Mine Tailings Geopolymers as a Waste Management Solution for A More Sustainable Habitat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Formulations
2.2. Characterization Methods
3. Results and Discussion
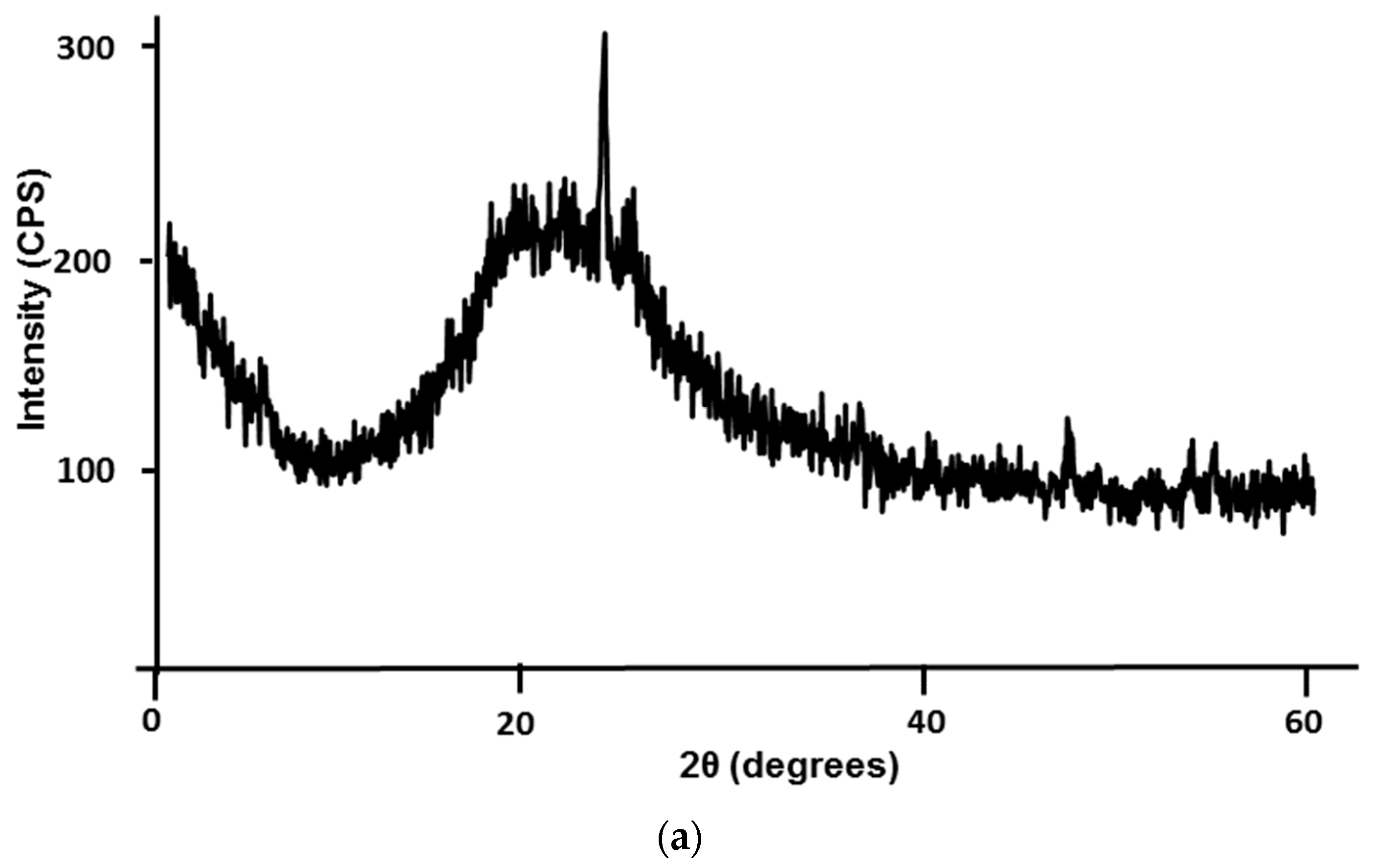
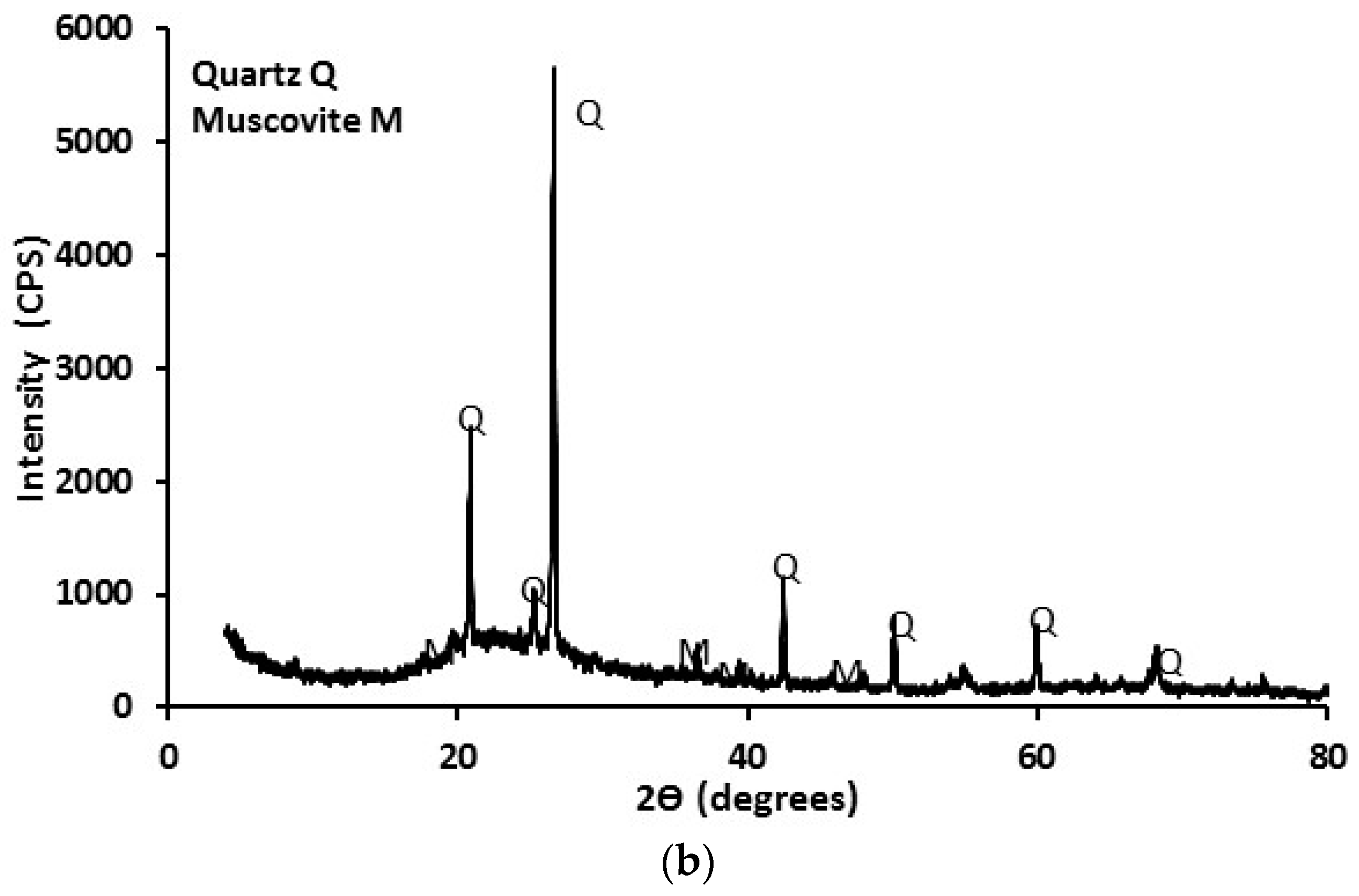
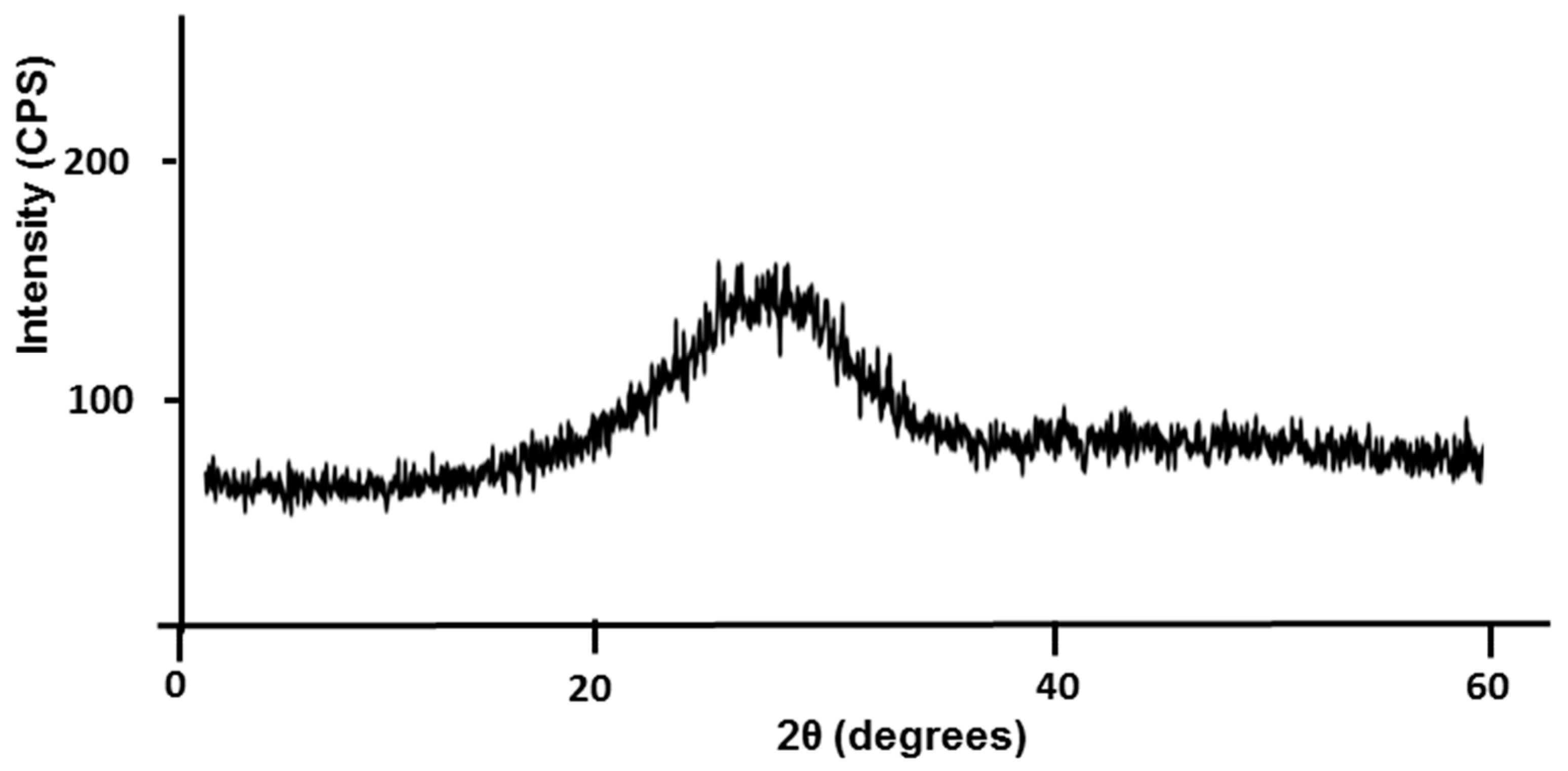
3.1. Mine Tailing Characterization
3.2. Metakaolin and Mine Tailing-Based Geopolymers
3.2.1. Metakaolin Characterization
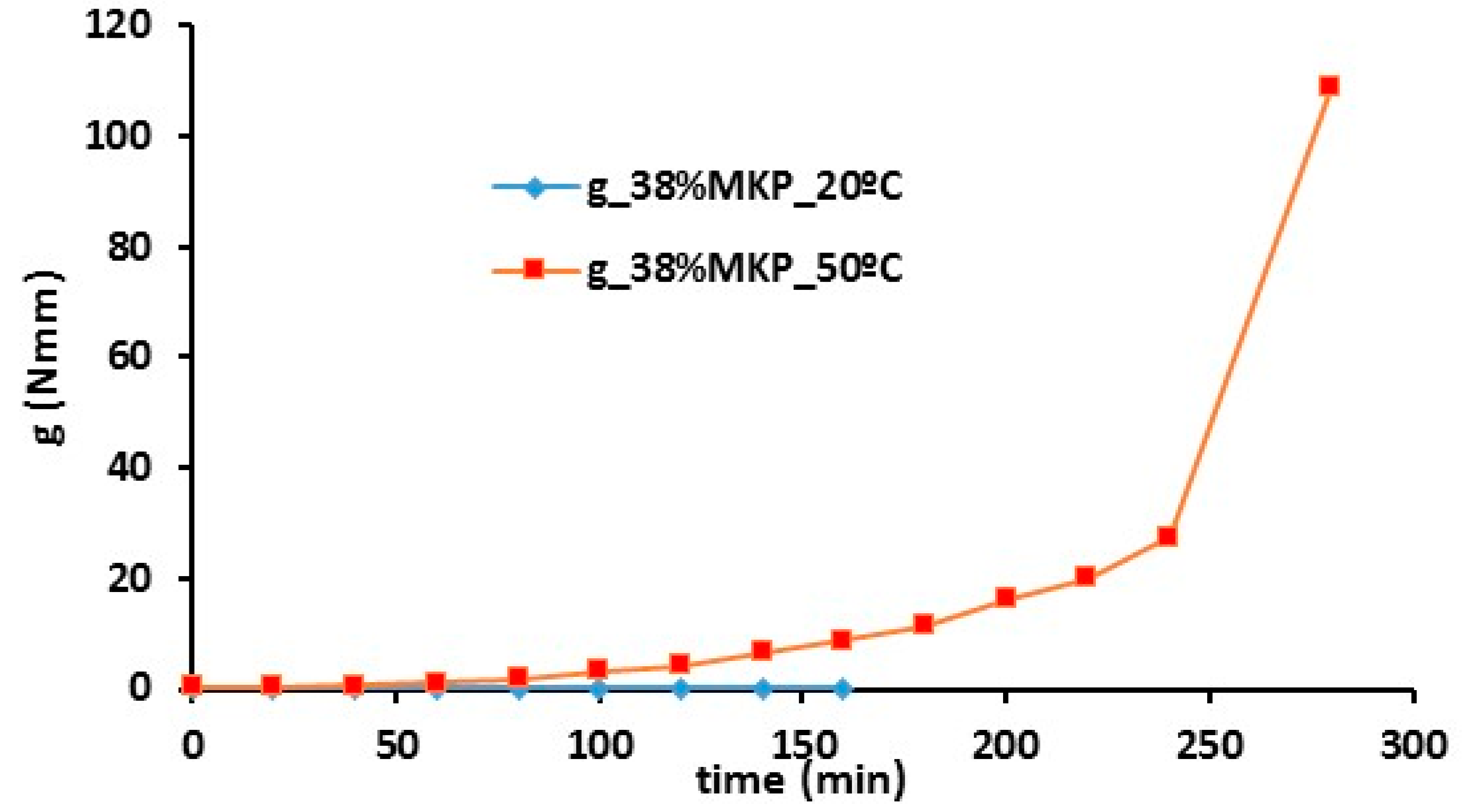
3.2.2. Metakaolin and Mine Tailing-Based Geopolymer Behaviour
3.3. Blast Furnace Slag and Mine Tailing Based Geopolymers
3.3.1. Blast Furnace Slag Characterization
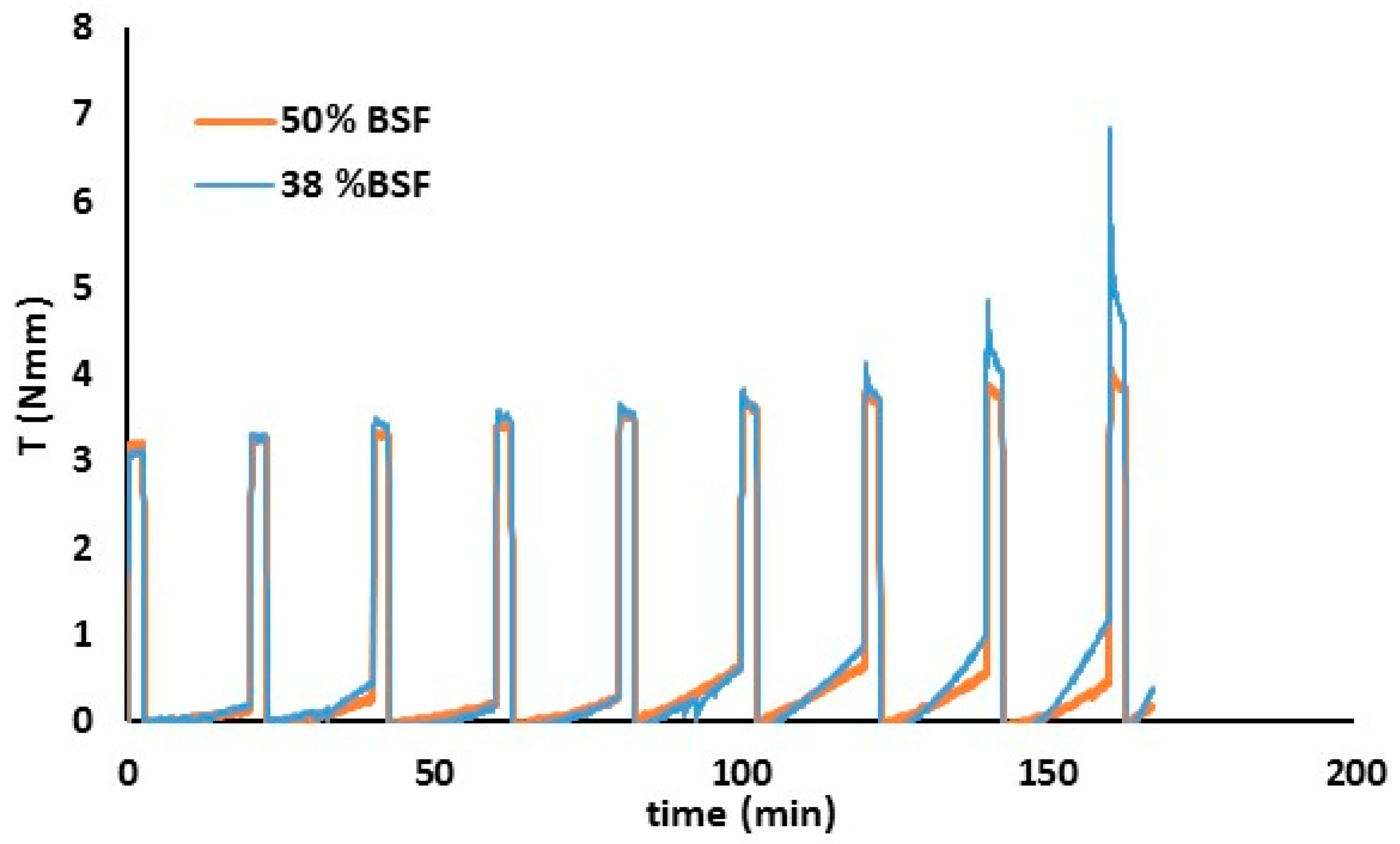
3.3.2. Blast Furnace Slag and Mine Tailing Based Geopolymer Behaviour
3.4. Geopolymer Chemical Durability
4. Conclusions
- -
- This particular mine tailing does not act as a geopolymer precursor (binder) because it is not reactive due to its composition; Nevertheless, it can be incorporated as a fine aggregate;
- -
- Metakaolin plus mine tailing geopolymers generates quite good compressive strength products (>20MPa) showing a faster reactive nature than the blast furnace slag plus mine tailing formulations.
- -
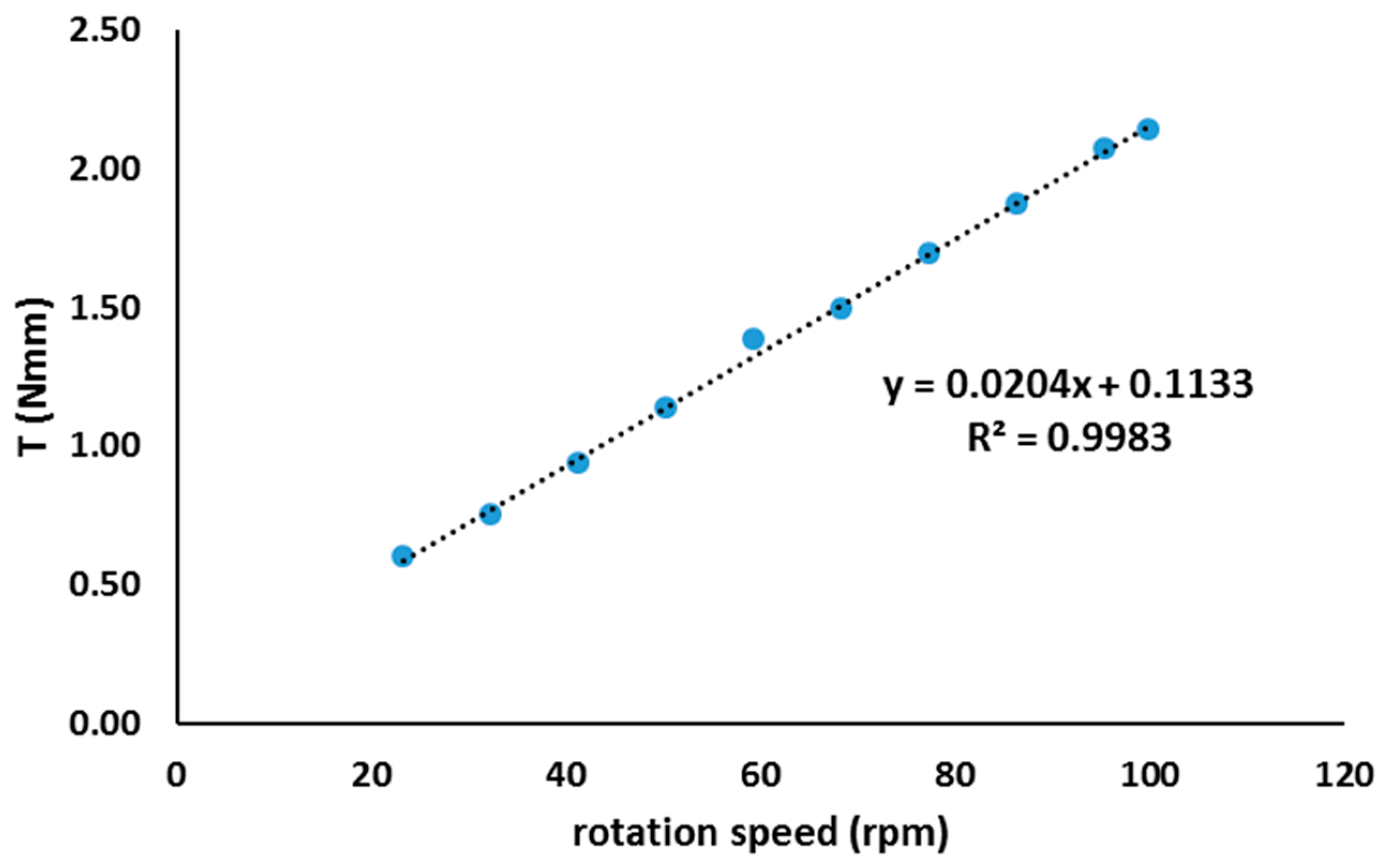
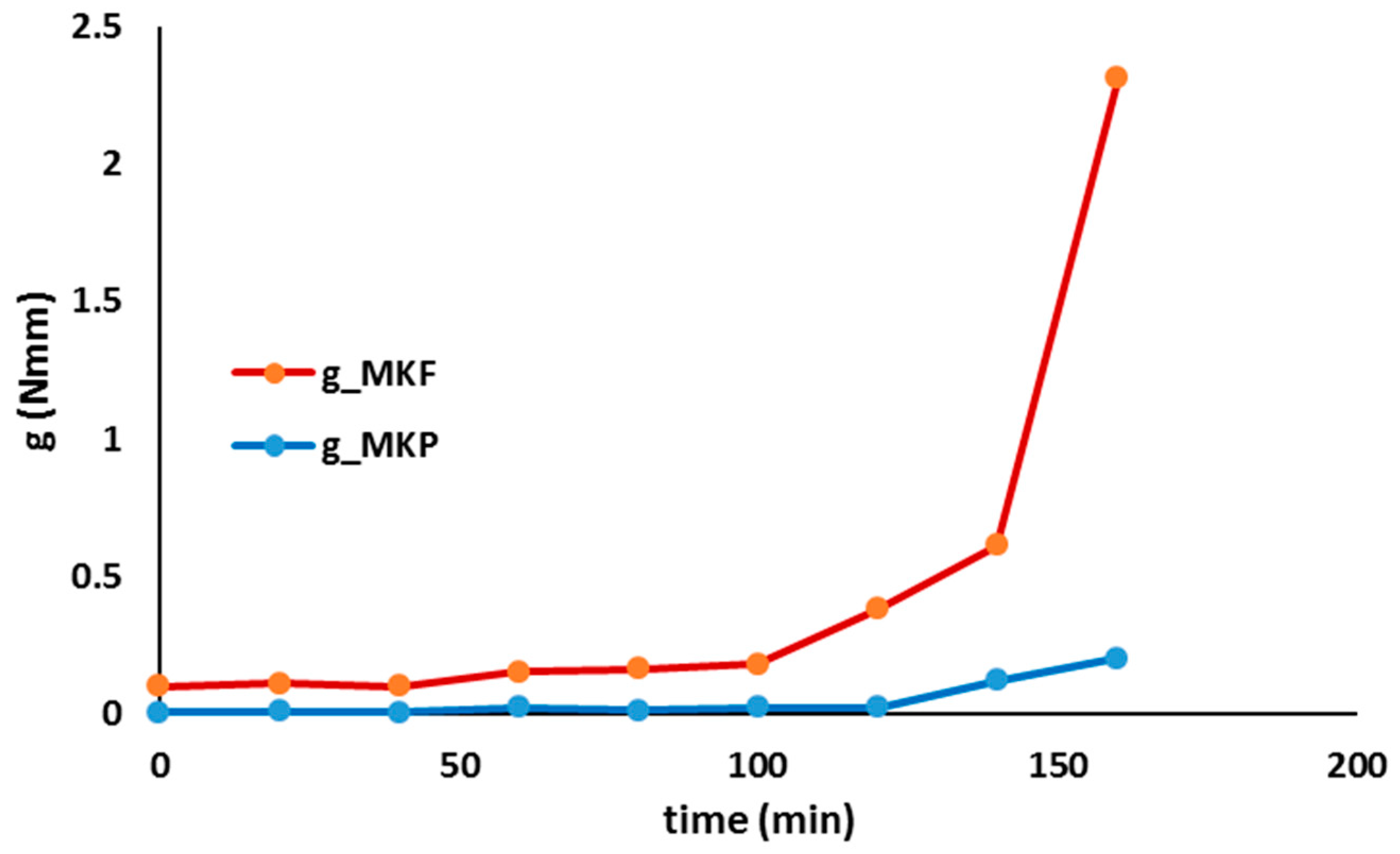
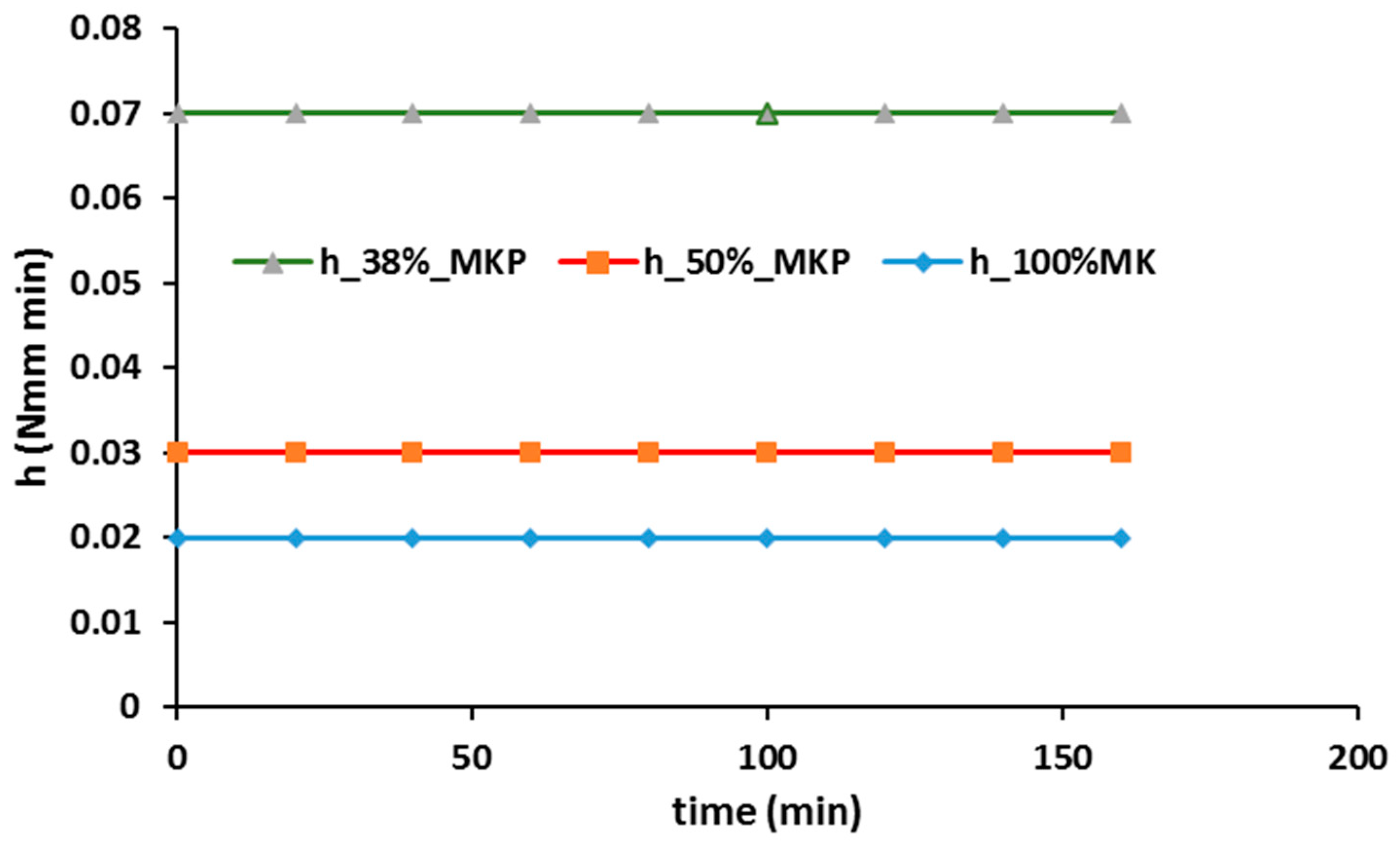
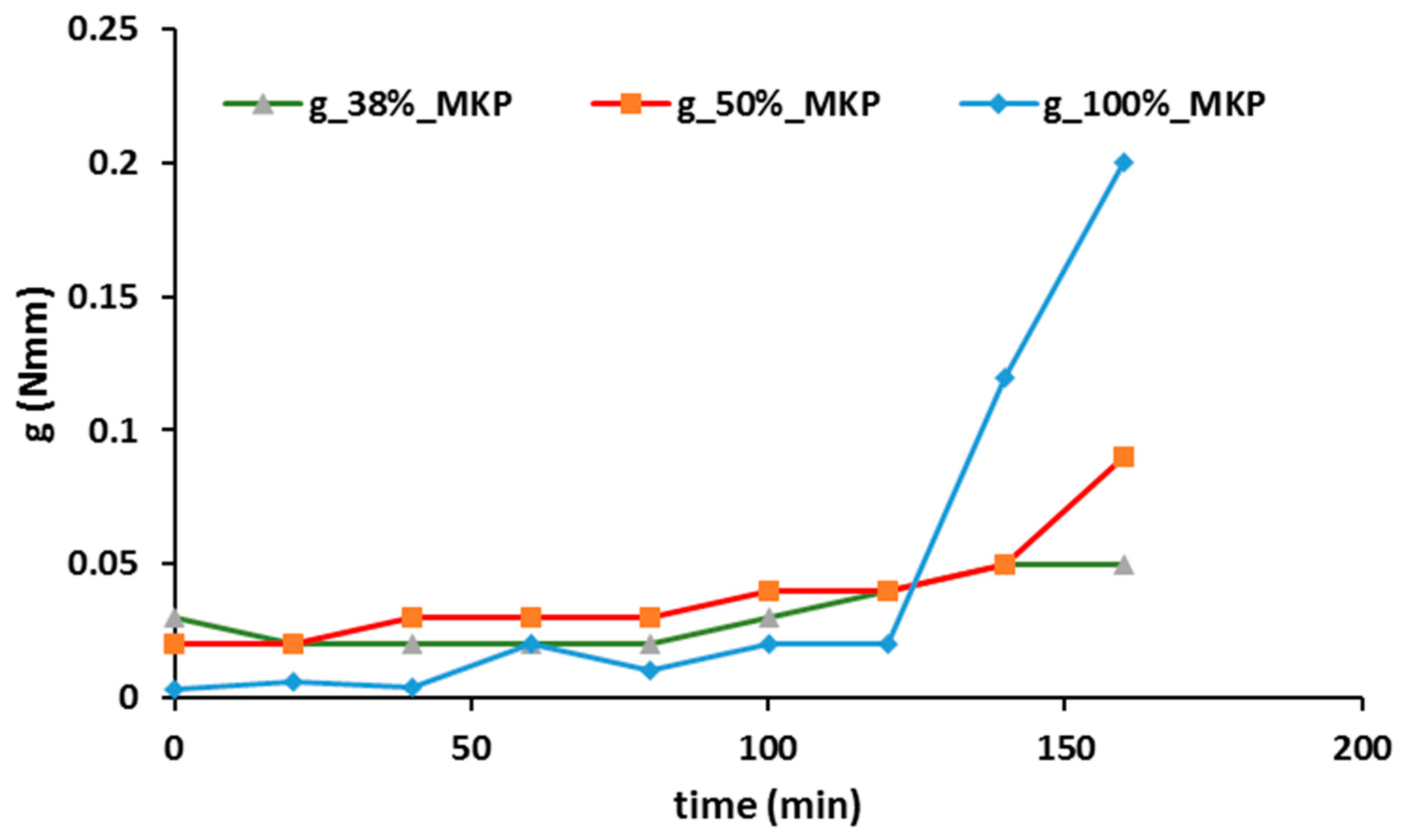
- Rheology proved to be an interesting approach to follow up the geopolymer process and even to control the proper curing conditions and components amount, when optimizing final properties such as mechanical strength:
- -
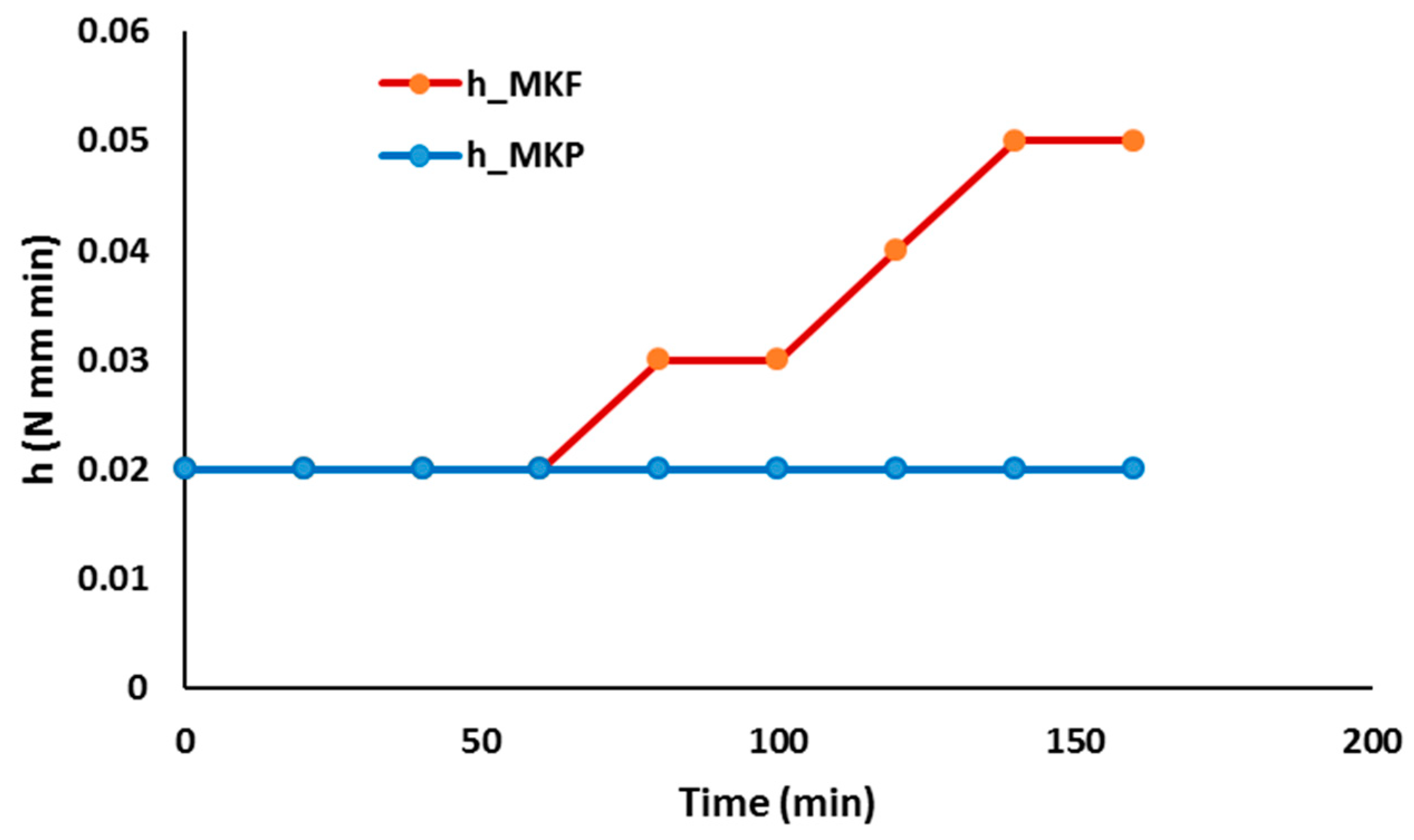
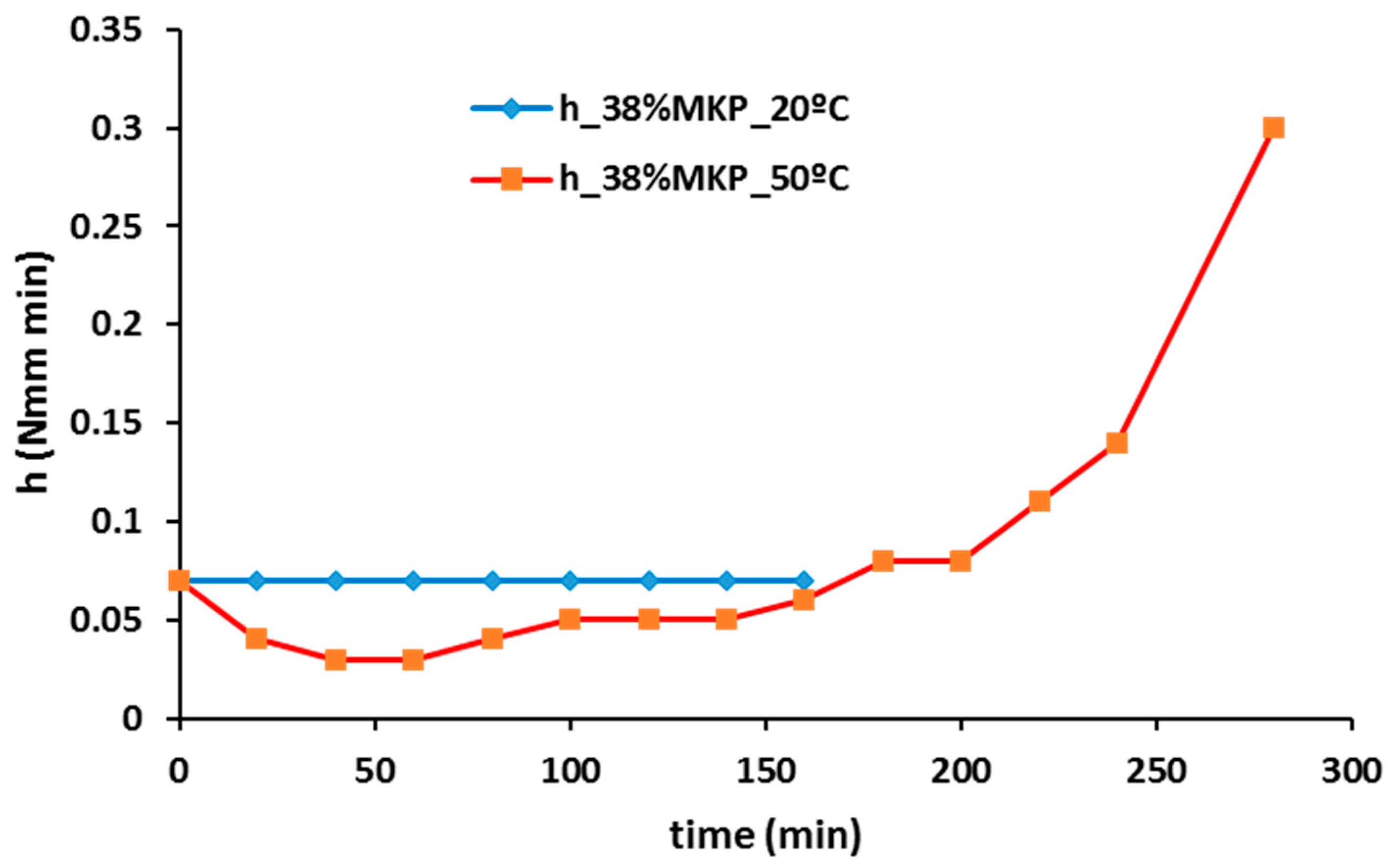
- The use of curing temperature to accelerate the geopolymer reaction is only effective, for a given temperature, until a certain time limit that depends on each precursor (metakaolin or blast furnace slag) and each composition.
- -
- Regarding chemical resistance, curing time is a very important factor in these formulations with small amount of binder (MK and BFS). These compositions, with high MT content, tested in very severe conditions (pH 4 and 7 during 40 days) show a significant chemical strength. In this way and, under normal weather condition (rain, water infiltrations), these compositions can be used in mines refilling.
- -
- Finally, one can say this waste-based geopolymer solution could be an interesting waste management solution, contributing for the sustainability of our habitat. Indeed, the working period of a mine is directly conditioned by the landfill capacity. Apart from an environmental contribution by preventing more landfill deposition, this waste valorization solution is also impacting in the economic and social aspects of mining activity which has a great local significance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Charbonnier, P. Management of Mining, Quarrying and Ore-Processing Waste in the European Union; BRGM Study made for DG Environment, European Commission: Orléans, France, 2001. [Google Scholar]
- Benhelal, E.; Zahedi, G.; Haslenda, H. A novel design for green and economical cement manufacturing. J. Clean. Prod. 2012, 22, 60–66. [Google Scholar] [CrossRef]
- Duxson, P.; Fernandez-Jimenez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: the current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Dyer, A. An Introduction to Zeolite Molecular Sieves; John Wiley & Sons: New York, NY, USA, 1988. [Google Scholar]
- Provis, J.L.; van Deventer, J.S.J. Alkali Activated Materials; Springer: London, UK, 2014. [Google Scholar]
- Rao, F.; Liu, Q. Geopolymerization and its potential application in mine tailings consolidation: A Review. Miner. Process. Extr. Metall. Rev. 2015, 36, 399–409. [Google Scholar] [CrossRef]
- Andrejkovičová, S.; Sudagar, A.; Rocha, J.; Patinha, C.; Hajjaji, W.; Ferreira da Silva, E.; Velosa, A.; Rocha, F. The effect of natural zeolite on microstructure, mechanical and heavy metals adsorption properties of metakaolin based geopolymers. Appl. Clay Sci. 2016, 126, 141–152. [Google Scholar] [CrossRef]
- Gomez-Zamorano, L.Y.; Vega-Cordero, E.; Struble, L. Composite geopolymers of metakaolin and geothermal nanosilica waste. Constr. Build. Mater. 2016, 115, 269–276. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Seabra, M.P.; Labrincha, J.A. Waste glass from end-of-life fluorescent lamps as raw material in geopolymers. Waste Manag. 2016, 52, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Purdon, A.O. The action of alkalis on blast-furnace slag. J. Soc. Chem. Ind. Trans. Commun. 1940, 59, 191–202. [Google Scholar]
- Provis, J.L.; Duxson, P.; van Deventer, J.S.J. The role of particle technology in developing sustainable construction materials. Adv. Power Technol. 2010, 21, 2–7. [Google Scholar] [CrossRef]
- Kürklü, G. The effect of high temperature on the design of blast furnace slag and coarse fly ash-based geopolymer mortar. Compos. Part B 2016, 92, 9–18. [Google Scholar] [CrossRef]
- Borges, P.H.R.; Banthia, N.; Himad, A.; Wander, A.; Vasconcelos, L.; Nunes, E.H.M. Performance of blended metakaolin/blast furnace slag alkali-activated mortars. Cem. Concr. Compos. 2016, 71, 42–52. [Google Scholar] [CrossRef]
- Hiemenz, P.C. Principles of Colloid and Surface Chemistry; Marcel Dekker Inc.: New York, NY, USA, 1977. [Google Scholar]
- Banfill, P.F.G. The rheology of cement and concrete—A review. In Proceedings of the 11th International Congress on the Chemistry of Cement, Durban, South Africa, 11–16 May 2003. [Google Scholar]
- Paiva, H.; Velosa, A.; Veiga, R.; Ferreira, V.M. Effect of maturation time on the fresh and hardened properties of an air lime mortar. Cem. Concr. Res. 2010, 40, 447–451. [Google Scholar] [CrossRef]
- Paiva, H.; Silva, L.M.; Labrincha, J.A.; Ferreira, V.M. Effects of a water-retaining agent on the rheological behaviour of a single-coat render mortar. Cem. Concr. Res. 2006, 36, 1257–1262. [Google Scholar] [CrossRef]
- Luukkonen, T.; Runtti, H.; Niskanen, M.; Tolonen, E.T.; Sarkkinen, M.; Kemppainen, K.; Ramo, J.; Lassi, U. Simultaneous removal of Ni(II), As(III), and Sb(III) from spiked mine effluent with metakaolin and blast-furnace-slag geopolymers. J. Environ. Manag. 2016, 166, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Duxsona, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven b, W.M.; van Deventer, J.S.J. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Coll. Surf. A: Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Banfill, P.F.G. Rheological methods for assessing the flow properties of mortar and related materials. Constr. Build. Mater. 1994, 8, 43–50. [Google Scholar] [CrossRef]
- Cheng, T.W.; Lee, M.L.; Ko, M.S.; Ueng, T.H.; Yang, S.F. The heavy metal adsorption characteristics on metakaolin-based geopolymer. Appl. Clay Sci. 2012, 56, 90–96. [Google Scholar] [CrossRef]
- Lopez, F.J.; Sugita, S.; Kobayashi, T. Cesium-adsorbent geopolymer foams based on silica from rice husk and metakaolin. Chem. Lett. 2014, 43, 128–130. [Google Scholar] [CrossRef]
- Lopez, F.J.; Sugita, S.; Tagaya, M.; Kobayashi, T. Metakaolin-based geopolymers for targeted adsorbents to heavy metal ion separation. J. Mater. Sci. Chem. Eng. 2014, 2, 16–27. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Durability and leaching behavior of mine tailings-based geopolymer bricks. Con. Build. Mat. 2013, 44, 743–750. [Google Scholar] [CrossRef]







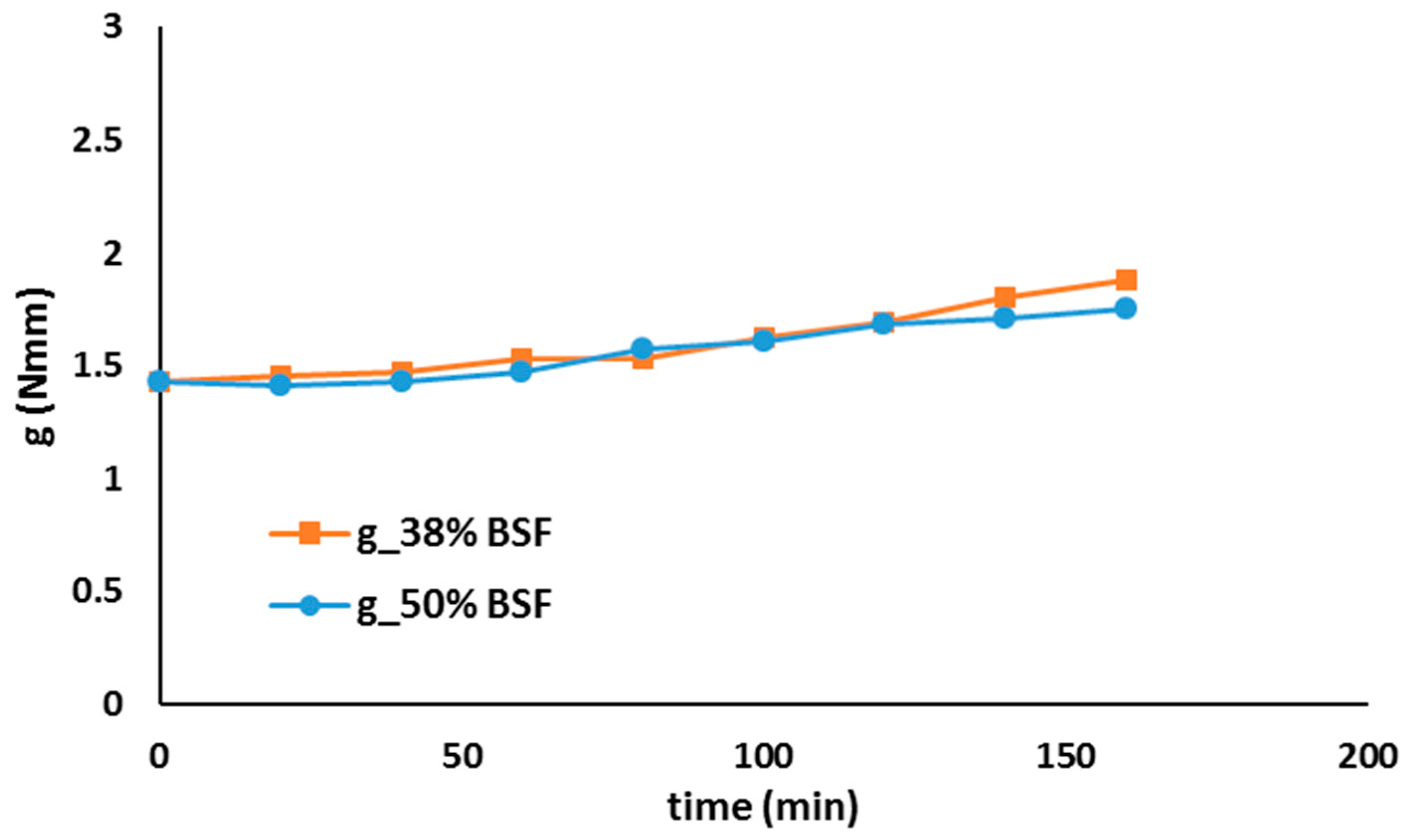
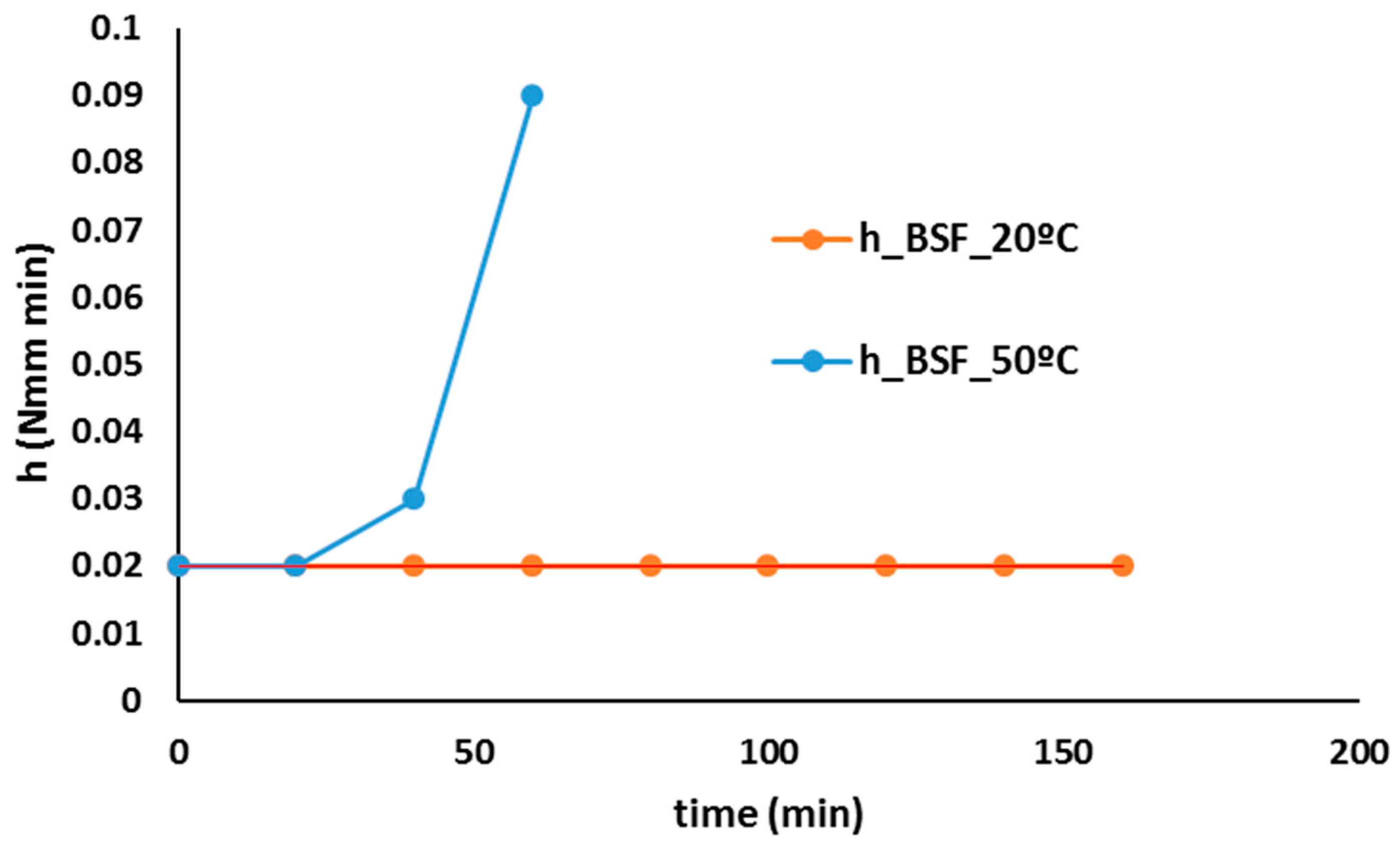
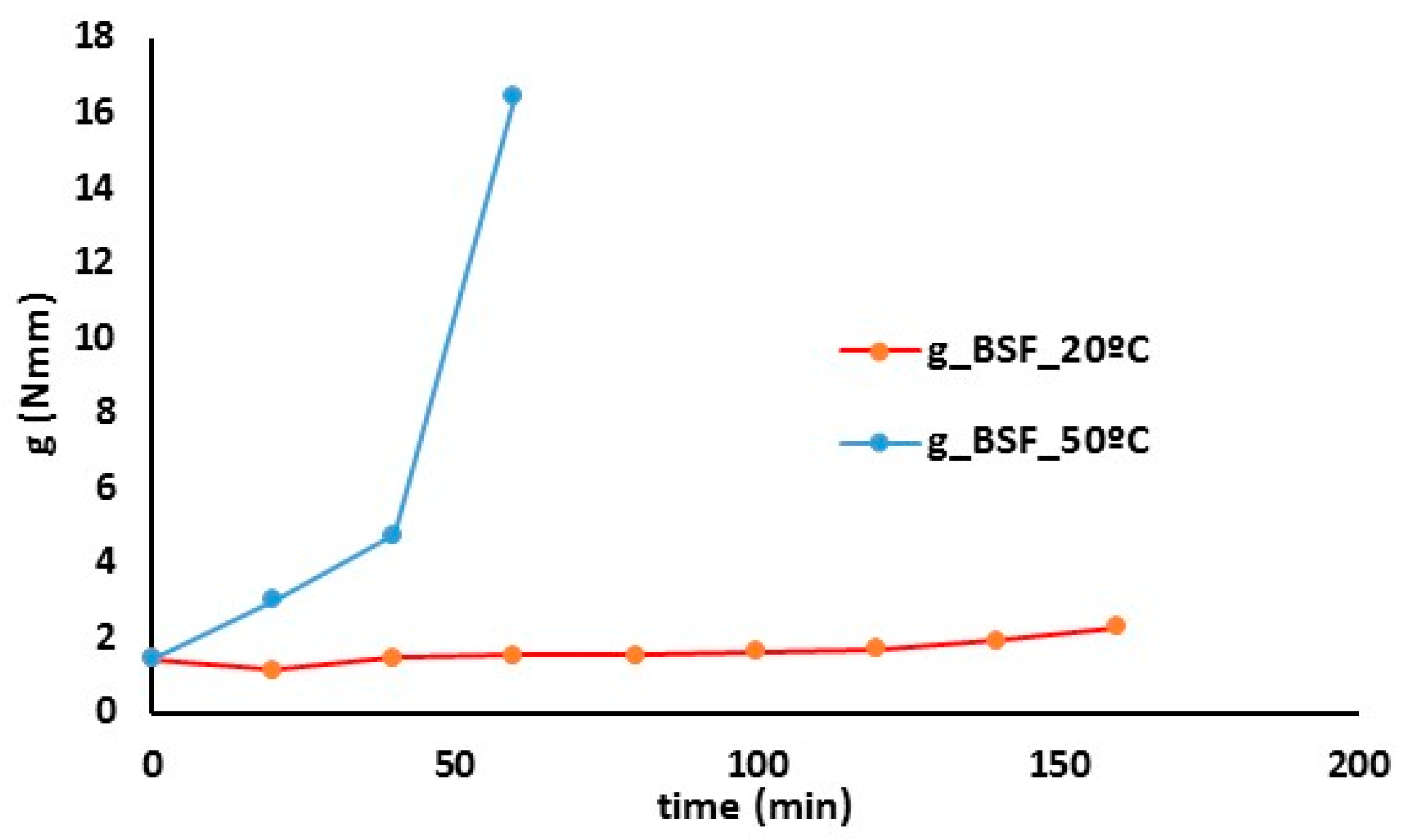
| Na2O (%) | MgO (%) | Al3O2 (%) | SiO2 (%) | P2O5 (%) | SO3 (%) | Cl (%) | K2O (%) | CaO (%) |
| 0.229 | 1.689 | 6.409 | 25.157 | 0.036 | 26.177 | 0.041 | 0.405 | 0.837 |
| TiO2 (%) | Cr (%) | MnO (%) | Fe2O3 (%) | Cu (%) | Zn (%) | Rb (%) | Sr (%) | Zr (%) |
| 0.094 | 0.015 | 0.068 | 19.237 | 0.192 | 0.310 | 0.001 | minor | 0.003 |
| Sb (%) | Ba (%) | Pb (%) | As (%) | Ce (%) | Co (%) | Sn (%) | V (%) | LOI* (%) |
| 0.003 | 0.008 | 0.140 | 0.196 | minor | 0.037 | 0.022 | minor | 18.680 |
| Sc (ppm) | V (ppm) | Cr (ppm) | Co (ppm) | Ni (ppm) | Cu (ppm) | Zn (ppm) | Ga (ppm) | Ge (ppm) |
| 4.3 | 50.9 | major | major | 18.1 | major | major | 19.4 | ND* |
| As (ppm) | Se (ppm) | Br (ppm) | Rb (ppm) | Sr (ppm) | Y (ppm) | Nb (ppm) | Mo (ppm) | Ag (ppm) |
| major | 56.8 | 11.1 | 24.2 | 21.4 | 18.8 | 4.4 | 5.8 | 21.3 |
| Cd (ppm) | Sn (ppm) | Sb (ppm) | Te (ppm) | I (ppm) | Cs (ppm) | La (ppm) | Ce (ppm) | Nd (ppm) |
| 19.2 | major | major | ND* | 27.1 | ND* | 17.8 | 44.6 | 16.6 |
| Sm (ppm) | Yb (ppm) | Hf (ppm) | Ta (ppm) | W (ppm) | Ti (ppm) | Pb (ppm) | Bi (ppm) | Th (ppm) |
| ND* | ND* | ND* | ND* | ND* | 7.9 | major | 51.4 | 3.7 |
| (%) | SiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MKP | 52.17 | 44.50 | 0.45 | ND* | ND* | 0.01 | ND* | 0.15 | 1.42 | 0.12 | 1.42 |
| MKF | 52.50 | 43.50 | 1.10 | ND* | ND* | 0.20 | 0.30 | 0.20 | 1.80 | 0.10 | 1.30 |
| Specific Surface Area (m2/g) | Apparent Density (g/cm3) | |
|---|---|---|
| MKP | 16.19 | 0.25 |
| MKF | 18.13 | 0.20 |
| Sample | Composition | Temperature (°C) | Time in Mould |
|---|---|---|---|
| G_MKP | 300 g MKP 580 g AD 80 g NaOH (10 M) | 20 °C | 7 days |
| G_MKF | 300 g MKF 580 g AD 80 g NaOH (10 M) | 20 °C | 7 days |
| G_50% MKP | 300 g MKP 300 g MT 580 g AD 80 g NaOH (10 M) | 20 °C | 7 days |
| G_38%MKP | 300g MKP 500g MT 580g AD 80g NaOH (10 M) | 20 °C 50 °C 50 °C (Sealed in plastic film) | 7 days 24 h 24 h |
| Composition | Compressive Strength (MPa) | Bulk Density (g/cm3) |
|---|---|---|
| MKP | 32 ± 0.02 | 1.67 ± 0.05 |
| MKF | 45 ± 0.02 | 1.95 ± 0.04 |
| Composition | Compressive Strength (MPa) | Bulk Density (g/cm3) |
|---|---|---|
| 100% MKP + 0%MT | 32 ± 002 | 1.70 ± 0.10 |
| 50% MKP + 50%MT | 22 ± 0.02 | 2.16 ± 0.02 |
| 38% MKP + 62%MT | 14 ± 0.03 | 1.94 ± 0.04 |
| Samples | Compressive Strength (MPa) | Bulk Density (g/m3) |
|---|---|---|
| 38%MKP_20 °C | 14 ± 0.06 | 1.9 ± 0.04 |
| 38%MKP_50 °C_24 h | 4 ± 0.04 | 1.8 ± 0.05 |
| 38%MKF_20 °C | 22 ± 0.02 | 1.9 ± 0.01 |
| 38%MKF_50 °C_24 h | 8 ± 0.01 | 1.6 ± 0.02 |
| Samples | Compressive Strength (MPa) | Bulk Density (g/m3) |
|---|---|---|
| 38%MKP_20 °C | 14 ± 0.06 | 1.9 ± 0.04 |
| 38%MKP_50 °C _24 h | 4 ± 0.04 | 1.8 ± 0.05 |
| 38%MKP_50 °C _4 h | 23 ± 0.02 | 2.0 ± 0.03 |
| 38%MKP_50 °C _24 h sealed | 22 ± 0.02 | 2.1 ± 0.01 |
| (%) | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BFS | 32.3 | 9.6 | 1.2 | 4.0 | 10.2 | 38.5 | 0.5 | 0.5 | 2.2 | 0.0 | 0.0 |
| Sample | Composition | Temperature (°C) | Time in Mould |
|---|---|---|---|
| G_50% BFS | 300 g BFS 300 g MT 198 g NaOH (10 M) 200 g H2O | 20 °C | 24 h |
| G_38% BFS | 300 g BFS 500 g MT 198 g NaOH (10 M) 100 g H2O | 20 °C 50 °C (for 40, 60 or, 90 min) | 24 h 24 h |
| Composition | Compressive Strength (MPa) | Bulk Density (g/cm3) |
|---|---|---|
| 38% BFS + 62% MT | 14.5 ± 0.02 | 1.9 ± 0.01 |
| 50% BSF + 50% MT | 15.4 ± 0.01 | 2.1 ± 0.01 |
| Samples | Compressive Strength (MPa) | Bulk Density (g/m3) |
|---|---|---|
| 38%BFS_20 °C | 15.4 ± 0.01 | 1.9 ± 0.01 |
| 38%BFS_50 °C _40 min | 18.0 ± 0.01 | 2.2 ± 0.02 |
| 38%BFS_50 °C _60 min | 10.0 ± 0.01 | 1.8 ± 0.01 |
| 38%BFS_50 °C _90 min | 9.4 ± 0.02 | 1.8 ± 0.01 |
| pH | Compositions | Mass Loss (%) |
|---|---|---|
| 4 | 50% MKP | 7.2 |
| 50% BFS | 9.4 | |
| 38% MKP | 9.0 | |
| 7 | 50% MKP | 4.2 |
| 50% BFS | 5.6 | |
| 38% MKP | 5.3 |
| Cr | Cu | Ni | Zn | V | As | Sb | Mn | Be | Co | Hg | Pb | SO3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | |
| MTP power | 15 | 192 | 18.1 | 0.310 | 50.9 | 200 | 3 | 10 | ND | 30 | 100 | 26180 | |
| pH 4 | |||||||||||||
| 50% MKP | 0.05 | 2.00 | 0.07 | 6.49 | 0.07 | 110 | 10.98 | 3.83 | <0.001 | 0.02 | 1.74 | 5819 | |
| 50% BFS | 1.71 | 4.72 | 0.2 | 22.21 | 0.24 | 200 | 21.12 | 33.6 | 0.01 | 0.28 | 9.98 | 7793 | |
| 38% MKP | 0.05 | 4.48 | 0.07 | 7.4 | 0.07 | 175 | 31.25 | 0.58 | <0.001 | 0.05 | 2.88 | 5267 | |
| pH 7 | |||||||||||||
| 50%MKP | 0.01 | 1.97 | 0.003 | <0.20 | <0.01 | 84 | 0.68 | 0.04 | <0.001 | <0.001 | <0.05 | 3679 | |
| 50% BFS | 0.15 | 3.15 | 0.14 | 18.89 | 0.1 | 93 | 1 | 0.30 | 0.21 | 2.77 | 11343 | ||
| 38% MKP | 0.01 | 2.17 | 0.03 | 6.48 | 0.030 | 93 | 0.97 | 0.19 | 0.02 | 2.44 | 7303 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, H.; Yliniemi, J.; Illikainen, M.; Rocha, F.; Ferreira, V.M. Mine Tailings Geopolymers as a Waste Management Solution for A More Sustainable Habitat. Sustainability 2019, 11, 995. https://doi.org/10.3390/su11040995
Paiva H, Yliniemi J, Illikainen M, Rocha F, Ferreira VM. Mine Tailings Geopolymers as a Waste Management Solution for A More Sustainable Habitat. Sustainability. 2019; 11(4):995. https://doi.org/10.3390/su11040995
Chicago/Turabian StylePaiva, Helena, Juho Yliniemi, Mirja Illikainen, Fernando Rocha, and Victor M. Ferreira. 2019. "Mine Tailings Geopolymers as a Waste Management Solution for A More Sustainable Habitat" Sustainability 11, no. 4: 995. https://doi.org/10.3390/su11040995
APA StylePaiva, H., Yliniemi, J., Illikainen, M., Rocha, F., & Ferreira, V. M. (2019). Mine Tailings Geopolymers as a Waste Management Solution for A More Sustainable Habitat. Sustainability, 11(4), 995. https://doi.org/10.3390/su11040995








