Recovery of Gold from Chloride Solution by TEMPO-Oxidized Cellulose Nanofiber Adsorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorbent Preparation
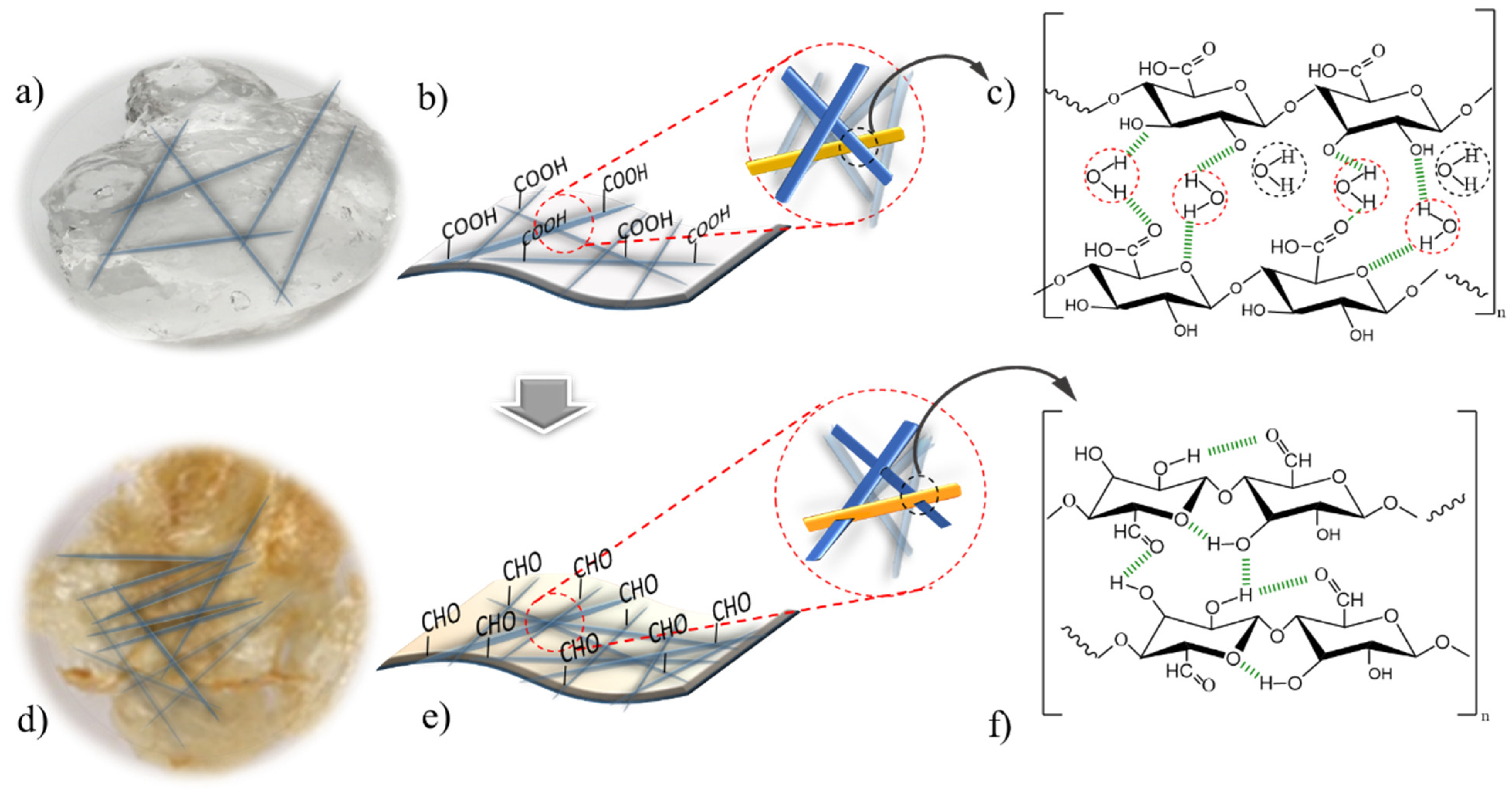
2.2.1. TEMPO-Oxidized Cellulose Nanofibers (TOCN)
2.2.2. Oven Dried TEMPO-Oxidized Cellulose Nanofibers (H-TOCN)
2.3. Batch Adsorption Study
2.4. Effect of Ionic Strength
2.5. Characterization of Materials
3. Results and Discussion
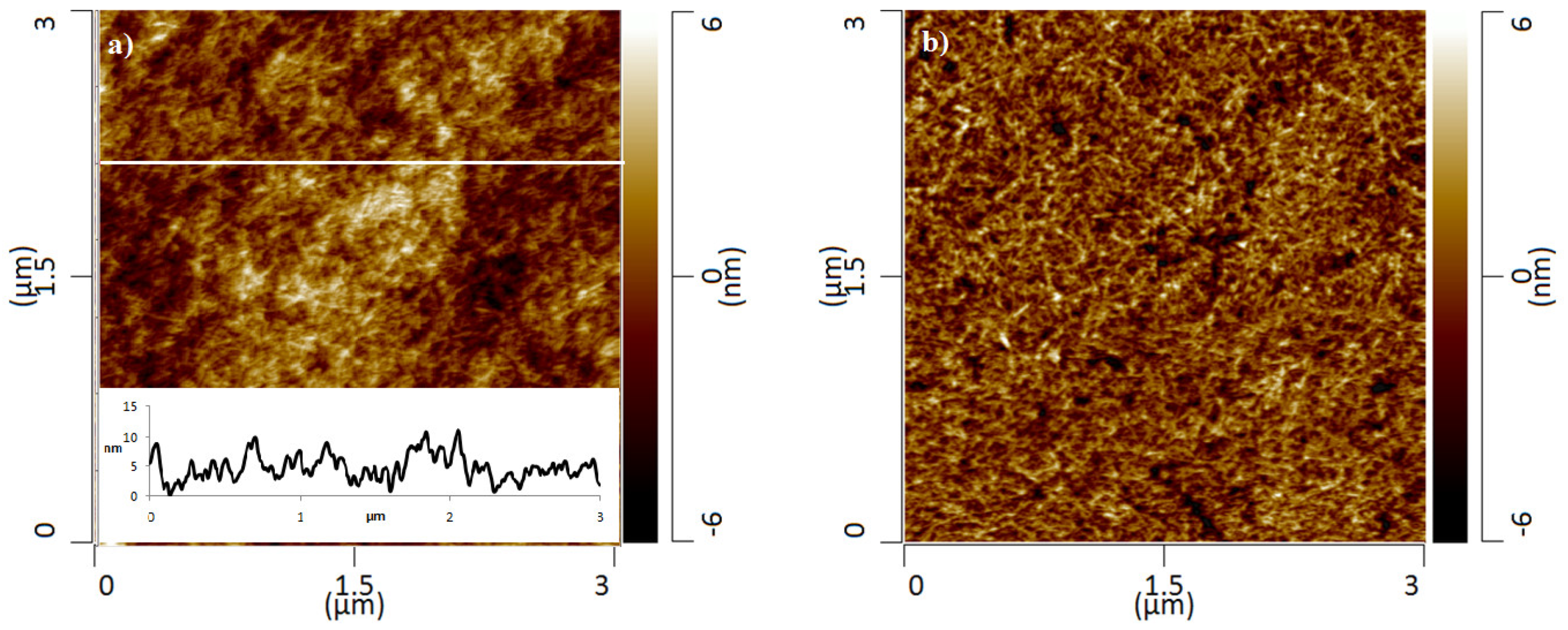
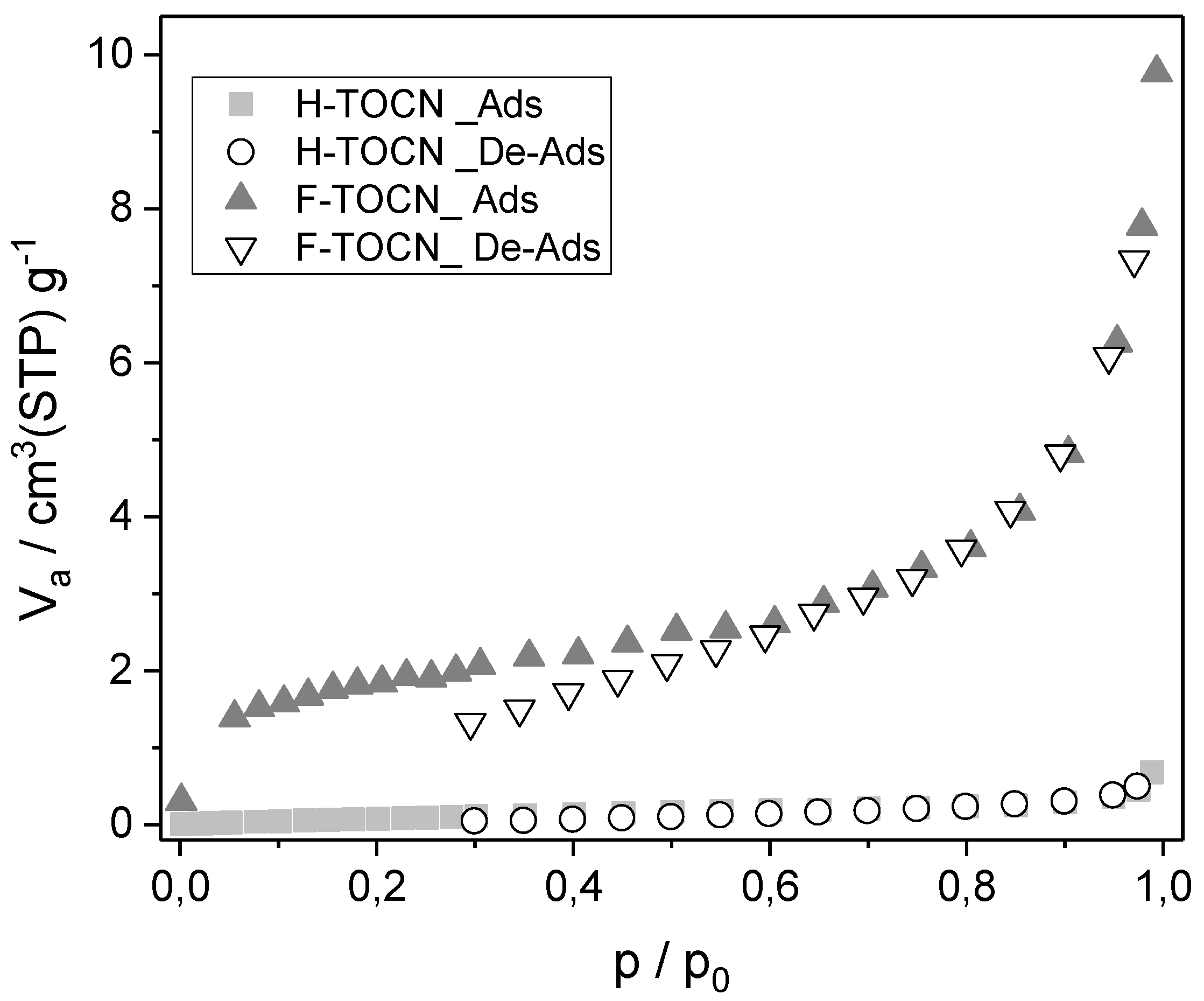
3.1. Adsorbent Characterization
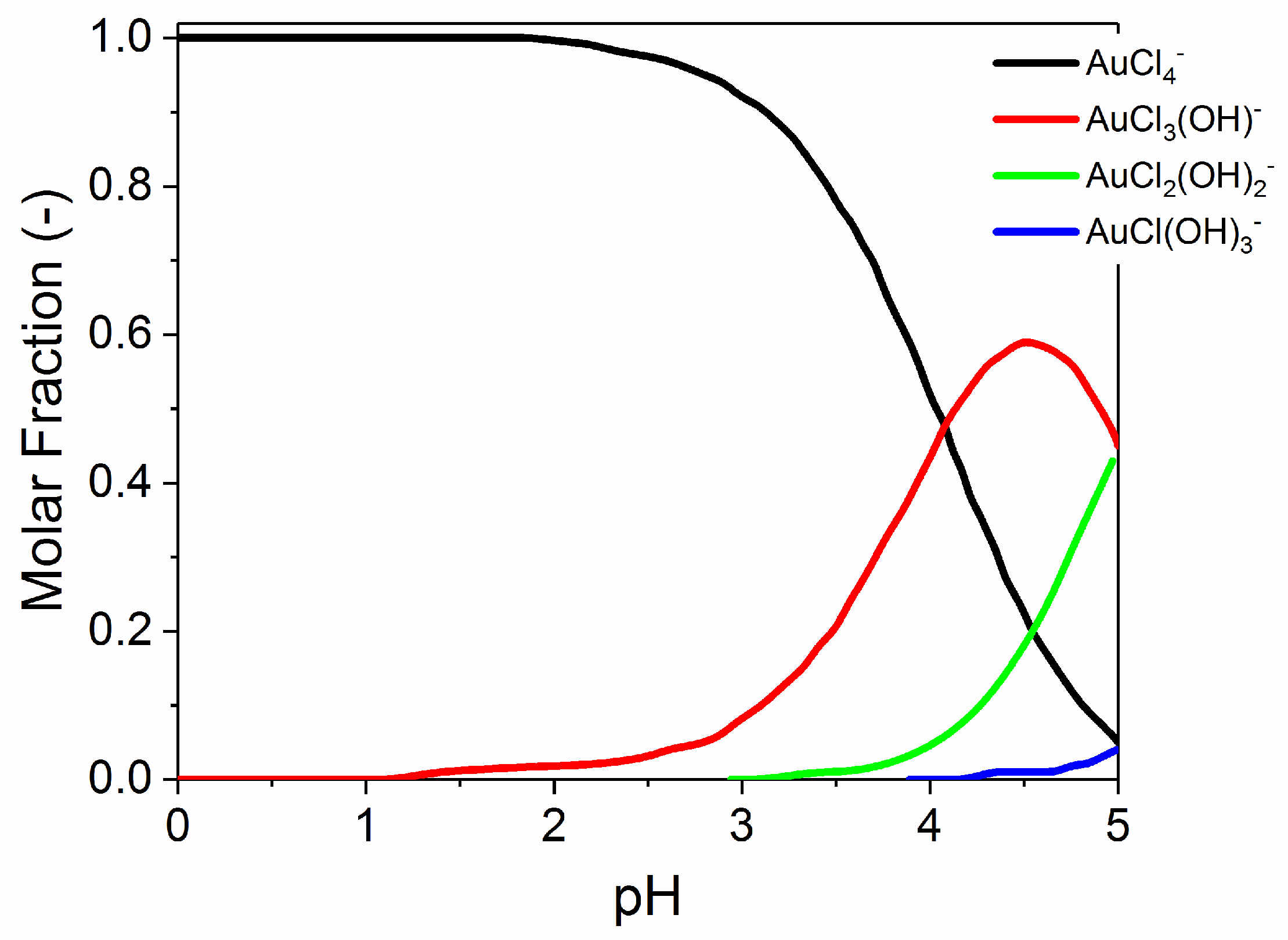
3.2. Recovery of Gold via the Adsorption Process
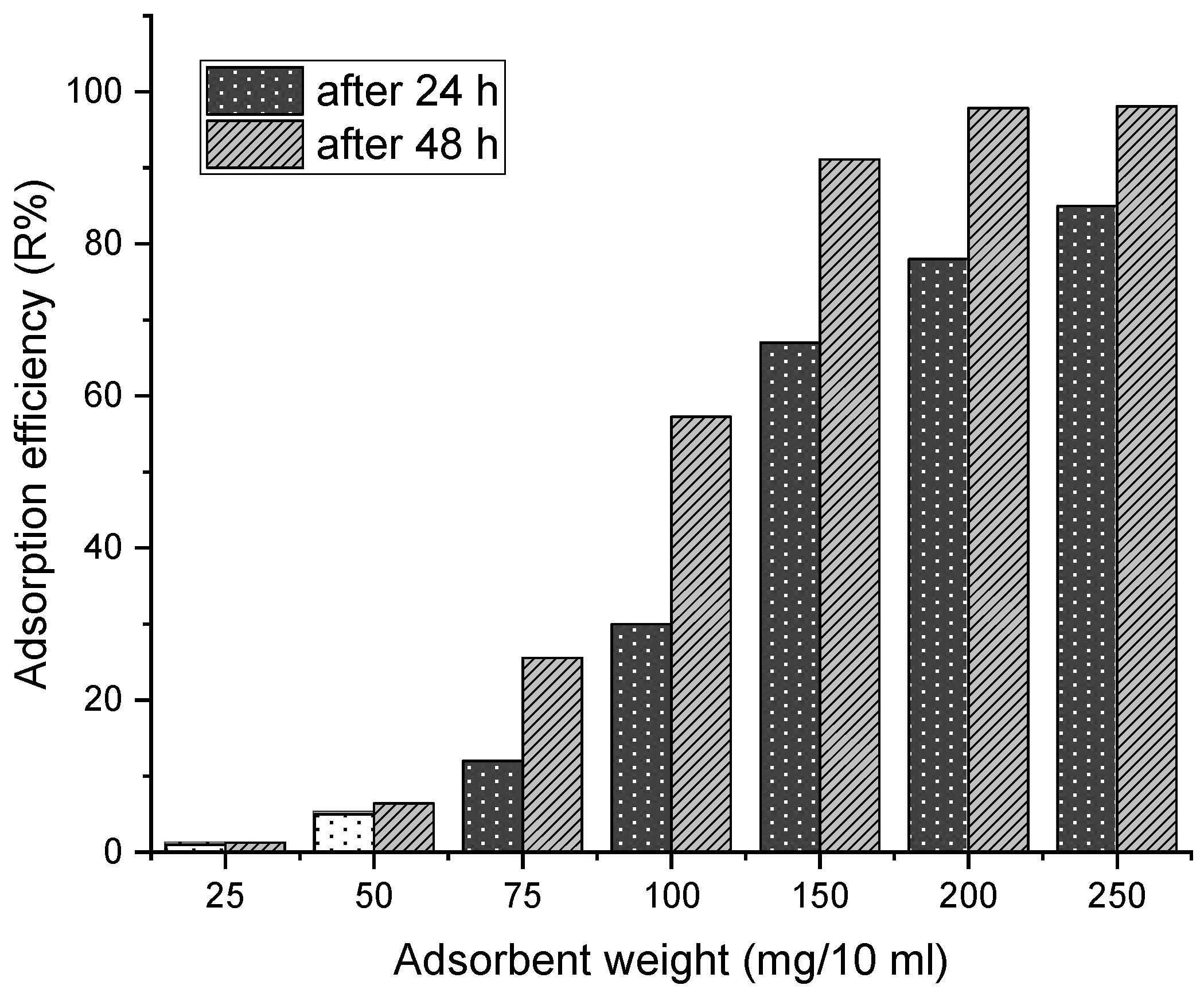
3.2.1. Effect of Adsorbent Dose
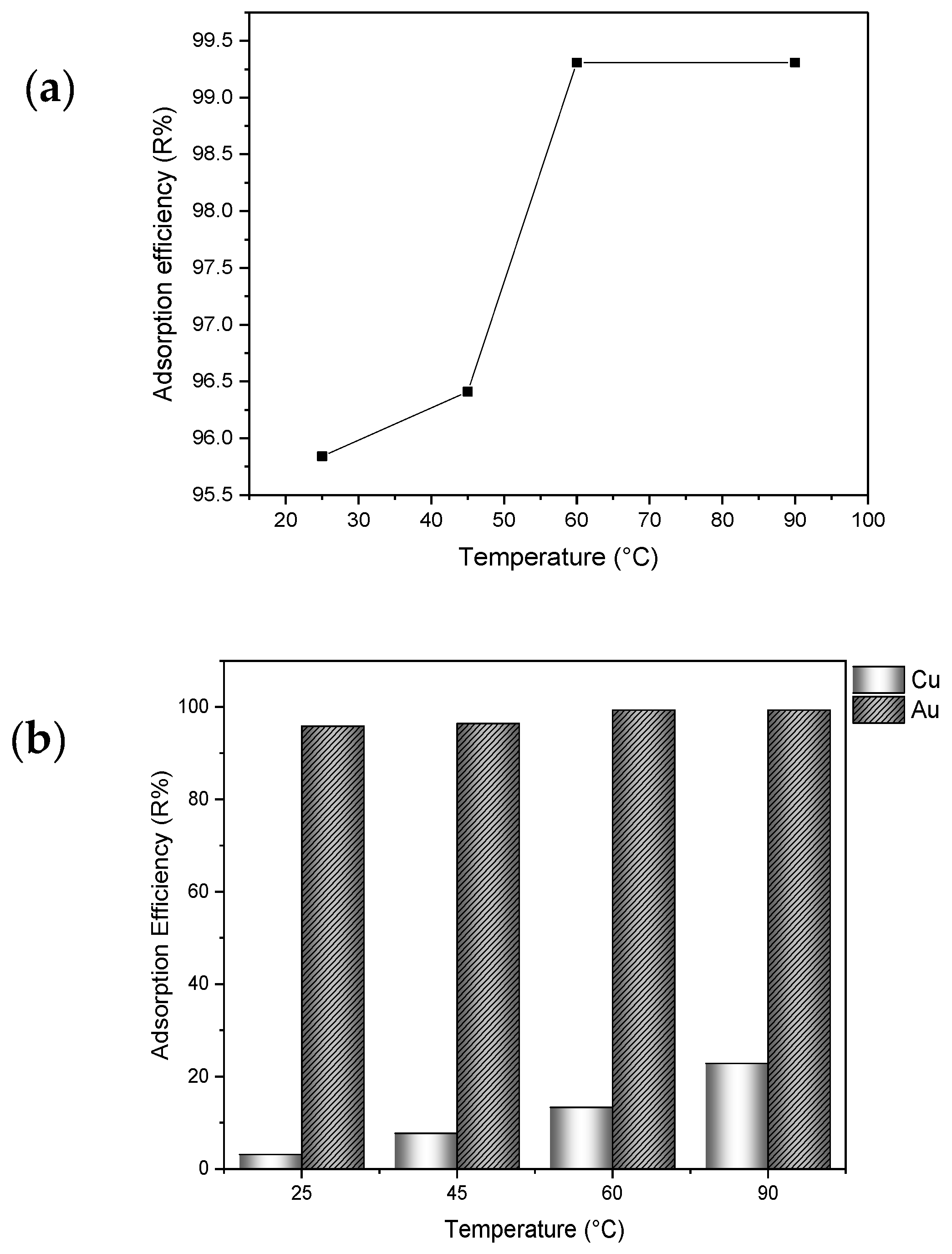
3.2.2. Effect of Temperature
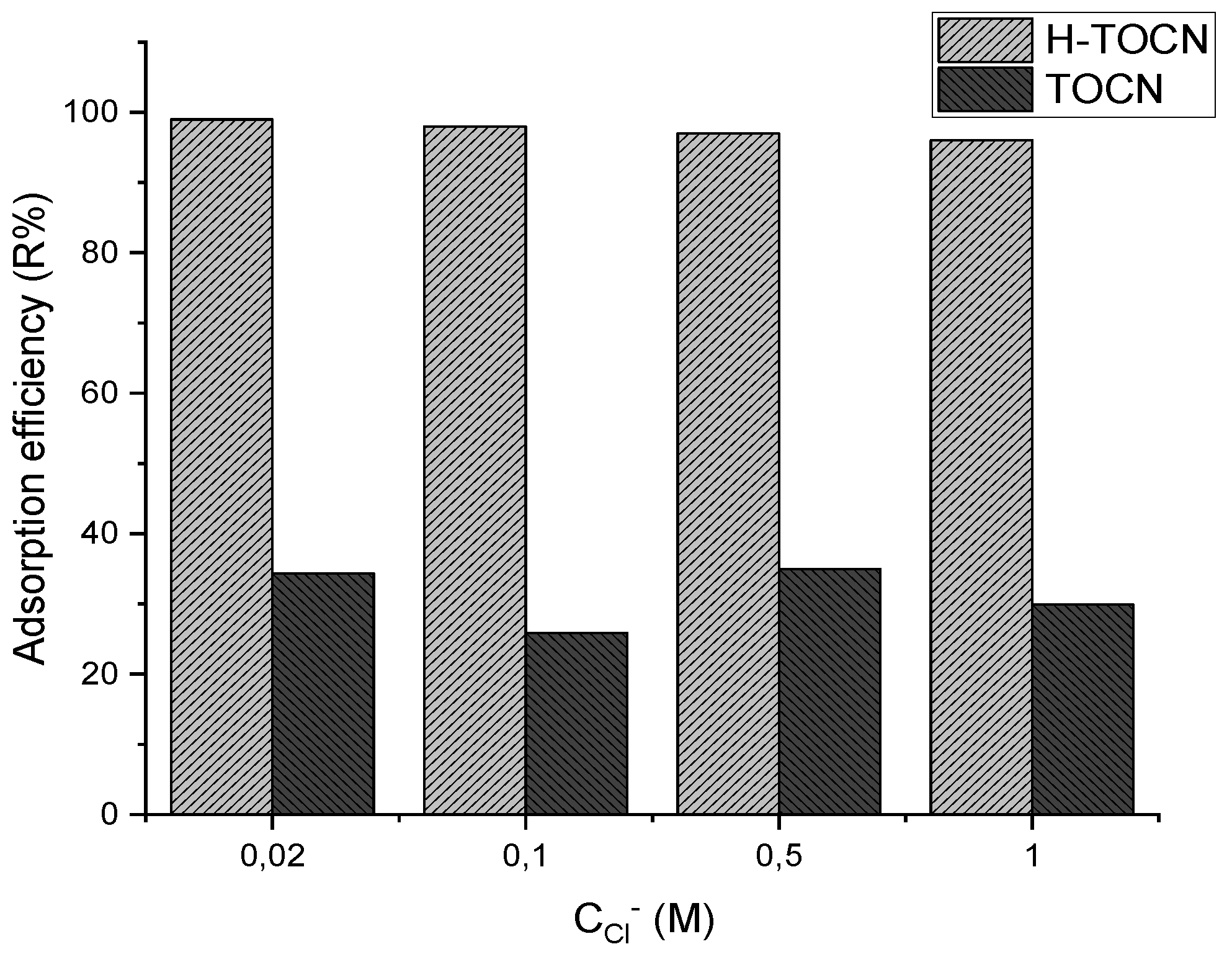
3.2.3. Effect of Ionic Strength
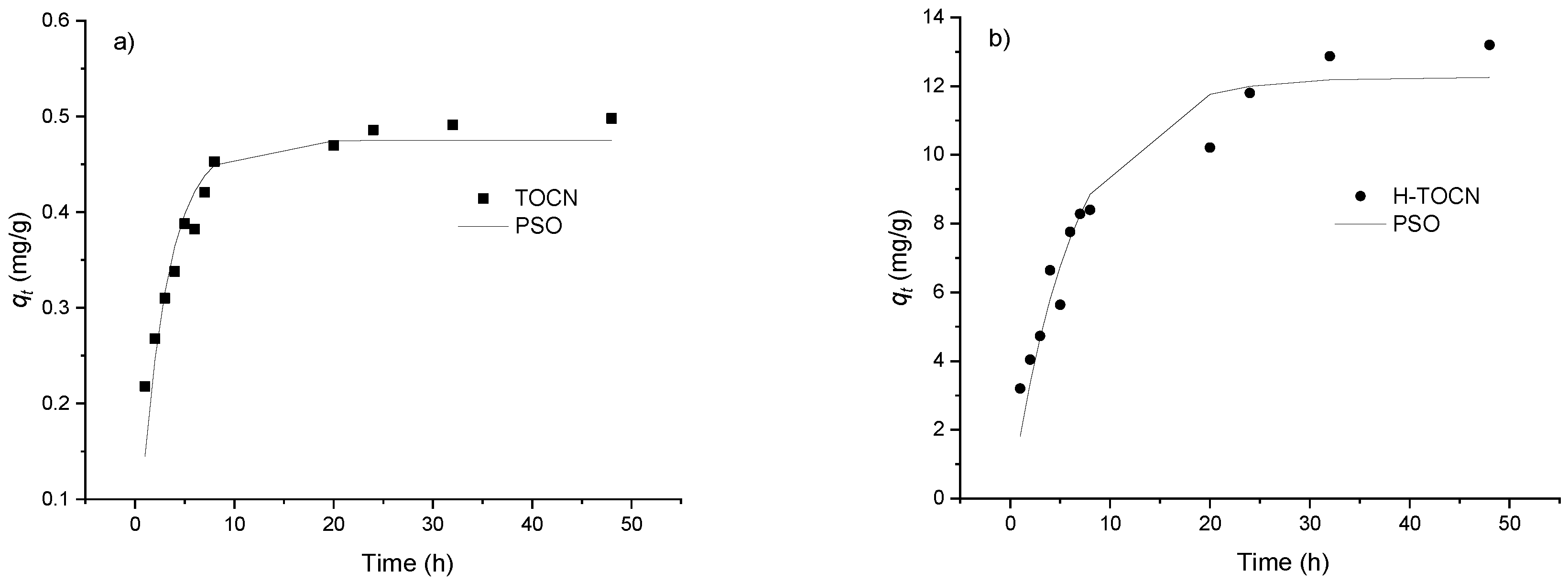
3.2.4. Kinetic Study
3.2.5. Equilibrium Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Natarajan, G.; Tay, S.B.; Yew, W.S.; Ting, Y. Engineered strains enhance gold biorecovery from electronic scrap. Miner. Eng. 2015, 75, 32–37. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef] [PubMed]
- Moats, M.; Wang, S.; Filzwieser, A.; Siegmund, A.; Davenport, W.; Robinson, T. Survey of Copper Electrorefining Operations; Copper, 2016; Available online: http://www.copper2016.jp/program/html/session-06.html (accessed on 6 March 2019).
- Lundström, M.; Ahtiainen, R.; Haakana, T.; O’callaghan, J. Techno-economical observations related to outotec gold chloride process. In Proceedings of the ALTA, Perth, Australia, 24–31 May 2014. [Google Scholar]
- Lampinen, M.; Seisko, S.; Forsström, O.; Laari, A.; Aromaa, J.; Lundström, M.; Koiranen, T. Mechanism and kinetics of gold leaching by cupric chloride. Hydrometallurgy 2017, 169, 103–111. [Google Scholar] [CrossRef]
- Hilson, G.; Monhemius, A.J. Alternatives to cyanide in the gold mining industry: What prospects for the future? J. Clean Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- Crundwell, F.; Moats, M.; Ramachandran, V.; Robinson, T.; Davenport, W.G. Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals; Elsevier: Oxford, UK, 2011; p. 610. [Google Scholar]
- Kurniawan, T.A.; Sillanpää, M.E.T.; Sillanpää, M. Nanoadsorbents for Remediation of Aquatic Environment: Local and Practical Solutions for Global Water Pollution Problems. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1233–1295. [Google Scholar] [CrossRef]
- Dubey, S.P.; Dwivedi, A.D.; Kim, I.; Sillanpää, M.; Kwon, Y.; Lee, C. Synthesis of graphene–carbon sphere hybrid aerogel with silver nanoparticles and its catalytic and adsorption applications. Chem. Eng. J. 2014, 244, 160–167. [Google Scholar] [CrossRef]
- Tasdelen, C.; Aktas, S.; Acma, E.; Guvenilir, Y. Gold recovery from dilute gold solutions using DEAE-cellulose. Hydrometallurgy 2009, 96, 253–257. [Google Scholar] [CrossRef]
- Ogata, T.; Nakano, Y. Mechanisms of gold recovery from aqueous solutions using a novel tannin gel adsorbent synthesized from natural condensed tannin. Water Res. 2005, 39, 4281–4286. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, S.; Repo, E.; Suopajärvi, T.; Liimatainen, H.; Niinimaa, J.; Sillanpää, M. Adsorption of Ni(II), Cu(II) and Cd(II) from aqueous solutions by amino modified nanostructured microfibrillated cellulose. Cellulose 2014, 21, 1471–1487. [Google Scholar] [CrossRef]
- Hokkanen, S.; Repo, E.; Sillanpää, M. Removal of heavy metals from aqueous solutions by succinic anhydride modified mercerized nanocellulose. Chem. Eng. J. 2013, 223, 40–47. [Google Scholar] [CrossRef]
- Gestranius, M.; Stenius, P.; Kontturi, E.; Sjöblom, J.; Tammelin, T. Phase behaviour and droplet size of oil-in-water Pickering emulsions stabilised with plant-derived nanocellulosic materials. Colloids Surf. Physicochem. Eng. Aspects 2017, 519, 60–70. [Google Scholar] [CrossRef]
- Salmon, S.; Hudson, S.M. Crystal Morphology, Biosynthesis, and Physical Assembly of Cellulose, Chitin, and Chitosan. J. Macromol. Sci. Part C 1997, 37, 199–276. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef] [PubMed]
- Isobe, N.; Chen, X.; Kim, U.J.; Kimura, S.; Wada, M.; Saito, T.; Isogai, A. TEMPO-oxidized cellulose hydrogel as a high-capacity and reusable heavy metal ion adsorbent. J. Hazard. Mater. 2013, 260, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Peyre, J.; Paakkonen, T.; Reza, M.; Kontturi, E. Simultaneous preparation of cellulose nanocrystals and micron-sized porous colloidal particles of cellulose by TEMPO-mediated oxidation. Green Chem. 2015, 17, 808–811. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Dubey, S.P.; Hokkanen, S.; Fallah, R.N.; Sillanpää, M. Recovery of gold from aqueous solutions by taurine modified cellulose: An adsorptive–reduction pathway. Chem. Eng. J. 2014, 255, 97–106. [Google Scholar] [CrossRef]
- Saito, T.; Nishiyama, Y.; Putaux, J.; Vignon, M.; Isogai, A. Homogeneous Suspensions of Individualized Microfibrils from TEMPO-Catalyzed Oxidation of Native Cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Borrell, P.F.; Božič, M.; Kokol, V.; Oksman, K.; Mathew, A.P. Nanocelluloses and their phosphorylated derivatives for selective adsorption of Ag+, Cu2+ and Fe3+ from industrial effluents. J. Hazard. Mater. 2015, 294, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Hubbe, M.; Rojas, O.; Lucia, L.; Sain, M. Cellulosic nanocomposites: A Review. BioResources 2008, 3, 929–980. [Google Scholar]
- Leikola, M.; Elomaa, H.; Jafari, S.H.; Rintala, L.; Wilson, B.; Lundström, M. Possibilities and challenges in gold chloride processing. In Proceedings of the Gold-Precious Metal Conference, Vienna, Austria, 18–20 October 2015. [Google Scholar]
- Vlassopoulos, D.; Wood, S.A.; Mucci, A. Gold speciation in natural waters: II. The importance of organic complexing—Experiments with some simple model ligands. Geochim. Cosmochim. Acta 1990, 54, 1575–1586. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Ahola, S.; Salmi, J.; Johansson, L.S.; Laine, J.; Österberg, M. Model Films from Native Cellulose Nanofibrils. Preparation, Swelling, and Surface Interactions. Biomacromolecules 2008, 9, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Nicol, M.J. Electrochemical and kinetic investigation of the behavior of gold in chloride solutions: The gold(III)-gold(I) reaction on platinum and the disproportionation of gold(I). Nati. Inst. for Met. 1981. [Google Scholar]
- Liimatainen, H.; Visanko, M.; Sirviä, J.A.; Hormi, O.E.O.; Niinimaki, J. Enhancement of the Nanofibrillation of Wood Cellulose through Sequential Periodate-Chlorite Oxidation. Biomacromolecules 2012, 13, 1592–1597. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y. Self-assembling of TEMPO Oxidized Cellulose Nanofibrils as Affected by Protonation of Surface Carboxyls and Drying Methods. ACS Sustain. Chem. Eng. 2016, 4, 1041–1049. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Adeva, P.; Alonso, M. Processing of residual gold (III) solutions via ion exchange. Gold Bull. 2005, 38, 9–13. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Yahyaei, B.; Kusiak-Nejman, E.; Sillanpää, M. The influence of carbonization temperature on the modification of TiO2 in the removal of methyl orange from aqueous solution by adsorption. Desalin. Water Treat. 2016, 57, 18825–18835. [Google Scholar] [CrossRef]
- Seki, A.; Ishiwata, F.; Takizawa, Y.; Asami, M. Crossed aldol reaction using cross-linked polymer-bound lithium dialkylamide. Tetrahedron 2004, 60, 5001–5011. [Google Scholar] [CrossRef]
- Jonathan, C.; Nick, G.; Stuart, W. Organic Chemistry, 2nd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Wasikiewicz, J.M.; Nagasawa, N.; Tamada, M.; Mitomo, H.; Yoshii, F. Adsorption of metal ions by carboxymethyl chitin and carboxymethyl chitosan hydrogels. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 236, 617–623. [Google Scholar] [CrossRef]
- Pangeni, B.; Paudyal, H.; Inoue, K.; Kawakita, H.; Ohto, K.; Alam, S. Selective recovery of gold(III) using cotton cellulose treated with concentrated sulfuric acid. Cellulose 2012, 19, 381–391. [Google Scholar] [CrossRef]
- Aktas, S.; Gozuak, B.; Acma, H.; Ozalp, M.R.; Acma, E. Gold recovery from chloride solutions using fallen leaves. Environ. Chem. Lett. 2011, 9, 47–53. [Google Scholar] [CrossRef]

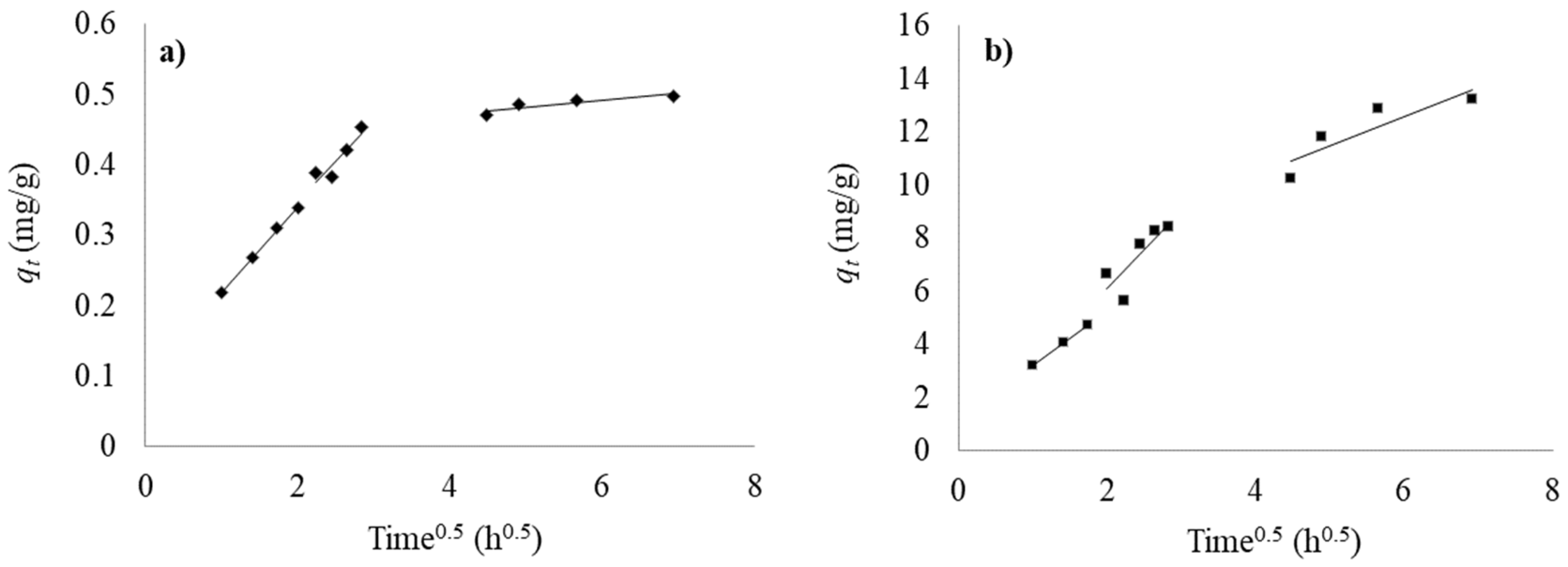

| Pseudo First-Order | Pseudo Second-Order | |||||
|---|---|---|---|---|---|---|
| r2 | k1(1/h) | qe(mg/gads) | r2 | k2(gads/mg.h) | qe(mg/gads) | |
| 0.95 | 0.57 | 0.3 | 0.984 | 0.364 | 0.475 | TOCN |
| 0.966 | 0.18 | 14.5 | 0.981 | 0.16 | 12.262 | H-TOCN |
| TOCN | H-TOCN | ||
|---|---|---|---|
| t (h) | βθ (g/L) | t (h) | βθ (g/L) |
| 4 | 5.80 | 4 | 4.95 |
| 18 | 7.11 | 9 | 6.88 |
| 23 | 7.52 | 19 | 7.40 |
| 33 | 7.90 | 31 | 9.08 |
| 48 | 8.43 | 48 | 9.57 |
| H-TOCN | TOCN | ||
|---|---|---|---|
| 0.135 | 0.005 | KL(L/mg) | Langmuir |
| 15.445 | 0.42 | qm(mg/g) | |
| 0.96 | 0.999 | r2 | |
| 2.03 | 0.002 | KF(Lmg(1-1/n))/g) | Freundlich |
| 1.53 | 0.6 | n | |
| 0.951 | 0.998 | r2 | |
| 0.012 | ---- | KL-F(L/mg) | Langmuir–Freundlich |
| 14.531 | 0.38 | qm(mg/g) | |
| 1.4 | 0.9 | n | |
| 0.953 | 0.786 | r2 | |
| Adsorbent | Adsorption Capacity (mg/g) | Contact Time (h) | Reference |
|---|---|---|---|
| Taurine-modified cellulose | 34.50 | 24 | [22] |
| Carboxymethyl chitin | 11.86 | 9 | [39] |
| Cotton cellulose | *1.22 | 96 | [40] |
| Fallen leaves | not reported | [41] | |
| H-TOCN | 15.44 (mg/g) | 48 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafari, S.; Wilson, B.P.; Hakalahti, M.; Tammelin, T.; Kontturi, E.; Lundström, M.; Sillanpää, M. Recovery of Gold from Chloride Solution by TEMPO-Oxidized Cellulose Nanofiber Adsorbent. Sustainability 2019, 11, 1406. https://doi.org/10.3390/su11051406
Jafari S, Wilson BP, Hakalahti M, Tammelin T, Kontturi E, Lundström M, Sillanpää M. Recovery of Gold from Chloride Solution by TEMPO-Oxidized Cellulose Nanofiber Adsorbent. Sustainability. 2019; 11(5):1406. https://doi.org/10.3390/su11051406
Chicago/Turabian StyleJafari, Shila, Benjamin P. Wilson, Minna Hakalahti, Tekla Tammelin, Eero Kontturi, Mari Lundström, and Mika Sillanpää. 2019. "Recovery of Gold from Chloride Solution by TEMPO-Oxidized Cellulose Nanofiber Adsorbent" Sustainability 11, no. 5: 1406. https://doi.org/10.3390/su11051406
APA StyleJafari, S., Wilson, B. P., Hakalahti, M., Tammelin, T., Kontturi, E., Lundström, M., & Sillanpää, M. (2019). Recovery of Gold from Chloride Solution by TEMPO-Oxidized Cellulose Nanofiber Adsorbent. Sustainability, 11(5), 1406. https://doi.org/10.3390/su11051406






