Development of Coupled Biokinetic and Thermal Model to Optimize Cold-Water Microbial Enhanced Oil Recovery (MEOR) in Homogenous Reservoir
Abstract
:1. Introduction
2. Materials and Methods
2.1. Stoichiometric Reactions of Microbial Growth and Biosurfactant Production
2.2. Nonisothermal Biokinetics
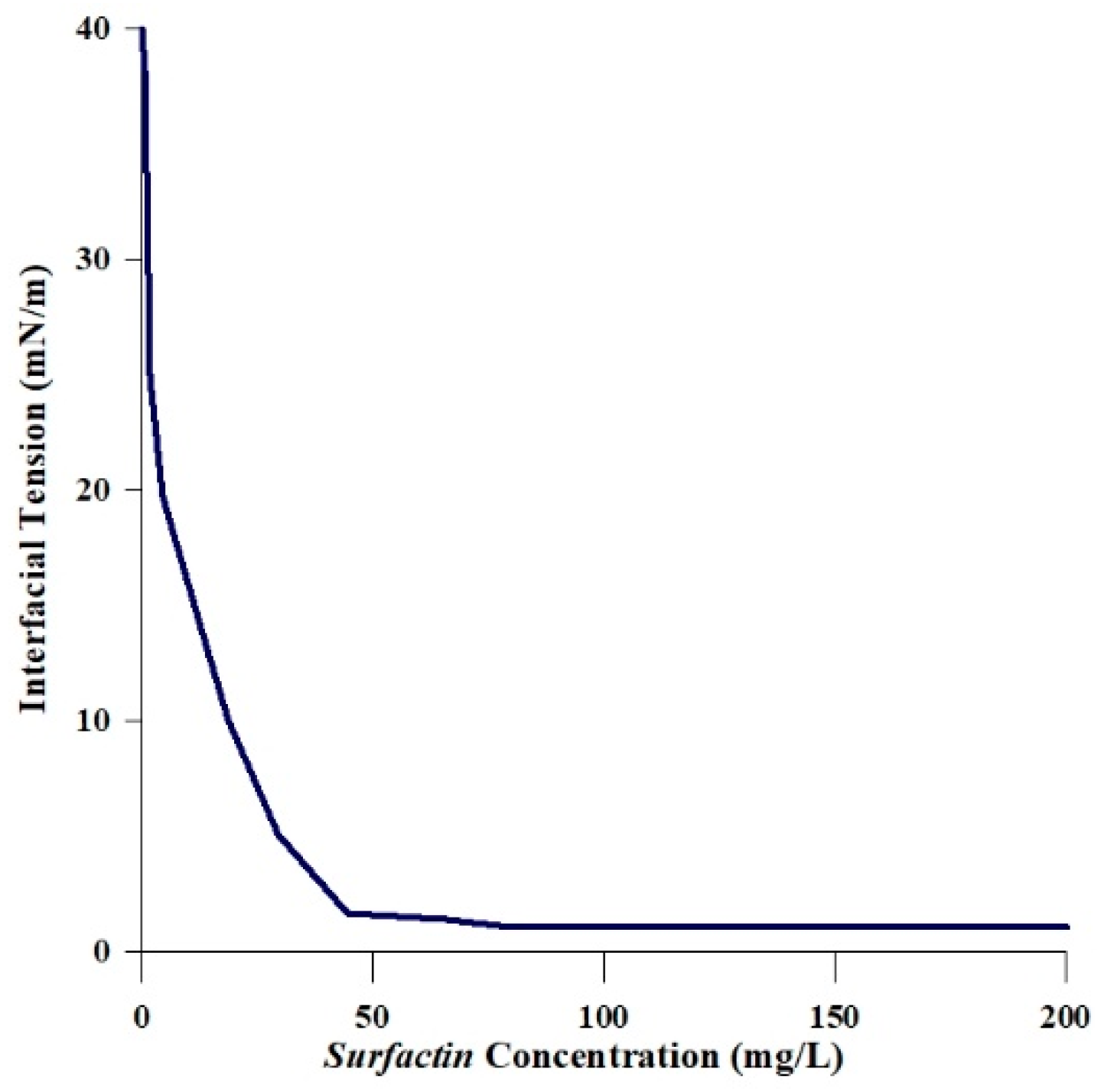
2.3. Wettability Alteration by Interfacial Tension
2.4. Mass and Energy Balance for Nonisothermal Model
2.5. Oil Viscosity Correlation
3. Results and Discussion
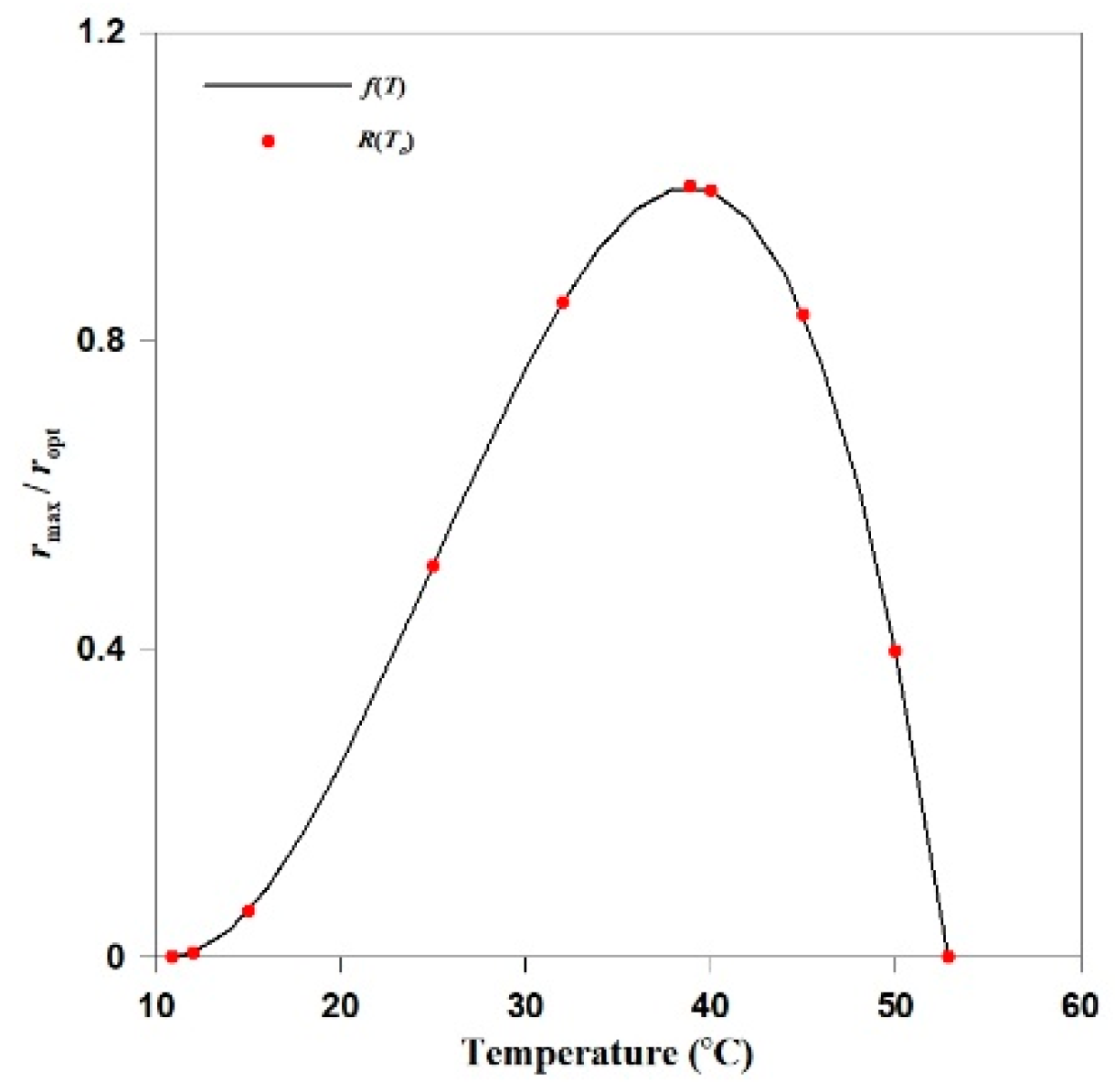
3.1. Calibration of The Temperature-Dependent Reaction Factor
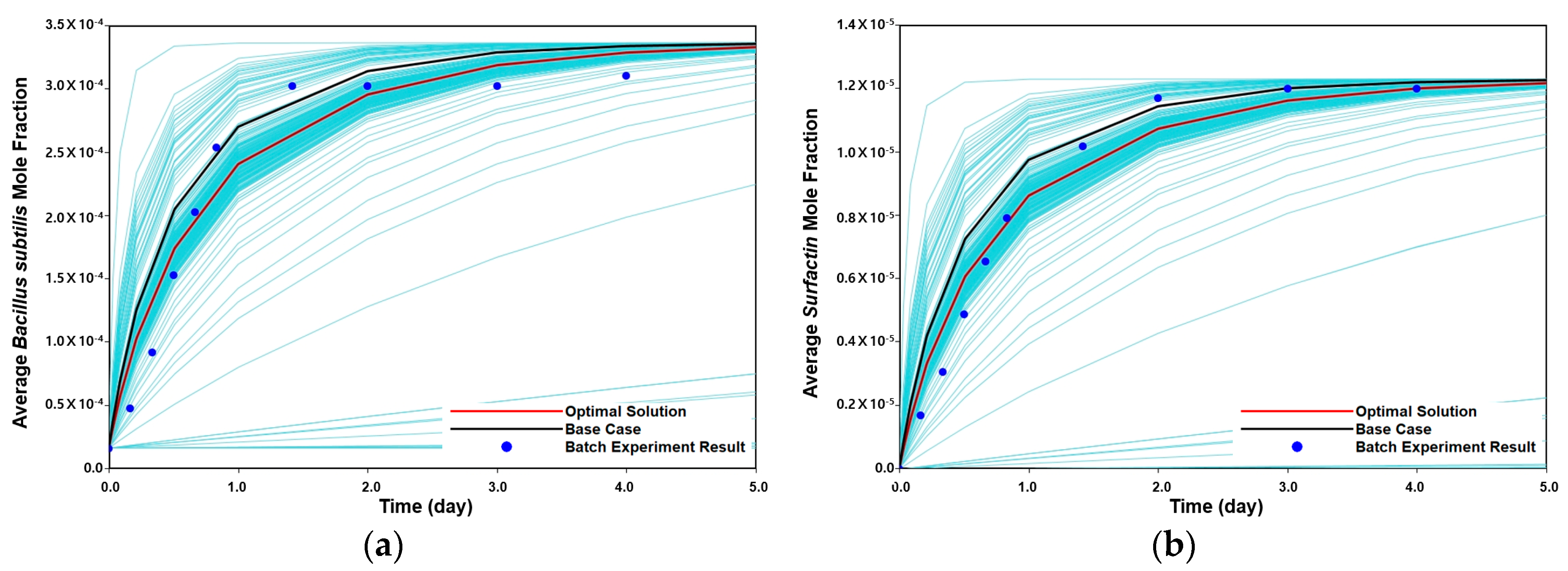
3.2. Validation of Stoichiometric Reaction and Biokinetics
3.3. Parametric Study of Injection Design
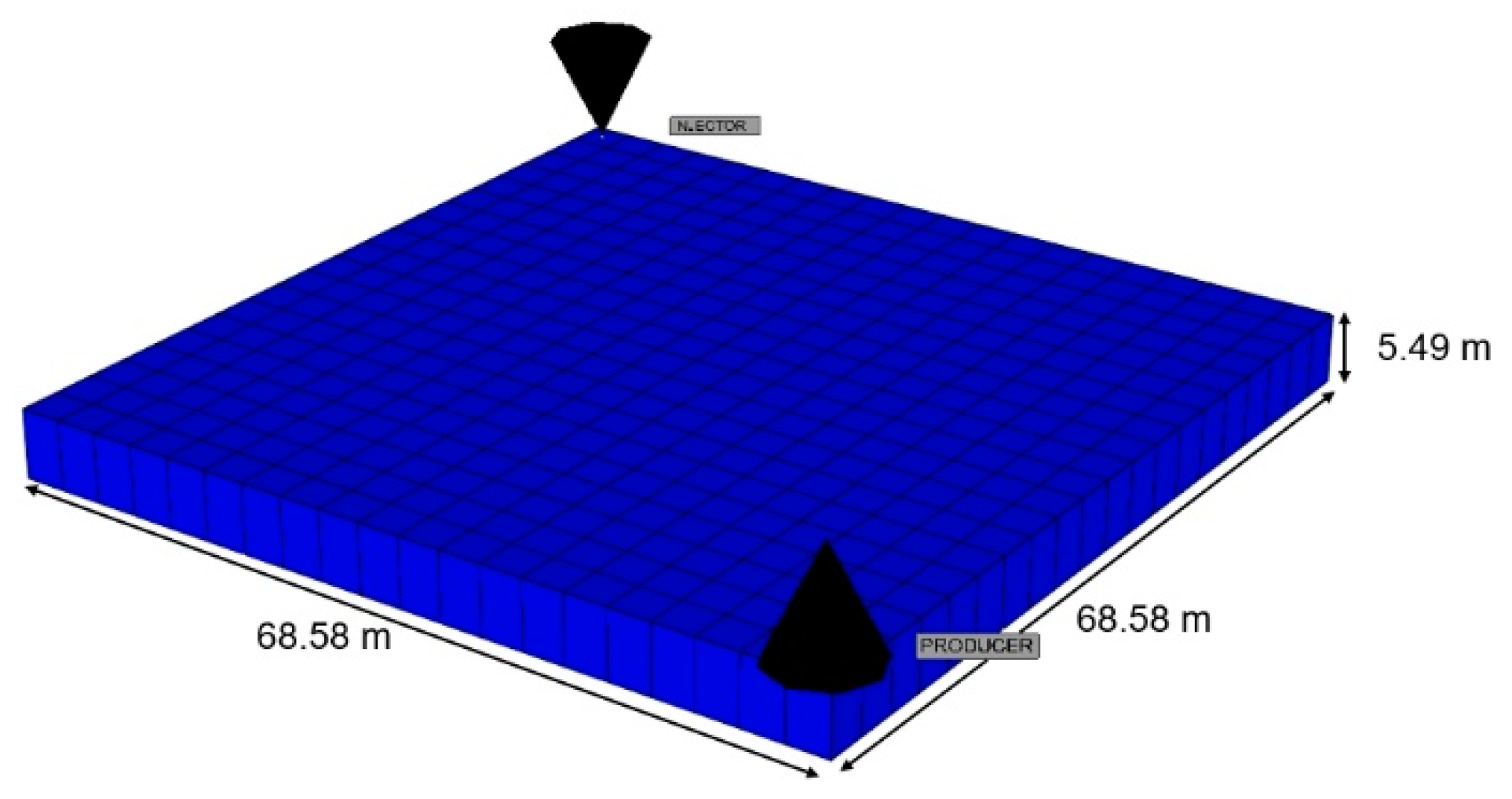
3.3.1. Model Description
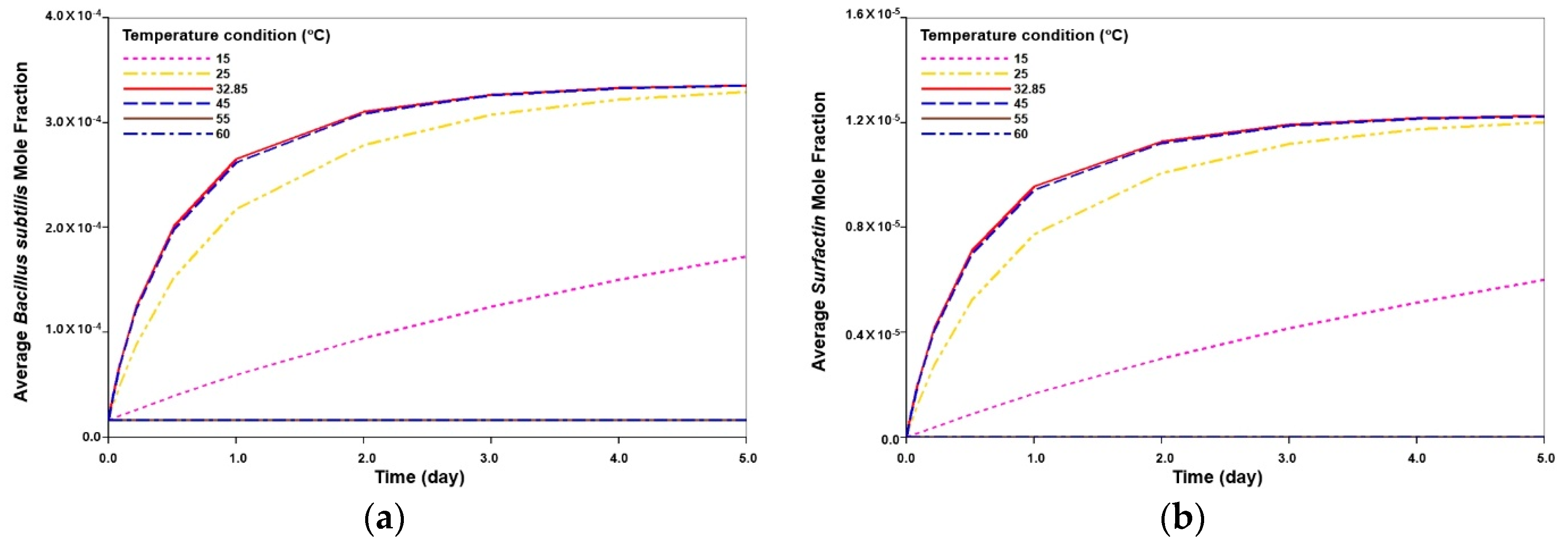
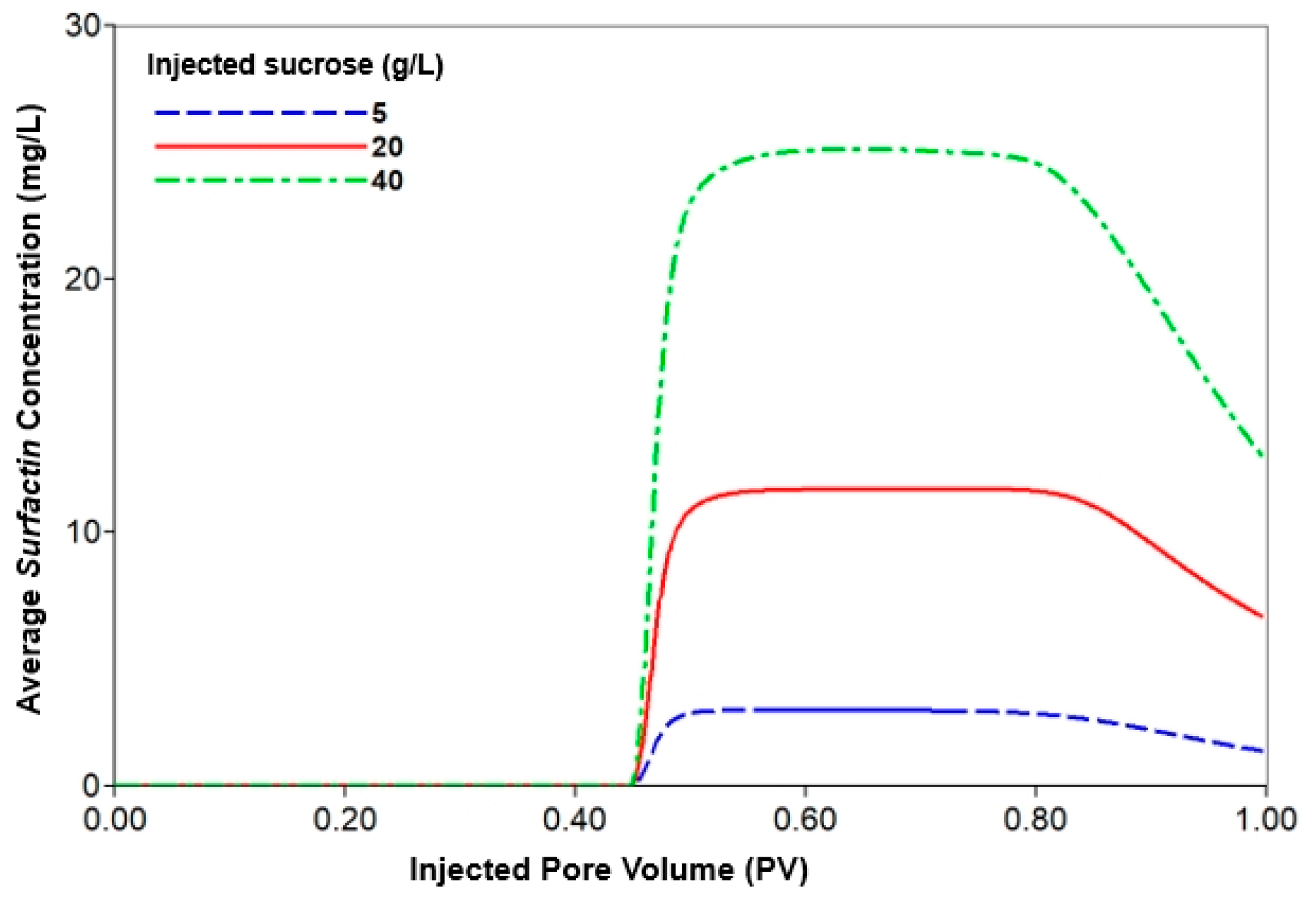
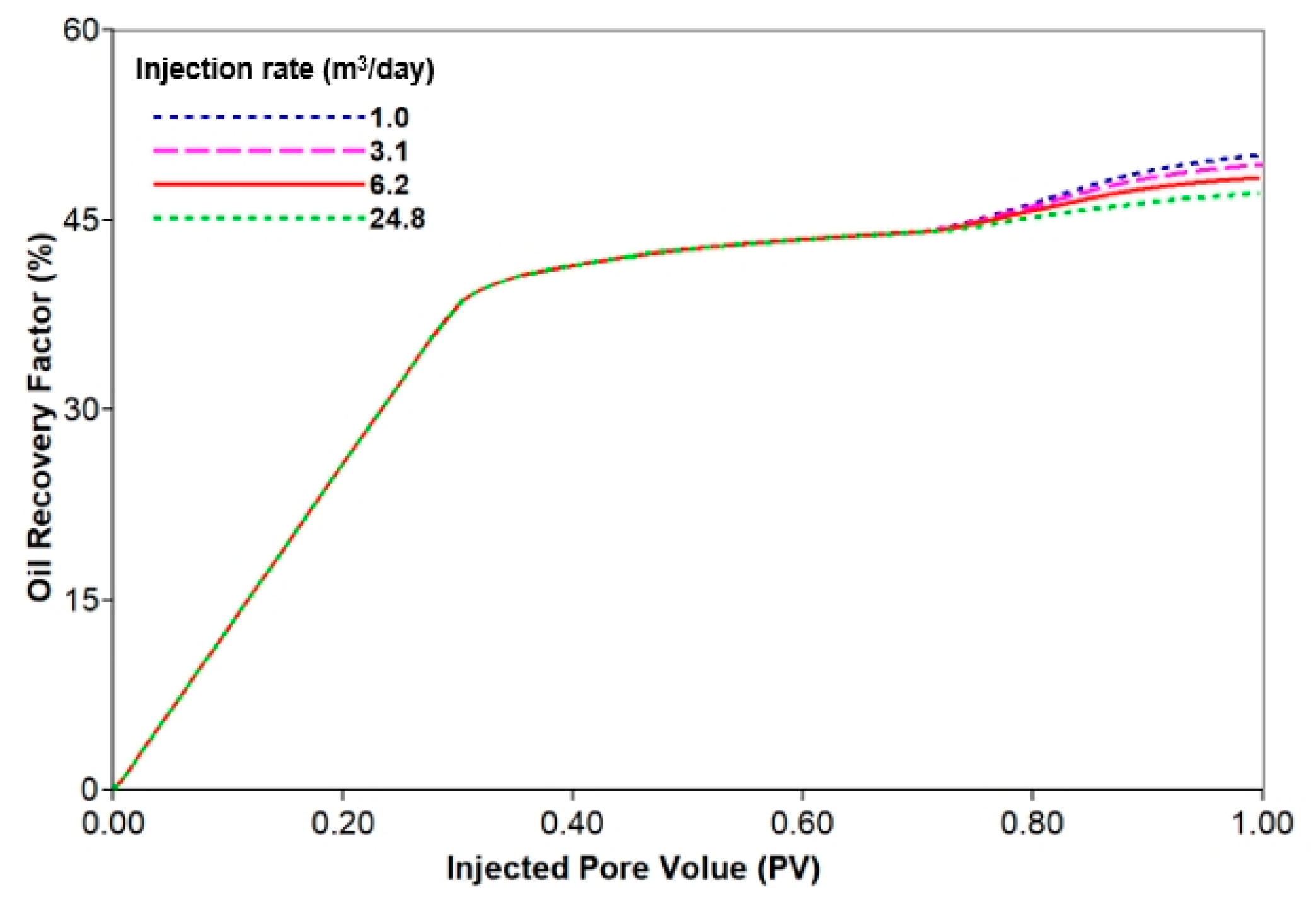
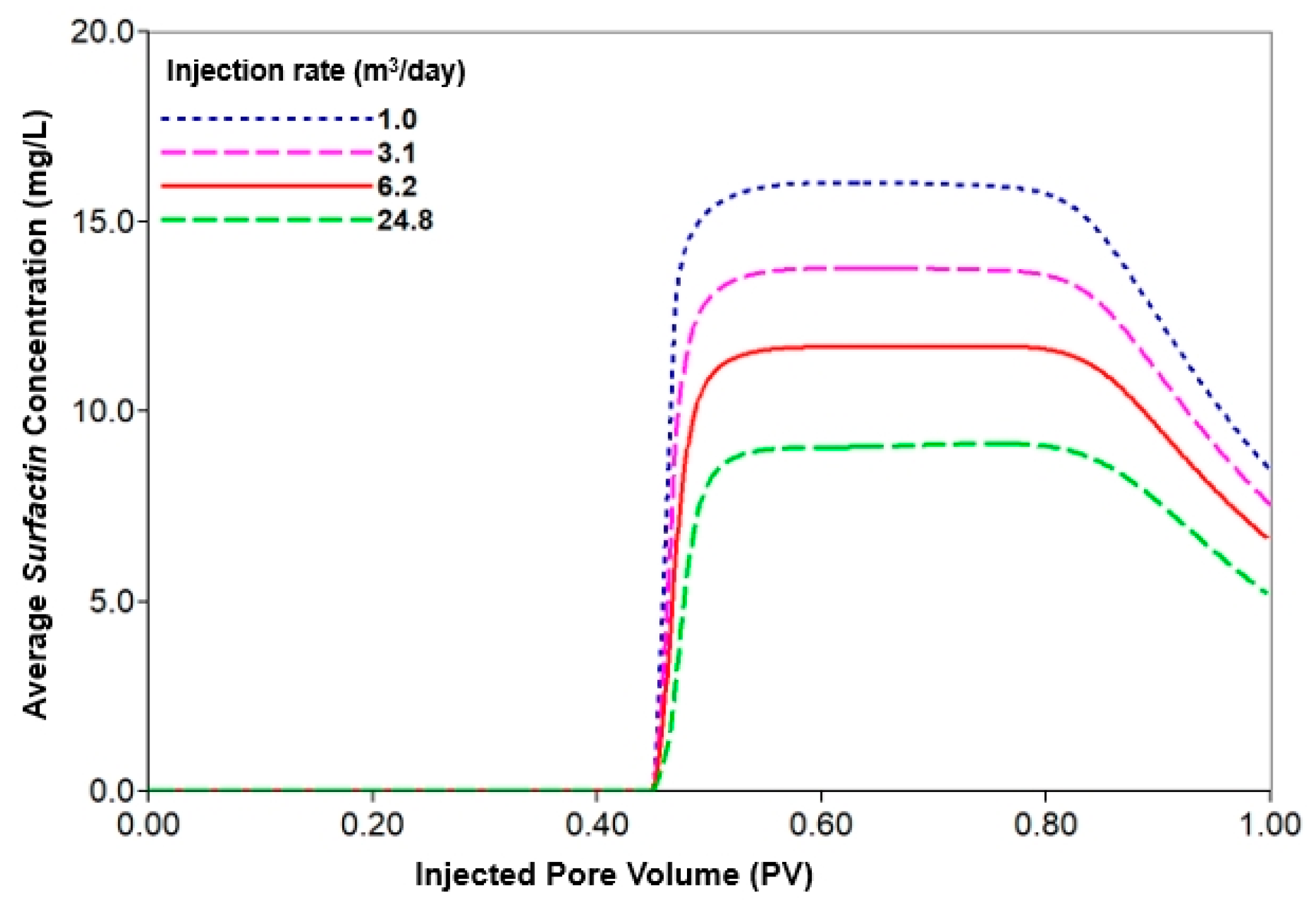
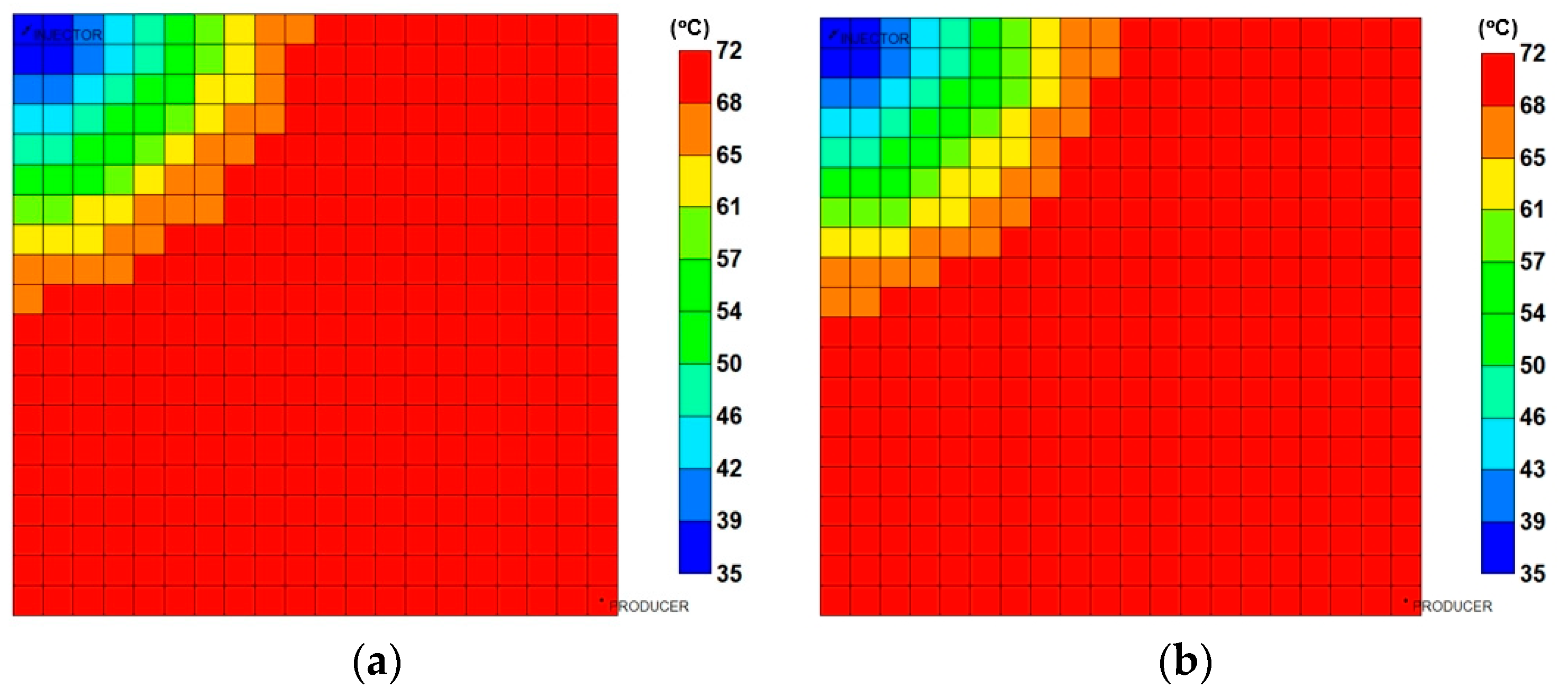
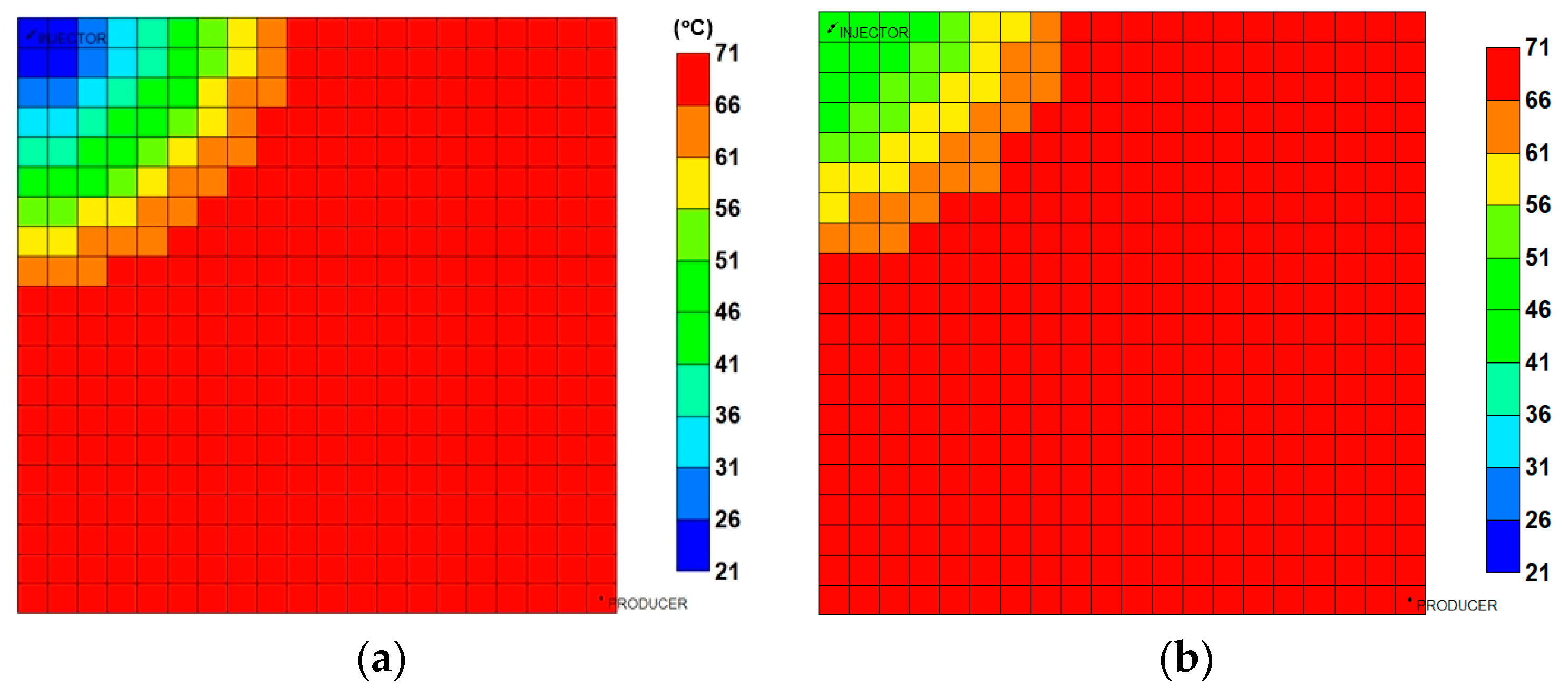
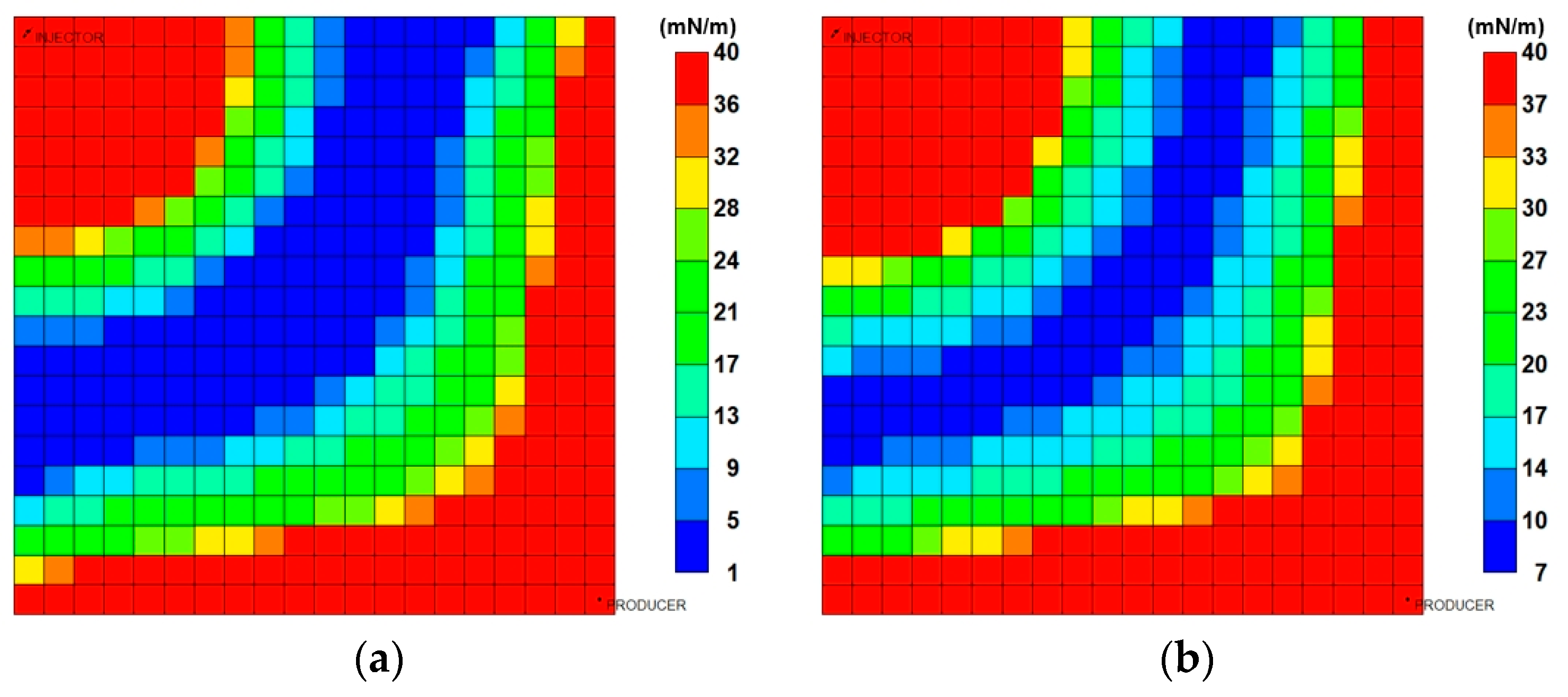
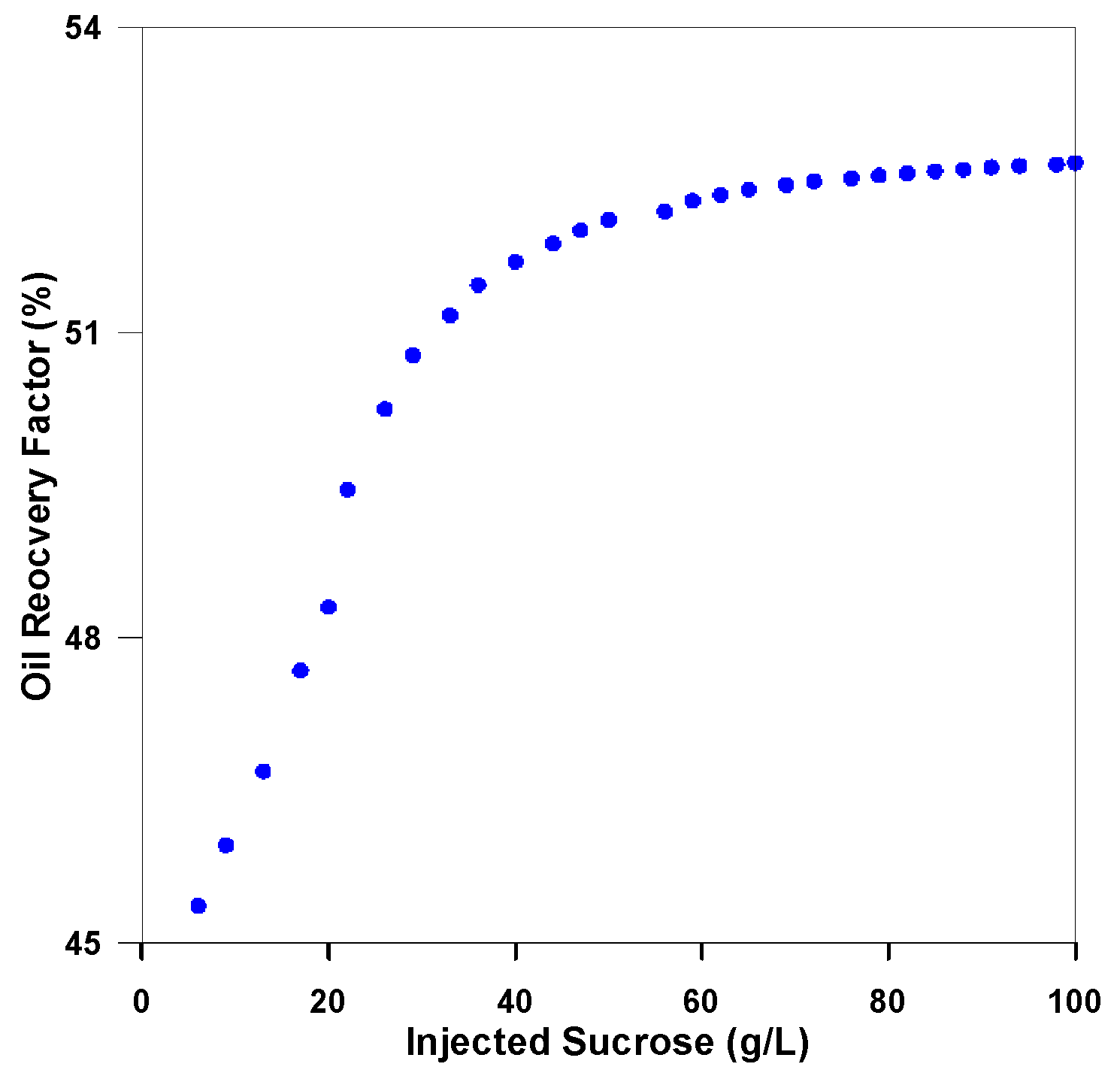
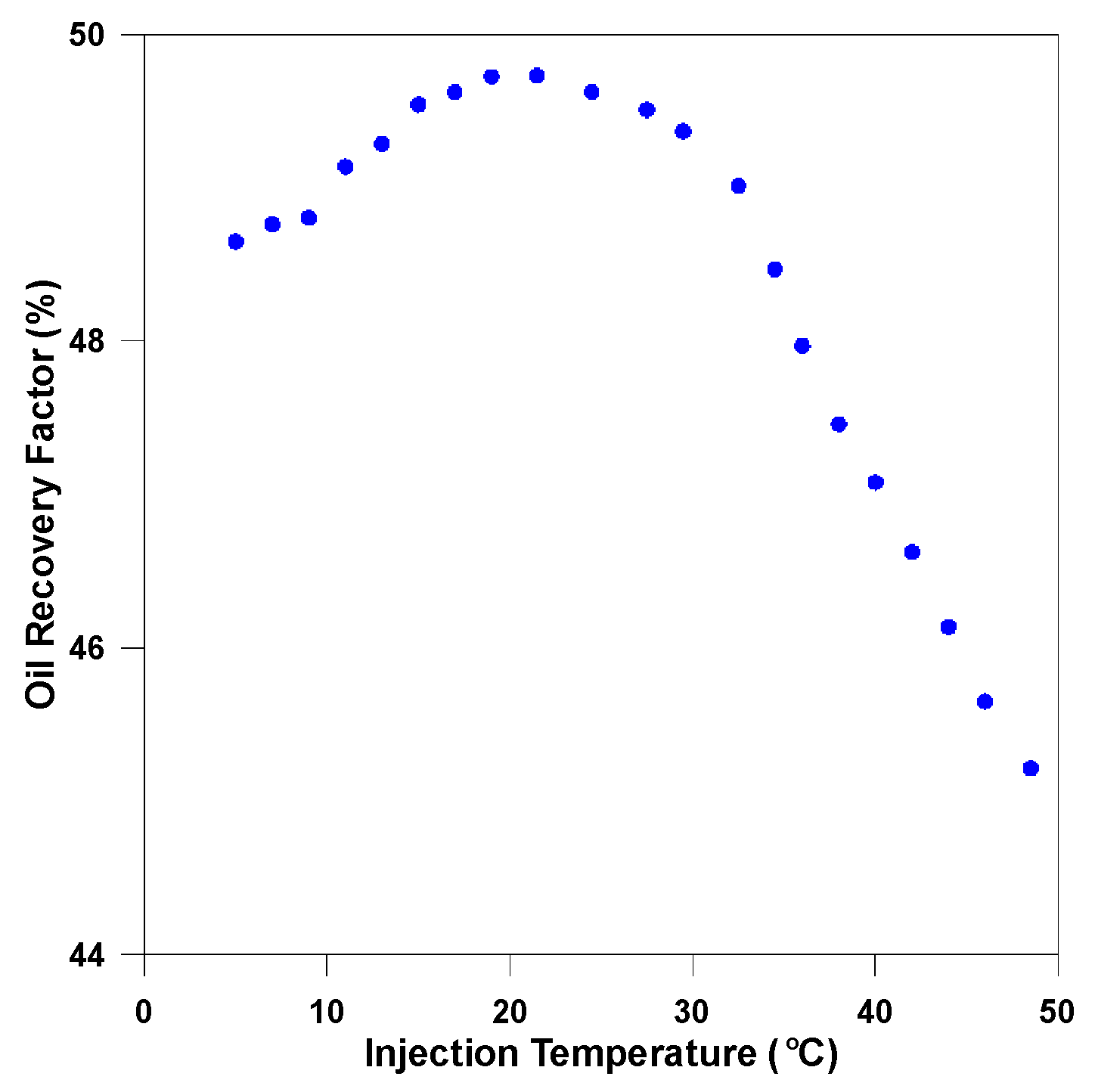
3.3.2. Analysis of Injection Parameters
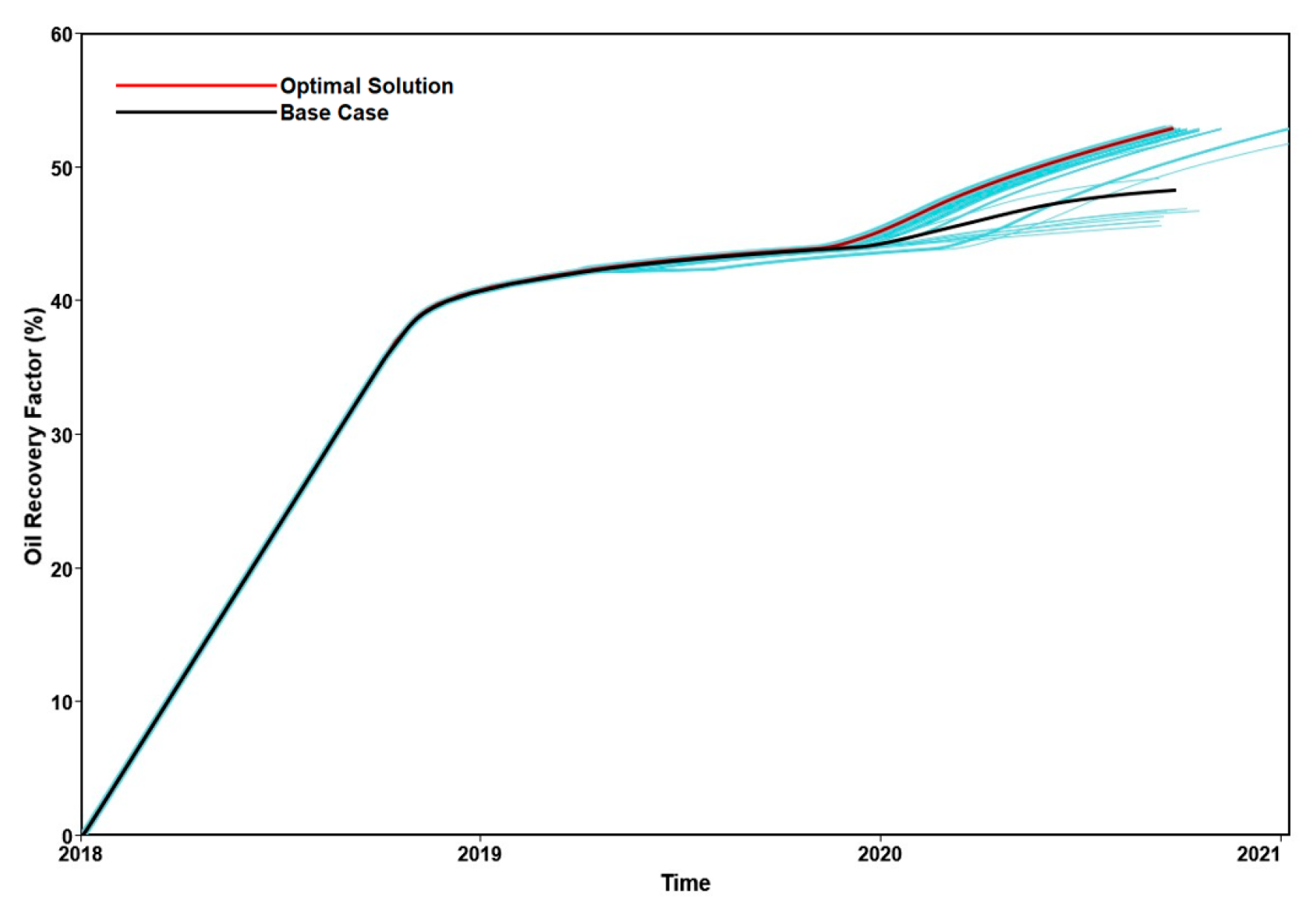
3.3.3. Parametric Analysis and Optimization Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nielsen, S.M. Microbial Enhanced Oil Recovery-Advanced Reservoir Simulation. Ph.D. Thesis, Technical University of Denmark (DTU), Lyngby, Denmark, July 2010. Available online: http://orbit.dtu.dk/files/5455607/61126_Sidsel%20Marie%20Nielsen_.pdf (accessed on 10 November 2017).
- Sen, R. Biotechnology in Petroleum Recovery: The microbial EOR. Prog. Energy Combust. 2008, 34, 714–724. [Google Scholar] [CrossRef]
- Patel, J.; Borgohain, S.; Kumar, M.; Rangarajan, V.; Somasundaran, P.; Sen, R. Recent Developments in Microbial Enhanced Oil Recovery. Renew. Sustain. Energy Rev. 2015, 52, 1539–1558. [Google Scholar] [CrossRef]
- Gray, M.; Yeung, A.; Foght, J.; Yarranton, H.W. Potential Microbial Enhanced Oil Recovery Processes: A Critical Analysis. Presented at the 2008 Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008. [Google Scholar] [CrossRef]
- Safdel, M.; Anbaz, M.A.; Daryasafar, A.; Jamialahmadi, M. Microbial Enhanced Oil Recovery, a Critical Review on Worldwide Implemented Field Trials in Different Countries. Renew. Sustain. Energy Rev. 2017, 74, 159–172. [Google Scholar] [CrossRef]
- Bryant, S.L.; Lockhart, T.P. Reservoir Engineering Analysis of Microbial Enhanced Oil Recovery. SPE Reserv. Eval. Eng. 2002, 5, 365–374. [Google Scholar] [CrossRef]
- Hosseininoosheri, P.; Lashgari, H.R.; Sepehrnoori, K. A Novel Method to Model and Characterize In-situ Bio-surfactant Production in Microbial Enhanced Oil Recovery. Fuel 2016, 183, 501–511. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, Z.Z.; Wang, J.X.; Zhang, G.Q.; Luo, Y.J.; Song, Z.Z.; Zhang, J.Y. Bacterial Community Diversity in a Low-Permeability Oil Reservoir and Its Potential for Enhancing Oil Recovery. Bioresour. Technol. 2013, 147, 110–116. [Google Scholar] [CrossRef]
- Amani, H.; Sarrahzadeh, M.H.; Haghighi, M.; Mehrnia, M.R. Comparative Study of Biosurfactant Producing Bacteria in MEOR Applications. J. Petrol. Sci. Eng. 2010, 75, 209–214. [Google Scholar] [CrossRef]
- Behesht, M.; Roostaazad, R.; Farhadpour, F.; Pishvaei, M.R. Model Development for MEOR Process in Conventional Nonfractured Reservoirs and Investigation of Physicochemical Parameter Effects. Chem. Eng. Technol. 2008, 31, 953–963. [Google Scholar] [CrossRef]
- Halim, A.Y.; Nielsen, S.M.; Nielsen, K.F.; Lantz, A.E. CPC TESTING: Towards the Understanding of Microbial Metabolism in Relation to Microbial Enhanced Oil Recovery. J. Petrol. Sci. Eng. 2017, 149, 151–160. [Google Scholar] [CrossRef]
- Heryani, H.; Putra, M.D. Kinetic Study and Modeling of Biosurfactant Production using Bacillus sp. Electron. J. Biotechnol. 2017, 27, 49–54. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Trefry, M.G.; Park, J.; Liu, K.; Haq, B.; Johnston, C.D.; Volk, H. Interactions of Microbial-Enhanced Oil Recovery Processes. Transp. Porous Med. 2011, 87, 77–104. [Google Scholar] [CrossRef]
- Makkar, R.S.; Cameotra, S.S. Biosurfactant Production by a Thermophilic Bacillus subtilis Strain. J. Ind. Microbiol. Biotechnol. 1997, 18, 37–42. [Google Scholar] [CrossRef]
- Nielsen, S.M.; Nesterov, I.; Shapiro, A.A. Simulations of Microbial-Enhanced Oil Recovery: Adsorption and Filtration. Transp. Porous Med. 2014, 102, 227–259. [Google Scholar] [CrossRef]
- Xia, W.J.; Dong, H.P.; Yu, L.; Yu, D.F. Comparative Study of Biosurfactant Produced by Microorganisms Isolated from Formation Water of Petroleum. Reserv. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 124–130. [Google Scholar] [CrossRef]
- Aditama, P.; Avbelj, E.; Reimann, S.; Mahler, E.; Poulsen, M.; Jelinek, W.; Alkan, H. Design and Execution of an MEOR Huff and Puff Pilot in a Wintershall Field. Presented at the 79th EAGE Conference and Exhibition, Paris, France, 12–15 June 2017. [Google Scholar] [CrossRef]
- Uzir, M.H.; Mat Don, M. Biochemical Engineering—A Concise Introduction; Universiti Sains Malaysia: Penang, Malaysia, 2007; pp. 15–26. [Google Scholar]
- Villadsen, J.; Nielsen, J.; Lidén, G. Bioreaction Engineering Principles; Springer: New York, NY, USA, 2011; pp. 119–382. [Google Scholar]
- Vilcáez, J.; Li, L.; Wu, D.; Hubbard, S.S. Reactive Transport Modeling of Induced Selective Plugging by Leuconostoc mesenteroides in Carbonate Formations. Geomicrobiol. J. 2013, 30, 813–828. [Google Scholar] [CrossRef]
- Arora, P.; Ranade, D.R.; Dhakephalkar, P.K. Development of a microbial process for the recovery of petroleum oil from depleted reservoirs at 91–96 °C. Bioresour. Technol. 2014, 165, 274–278. [Google Scholar] [CrossRef]
- Sivasankar, P.; Kumar, G.S. Numerical Modelling of Enhanced Oil Recovery by Microbial Flooding under Nonisothermal Conditions. J. Petrol. Sci. Eng. 2014, 124, 161–172. [Google Scholar] [CrossRef]
- Computer Modelling Group (CMG). STARS User Guide; Computer Modelling Group Ltd: Calgary, AB, Canada, 2017; pp. 373–1531. [Google Scholar]
- Computer Modelling Group (CMG). CMOST User Guide; Computer Modelling Group Ltd.: Calgary, AB, Canada, 2017; pp. 25–361. [Google Scholar]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications; Tata McGraw-Hill Education: Boston, MA, USA, 2012; pp. 144–224. [Google Scholar]
- Han, K.; Levenspiel, O. Extended Monod Kinetics for Substrate, Product, and Cell Inhibition. Biotechnol. Bioeng. 1988, 32, 430–447. [Google Scholar] [CrossRef]
- Merchuk, J.C.; Asenjo, J.A. The Monod Equation and Mass Transfer. Biotechnol. Bioeng. 1995, 45, 91–94. [Google Scholar] [CrossRef]
- Rosso, L.; Lobry, J.R.; Bajard, S.; Flandrois, J.P. Convenient Model to Describe the Combined Effects of Temperature and pH on Microbial Growth. Appl. Environ. Microbiol. 1995, 61, 610–616. Available online: https://aem.asm.org/content/aem/61/2/610.full.pdf (accessed on 27 November 2017).
- Bueltemeier, H.; Alkan, H.; Amro, M. A New Modeling Approach to MEOR Calibrated by Microbial Growth and Metabolite Curves. Presented at SPE EOR Conference of Oil and Gas West Asia, Muscat, Oman, 31 March 31–2 April 2014. [Google Scholar] [CrossRef]
- Brooks, R.H.; Corey, A.T. Properties of Porous Media Affecting Fluid Flow. J. Irrig. Drain. Divis. 1966, 92, 61–90. [Google Scholar]
- Vinsome, P.K.W.; Westerveld, J. A Simple Method for Predicting Cap and Base Rock Heat Losses in Thermal Reservoir Simulators. J. Can. Petrol. Technol. 1980, 19, 87–90. [Google Scholar] [CrossRef]
- Standing, M.B. Volumetric and Phase Behavior of Oil Field Hydrocarbon Systems: PVT for Engineers; California Research Corp: California, CA, USA, 1951; pp. 121–127. [Google Scholar]
- Beal, C. The Viscosity of Air, Water, Natural gas, Crude Oil and Its Associated Gases at Oil Field Temperatures and Pressures. Trans. AIME 1946, 165, 94–115. [Google Scholar] [CrossRef]
- Whitson, C.H.; Brulé, M.R. Phase Behavior; Henry L. Doherty Memorial Fund of AIME, Society Petroleum Engineers: Richardson, TX, USA, 2000; pp. 109–120. [Google Scholar]
- Ratkowsky, D.A.; Lowry, R.K.; McMeekin, T.A.; Stokes, A.N.; Chandler, R.E. Model for Bacterial Culture Growth Rate throughout the Entire Biokinetic Temperature Range. J. Bacteriol. 1983, 154, 1222–1226. Available online: https://jb.asm.org/content/jb/154/3/1222.full.pdf (accessed on 15 December 2017).
- Robertson, E.C. Thermal Properties of Rocks (No. 88-441); US Geological Survey: Reston, VA, USA, 1988; pp. 5–59. Available online: http://pubs.er.usgs.gov/publication/ofr88441 (accessed on 14 January 2018).





| Electron Acceptor | Moles | -eq/mol | Moles -eq/mol | = (Moles × -eq/mol)/sum |
|---|---|---|---|---|
| Lactate | 0.5 | 12 | 6 | 0.141 |
| Acetate | 0.5 | 8 | 4 | 0.094 |
| Mannitol | 0.1 | 26 | 2.6 | 0.0610 |
| Ethanol | 0.5 | 12 | 6 | 0.141 |
| Fructose | 1 | 24 | 24 | 0.563 |
| Sum = 42.6 |
| Temperature Range | Value (°C) |
|---|---|
| Minimum temperature | 10.85 |
| Optimal temperature | 37.85 |
| Maximum temperature | 52.85 |
| Types | Temperature (°C) | Activation Energy (J/gmol) |
|---|---|---|
| derived | 10.85 | 16,324,518.55 |
| 12 | 567,091.09 | |
| 15 | 153,312.32 | |
| 25 | 55,582.38 | |
| 32 | 18,816.06 | |
| 37.85 | −3681.10 | |
| 40 | −29,192.22 | |
| 45 | −126,491.17 | |
| 50 | −5,939,661.80 | |
| reference | 52.85 | 54,881.64 |
| Property | Value |
|---|---|
| Volume (L) | 1 |
| Pressure (atm) | 1 |
| Temperature (°C) | 45 |
| Initial sucrose (g/L) | 20.0 |
| Initial (g/L) | 3.0 |
| Initial (g/L) | 2.8 |
| Initial microbes (g/L) | 0.1 |
| Reservoir Property | Value |
|---|---|
| Number of grid blocks | 20 20 1 |
| Thickness (m) | 5.49 |
| Grid block size (m) | 3.43 3.43 3.43 |
| Porosity | 0.24 |
| Permeability (md) | 30 |
| Initial water saturation | 0.23 |
| Initial oil saturation | 0.77 |
| Oil compressibility (1/kPa) | 4.35 10−7 |
| Rock compressibility (1/kPa) | 1.45 10−7 |
| Initial reservoir pressure (kPa) | 38,611 |
| Initial reservoir temperature (°C) | 71 |
| Initial interfacial tension (mN/m) | 40 |
| Rock heat capacity (J/m−3·°C) | 2,511,000 |
| Rock heat conductivity (J/m·d·°C) | 258,109.29 |
| Parameter | Phase | Capillary Number | Value |
|---|---|---|---|
| Residual saturation | Water () | Low | 0.23 |
| High | 0 | ||
| Oil () | Low | 0.39 | |
| High | 0 | ||
| Relative permeability at residual saturation | Water () | Low | 0.138 |
| High | 1 | ||
| Oil () | Low | 0.515 | |
| High | 1 | ||
| Exponent for calculating relative permeability | Water () | Low | 3.182 |
| High | 1 | ||
| Oil () | Low | 2.405 | |
| High | 1 |
| Case | Stage | Injection Temperature (°C) | Injection Rate (m3/d) | Injected Sucrose (g/L) |
|---|---|---|---|---|
| Injection nutrient concentration | 1st and 2nd WF | 35 | 6.2 | 0.0 |
| MEOR | 35 | 6.2 | 5 | |
| 20 | ||||
| 40 | ||||
| Injection rate | 1st and 2nd WF | 35 | 6.2 | 0 |
| MEOR | 35 | 1.0 | 20 | |
| 3.1 | ||||
| 6.2 | ||||
| 24.8 | ||||
| Injection temperature | 1st and 2nd WF | Same as MEOR | 6.2 | 0 |
| MEOR | 5 | 6.2 | 20 | |
| 20 | ||||
| 35 | ||||
| 45 |
| Parameter | Range |
|---|---|
| Injected sucrose and microbe concentration (g/L) | 1st and 2nd WF: sucrose 0 + microbe 0 MEOR: sucrose 5–40+microbe 0.1 (Base case MEOR: sucrose 20+microbe 0.1) |
| Injection Rate (m3/d) | 1st and 2nd WF: 6.2 MEOR: 1–25 (Base case MEOR: 6.2) |
| Injection Temperature (°C) | 1st and 2nd WF: same as MEOR MEOR: 5–50 (Base case MEOR: 35) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, E.; Jeong, M.S.; Kim, T.H.; Lee, J.H.; Cho, J.H.; Lee, K.S. Development of Coupled Biokinetic and Thermal Model to Optimize Cold-Water Microbial Enhanced Oil Recovery (MEOR) in Homogenous Reservoir. Sustainability 2019, 11, 1652. https://doi.org/10.3390/su11061652
Hong E, Jeong MS, Kim TH, Lee JH, Cho JH, Lee KS. Development of Coupled Biokinetic and Thermal Model to Optimize Cold-Water Microbial Enhanced Oil Recovery (MEOR) in Homogenous Reservoir. Sustainability. 2019; 11(6):1652. https://doi.org/10.3390/su11061652
Chicago/Turabian StyleHong, Eunji, Moon Sik Jeong, Tae Hong Kim, Ji Ho Lee, Jin Hyung Cho, and Kun Sang Lee. 2019. "Development of Coupled Biokinetic and Thermal Model to Optimize Cold-Water Microbial Enhanced Oil Recovery (MEOR) in Homogenous Reservoir" Sustainability 11, no. 6: 1652. https://doi.org/10.3390/su11061652
APA StyleHong, E., Jeong, M. S., Kim, T. H., Lee, J. H., Cho, J. H., & Lee, K. S. (2019). Development of Coupled Biokinetic and Thermal Model to Optimize Cold-Water Microbial Enhanced Oil Recovery (MEOR) in Homogenous Reservoir. Sustainability, 11(6), 1652. https://doi.org/10.3390/su11061652





