Evaluation of Matricaria aurea Extracts as Effective Anti-Corrosive Agent for Mild Steel in 1.0 M HCl and Isolation of Their Active Ingredients
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.1.1. Collection and Identification of Plant Material
2.1.2. Test Specimen Preparation
2.2. Methods
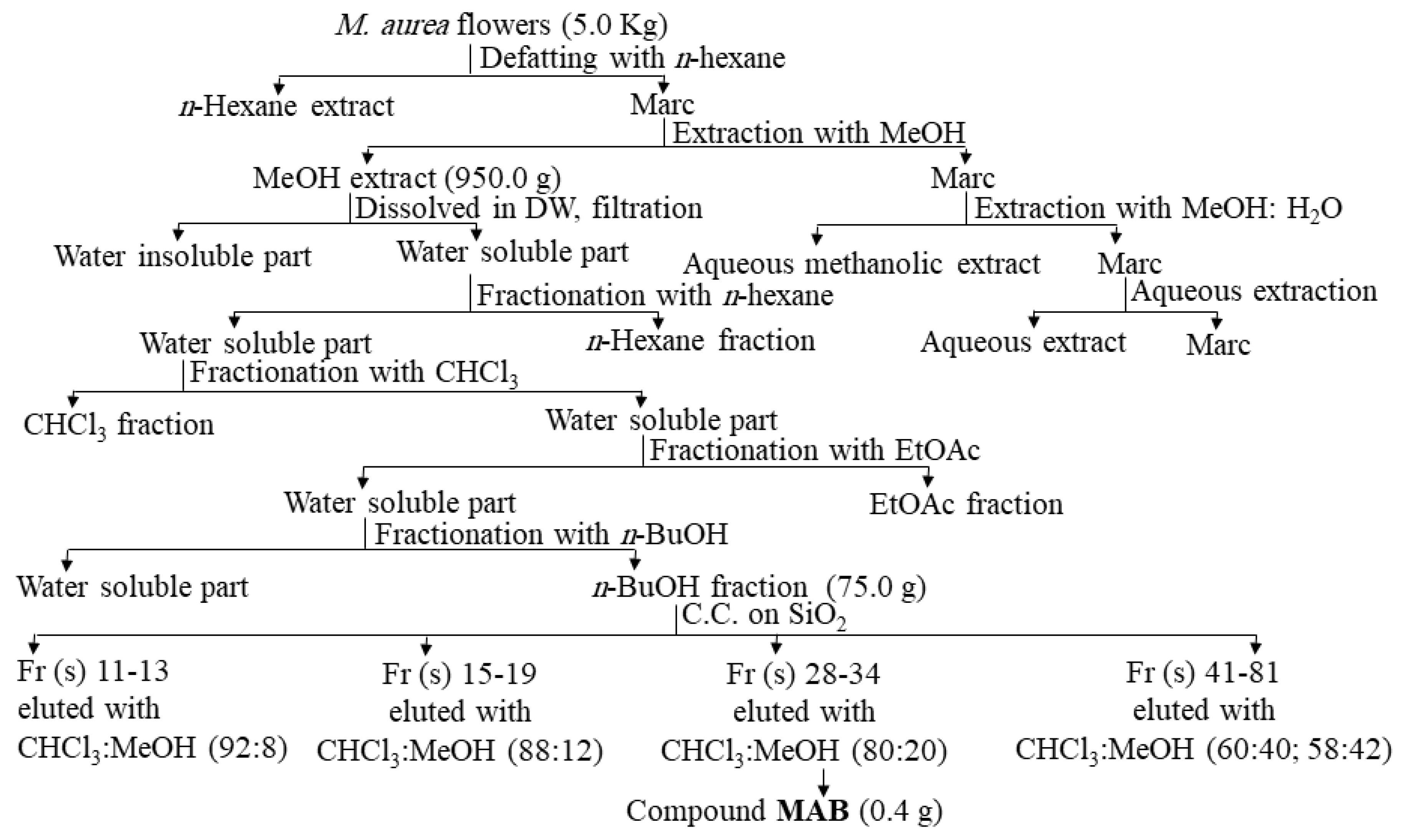
2.2.1. Purification of the Active Phytomolecule
2.2.2. 1D and 2D Nuclear Magnetic Resonance (NMR) Analysis
2.2.3. UV-Visible Spectroscopy
2.2.4. Mass Spectrometry (MS) Analysis
2.2.5. Chemical Structure Identification of MAB
2.2.6. Weight Loss Experiments
2.2.7. Electrochemical Studies
2.2.8. SEM and EDS Measurements
3. Results and Discussion
3.1. Screening of M. aurea Extracts for Their Corrosion Inhibitive Properties
3.2. Identification of the Compound MAB Isolated from M. aurea
3.3. Detailed Study of Corrosion Inhibitive Properties of MAB
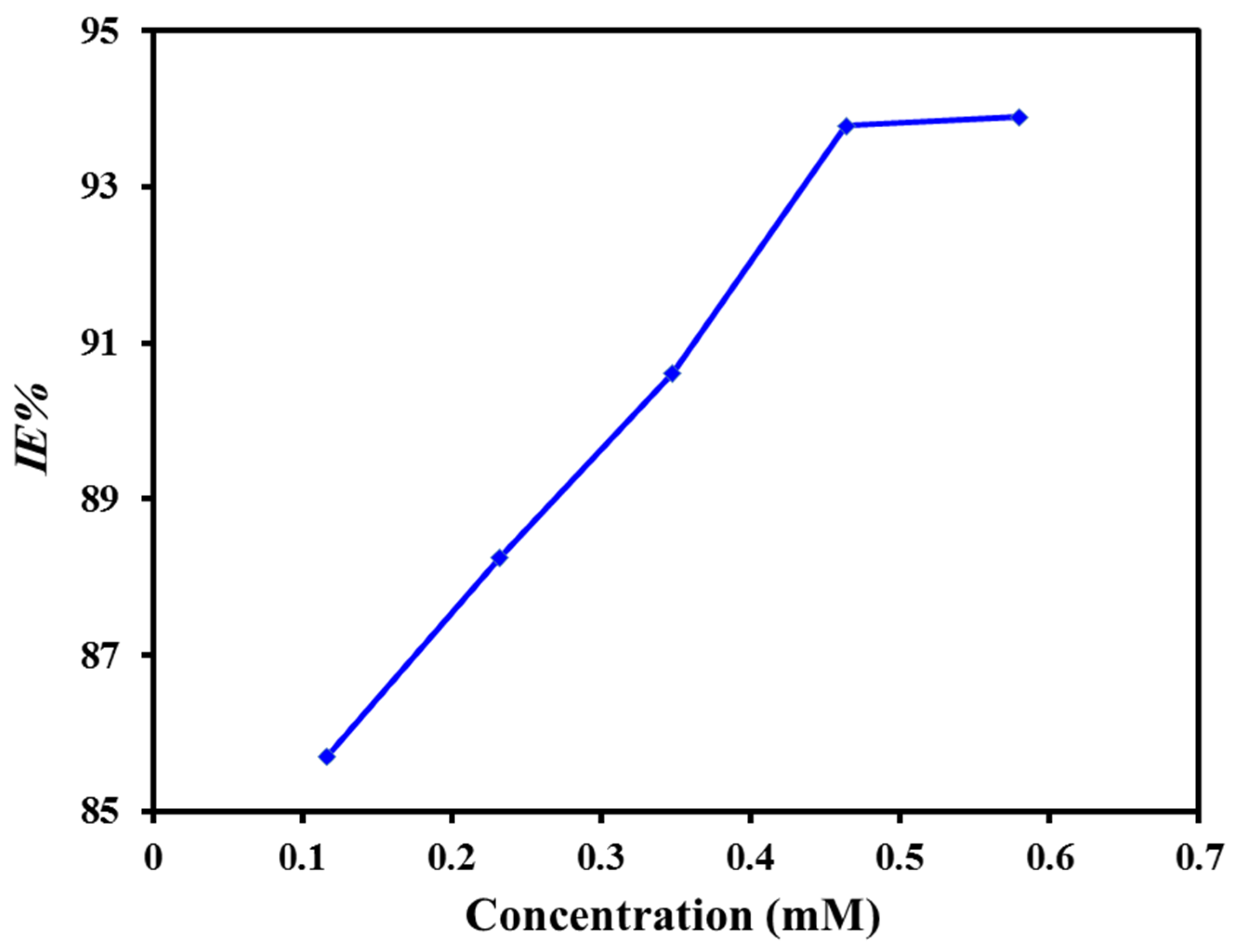
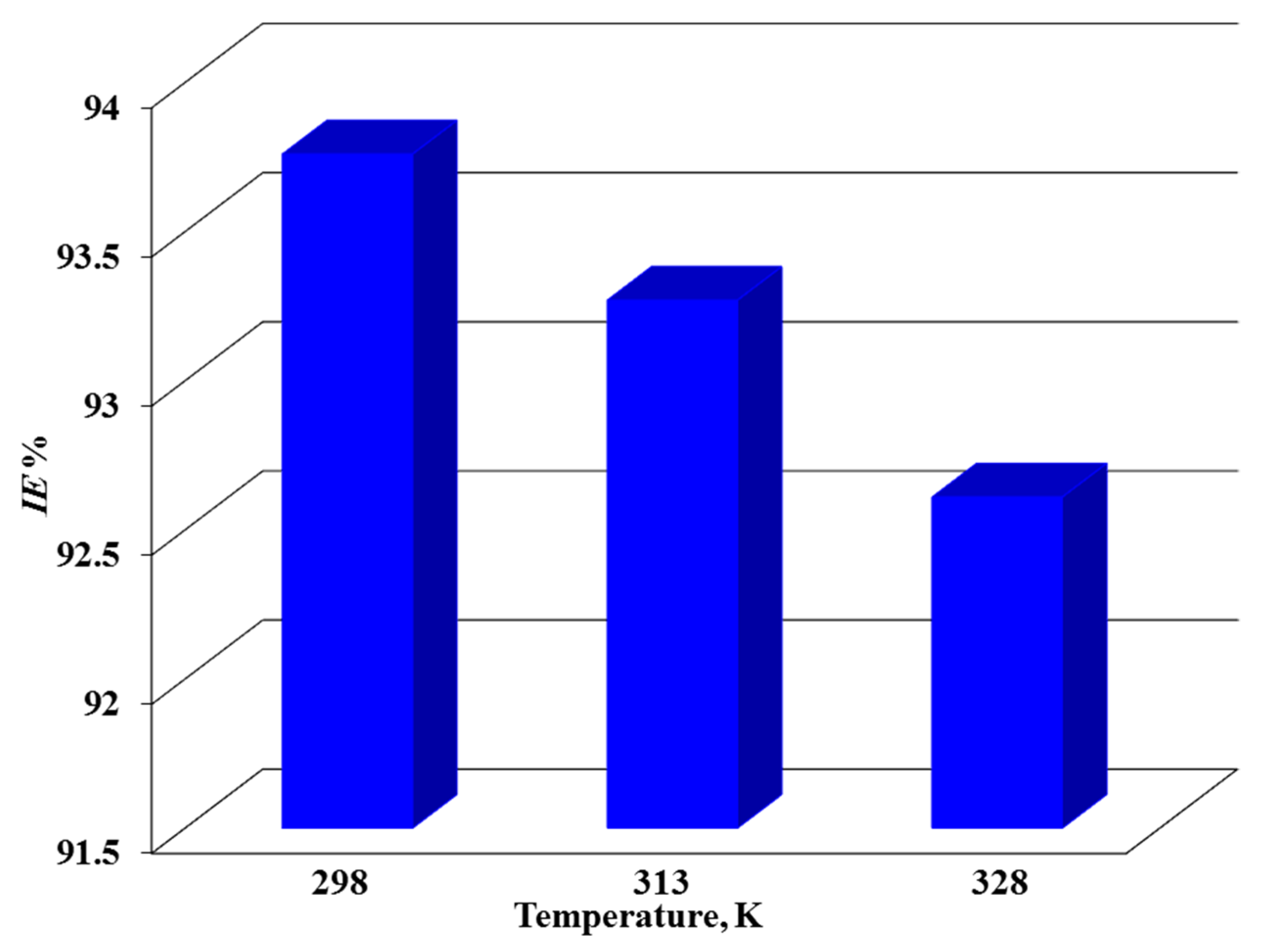
3.3.1. Weight Loss Study
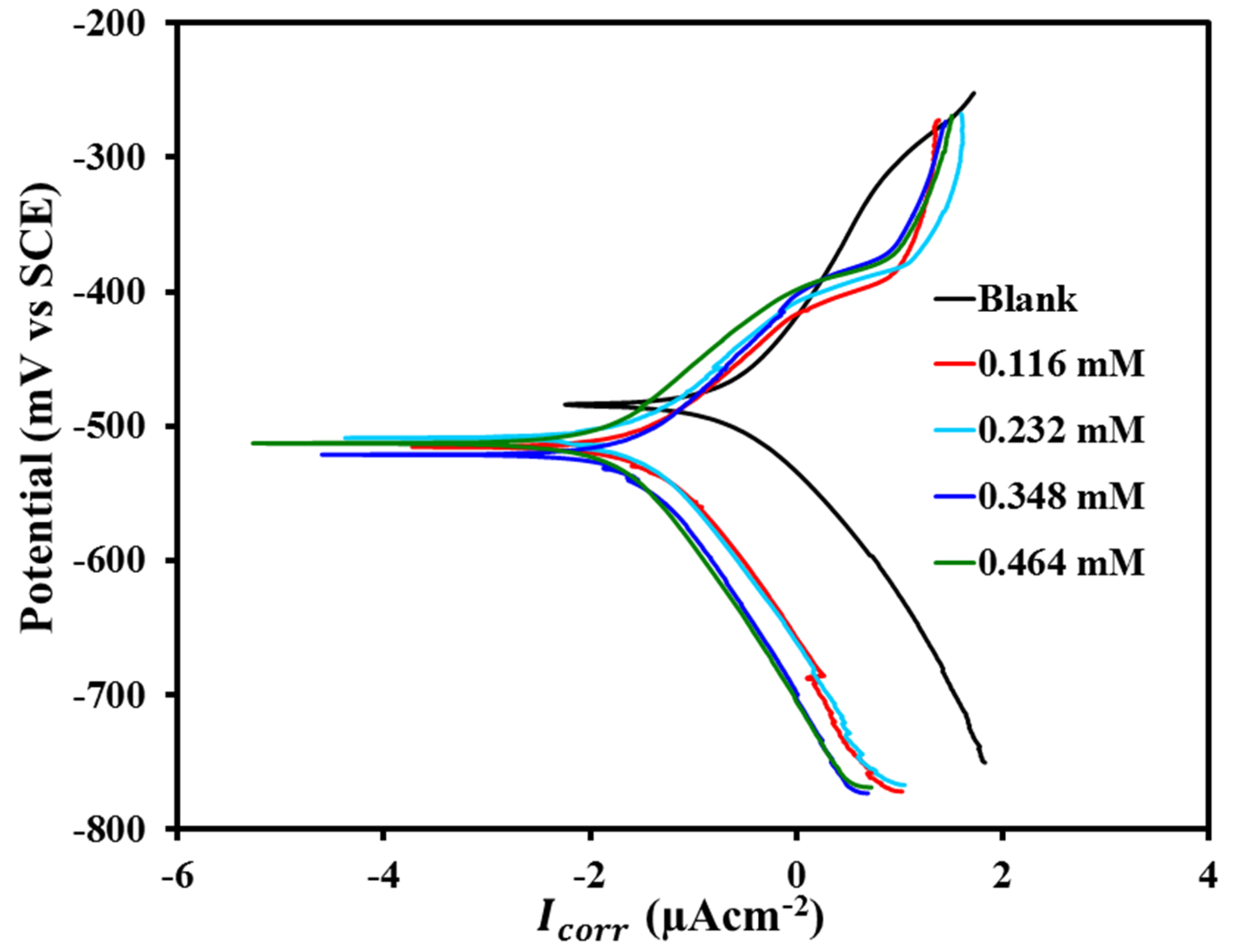
3.3.2. Tafel Plots Measurements
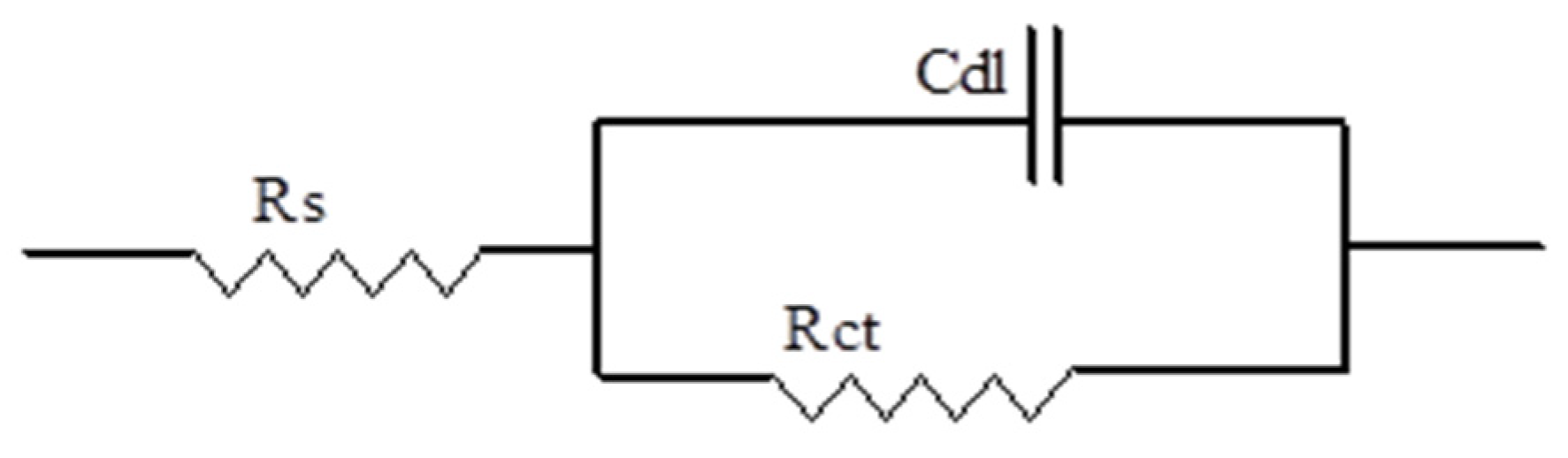
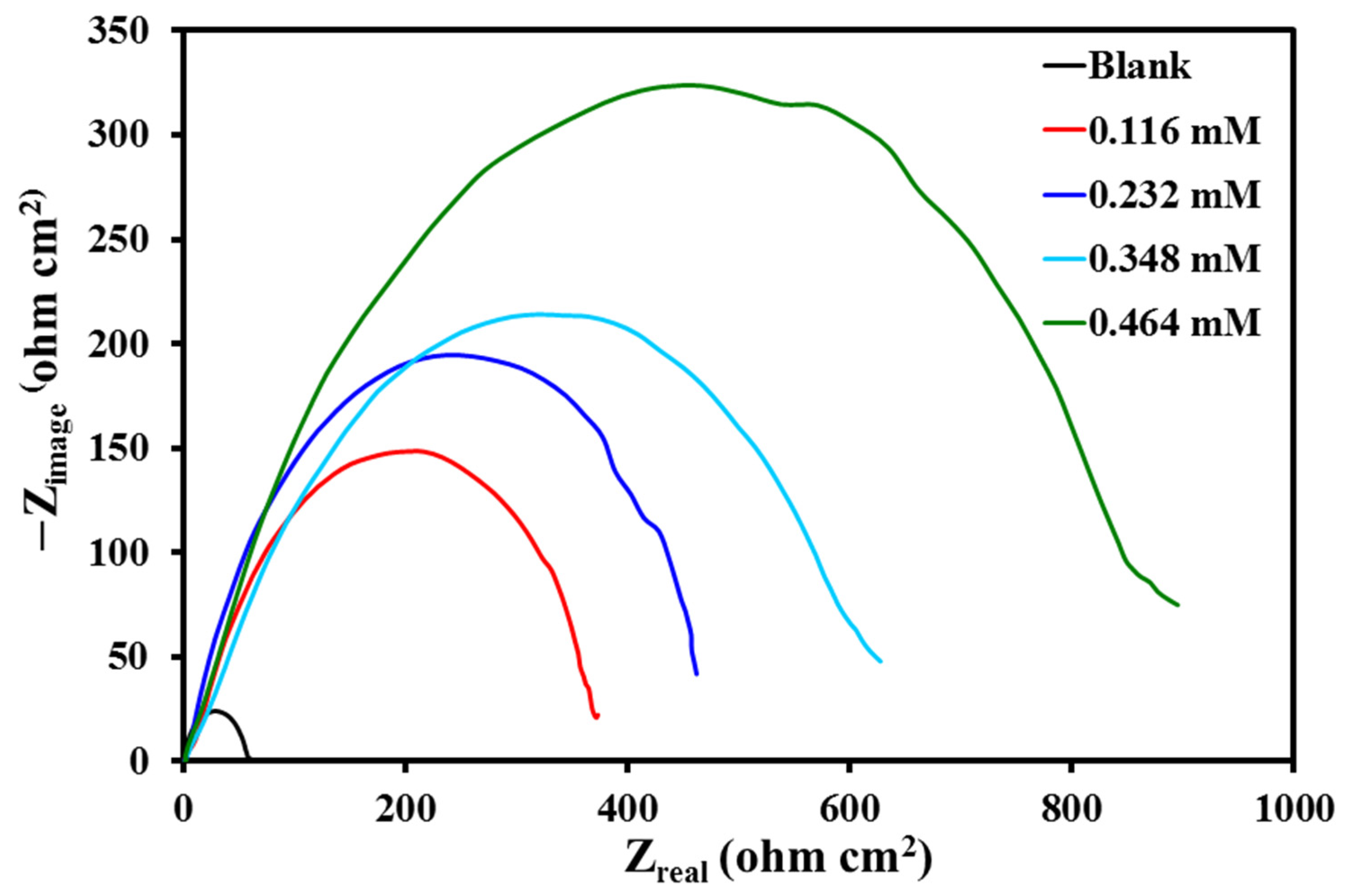
3.3.3. EIS Measurements
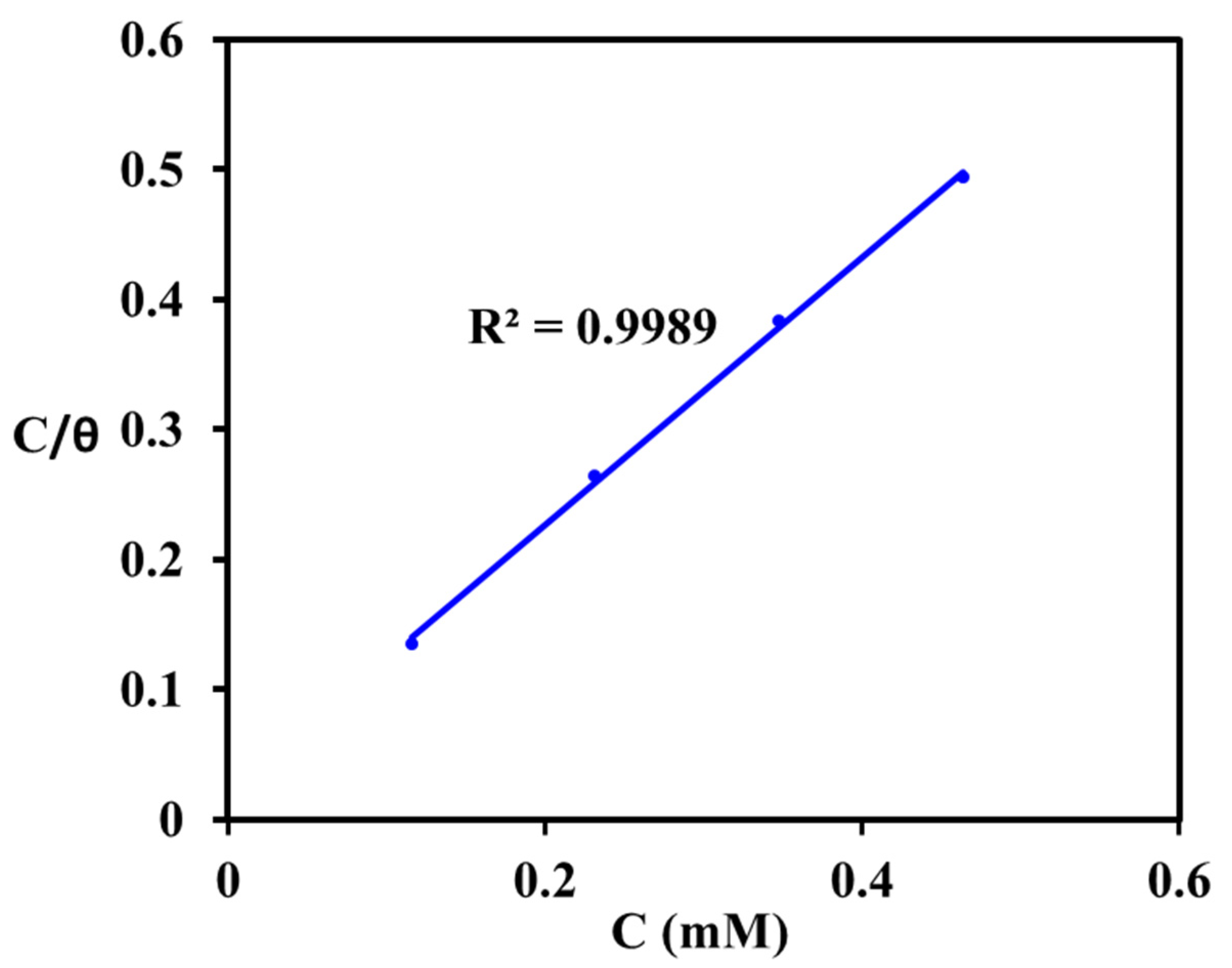
3.3.4. Adsorption Isotherm and Mechanism of Inhibition
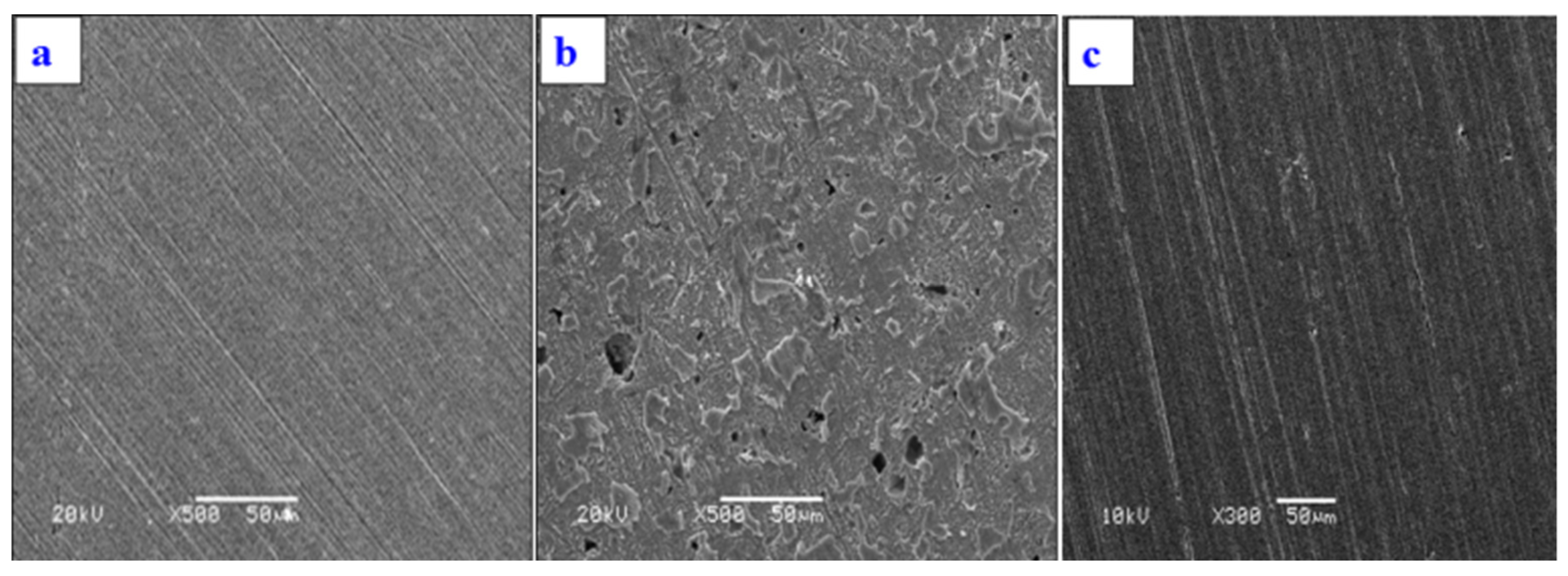
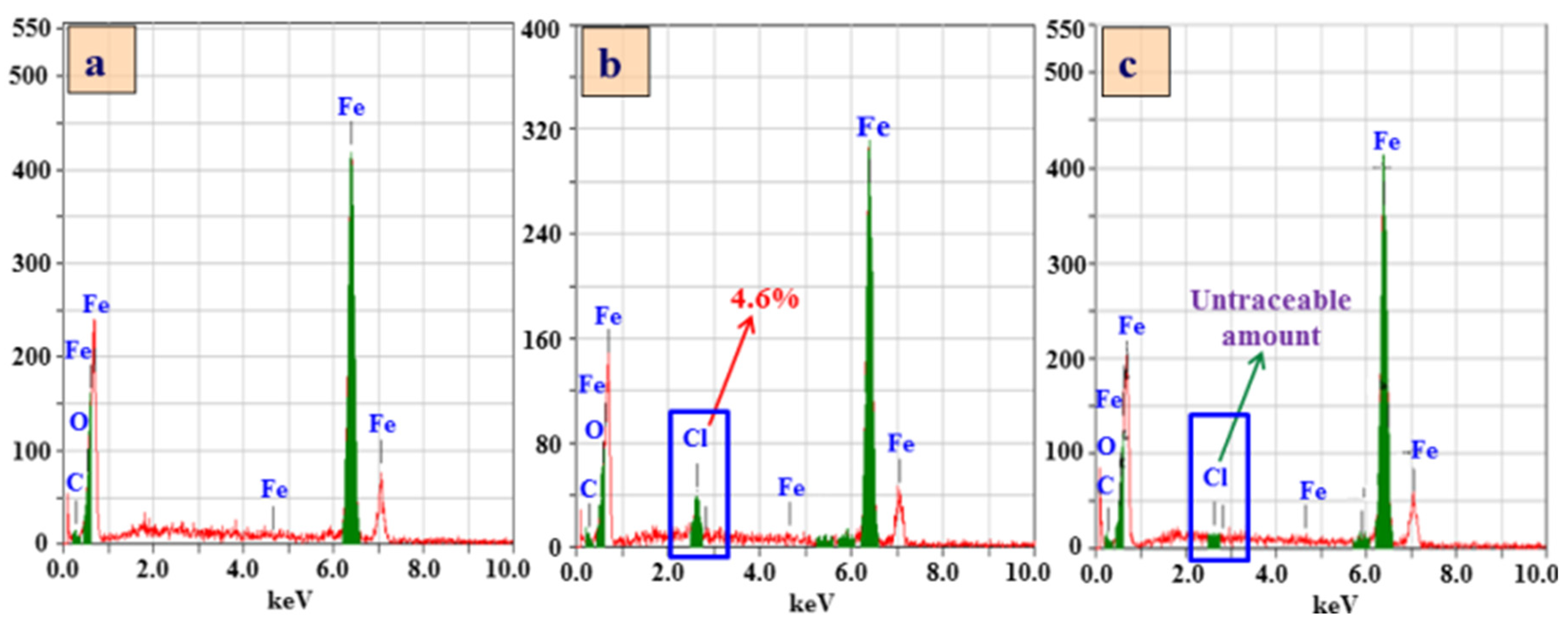
3.3.5. EDS and SEM Analysis
4. Conclusions
- The corrosion inhibitive capability of MAB increases with increasing the concentration of MAB, and highest IE (94.0%) was achieved at 0.464 mM.
- Electrochemical studies demonstrated a mixed-type inhibitor for MAB with the inclining cathodic efficacy.
- This novel green inhibitor MAB obeys Langmuir adsorption method isotherm, protects MS specimen through physical adsorption route, and process was exothermic and spontaneous.
- EDS and SEM analysis showed highly improved surface of MS specimen with MAB in corrosive media.
- Corrosion inhibitive properties evaluation of MAB using different methods such as weight loss, Tafel plots, EIS, SEM, and EDS analysis revealed that MAB has excellent inhibitive properties for MS sample in HCl corrosive environment.
- Corrosion inhibitive properties of M. aurea extracts and its isolated compound MAB is being reported here for the first time.
- The consistent results of the electrochemical and weight loss experiments is evident that this green inhibitor can be effectively utilized as a potent corrosion inhibitor for MS in aggressive solution (1.0 M HCl).
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raja, P.B.; Sethuraman, M.G. Natural products as corrosion inhibitor for metals in corrosive media—A review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.; Ebenso, E.E.; Bahadur, I. A green and sustainable approach for mild steel acidic corrosion inhibition using leaves extract: experimental and DFT studies. J. Bio- Tribo-Corros. 2018, 4, 33. [Google Scholar] [CrossRef]
- Zaferani, S.H.; Sharifi, M.; Zaarei, D.; Shishesaz, M.R. Application of eco-friendly products as corrosion inhibitors for metals in acid pickling processes–A review. J. Environ. Chem. Eng. 2013, 1, 652–657. [Google Scholar] [CrossRef]
- Grassino, A.N.; Halambek, J.; Djaković, S.; Brnčić, S.R.; Dent, M.; Grabarić, Z. Utilization of tomato peel waste from canning factory as a potential source for pectin production and application as tin corrosion inhibitor. Food Hydrocoll. 2016, 52, 265–274. [Google Scholar] [CrossRef]
- Elgahawi, H.; Gobara, M.; Baraka, A.; Elthalabawy, W. Eco-friendly corrosion inhibition of AA2024 in 3.5% NaCl using the extract of Linum usitatissimum seeds. J. Bio- Tribo-Corros. 2017, 3, 55. [Google Scholar] [CrossRef]
- El-Etre, A.; Abdallah, M.; El-Tantawy, Z. Corrosion inhibition of some metals using lawsonia extract. Corros. Sci. 2005, 47, 385–395. [Google Scholar] [CrossRef]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.H.; Hemapriya, V.; Gopiraman, M.; Kim, I.S.; Chung, I.M. Rhus verniciflua as a green corrosion inhibitor for mild steel in 1 M H2SO4. RSC Adv. 2016, 6, 57144–57153. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.H.; Sasireka, A.; Hemapriya, V.; Chung, I.M. β-Sitosterol isolated from rice hulls as an efficient corrosion inhibitor for mild steel in acidic environments. New J. Chem. 2017, 41, 3900–3907. [Google Scholar] [CrossRef]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green corrosion inhibitors from natural sources and biomass wastes. Molecules 2019, 24, 48. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Tan, B.; Chen, S. Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corros. Sci. 2018, 133, 6–16. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Obot, I.B.; Suleiman, R.K. Comparative studies on the corrosion inhibition efficacy of ethanolic extracts of date palm leaves and seeds on carbon steel corrosion in 15% HCl solution. J. Adhes. Sci. Technol. 2018, 32, 1934–1951. [Google Scholar] [CrossRef]
- Ehsani, A.; Mahjani, M.; Hosseini, M.; Safari, R.; Moshrefi, R.; Shiri, H.M. Evaluation of Thymus vulgaris plant extract as an eco-friendly corrosion inhibitor for stainless steel 304 in acidic solution by means of electrochemical impedance spectroscopy, electrochemical noise analysis and density functional theory. J. Colloid Interface Sci. 2017, 490, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Deyab, M.; Osman, M.; Elkholy, A.; Heakal, F.E.T. Green approach towards corrosion inhibition of carbon steel in produced oilfield water using lemongrass extract. RSC Adv. 2017, 7, 45241–45251. [Google Scholar] [CrossRef]
- Anupama, K.; Ramya, K.; Shainy, K.; Joseph, A. Adsorption and electrochemical studies of Pimenta dioica leaf extracts as corrosion inhibitor for mild steel in hydrochloric acid. Mater. Chem. Phys. 2015, 167, 28–41. [Google Scholar] [CrossRef]
- Lecante, A.; Robert, F.; Blandinières, P.; Roos, C. Anti-corrosive properties of S. tinctoria and G. ouregou alkaloid extracts on low carbon steel. Curr. Appl. Phys. 2011, 11, 714–724. [Google Scholar] [CrossRef]
- Li, L.; Xu, W.; Lei, J.; Wang, J.; He, J.; Li, N.; Pan, F. Experimental and theoretical investigations of Michelia alba leaves extract as a green highly-effective corrosion inhibitor for different steel materials in acidic solution. RSC Adv. 2015, 5, 93724–93732. [Google Scholar] [CrossRef]
- Chaussemier, M.; Pourmohtasham, E.; Gelus, D.; Pécoul, N.; Perrot, H.; Lédion, J.; Cheap-Charpentier, H.; Horner, O. State of art of natural inhibitors of calcium carbonate scaling. A review article. Desalination 2015, 356, 47–55. [Google Scholar] [CrossRef]
- Rani, B.; Basu, B.B.J. Green inhibitors for corrosion protection of metals and alloys: an overview. Int. J. Corros. 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- Alkhathlan, H.; Khan, M.; Abdullah, M.; AlMayouf, A.; Badjah-Hadj-Ahmed, A.Y.; AlOthman, Z.; Mousa, A. Anticorrosive assay-guided isolation of active phytoconstituents from Anthemis pseudocotula extracts and a detailed study of their effects on the corrosion of mild steel in acidic media. RSC Adv. 2015, 5, 54283–54292. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, N.A.; Mahmood, A.; Al-Kedhairy, A.A.; Alkhathlan, H.Z. The composition of the essential oil and aqueous distillate of Origanum vulgare L. growing in Saudi Arabia and evaluation of their antibacterial activity. Arab. J. Chem. 2018, 11, 1189–1200. [Google Scholar] [CrossRef]
- Khan, M.; Garg, A.; Srivastava, S.; Darokar, M. A cytotoxic agent from Strychnos nux-vomica and biological evaluation of its modified analogues. Med. Chem. Res. 2012, 21, 2975–2980. [Google Scholar] [CrossRef]
- Alkhathlan, H.Z.; Khan, M.; Abdullah, M.M.; Al-Mayouf, A.M. Method of Protecting Metal From Corrosion Using Plant Derived Anti-Corrosion Agent. U.S. Patent No. 9,988,726, 5 June 2018. [Google Scholar]
- Hudaib, M.; Mohammad, M.; Bustanji, Y.; Tayyem, R.; Yousef, M.; Abuirjeie, M.; Aburjai, T. Ethnopharmacological survey of medicinal plants in Jordan, Mujib Nature Reserve and surrounding area. J. Ethnopharmacol. 2008, 120, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Qnais, E. The analgesic effect of the ethanolic extract of Matricaria aurea. Turk. J. Biol. 2011, 35, 347–352. [Google Scholar]
- Moussaoui, F.; Zellagui, A.; Segueni, N.; Touil, A.; Rhouati, S. Flavonoid constituents from Algerian Launaea resedifolia (OK) and their antimicrobial activity. Rec. Nat. Prod. 2010, 4, 91–95. [Google Scholar]
- Bothi Raja, P.; Sethuraman, M. Strychnos nux-vomica an eco-friendly corrosion inhibitor for mild steel in 1 M sulfuric acid medium. Mater. Corros. 2009, 60, 22–28. [Google Scholar] [CrossRef]
- Yurt, A.; Ulutas, S.; Dal, H. Electrochemical and theoretical investigation on the corrosion of aluminium in acidic solution containing some Schiff bases. Appl. Surf. Sci. 2006, 253, 919–925. [Google Scholar] [CrossRef]
- Ahamad, I.; Prasad, R.; Quraishi, M. Adsorption and inhibitive properties of some new Mannich bases of Isatin derivatives on corrosion of mild steel in acidic media. Corros. Sci. 2010, 52, 1472–1481. [Google Scholar] [CrossRef]
- Gopiraman, M.; Sakunthala, P.; Kesavan, D.; Alexramani, V.; Kim, I.; Sulochana, N. An investigation of mild carbon steel corrosion inhibition in hydrochloric acid medium by environment friendly green inhibitors. J. Coat. Technol. Res. 2012, 9, 15–26. [Google Scholar] [CrossRef]
- Elayyachy, M.; El Idrissi, A.; Hammouti, B. New thio-compounds as corrosion inhibitor for steel in 1 M HCl. Corros. Sci. 2006, 48, 2470–2479. [Google Scholar] [CrossRef]
- Khaled, K.; Al-Qahtani, M. The inhibitive effect of some tetrazole derivatives towards Al corrosion in acid solution: Chemical, electrochemical and theoretical studies. Mater. Chem. Phys. 2009, 113, 150–158. [Google Scholar] [CrossRef]
- Quraishi, M.; Singh, A.; Singh, V.K.; Yadav, D.K.; Singh, A.K. Green approach to corrosion inhibition of mild steel in hydrochloric acid and sulphuric acid solutions by the extract of Murraya koenigii leaves. Mater. Chem. Phys. 2010, 122, 114–122. [Google Scholar] [CrossRef]
- Bentiss, F.; Lagrenée, M.; Elmehdi, B.; Mernari, B.; Traisnel, M.; Vezin, H. Electrochemical and quantum chemical studies of 3, 5-di (n-tolyl)-4-amino-1, 2, 4-triazole adsorption on mild steel in acidic media. Corrosion 2002, 58, 399–407. [Google Scholar] [CrossRef]
- Ghali, E.; Sastri, V.S.; Elboujdaini, M. Corrosion Prevention and Protection: Practical Solutions; John Wiley & Sons: West Sussex, UK, 2007. [Google Scholar]
- Cheng, S.; Chen, S.; Liu, T.; Chang, X.; Yin, Y. Carboxymenthylchitosan as an ecofriendly inhibitor for mild steel in 1 M HCl. Mater. Lett. 2007, 61, 3276–3280. [Google Scholar] [CrossRef]
- Özcan, M. AC impedance measurement of cystine adsorption at mild steel/sulfuric acid interface as corrosion inhibitor. J. Solid State Electrochem. 2008, 12, 1653–1661. [Google Scholar] [CrossRef]
- Hussin, M.H.; Kassim, M.J. The corrosion inhibition and adsorption behavior of Uncaria gambir extract on mild steel in 1 M HCl. Mater. Chem. Phys. 2011, 125, 461–468. [Google Scholar] [CrossRef]



| Concentration of Inhibitor (mM) | Weight Loss (g) | Surface Coverage | Corrosion Rate | |
|---|---|---|---|---|
| 0 (Blank) | 0.2587 | - | 0.1354 | - |
| 0.116 | 0.0370 | 0.86 | 0.0194 | 85.70 ± 0.84 |
| 0.232 | 0.0304 | 0.88 | 0.0159 | 88.25 ± 0.98 |
| 0.348 | 0.0243 | 0.91 | 0.0127 | 90.61 ± 0.83 |
| 0.464 | 0.0161 | 0.94 | 0.0084 | 93.78 ± 0.91 |
| 0.580 | 0.0158 | 0.94 | 0.0083 | 93.89 ± 0.95 |
| Temperatures (K) | Weight Loss (g) | Surface Coverage | Corrosion Rate | |
|---|---|---|---|---|
| 298 ± 1 | 0.0161 | 0.94 | 0.0084 | 93.78 ± 0.91 |
| 313 ± 1 | 0.0174 | 0.93 | 0.0091 | 93.27 ± 0.85 |
| 328 ± 1 | 0.0258 | 0.93 | 0.0135 | 90.03 ± 1.02 |
| MAB Concentration (mM) | (mV) | (µAcm−) | (mV/dec) | (mV/dec) | Tafel | LPR | |
|---|---|---|---|---|---|---|---|
| 0 (Blank) | 486.6 | 213.0 | 99.85 | 110.73 | 54.5 | - | - |
| 0.116 | 515.5 | 29.0 | 57.31 | 73.42 | 339.64 | 86.38 | 83.95 |
| 0.232 | 508.7 | 23.4 | 61.16 | 75.90 | 499.18 | 89.01 | 89.08 |
| 0.348 | 521.8 | 19.8 | 59.25 | 80.71 | 576.39 | 90.70 | 90.54 |
| 0.464 | 513.5 | 10.2 | 63.41 | 83.62 | 907.02 | 95.21 | 94.00 |
| Concentration (mM) | ||||
|---|---|---|---|---|
| 0 (Blank) | 57.1 | 533.0 | - | - |
| 0.116 | 385.0 | 352.3 | 0.85 | 85.17 |
| 0.232 | 479.7 | 348.2 | 0.88 | 88.10 |
| 0.348 | 634.0 | 222.3 | 0.91 | 91.00 |
| 0.464 | 886.4 | 118.4 | 0.94 | 93.56 |
| Compound | |||
|---|---|---|---|
| MAB | 19.66 | 10.23 | 31.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.; Abdullah, M.M.S.; Mahmood, A.; Al-Mayouf, A.M.; Alkhathlan, H.Z. Evaluation of Matricaria aurea Extracts as Effective Anti-Corrosive Agent for Mild Steel in 1.0 M HCl and Isolation of Their Active Ingredients. Sustainability 2019, 11, 7174. https://doi.org/10.3390/su11247174
Khan M, Abdullah MMS, Mahmood A, Al-Mayouf AM, Alkhathlan HZ. Evaluation of Matricaria aurea Extracts as Effective Anti-Corrosive Agent for Mild Steel in 1.0 M HCl and Isolation of Their Active Ingredients. Sustainability. 2019; 11(24):7174. https://doi.org/10.3390/su11247174
Chicago/Turabian StyleKhan, Merajuddin, Mahmood M. S. Abdullah, Adeem Mahmood, Abdullah M. Al-Mayouf, and Hamad Z. Alkhathlan. 2019. "Evaluation of Matricaria aurea Extracts as Effective Anti-Corrosive Agent for Mild Steel in 1.0 M HCl and Isolation of Their Active Ingredients" Sustainability 11, no. 24: 7174. https://doi.org/10.3390/su11247174
APA StyleKhan, M., Abdullah, M. M. S., Mahmood, A., Al-Mayouf, A. M., & Alkhathlan, H. Z. (2019). Evaluation of Matricaria aurea Extracts as Effective Anti-Corrosive Agent for Mild Steel in 1.0 M HCl and Isolation of Their Active Ingredients. Sustainability, 11(24), 7174. https://doi.org/10.3390/su11247174







