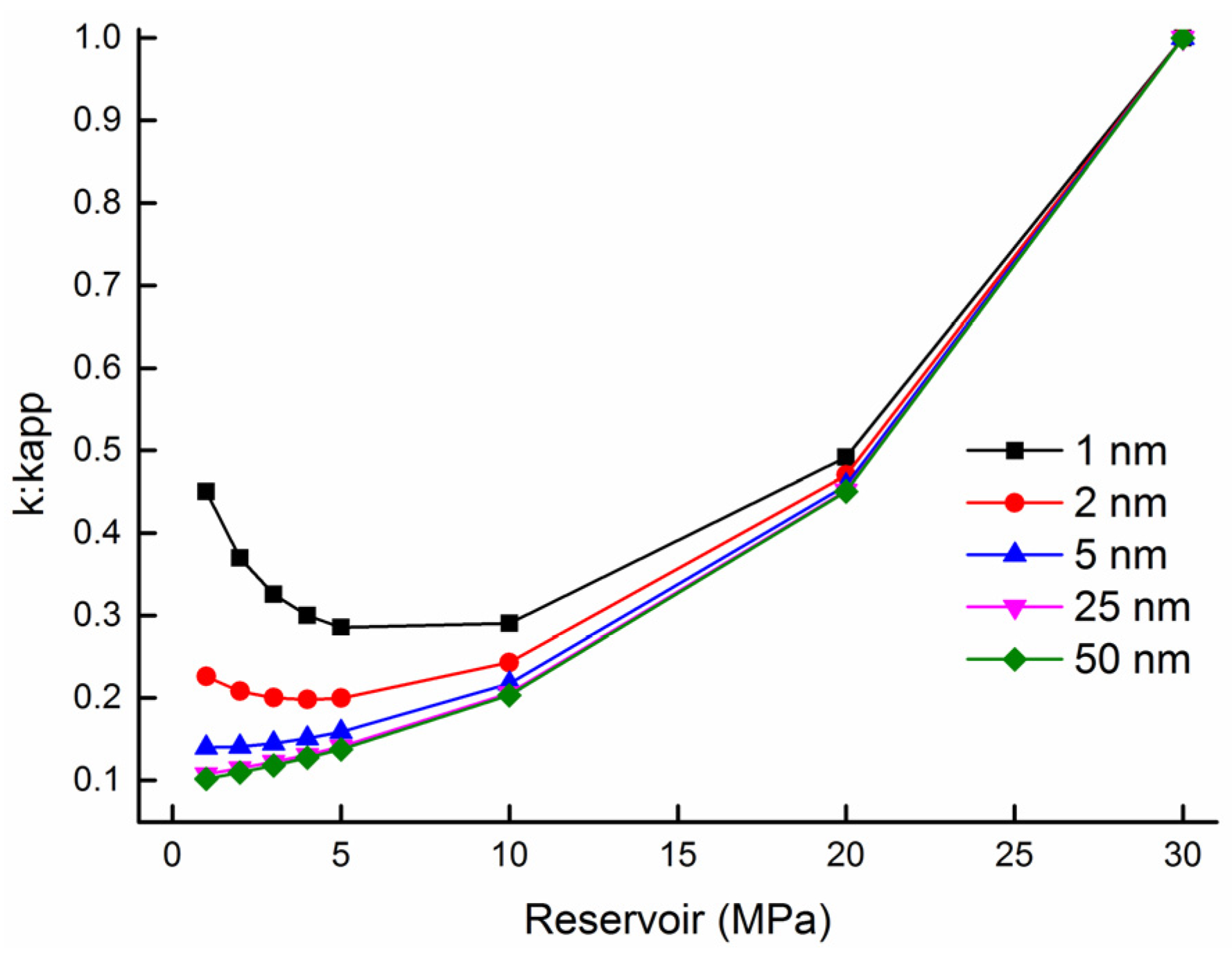
| Parameter | Value |
|---|---|
| Young’s modulus of shale, E() | |
| Poisson’s ratio of shale, | |
| Density of shale, () | |
| Average pore diameter of matrix, () | |
| Density of gas, () | |
| Viscosity of gas, µ() | 2 × 104 |
| Molar mass of gas, M(kg/mol) | |
| Gas molecular diameter, () | |
| Reservoir initial pressure, () | |
| Overburden pressure, () | |
| Confining pressure, () | |
| Boundary pressure, () | |
| Langmuir pressure constant, () | |
| Langmuir volume constant, () | |
| Initial porosity of matrix, | |
| Initial permeability of matrix, () | |
| Pore curvature, | |
| Standard molar volume, () | |
| Avogadro constant, () |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Ma, F.; Zhao, H.; Liu, G.; Guo, J. Gas Multiple Flow Mechanisms and Apparent Permeability Evaluation in Shale Reservoirs. Sustainability 2019, 11, 2114. https://doi.org/10.3390/su11072114
Feng X, Ma F, Zhao H, Liu G, Guo J. Gas Multiple Flow Mechanisms and Apparent Permeability Evaluation in Shale Reservoirs. Sustainability. 2019; 11(7):2114. https://doi.org/10.3390/su11072114
Chicago/Turabian StyleFeng, Xuelei, Fengshan Ma, Haijun Zhao, Gang Liu, and Jie Guo. 2019. "Gas Multiple Flow Mechanisms and Apparent Permeability Evaluation in Shale Reservoirs" Sustainability 11, no. 7: 2114. https://doi.org/10.3390/su11072114
APA StyleFeng, X., Ma, F., Zhao, H., Liu, G., & Guo, J. (2019). Gas Multiple Flow Mechanisms and Apparent Permeability Evaluation in Shale Reservoirs. Sustainability, 11(7), 2114. https://doi.org/10.3390/su11072114




