Effects of N Addition Frequency and Quantity on Hydrocotyle vulgaris Growth and Greenhouse Gas Emissions from Wetland Microcosms
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Microcosm Set-up
2.2. Experimental Design
2.3. Measurement of Growth Index
2.4. Measurement of Greenhouse Gas Emissions
Estimation of CO2 Equivalent
2.5. Data Analysis
3. Results
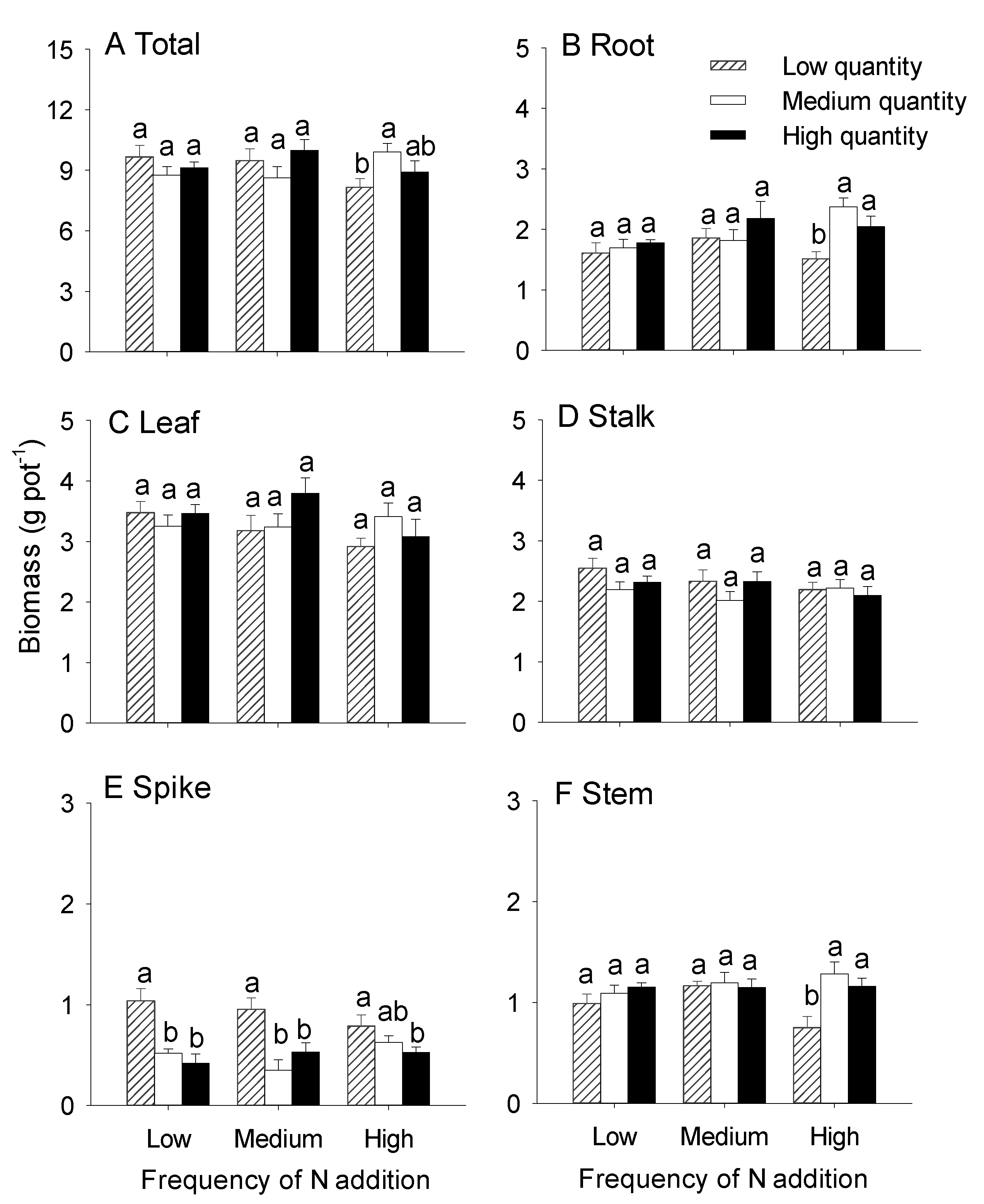
3.1. Effects of the Quantity and Frequency of N Addition on the Growth of Hydrocotyle Vulgaris
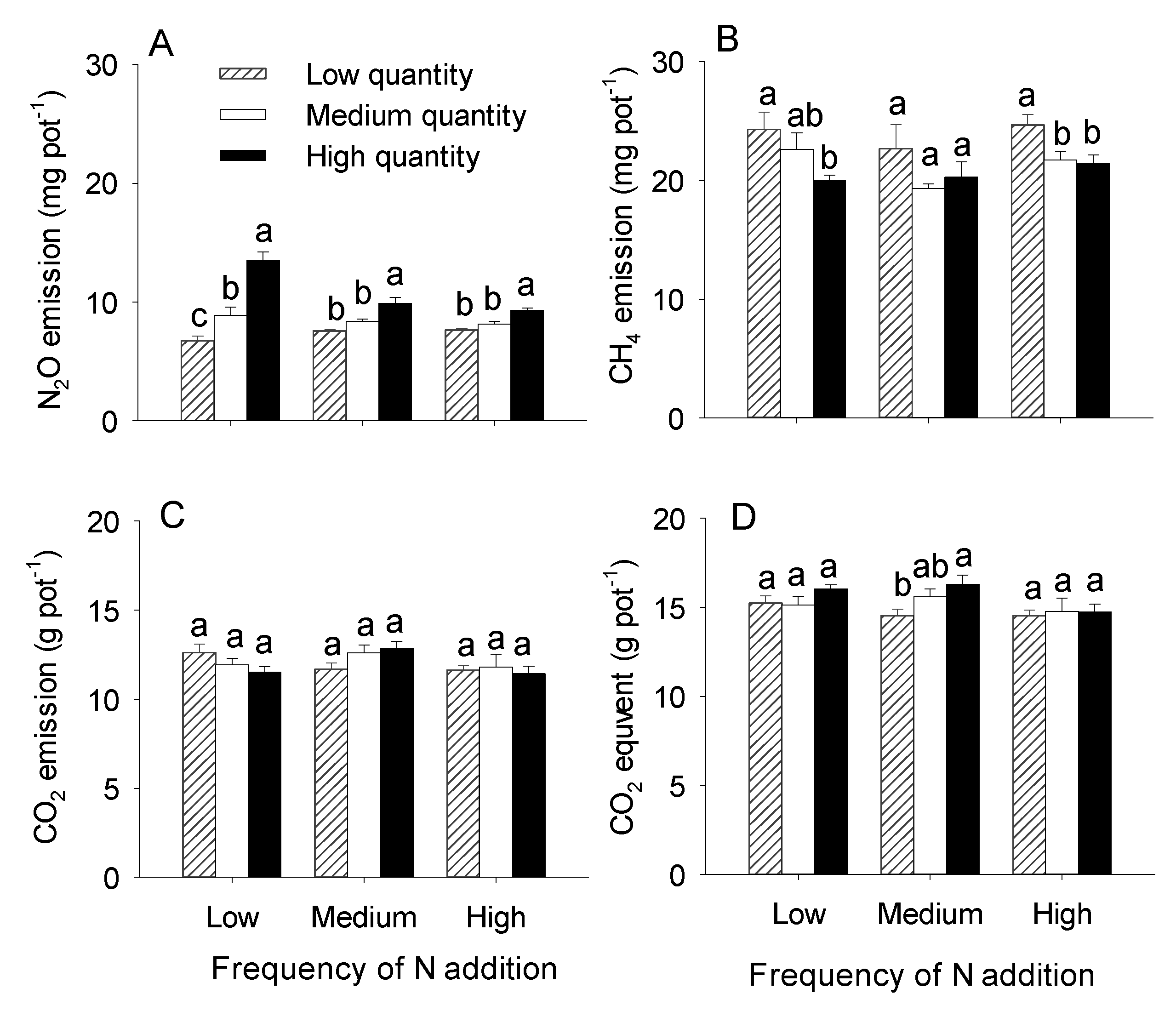
3.2. Effects of N addition Quantity and Frequency on Greenhouse Gas Emissions
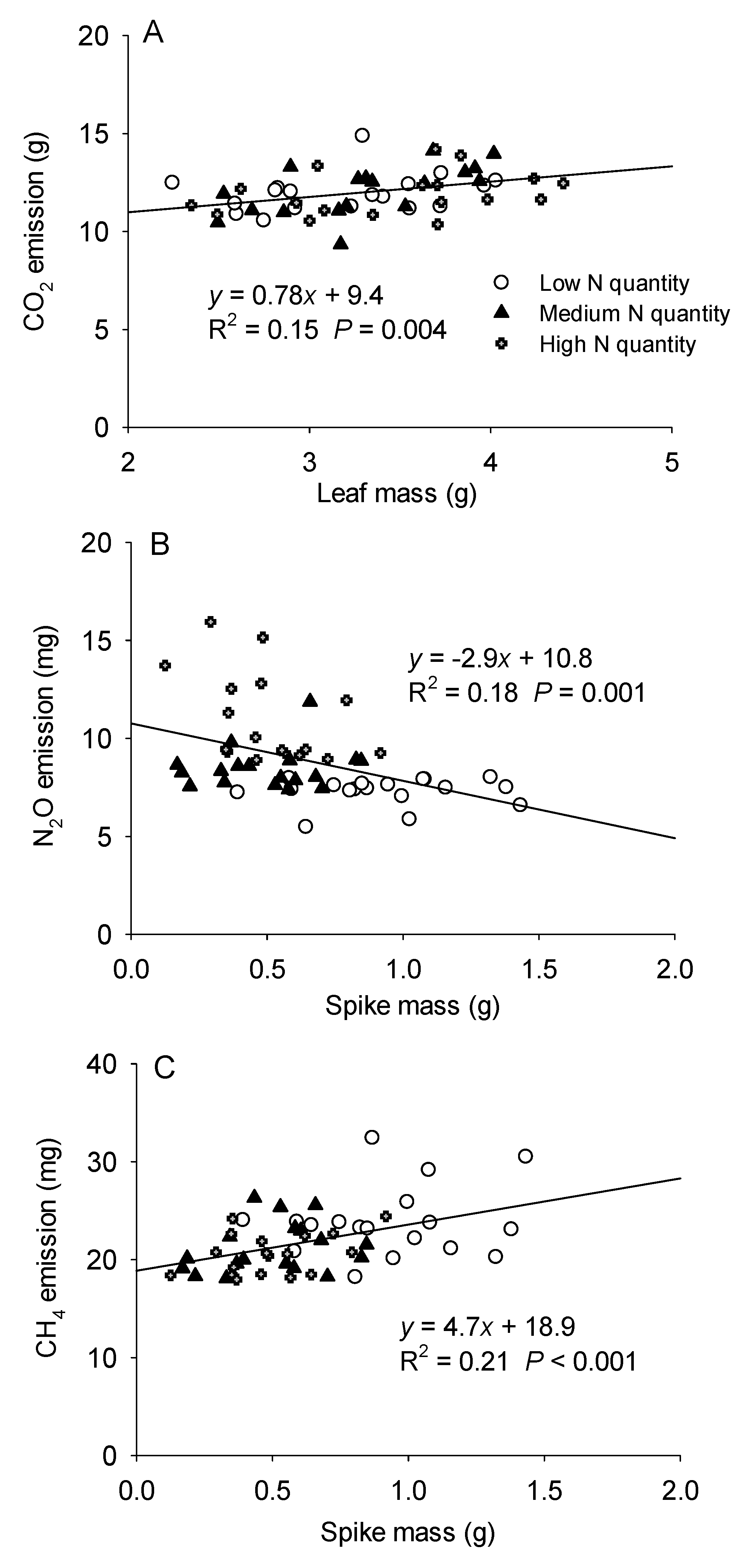
3.3. The Relationship between Greenhouse Gas Emissions and the Biomass of H. Vulgaris
4. Discussion
4.1. Effects of N Addition Quantity and Frequency on the Growth of H. Vulgaris
4.2. Effects of the Quantity and Frequency of N Addition on Greenhouse Gas Emissions from Microcosms
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kanakidou, M.; Myriokefalitakis, S.; Daskalakis, N.; Fanourgakis, G.; Nenes, A.; Baker, A.R.; Tsigaridis, K.; Mihalopoulos, N. Past, Present, and Future Atmospheric Nitrogen Deposition. J. Atmos. Sci. 2016, 73, 2039–2047. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.C.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, L.F.; Zhang, Z.H.; Liu, W.; Chen, L.T.; Cao, G.M.; Yue, H.W.; Zhou, J.Z.; Yang, Y.F.; Tang, Y.H.; et al. Molecular mechanisms of water table lowering and nitrogen deposition in affecting greenhouse gas emissions from a Tibetan alpine wetland. Glob. Chang. Biol. 2017, 23, 815–829. [Google Scholar] [CrossRef]
- Xiao, L.L.; Xie, B.H.; Liu, J.C.; Zhang, H.X.; Han, G.X.; Wang, O.M.; Liu, F.H. Stimulation of long-term ammonium nitrogen deposition on methanogenesis by Methanocellaceae in a coastal wetland. Sci. Total Environ. 2017, 595, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.A.; Chen, H.Y.H.; Ruan, H.H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, X.Y.; Wang, S.Q.; Lu, X.H.; Ouyang, X.Y. A review of spatial variation of inorganic nitrogen (N) wet deposition in China. PLoS ONE 2016, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Zhang, Y.; Han, W.X.; Tang, A.H.; Shen, J.L.; Cui, Z.L.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Matcha, S.K. Quantifying nitrogen effects on castor bean (Ricinus communis L.) development, growth, and photosynthesis. Ind. Crop. Prod. 2010, 31, 185–191. [Google Scholar] [CrossRef]
- Romme, W.H.; Tinker, D.B.; Stakes, G.K.; Turner, M.G. Does inorganic nitrogen limit plant growth 3–5 years after fire in a Wyoming, USA, lodgepole pine forest? For. Ecol. Manag. 2009, 257, 829–835. [Google Scholar] [CrossRef]
- Wang, D.; He, H.L.; Gao, Q.; Zhao, C.Z.; Zhao, W.Q.; Yin, C.Y.; Chen, X.L.; Ma, Z.L.; Li, D.D.; Sun, D.D.; et al. Effects of short-term N addition on plant biomass allocation and C and N pools of the Sibiraea angustata scrub ecosystem. Eur. J. Soil Sci. 2017, 68, 212–220. [Google Scholar] [CrossRef]
- Walker, J.T.; James, J.J.; Drenovsky, R.E. Competition from Bromus tectorum removes differences between perennial grasses in N capture and conservation strategies. Plant Soil 2017, 412, 177–188. [Google Scholar] [CrossRef]
- Song, M.H.; Jiang, J.; Cao, G.M.; Xu, X.L. Effects of temperature, glucose and inorganic nitrogen inputs on carbon mineralization in a Tibetan alpine meadow soil. Eur. J. Soil Biol. 2010, 46, 375–380. [Google Scholar] [CrossRef]
- Liu, L.L.; Greaver, T.L. A review of nitrogen enrichment effects on three biogenic GHGs: The CO2 sink may be largely offset by stimulated N2O and CH4 emission. Ecol. Lett. 2009, 12, 1103–1117. [Google Scholar] [CrossRef]
- Mo, Y.; Deng, Z.H.; Gao, J.Q.; Guo, Y.X.; Yu, F.H. Does richness of emergent plants affect CO2 and CH4 emissions in experimental wetlands? Freshw. Biol. 2015, 60, 1537–1544. [Google Scholar] [CrossRef]
- Hu, M.J.; Tong, C. Effects of nitrogen enrichment on the greenhouse gas fluxes in natural wetlands and the associated mechanism: A review. Chin. J. Ecol. 2014, 33, 1969–1976. [Google Scholar]
- Yan, Y.L.; Ganjurjav, H.; Hu, G.Z.; Liang, Y.; Li, Y.; He, S.C.; Danjiu, L.B.; Yang, J.; Gao, Q.Z. Nitrogen deposition induced significant increase of N2O emissions in an dry alpine meadow on the central Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2018, 265, 45–53. [Google Scholar] [CrossRef]
- Osone, Y.; Yazaki, K.; Masaki, T.; Ishida, A. Responses to nitrogen pulses and growth under low nitrogen availability in invasive and native tree species with differing successional status. J. Plant Res. 2014, 127, 315–328. [Google Scholar] [CrossRef]
- Wang, A.O.; Jiang, X.X.; Zhang, Q.Q.; Zhou, J.; Li, H.L.; Luo, F.L.; Zhang, M.X.; Yu, F.H. Nitrogen addition increases intraspecific competition in the invasive wetland plant Alternanthera philoxeroides, but not in its native congener Alternanthera Sessilis. Plant Spec. Biol. 2015, 30, 176–183. [Google Scholar] [CrossRef]
- Yuan, Q.Y.; Wang, P.; Liu, L.; Dong, B.C.; Yu, F.H. Root responses to nitrogen pulse frequency under different nitrogen amounts. Acta Ecol. 2017, 80, 32–38. [Google Scholar] [CrossRef]
- Ma, Y.; Qian, C.; Sun, D.; Deng, L.; Huang, G.; Lu, W. Effect of nitrogen fertilizer application on greenhouse gas emissions from soil in paddy field. Trans. Chin. Soc. Agric. Eng. 2016, 32, 128–134. [Google Scholar]
- Sapkota, T.B.; Aryal, J.P.; Khatri-Chhetri, A.; Shirsath, P.B.; Arumugam, P.; Stirling, C.M. Identifying high-yield low-emission pathways for the cereal production in South Asia. Mitig. Adapt. Strateg. Glob. Chang. 2018, 23, 621–641. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.J.; Rørslett, B.; Springuel, I. Strategy analysis of submerged lake macrophyte communities: An international example. Aquat. Bot. 1990, 36, 303–323. [Google Scholar] [CrossRef]
- Ma, J.S. The Checklist of the Chinese Invasive Plants; Higher Education Press: Beijing, China, 2013. [Google Scholar]
- Miao, L.H.; Ji, M.C.; Wang, Y.Y.; Qiao, D.D.; Chen, Y.C. Study on invasion risk of Hydrocotyle vulgaris as an alien species in wetlands. J. Zhejiang Univ. 2011, 37, 425–431. [Google Scholar]
- Zhou, J.; Li, H.L.; Luo, F.L.; Huang, W.J.; Zhang, M.X.; Yu, F.H. Effects of nitrogen addition on interspecific competition between Alternanthera philoxeroides and Alternanthera Sessilis. Acta Ecol. Sin. 2015, 35, 8258–8267. [Google Scholar]
- Gao, J.Q.; Duan, M.Y.; Zhang, X.Y.; Li, Q.W.; Yu, F.H. Effects of frequency and intensity of drying-rewetting cycles on Hydrocotyle vulgaris growth and greenhouse gas emissions from wetland microcosms. Catena 2018, 164, 44–49. [Google Scholar] [CrossRef]
- Wang, J.Y.; Song, C.C.; Miao, Y.Q.; Meng, H.N. Greenhouse Gas Emissions from Southward Transplanted Wetlands During Freezing-Thawing Periods in Northeast China. Wetlands 2013, 33, 1075–1081. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Quan, H.; Dong, B.C.; Liu, L.; Li, H.L. The effects of habitat type and nitrogen deposition on the invasion of Hydrocotyle vulgaris in wetland plant communities. Acta Ecol. Sin. 2016, 36, 4045–4054. [Google Scholar]
- Bloom, A.J.; Chapin, F.S., III; Mooney, H.A. Resource Limitation in plants-an economic analogy. Annu. Rev. Ecol. Evol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Niklas, K.J. Modelling below- and above-ground biomass for non-woody and woody plants. Ann. Bot. 2005, 95, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B. Root-shoot relations: Optimality in acclimation and adaptation or the Emperor s new clothes? In Plant Roots: The Hidden Half; CRC Press: New York, NY, USA, 2002; pp. 205–220. [Google Scholar]
- King, J.S.; Albaugh, T.J.; Allen, H.L.; Kress, L.W. Stand-level allometry in Pinus taeda as affected by irrigation and fertilization. Tree Physiol. 1999, 19, 769–778. [Google Scholar] [CrossRef]
- Coyle, D.R.; Coleman, M.D.; Aubrey, D.P. Above- and below-ground biomass accumulation, production, and distribution of sweetgum and loblolly pine grown with irrigation and fertilization. Can. J. For. Res. 2008, 38, 1335–1348. [Google Scholar] [CrossRef]
- Herben, T.; Sera, B.; Klimesova, J. Clonal growth and sexual reproduction: Tradeoffs and environmental constraints. Oikos 2015, 124, 469–476. [Google Scholar] [CrossRef]
- Kleunen, M.V.; Fischer, M.; Schmid, B. Effects of intra-specific competition on size variation and reproductive allocation in a clonal plant. Oikos 2001, 94, 515–524. [Google Scholar] [CrossRef]
- Tang, W.G.; Chen, D.X.; Phillips, O.L.; Liu, X.; Zhou, Z.; Li, Y.D.; Xi, D.; Zhu, F.F.; Fang, J.Y.; Zhang, L.M.; et al. Effects of long-term increased N deposition on tropical montane forest soil N2 and N2O emissions. Soil Biol. Biomchem. 2018, 126, 194–203. [Google Scholar] [CrossRef]
- Fang, Y.T.; Koba, K.; Makabe, A.; Takahashi, C.; Zhu, W.X.; Hayashi, T.; Hokari, A.A.; Urakawa, R.; Bai, E.; Houlton, B.Z.; et al. Microbial denitrification dominates nitrate losses from forest ecosystems. Proc. Natl. Acad. Sci. USA 2015, 112, 1470–1474. [Google Scholar] [CrossRef]
- Xie, D.N.; Si, G.Y.; Zhang, T.; Mulder, J.; Duan, L. Nitrogen deposition increases N2O emission from an N-saturated subtropical forest in southwest China. Environ. Pollut. 2018, 243, 1818–1824. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.J.; Li, Z.Y.; Wang, S.N.; Qu, Y.K. Effects of irrigation regime and nitrogen fertilizer management on CH4, N2O and CO2 emissions from saline-alkaline paddy Fields in Northeast China. Sustainability 2018, 10, 475. [Google Scholar] [CrossRef]
- Li, K.H.; Gong, Y.M.; Song, W.; Lv, J.L.; Chang, Y.H.; Hu, Y.K.; Tian, C.Y.; Christie, P.; Liu, X.J. No significant nitrous oxide emissions during spring thaw under grazing and nitrogen addition in an alpine grassland. Glob. Chang. Biol. 2012, 18, 2546–2554. [Google Scholar] [CrossRef]
- Lohila, A.; Aurela, M.; Hatakka, J.; Pihlatie, M.; Minkkinen, K.; Penttila, T.; Laurila, T. Responses of N2O fluxes to temperature, water table and N deposition in a northern boreal fen. Eur. J. Soil Sci. 2010, 61, 651–661. [Google Scholar] [CrossRef]
- Angel, R.; Claus, P.; Conrad, R. Methanogenic archaea are globally ubiquitous in aerated soils and become active under wet anoxic conditions. ISME J. 2012, 6, 847–862. [Google Scholar] [CrossRef]
- Tian, X.F.; Hu, H.W.; Ding, Q.; Song, M.H.; Xu, X.L.; Zheng, Y.; Guo, L.D. Influence of nitrogen fertilization on soil ammonia oxidizer and denitrifier abundance, microbial biomass, and enzyme activities in an alpine meadow. Biol. Fert. Soils 2014, 50, 703–713. [Google Scholar] [CrossRef]
- Hu, M.J.; Wilson, B.J.; Sun, Z.G.; Huang, J.F.; Tong, C. Effects of nitrogen and sulphate addition on methane oxidation in the marsh soil of a typical subtropical estuary (Min River) in China. Chem. Ecol. 2018, 34, 610–623. [Google Scholar] [CrossRef]
- Wang, J.; Xu, T.T.; Yin, L.C.; Han, C.; Deng, H.; Jiang, Y.B.; Zhong, W.H. Nitrate addition inhibited methanogenesis in paddy soils under long-term managements. Plant Soil Environ. 2018, 64, 393–399. [Google Scholar]
- Joabsson, A.; Christensen, T.R. Methane emissions from wetlands and their relationship with vascular plants: An Arctic example. Glob. Chang. Biol. 2010, 7, 919–932. [Google Scholar] [CrossRef]
- Khahil, M.I.; Baggs, E.M. CH4 oxidation and N2O emissions at varied soil water-filled pore spaces and headspace CH4 concentrations. Soil Biol. Biomchem. 2005, 37, 1785–1794. [Google Scholar] [CrossRef]
- Hirota, M.; Zhang, P.C.; Gu, S.; Shen, H.H.; Kuriyama, T.; Li, Y.N.; Tang, Y.H. Small-scale variation in ecosystem CO2 fluxes in an alpine meadow depends on plant biomass and species richness. J. Plant Res. 2010, 123, 531–541. [Google Scholar] [CrossRef]
- Li, F.L.; Gao, S.M. Plant Biology; China Forestry Publishing House: Beijing, China, 2008; p. 103. [Google Scholar]

| Variable | Frequency (F) | Quantity (Q) | F × Q | |||
|---|---|---|---|---|---|---|
| F2,45 | p | F2,45 | p | F4,45 | p | |
| Total mass | 0.43 | 0.653 | 0.25 | 0.780 | 2.88 | 0.033 |
| Root mass | 2.60 | 0.085 | 3.73 | 0.032 | 2.32 | 0.071 |
| Leaf mass | 1.52 | 0.230 | 1.08 | 0.348 | 1.56 | 0.202 |
| Stalk mass | 1.24 | 0.298 | 1.65 | 0.203 | 0.84 | 0.507 |
| Stem mass | 1.27 | 0.29 | 5.45 | 0.008 | 2.73 | 0.041 |
| Spike mass | 0.20 | 0.819 | 21.78 | <0.001 | 2.20 | 0.084 |
| Root/shoot | 4.02 | 0.025 | 5.30 | 0.009 | 0.71 | 0.59 |
| No. of ramets | 0.88 | 0.422 | 20.84 | <0.001 | 0.18 | 0.948 |
| Leaf area | 0.85 | 0.433 | 9.51 | <0.001 | 0.93 | 0.455 |
| SLA | 0.62 | 0.542 | 5.06 | 0.010 | 1.14 | 0.348 |
| Variable | Frequency (F) | Quantity (Q) | F × Q | |||
|---|---|---|---|---|---|---|
| F3,56 | p | F3,56 | p | F3,56 | p | |
| N2O emission | 8.45 | 0.001 | 55.75 | <0.001 | 11.14 | <0.001 |
| CH4 emission | 2.20 | 0.122 | 6.75 | 0.003 | 0.60 | 0.665 |
| CO2 emission | 2.31 | 0.111 | 0.14 | 0.871 | 1.82 | 0.141 |
| CO2 equivalent | 2.56 | 0.089 | 0.66 | 0.524 | 1.25 | 0.304 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.-W.; Zhang, X.-Y.; Gao, J.-Q.; Song, M.-H.; Liang, J.-F.; Yue, Y. Effects of N Addition Frequency and Quantity on Hydrocotyle vulgaris Growth and Greenhouse Gas Emissions from Wetland Microcosms. Sustainability 2019, 11, 1520. https://doi.org/10.3390/su11061520
Li Q-W, Zhang X-Y, Gao J-Q, Song M-H, Liang J-F, Yue Y. Effects of N Addition Frequency and Quantity on Hydrocotyle vulgaris Growth and Greenhouse Gas Emissions from Wetland Microcosms. Sustainability. 2019; 11(6):1520. https://doi.org/10.3390/su11061520
Chicago/Turabian StyleLi, Qian-Wei, Xiao-Ya Zhang, Jun-Qin Gao, Ming-Hua Song, Jin-Feng Liang, and Yi Yue. 2019. "Effects of N Addition Frequency and Quantity on Hydrocotyle vulgaris Growth and Greenhouse Gas Emissions from Wetland Microcosms" Sustainability 11, no. 6: 1520. https://doi.org/10.3390/su11061520
APA StyleLi, Q.-W., Zhang, X.-Y., Gao, J.-Q., Song, M.-H., Liang, J.-F., & Yue, Y. (2019). Effects of N Addition Frequency and Quantity on Hydrocotyle vulgaris Growth and Greenhouse Gas Emissions from Wetland Microcosms. Sustainability, 11(6), 1520. https://doi.org/10.3390/su11061520




