Utilization of Lime Mud Waste from Paper Mills for Efficient Phosphorus Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Green Ca(OH)2 Preparation
2.2. Phosphorus Removal Study
2.3. Physical Characterization
3. Results
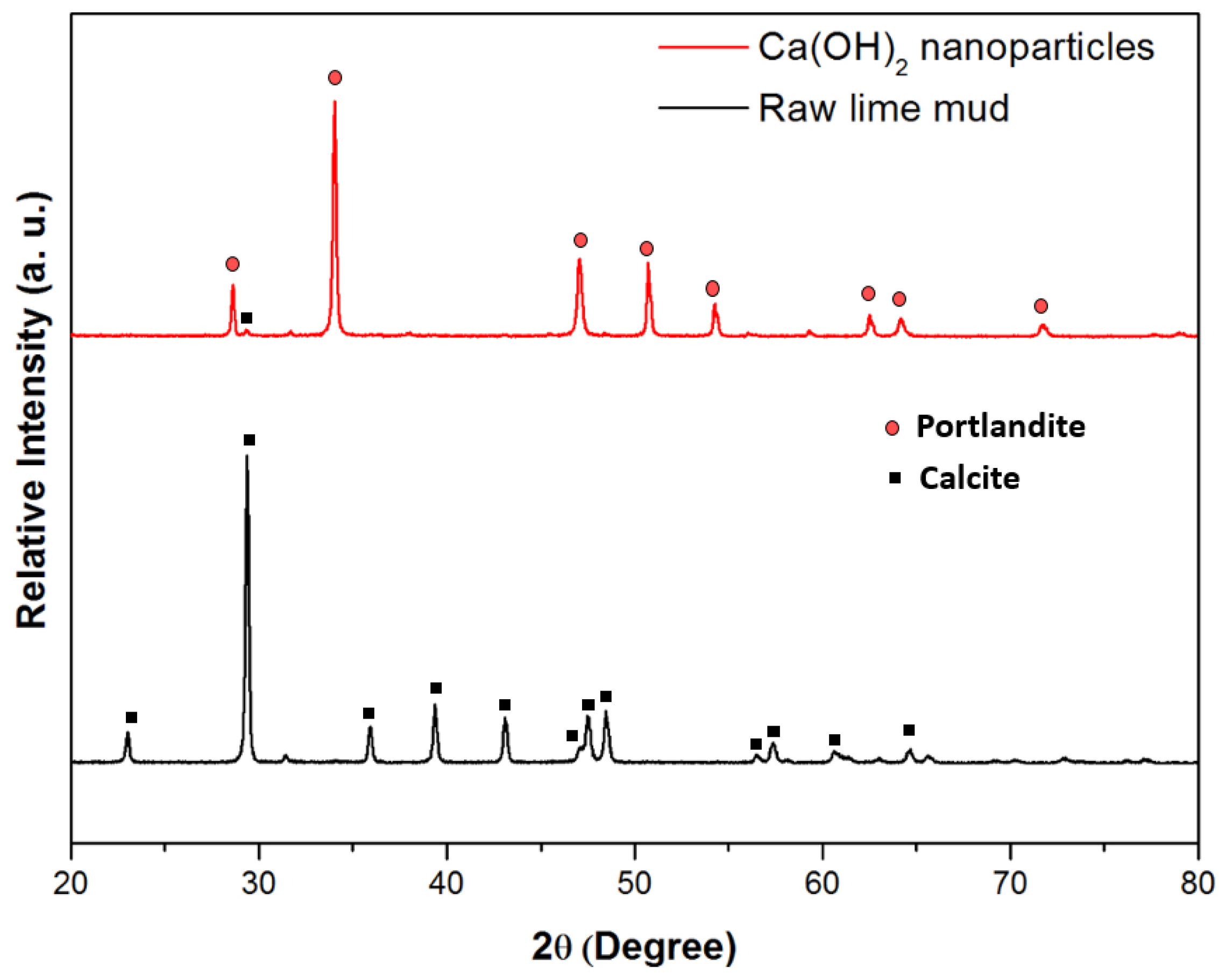
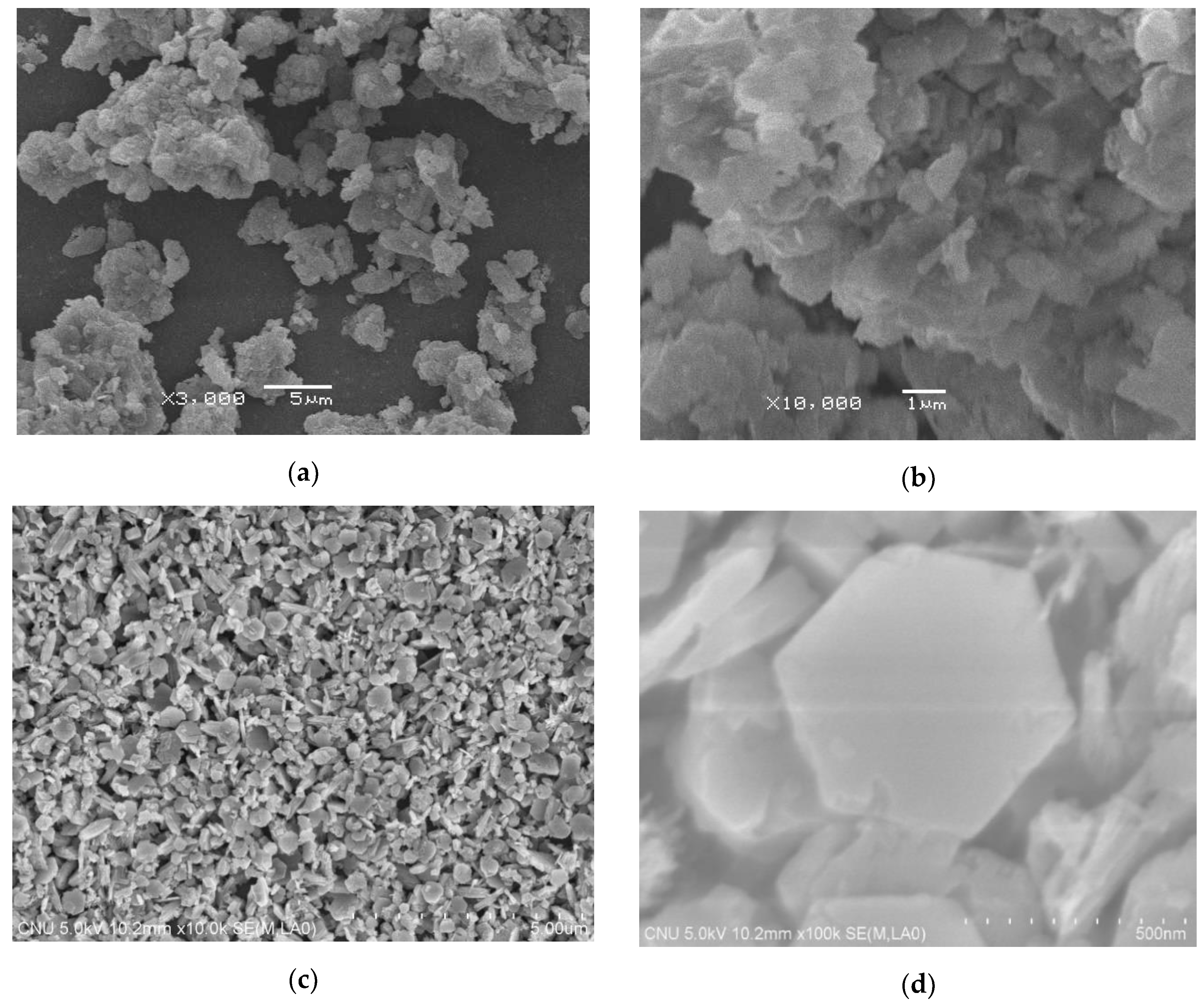
3.1. Characteristics of Green Ca(OH)2
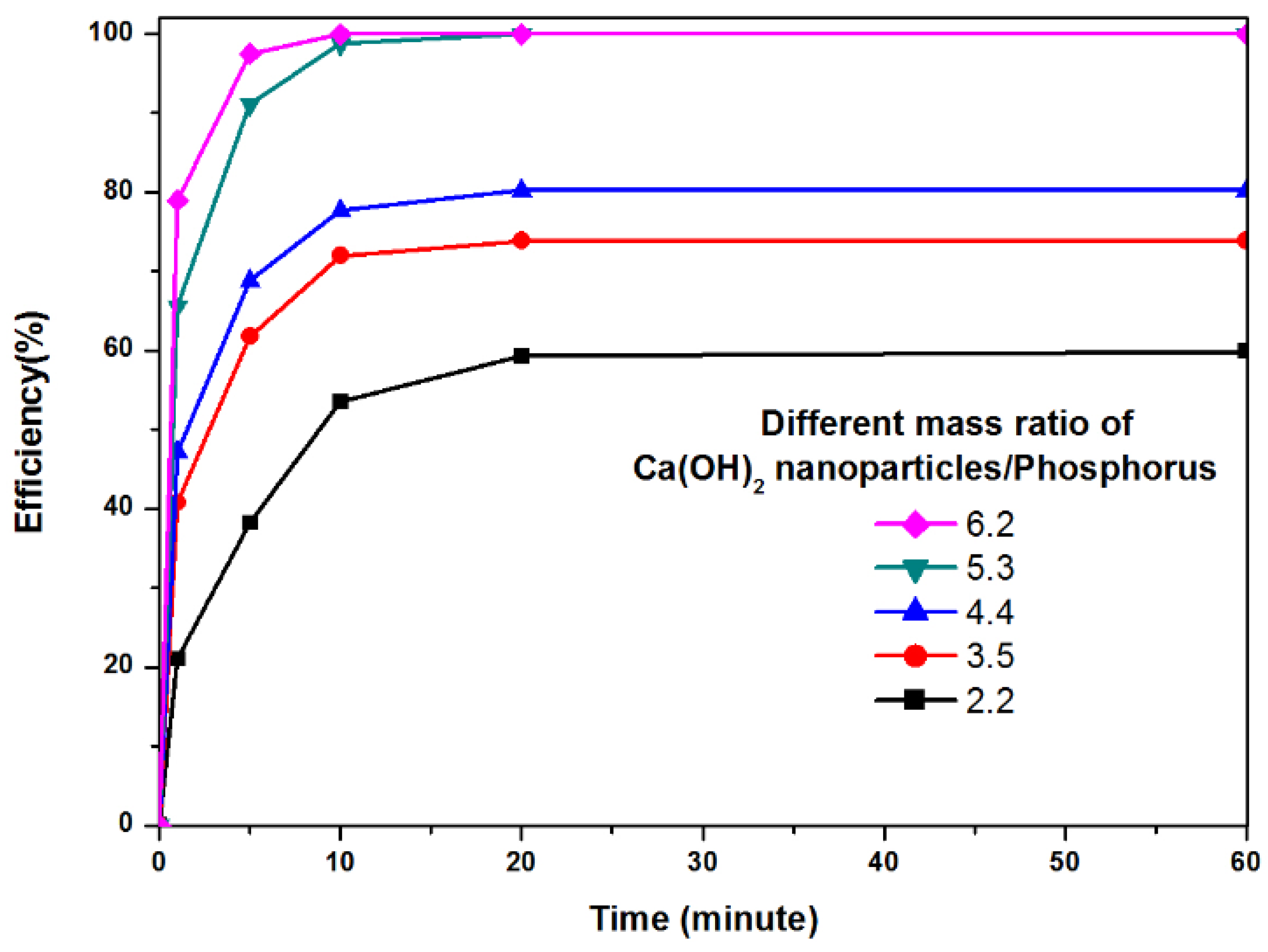
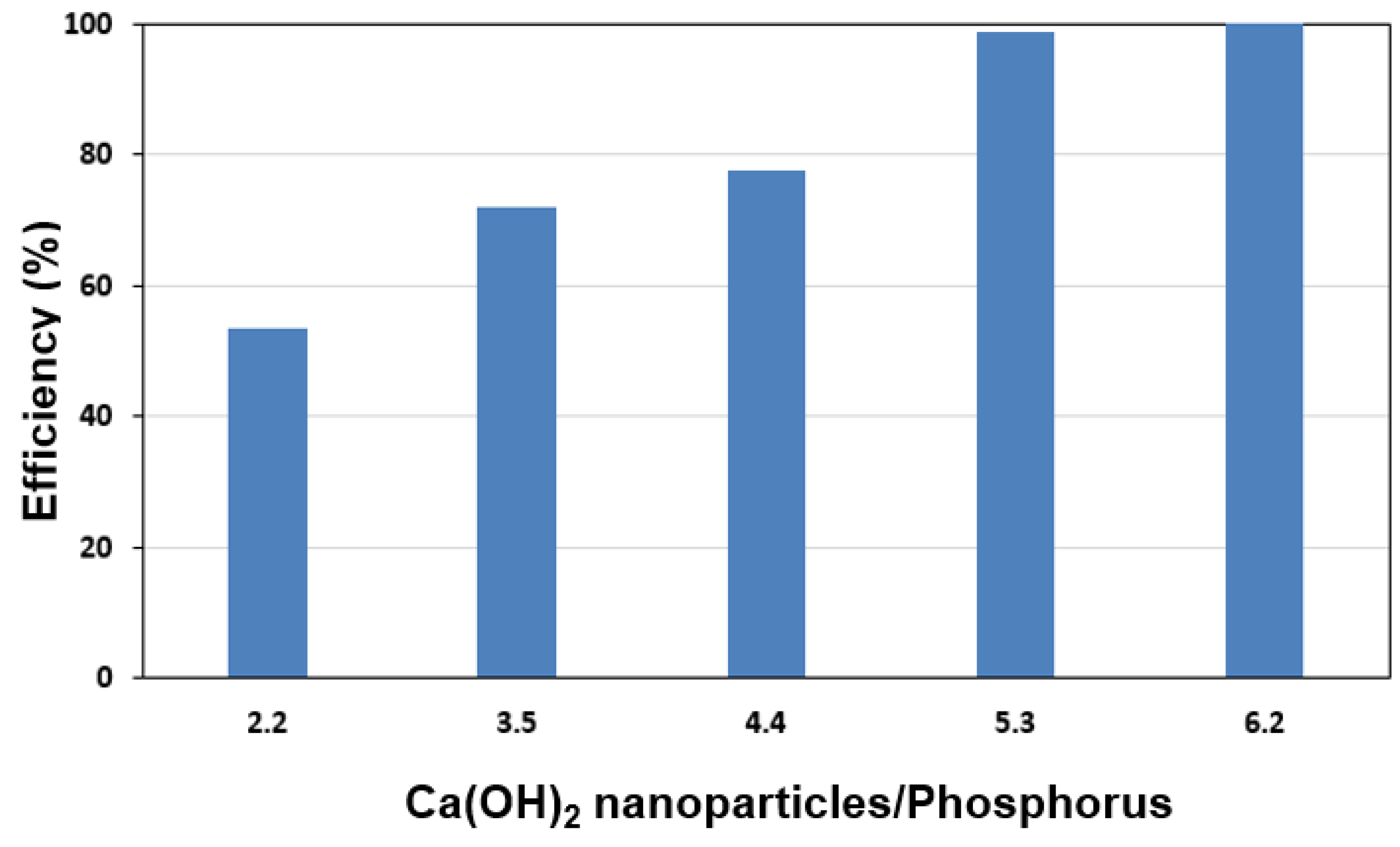
3.2. Phosphorus Treatment Application
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, J.J.; Lange, C.R.; Dougherty, M. Laboratory study using paper mill lime mud for agronomic benefit. Process Saf. Environ. 2009, 87, 401–405. [Google Scholar] [CrossRef]
- Nurmesniemi, H.; Poykio, R.; Keiski, R.L. A case study of waste management at the Northern Finnish pulp and paper mill complex of Stora Enso Veitsiluoto Mills. Waste Manag. 2007, 27, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.T.; Zhao, H.; Bai, H.L.; Zhang, L.P.; Tang, H.W. Papermaking effluent treatment: A new cellulose nanocrystalline/polysulfone composite membrane. Proc. Environ. Sci. 2012, 16, 145–151. [Google Scholar] [CrossRef]
- Paper Industry—Statistics & Facts. Available online: https://www.statista.com/topics/1701/paper-industry/ (accessed on 30 January 2019).
- Production Volume of Paper and Cardboard in Major Countries from 2009 to 2016 (in 1000 Metric Tons). Available online: https://www.statista.com/statistics/240598/production-of-paper-and-cardboard-in-selected-countries/ (accessed on 30 January 2019).
- Wirojanagud, W.; Tantemsapya, N.; Tantriratna, P. Precipitation of heavy metals by lime mud waste of pulp and paper mill. Songklanakarin J. Sci. Technol. 2004, 26, 45–53. [Google Scholar]
- Ren, X. Cleaner production in China’s pulp and paper industry. J. Clean. Prod. 1998, 6, 349–355. [Google Scholar] [CrossRef]
- Liu, Y.J.; Naidu, R.; Ming, H. Red mud as an amendment for pollutants in solid and liquid phases. Geoderma 2011, 163, 1–12. [Google Scholar] [CrossRef]
- Monte, M.C.; Fuente, E.; Blanco, A.; Negro, C. Waste management from pulp and paper production in the European Union. Waste Manag. 2009, 29, 293–308. [Google Scholar] [CrossRef]
- Madrid, M.; Orbe, A.; Carre, H.; Garcia, Y. Thermal performance of sawdust and lime-mud concrete masonry units. Constr. Build. Mater. 2018, 169, 113–123. [Google Scholar] [CrossRef]
- Hartley, W.; Edwards, R.; Lepp, N.W. Arsenic and heavy metal mobility in iron oxide-amended contaminated soils as evaluated by short- and long-term leaching tests. Environ. Pollut. 2004, 131, 495–504. [Google Scholar] [CrossRef]
- Bellaloui, A.; Chtaini, A.; Ballivy, G.; Narasiah, S. Laboratory investigation of the control of acid mine drainage using alkaline paper mill waste. Water Air Soil Pollut. 1999, 11, 57–73. [Google Scholar] [CrossRef]
- Correa, T.H.A.; Toledo, R.; Silva, N.S.; Holanda, J.N.F. Novel nano-sized biphasic calcium phosphate bioceramics (β-CPP/β-TCP) derived of lime mud waste. Mater. Lett. 2019, 243, 17–20. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, P.; Wang, Q. Lime mud from papermaking process as a potential ameliorant for pollutants at ambient conditions: A review. J. Clean. Prod. 2015, 103, 828–836. [Google Scholar] [CrossRef]
- Qin, J.; Cui, C.; Cui, X.; Hussain, A.; Yang, C. Preparation and characterization of ceramite from lime mud and coal fly ash. Constr. Build. Mater. 2015, 95, 10–17. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Chaudhary, M.; Maiti, A. Defluoridation by highly efficient calcium hydroxide nanorods from synthetic and industrial wastewater. Colloids Surf. A. Physicochem. Eng. Asp. 2019, 561, 79–88. [Google Scholar] [CrossRef]
- Hu, H.; Li, X.; Huang, P.; Zhang, Q.; Yuan, W. Efficient removal of copper from wastewater by using mechanically activated calcium carbonate. J. Envron. Manag. 2017, 203, 1–7. [Google Scholar] [CrossRef]
- Krishna, K.C.B.; Niaz, M.R.; Sarkar, D.C.; Jansen, T. Phosphorus removal from aqueous solution can be enhanced through the calcination of lime sludge. J. Envron. Manag. 2017, 200, 359–365. [Google Scholar] [CrossRef]
- Baraka, A.M.; El-Tayieb, M.M.; Shafai, M.E.; Mahamed, N.Y. Sorptive removal of phosphate from wastewater using activated red mud. Aust. J. Basic Appl. Sci. 2012, 6, 500–510. [Google Scholar]
- Ni, F.; He, J.; Wang, Y.; Luan, Z. Preparation and characterization of a cost-effective red mud/polyaluminum chloride composite coagulant for enhanced phosphate removal from aqueous solutions. J. Water Process Eng. 2015, 6, 158–165. [Google Scholar] [CrossRef]
- Kruger, O.; Adam, C. Phosphorus in recycling fertilizers—Analytical challenges. Environ. Res. 2017, 155, 353–358. [Google Scholar] [CrossRef]
- Eussen, S.R.; Verhagen, H.; Klungel, O.H.; Garssen, J.; van Loveren, H.; van Kranen, H.J.; Rompelberg, C.J. Functional foods and dietary supplements: Products at the interface between pharma and nutrition. Eur. J. Pharmacol. 2011, 668, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Puijenbroek, P.J.T.M.V.; Beusen, A.H.W.; Bouwman, A.F. Datasets of the phosphorus content in laundry and dishwasher detergents. Data Brief 2018, 21, 2284–2289. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Degg, A.; Walke, P.; Reiter, F.; Nilges, T. Influence of copper on the capacity of phosphorus-anode in sodium-ion-batteries. J. Solid State Chem. 2019, 270, 636–641. [Google Scholar] [CrossRef]
- Harrison, R.M. Pollution: Causes, Effects, and Control, 3rd ed.; The Royal Society of Chemistry: London, UK, 1996. [Google Scholar]
- Kiely, G. Environmental Engineering; McGrawHill: New York, NY, USA, 1997. [Google Scholar]
- Hosni, K.; Ben, M.S.; Chachi, A.; Ben Amor, M. The removal of PO43− by calcium hydroxide from synthetic wastewater: Optimisation of the operating conditions. Desalination 2008, 223, 337–343. [Google Scholar] [CrossRef]
- Yang, M.; Shi, J.; Xu, Z.; Zhu, S.; Cui, Y. Phosphorus removal and recovery from fosfomycin pharmaceutical wastewater by the induced crystallization process. J. Environ. Manag. 2019, 231, 207–212. [Google Scholar] [CrossRef]
- Zhang, J.; Bligh, M.W.; Liang, P.; Waite, T.D.; Huang, X. Phosphorus removal by in situ generated Fe(II): Efficacy, kinetics and mechanism. Water Res. 2018, 136, 120–130. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sakoda, A.; Suzuki, M. Technical Note: Removal of phosphate by adsorption onto oyster shell powder-kinetic studies. J. Chem. Technol. Biotechnol. 2011, 80, 356–358. [Google Scholar] [CrossRef]
- Xiong, J.; Quin, Y.; Islam, E.; Yue, M.; Wang, W. Phosphate removal from solution using powdered freshwater mussel shells. Desalination 2011, 276, 317–321. [Google Scholar] [CrossRef]
- Penn, C.J.; Bryant, R.B.; Callahan, M.P.; McGrath, J.M. Use of Industrial By-products to Sorb and Retain Phosphorus. Commun. Soil Sci. Plant Anal. 2011, 42, 633–644. [Google Scholar] [CrossRef]
- Khan, M.D.; Ahn, J.W.; Nam, G. Environmental benign synthesis, characterization and mechanism studies of green calcium hydroxide nano-plates derived from waste oyster shells. J Environ. Manag. 2018, 223, 947–951. [Google Scholar] [CrossRef]
- Madrid, J.A.; Lanzon, M. Synthesis and morphological examination of high-purity Ca(OH)2 nanoparticles suitable to consolidate porous surfaces. Appl. Surf. Sci. 2017, 424, 2–8. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Sandhyarani, M.; Nellaippan, T.A.; Rameshbabu, N. Estimation of crystallite size, lattice strain and dislocation density of nanocrystalline carbonate substituted hydroxyapatite by V-ray peak variance analysis. Proc. Mater. Sci. 2014, 5, 212–221. [Google Scholar] [CrossRef]
- Shafi, P.M.; Bose, A.C. Impact of crystalline defects and size on X-ray line broadening: A phenomenological approach for tetragonal SnO2 nanocrystals. AIP Adv. 2015, 5, 057137. [Google Scholar] [CrossRef]
- Darroudi, M.; Bagherpour, M.; Hosseini, H.A.; Ebrahimi, M. Biopolymer-assisted green synthesis and characterization of calcium hydroxide nanoparticles. Ceram. Int. 2016, 42, 3816–3819. [Google Scholar] [CrossRef]
- Pereira, M.R.N.; Salviano, A.B.; Medeiros, T.P.; Santos, M.R.D.; Cibaka, T.E.; Andrade, M.H.C.; Porto, A.O.; Lago, R.M. Ca(OH)2 nanoplates support on activated carbon for the neutralization/removal of free fatty acids during biodiesel production. Fuel 2018, 221, 469–475. [Google Scholar] [CrossRef]
- Makreski, P.; Jovanovski, G. Minerals from Macedonia IX. Distinction between some rhombohedral carbonates by FT IR spectroscopy. Bull. Chem. Technol. Macedonia 2003, 22, 25–32. [Google Scholar]
- Asikin-Mijan, N.; Taufiq-Yap, Y.H.; Lee, H.V. Synthesis of clamshell derived Ca(OH)2 nano-particles via simple surfactant-hydration treatment. Chem. Eng. J. 2015, 262, 1043–1051. [Google Scholar] [CrossRef]
- Torit, J.; Phihusut, D. Phosphorus removal from waste water using eggshell ash. Environ. Sci. Pollut. Res. Int. 2018, 25, 1–9. [Google Scholar]
- Deng, Y.; Wheatley, A. Mechanisms of phosphorus removal by recycled crushed concrete. Int. J. Environ. Res. Public Health 2018, 15, 357. [Google Scholar] [CrossRef]
- Nawar, N.; Ahmad, M.E.; El Said, W.M.; Moalla, S.M.N. Adsorptive removal of phosphorus from wastewater using drinking water treatment-alum sludge (DWT-AS) as low cost adsorbent. Am. J. Chem. Appl. 2015, 2, 79–85. [Google Scholar]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, H.H.T.; Khan, M.D.; Chilakala, R.; Lai, T.Q.; Thenepalli, T.; Ahn, J.W.; Park, D.U.; Kim, J. Utilization of Lime Mud Waste from Paper Mills for Efficient Phosphorus Removal. Sustainability 2019, 11, 1524. https://doi.org/10.3390/su11061524
Vu HHT, Khan MD, Chilakala R, Lai TQ, Thenepalli T, Ahn JW, Park DU, Kim J. Utilization of Lime Mud Waste from Paper Mills for Efficient Phosphorus Removal. Sustainability. 2019; 11(6):1524. https://doi.org/10.3390/su11061524
Chicago/Turabian StyleVu, Hong Ha Thi, Mohd Danish Khan, Ramakrishna Chilakala, Tuan Quang Lai, Thriveni Thenepalli, Ji Whan Ahn, Dong Un Park, and Jeongyun Kim. 2019. "Utilization of Lime Mud Waste from Paper Mills for Efficient Phosphorus Removal" Sustainability 11, no. 6: 1524. https://doi.org/10.3390/su11061524
APA StyleVu, H. H. T., Khan, M. D., Chilakala, R., Lai, T. Q., Thenepalli, T., Ahn, J. W., Park, D. U., & Kim, J. (2019). Utilization of Lime Mud Waste from Paper Mills for Efficient Phosphorus Removal. Sustainability, 11(6), 1524. https://doi.org/10.3390/su11061524





