Environmental Assessment of Magnesium Oxychloride Cement Samples: A Case Study in Europe
Abstract
1. Introduction
2. Materials and Methodology
2.1. Materials, Mix Design and Sample Preparation
2.2. Methodology
2.2.1. Compressive and Flexural Strength
2.2.2. Environmental Assessment
- Inventories: The inventories of three types of alkali used in this study, i.e., NaOH, CaO and NH4OH, which are the most frequently used bases in terms of Mg(OH)2 precipitation in industry [20]. In practice, CaO is more commonly used as an alkali base during the precipitation of Mg(OH)2 due to its cheaper price and larger availability than Ca(OH)2; therefore, CaO was selected in this study instead of Ca(OH)2. Meanwhile, the hydrochloric (HCl) and background data were obtained from the Ecoinvent database developed by the Swiss Centre for Life Cycle Inventories [38] and the ETH-ESU database, provided by Eidgenössische Technische Hochschule [39]. The electricity consumption during the calcination of Mg(OH)2 at the temperature of 650 °C for 1 h was recorded using a plug-in electric energy power meter (Floureon, Shenzhen, China). The inventories of MgO production from the calcination of magnesite were acquired from previous studies [40,41] (Table 2). The inventories of PC were obtained from a number of references [42,43,44,45,46], the details of which can be found in [40] (Table 3). When comparing MOC and PC concrete, the inventories of aggregates and pulverized fly ash were from Ecoinvent database and [47] (Table 4). The quantity of each component used to produce 1 kg of MOC and PC concrete is shown in Table 5.
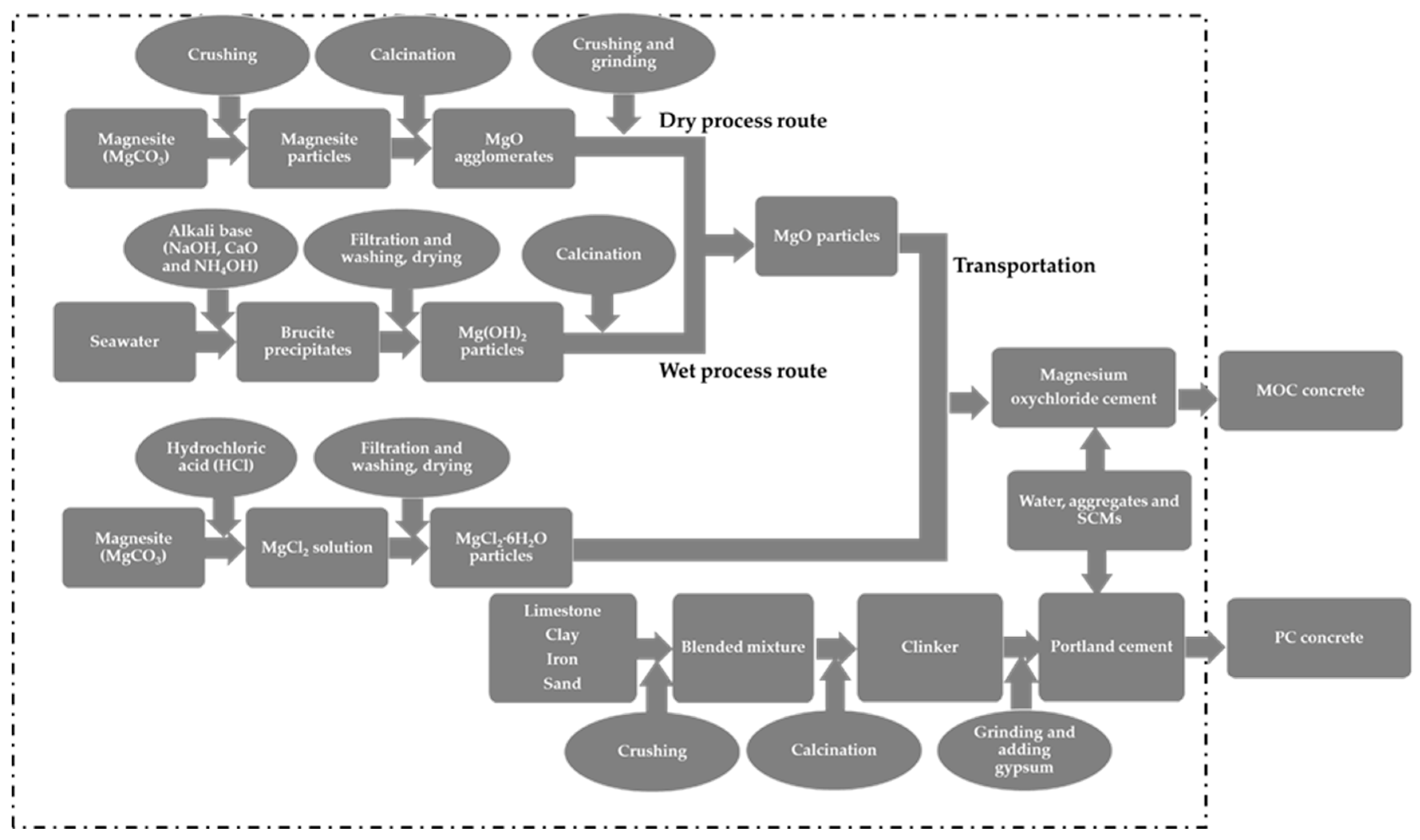
- Goal, functional unit and scope: This goal of this study was to perform a comparative assessment of the environmental burdens posed by the preparation of MOC paste prepared using three different molar ratios of MgO/MgCl2∙6H2O, where NaOH was used as the alkali base during the synthesis of MgO, then a comparative environmental assessment of MOC paste, in which different alkali bases were used including CaO, NaOH and NH4OH. Finally, the environmental impacts of MOC concrete prepared with two sources of reactive MgO from the wet (i.e., synthesis from seawater) and dry process routes (i.e., calcination of magnesite) were analysed, and then the results were compared with the environmental burdens related to the preparation of PC concrete with the same w/c ratio (i.e., 0.34) afterwards; meanwhile, the transportation of reactive MgO from China to Europe was also taken into account. Therefore, the system boundary used in this study, incorporating the production and utilization phases of MOC and PC samples, is shown in Figure 2. The functional unit of assessment used was 1.0 kg of MOC paste prepared according to the mix compositions listed in Table 1; however, the final part of this study concerns a comparative environmental assessment of 1.0 kg of MOC concrete (molar ratio: 9) and PC concrete with the same w/c ratio (i.e., 0.34).
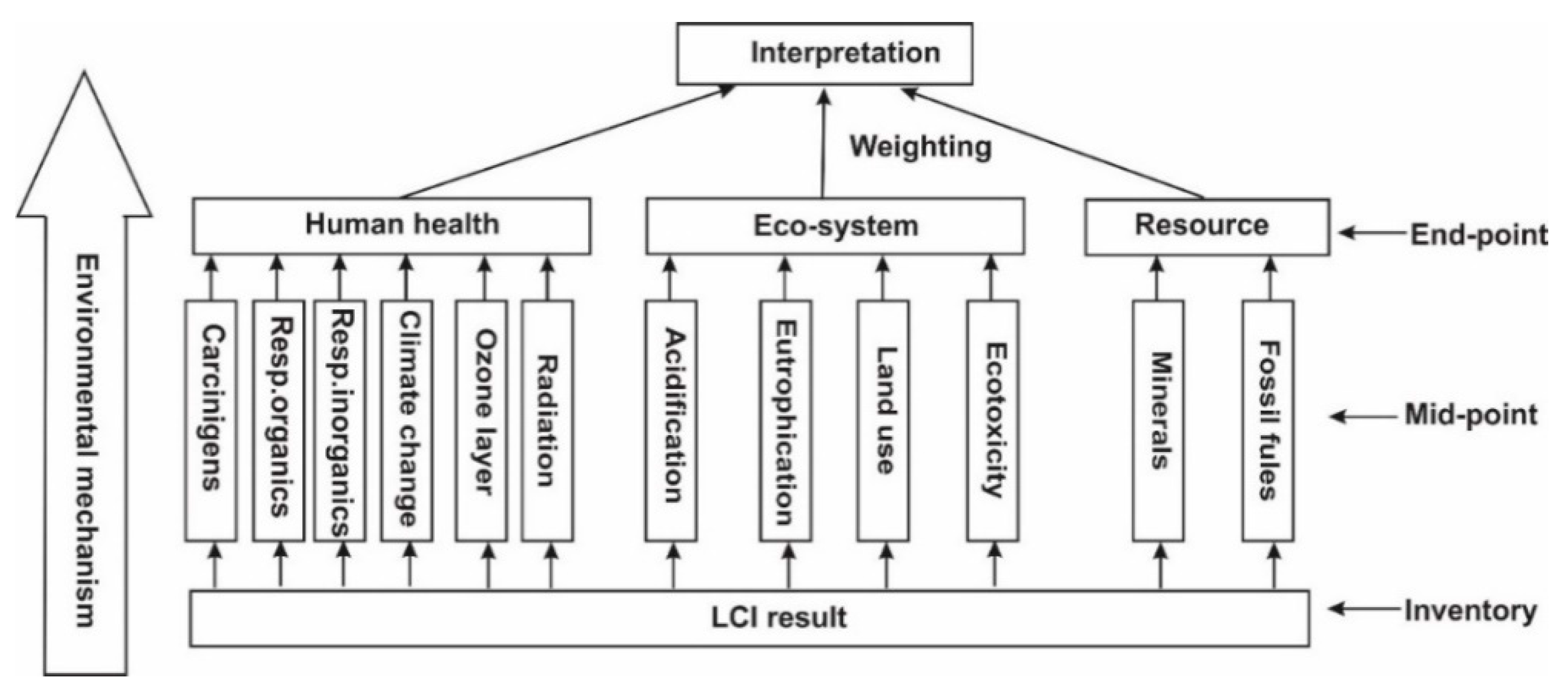
- Impact assessment: In this LCA study, Eco-indicator 99 was employed as it facilitates the selection of a series of environmental indicators to acquire the environmental loads associated with the production of various binders quantitatively, reflected by several categories. Several key categories such as climate change, affected by CO2 emissions, were also incorporated in this method, and these categories provided a comprehensive profile of the ecological impacts of MOC and PC sample preparation. Meanwhile, these categories also gave a clear representation of the impacts related to human health, ecosystem quality and resources [27,48,49]. Furthermore, the sum of various scores associated with a series of environmental impacts into a single score facilitates a useful comparison of various products and processes, and the weighting and interpretation procedures of environmental burdens used in Eco-indicator 99 [50] are also shown in Figure 3. Meanwhile, a mixing triangle was employed to tackle the weighting issues for the two products/processes, in which the indifference curves were calculated by the determination of the points where they cross the boundaries of the triangle [51]. Furthermore, the global warming potential (GWP) of MOC and PC samples was also investigated by IPCC 2007 within a timeframe of 100 years.
- Description of allocation: Allocation is of great importance, as sometimes a production process may produce more than one end-product. In this study, during the synthesis of Mg(OH)2 from seawater, which is the main product, some by-products also formed simultaneously, such as NaCl. Therefore, the environmental burdens have to be divided between these two final products in a way that reflects the underlying relationships between them, e.g., allocation by mass or by economic value [35]. Figure 4 shows the illustration of allocation in this study. The mass allocation coefficient CM and economical allocation coefficient CE can be calculated as the mass and economical value ratio between main product and by-product, respectively, shown in Equations (3)–(4):CM = Mmain product/(Mmain product + Mby-product)CE = Emain product/(Emain product + Eby-product).
3. Results
3.1. Environmental Assessment of MOC Paste Prepared with Various Molar Ratios of MgO/MgCl2∙6H2O
3.1.1. CO2 Sequestration Potential of MOC Paste Prepared with Various Molar Ratios of MgO/MgCl2∙6H2O
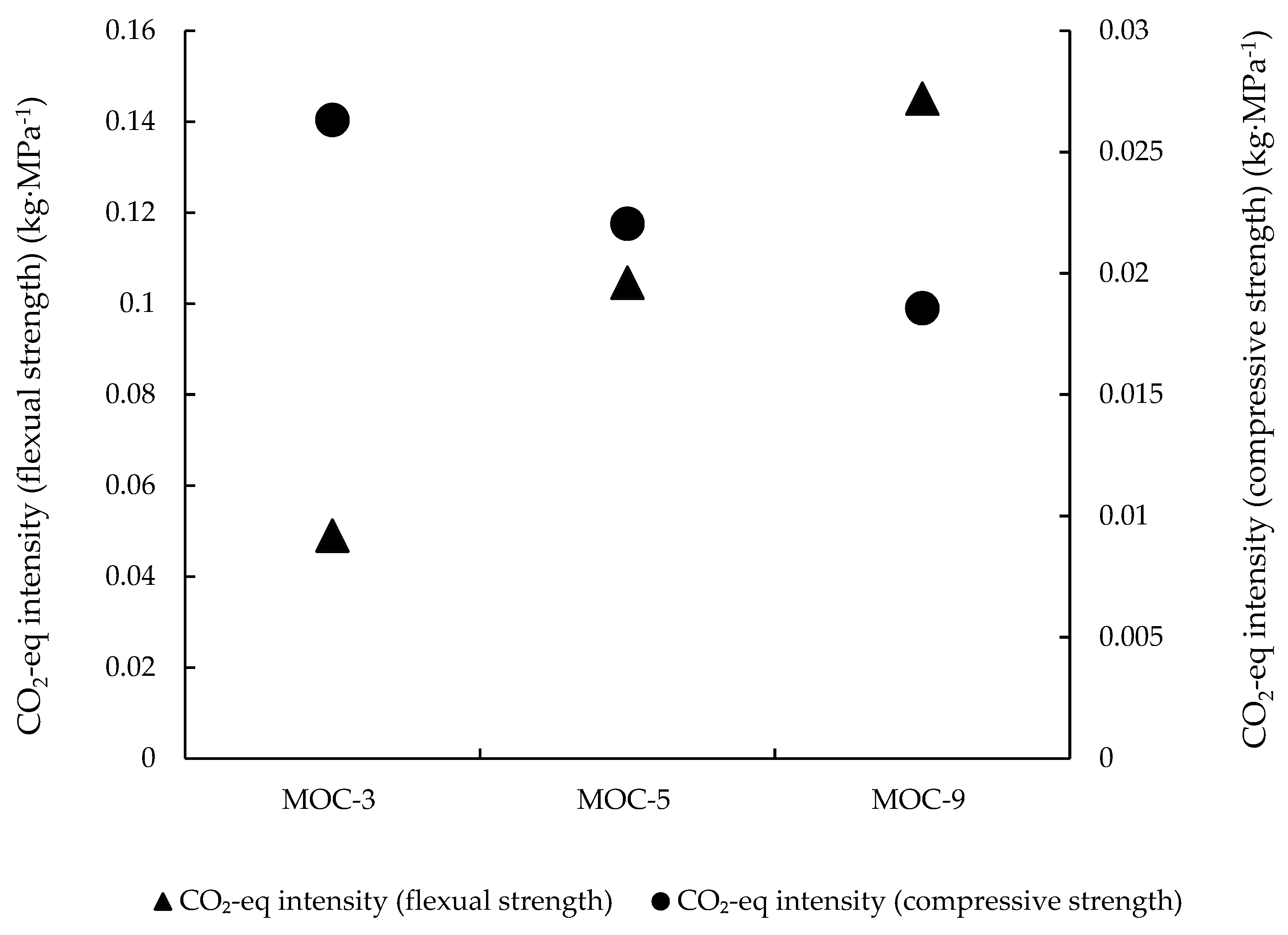
3.1.2. CO2 Intensities of MOC Paste Prepared with Various Molar Ratios of MgO/MgCl2∙6H2O
3.2. Environmental Assessment of MOC Paste Prepared with MgO Synthesized by Various Alkali Bases
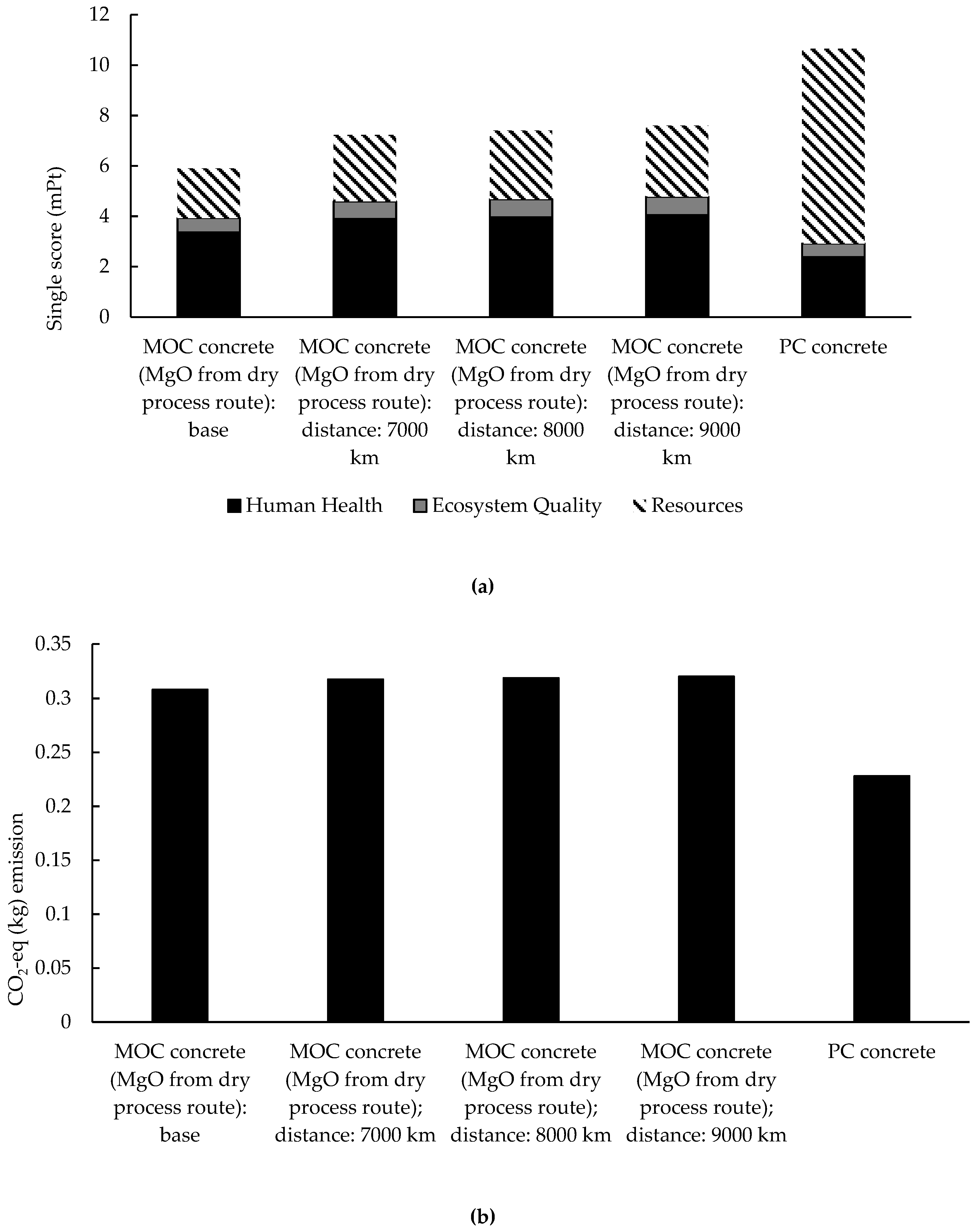
3.3. Comparative Environmental Assessment of MOC Concrete Prepared with Different MgO Sources and PC Concrete
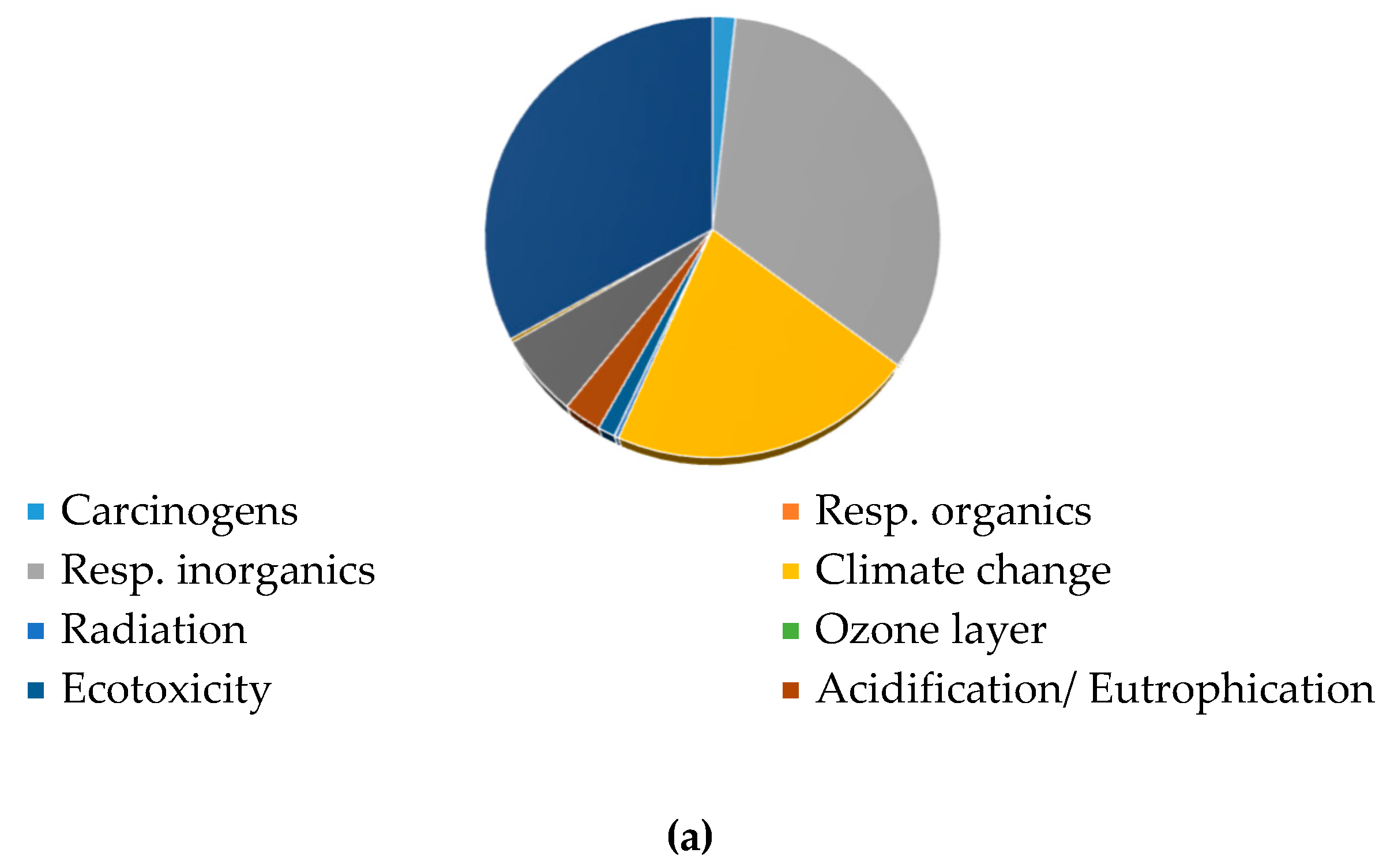
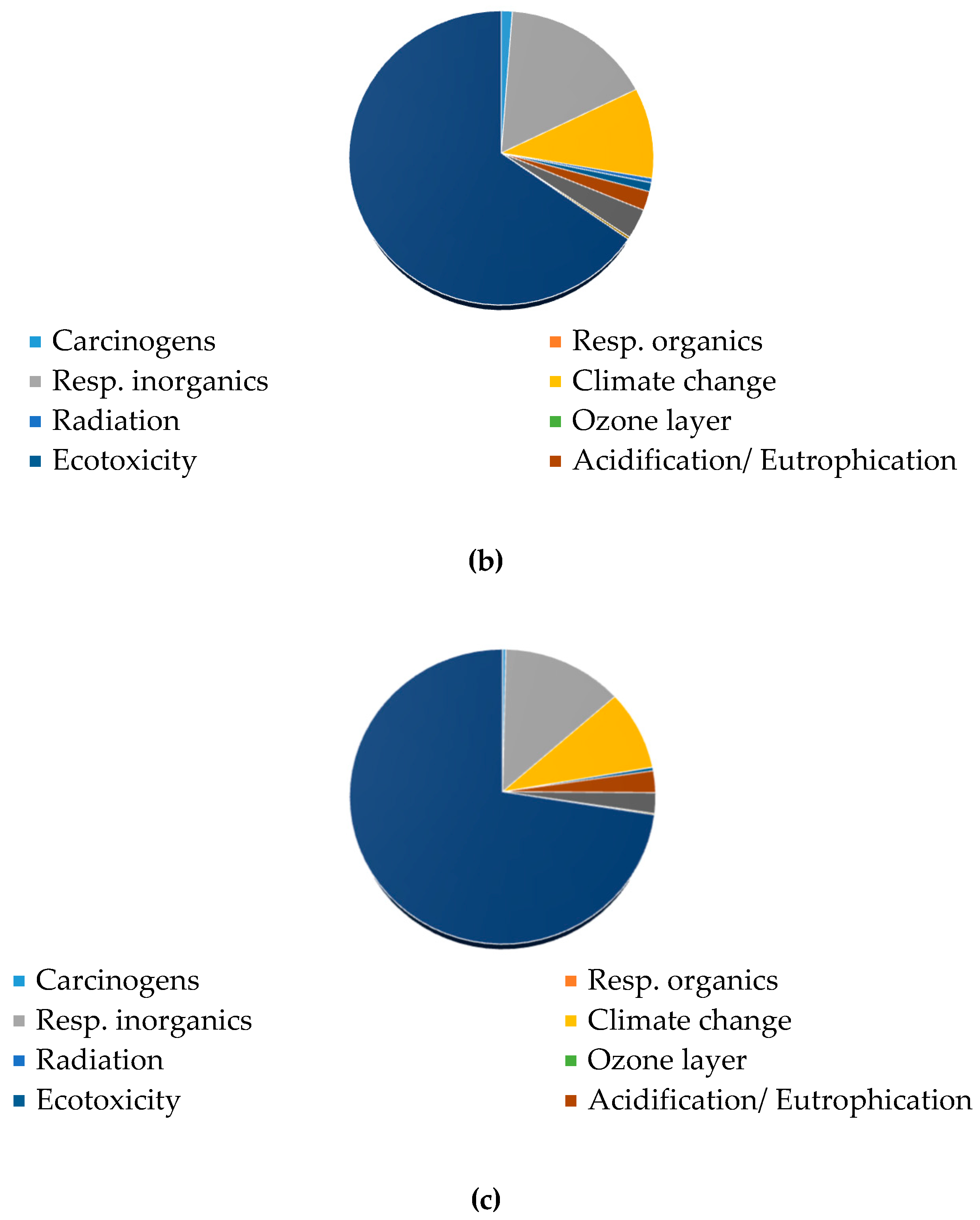
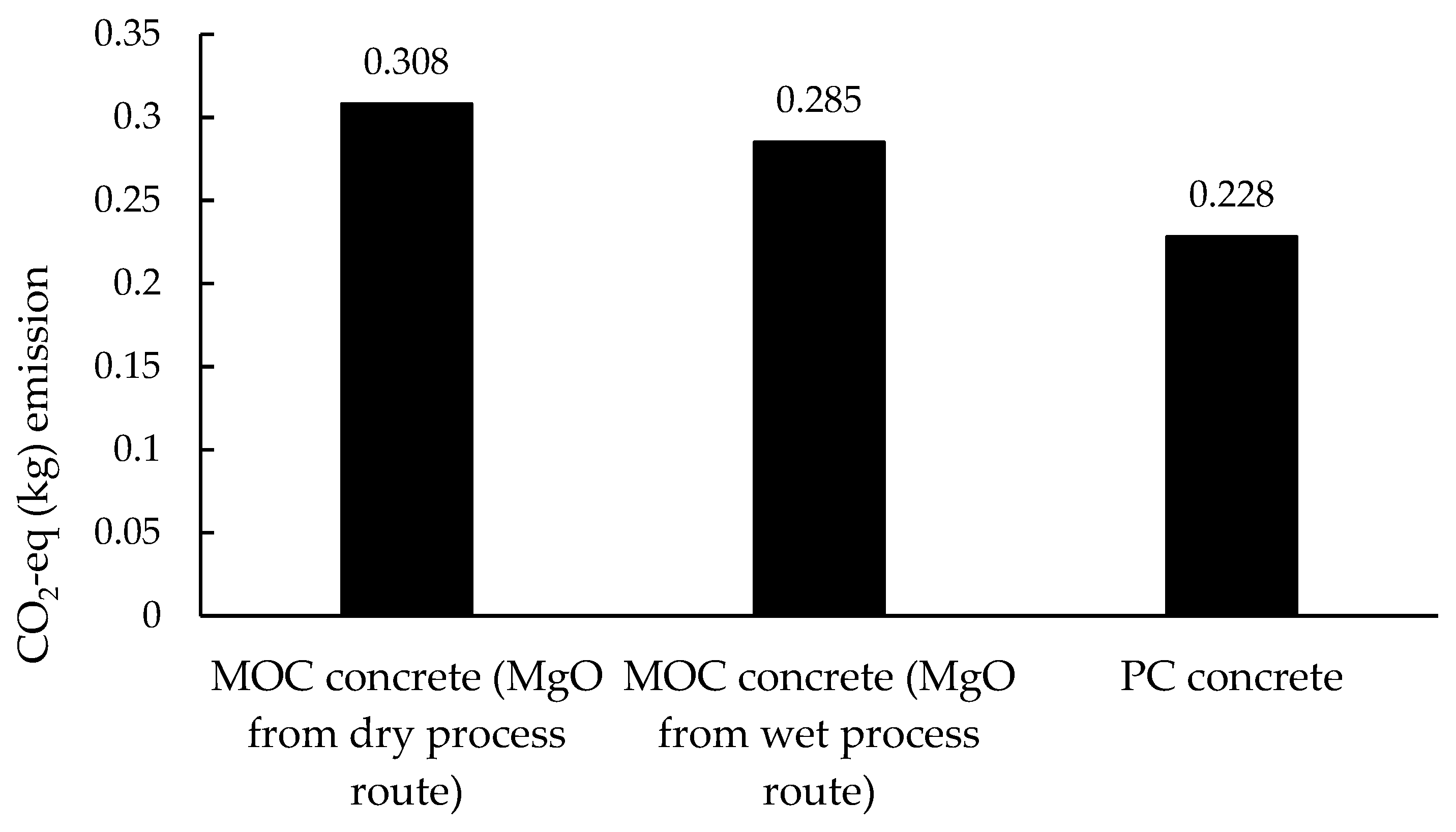
3.3.1. Contribution Analysis and CO2-eq Emissions
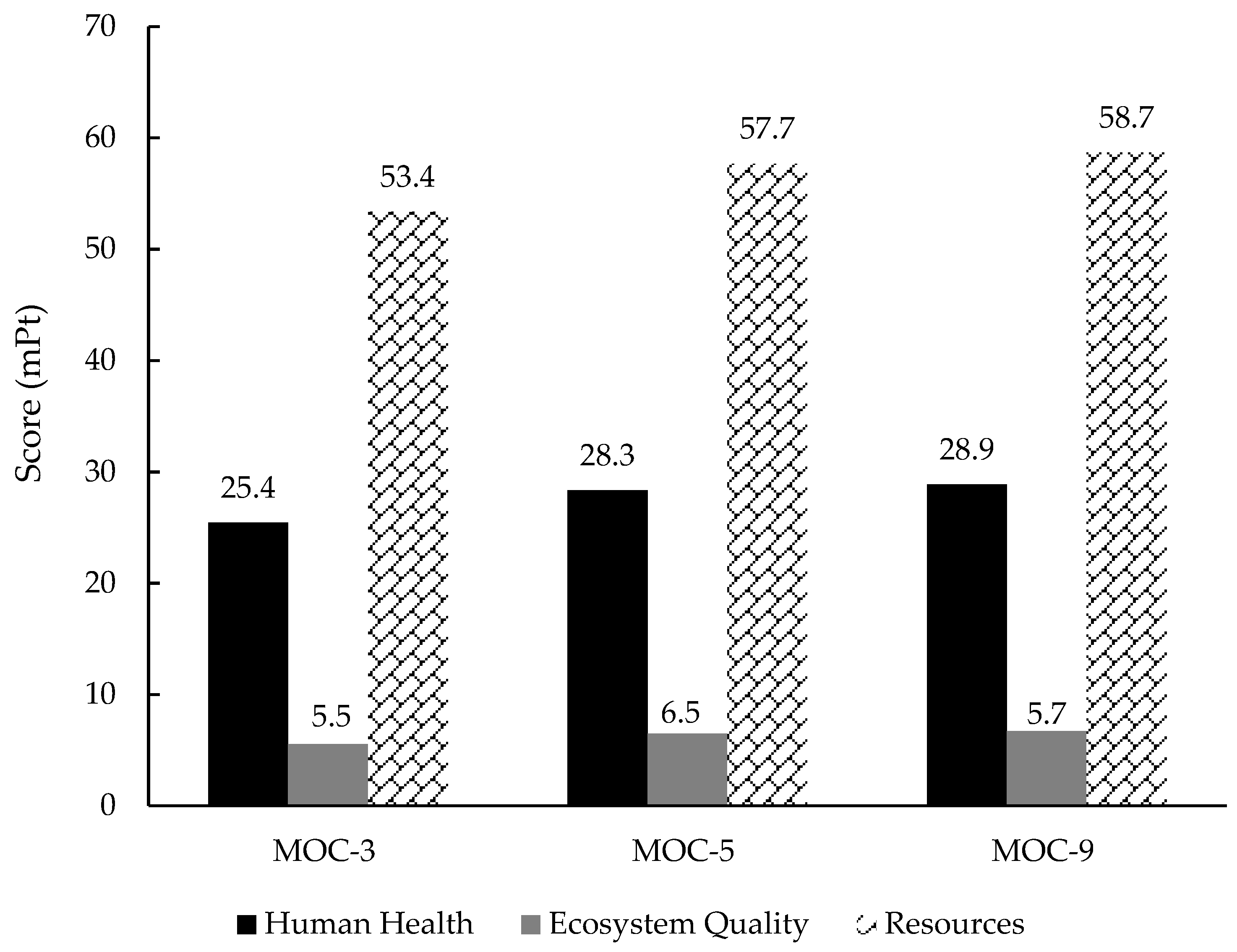
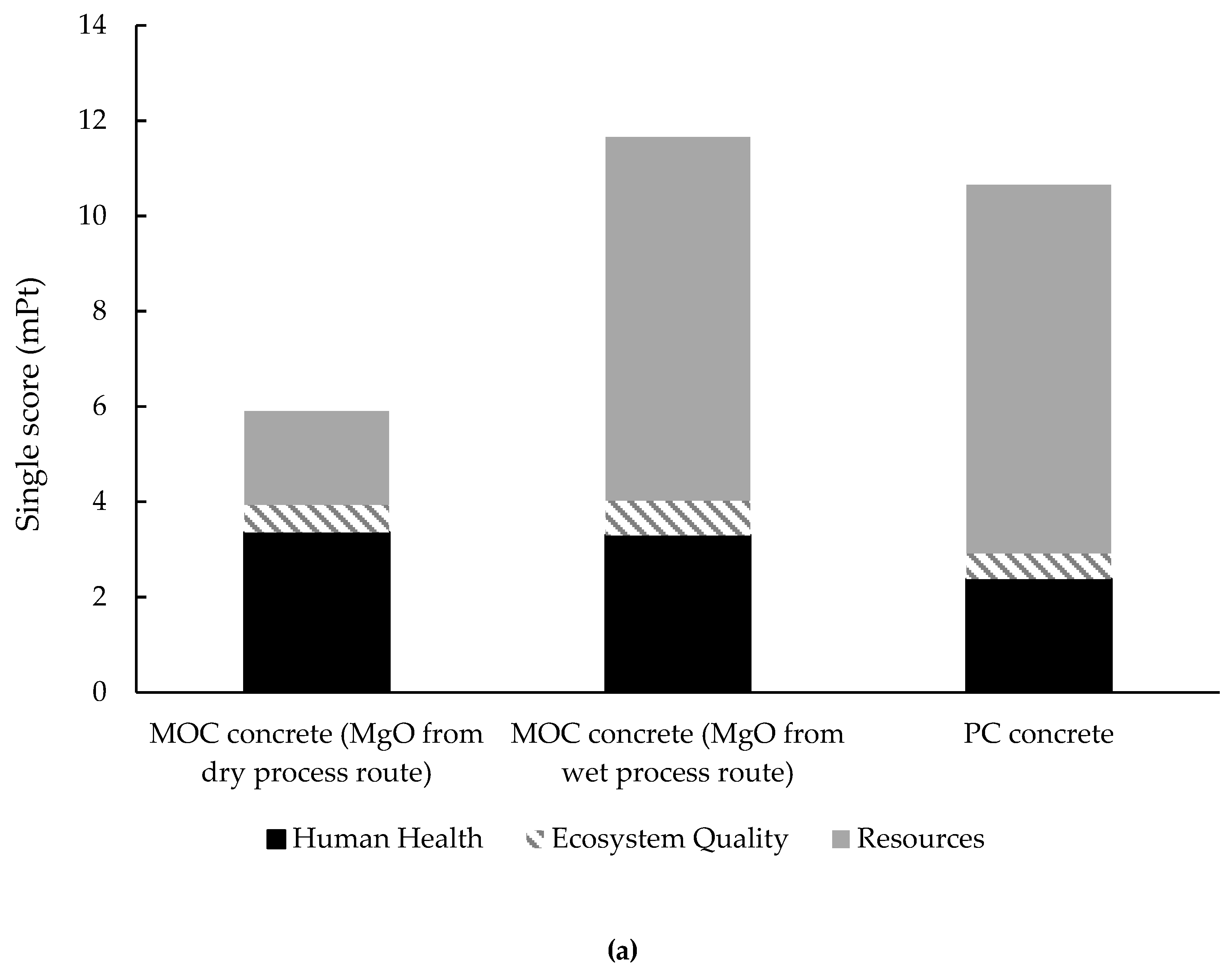
3.3.2. Single Score and Weighting Process
4. Discussion
5. Conclusions
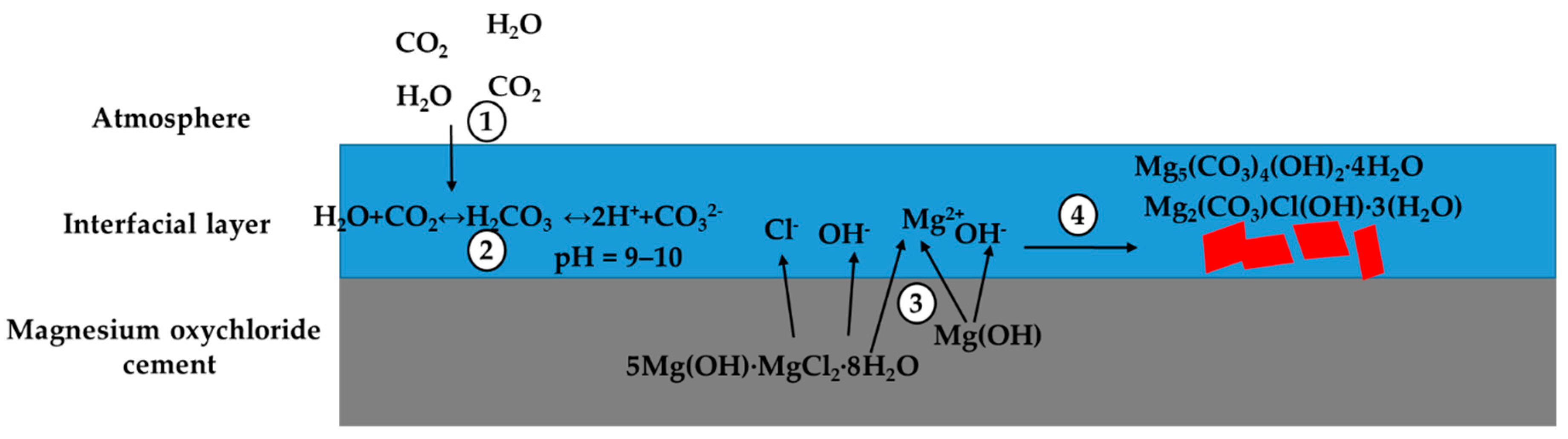
- Increasing the molar ratios in MOC paste led to more severe environmental impacts and greater CO2 sequestration potentials, which are related to the difference between MgO and MgCl2∙6H2O in terms of their energy requirements for production and their CO2 binding capacities.
- In terms of environmental efficiency, as reflected by CO2 intensities, the molar ratios of MOC paste should be carefully selected depending on the strength requirements of the applications, and allocating the environmental impacts of MgO production by mass is more favourable.
- The transportation of reactive MgO from China to Europe has a negligible influence on the environmental impacts of MOC concrete preparation when MgO is from the dry process route, and MOC concrete cannot be categorized as a type of ‘low-carbon’ binder, by contrast with PC concrete. Meanwhile, in terms of the overall environmental impacts, MOC concrete can only be recognized as a more sustainable binder than PC concrete when the main component, MgO, is obtained through the dry process route rather than the wet process route in MOC concrete preparation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Altiner, M.; Yildirim, M. Study of using dolomite as starting material resource to produce magnesium oxychloride cement. J. Adv. Concr. Technol. 2017, 15, 269–277. [Google Scholar] [CrossRef][Green Version]
- Góchez, R.R.; Vreeland, T.; Wambaugh, J.; Kitchens, C.L. Conversion of magnesium oxychloride to chlorartinite and resulting increased water resistance. Mater. Lett. 2017, 207, 1–3. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Hu, C.; Yang, S.; Li, Z. Influence of curing regimes on mechanical properties of magnesium oxychloride cement-based composites. Constr. Build. Mater. 2016, 102, 613–619. [Google Scholar] [CrossRef]
- Tang, S.; Hu, Y.; Ren, W.; Yu, P.; Huang, Q.; Qi, X.; Li, Y.; Chen, E. Modeling on the hydration and leaching of eco-friendly magnesium oxychloride cement paste at the micro-scale. Constr. Build. Mater. 2019, 204, 684–690. [Google Scholar] [CrossRef]
- Barnes, P.; Bensted, J. Structure and Performance of Cements; CRC Press: London, UK, 2002. [Google Scholar]
- Li, Z.; Chau, C. Influence of molar ratios on properties of magnesium oxychloride cement. Cem. Concr. Res. 2007, 37, 866–870. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Zheng, L.; Wen, J.; Wu, C.; Tan, Y. Compressive strength of fly ash magnesium oxychloride cement containing granite wastes. Constr. Build. Mater. 2013, 38, 1–7. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C. Comparison of glass powder and pulverized fuel ash for improving the water resistance of magnesium oxychloride cement. Cem. Concr. Compos. 2018, 86, 98–109. [Google Scholar] [CrossRef]
- Zhang, R.; Panesar, D.K. Sulfate resistance of carbonated ternary mortar blends: Portland cement, reactive MgO and supplementary cementitious materials. J. Clean. Prod. 2019, 238. [Google Scholar] [CrossRef]
- Zhang, R.; Panesar, D.K. Design of carbonated r–MgO–PC binder system: effect of r–MgO replacement level and water–to–binder ratio. J. Am. Ceram. Soc. 2019. [Google Scholar] [CrossRef]
- Zhang, R.; Bassim, N.; Panesar, D.K. Characterization of Mg components in reactive MgO–Portland cement blends during hydration and carbonation. J. CO2. Util. 2018, 27, 518–527. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Cho, D.-W.; Tsang, D.C.; Yang, J.; Hou, D.; Baek, K.; Kua, H.W.; Poon, C.-S. Novel synergy of Si-rich minerals and reactive MgO for stabilisation/solidification of contaminated sediment. J. Hazard. Mater. 2019, 365, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Tsang, D.C.; Kua, H.W.; Yang, J.; Ok, Y.S.; Ding, S.; Hou, D.; Poon, C.S. The roles of biochar as green admixture for sediment-based construction products. Cem. Concr. Compos. 2019. [Google Scholar] [CrossRef]
- Wang, L.; Iris, K.; Tsang, D.C.; Yu, K.; Li, S.; Poon, C.S.; Dai, J.-G. Upcycling wood waste into fibre-reinforced magnesium phosphate cement particleboards. Constr. Build. Mater. 2018, 159, 54–63. [Google Scholar] [CrossRef]
- Shand, M.A. The Chemistry and Technology of Magnesia; John Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kramer, D.A. Magnesium, its Alloys and Compounds. Industrial Minerals and Rocks; U.S. Geological Survey: Reston, VA, USA, 2001; pp. 1258–2331. [Google Scholar]
- Jin, F.; Al-Tabbaa, A. Characterisation of different commercial reactive magnesia. Adv. Cem. Res. 2014, 26, 101–113. [Google Scholar] [CrossRef]
- Dong, H.; Unluer, C.; Yang, E.-H.; Al-Tabbaa, A. Synthesis of reactive MgO from reject brine via the addition of NH4OH. Hydrometallurgy 2017, 169, 165–172. [Google Scholar] [CrossRef]
- Hassan, D. Environmental Sustainability Assessment and Associated Experimental Investigations of Magnesia Production Routes; University of Cambridge: Cambridge, UK, 2014. [Google Scholar]
- Dong, H.; Yang, E.-H.; Unluer, C.; Jin, F.; Al-Tabbaa, A. Investigation of the properties of MgO recovered from reject brine obtained from desalination plants. J. Clean. Prod. 2018, 196, 100–108. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of Mineralogy, Mineralogical Society of America; Mineral Data Publishing: Chantilly, VA, USA, 2003. [Google Scholar]
- CFR. Code of Federal Regulations Title 21; Food and Drug Administration: Washington, DC, USA, 2018. [Google Scholar]
- Sinka, M.; Van den Heede, P.; De Belie, N.; Bajare, D.; Sahmenko, G.; Korjakins, A. Comparative life cycle assessment of magnesium binders as an alternative for hemp concrete. Resour. Conserv. Recy. 2018, 133, 288–299. [Google Scholar] [CrossRef]
- Al-Breiki, M.; Bicer, Y. Thermodynamic analysis of theoretical dolomite formation from seawater and captured carbon dioxide. SN Appl. Sci. 2019, 1, 1272. [Google Scholar] [CrossRef]
- Ruan, S.; Unluer, C. Effect of air entrainment on the performance of reactive MgO and PC mixes. Constr. Build. Mater. 2017, 142, 221–232. [Google Scholar] [CrossRef]
- Ruan, S.; Liu, J.; Yang, E.-H.; Unluer, C. Performance and microstructure of calcined dolomite and reactive magnesia-based concrete samples. J. Mater. Civ. Eng. 2017, 29. [Google Scholar] [CrossRef]
- Ruan, S.; Unluer, C. Influence of supplementary cementitious materials on the performance and environmental impacts of reactive magnesia cement concrete. J. Clean. Prod. 2017, 159, 62–73. [Google Scholar] [CrossRef]
- Ruan, S.; Unluer, C. Influence of mix design on the carbonation, mechanical properties and microstructure of reactive MgO cement-based concrete. Cem. Concr. Compos. 2017, 80, 104–114. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C. Effect of pulverized fuel ash and CO2 curing on the water resistance of magnesium oxychloride cement (MOC). Cem. Concr. Res. 2017, 97, 115–122. [Google Scholar] [CrossRef]
- Power, I.M.; Dipple, G.M.; Francis, P.S. Assessing the carbon sequestration potential of magnesium oxychloride cement building materials. Cem. Concr. Compos. 2017, 78, 97–107. [Google Scholar] [CrossRef]
- Dong, H.; Unluer, C.; Yang, E.-H.; Al-Tabbaa, A. Recovery of reactive MgO from reject brine via the addition of NaOH. Desalination 2018, 429, 88–95. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Dong, J.; Wen, J.; Tan, Y. Reseach development on hydration product, phase transformation and water resistance evaluation method of magnesium oxychloride cement. J. Chin. Ceram. Soc. 2013, 41, 1465–1473. [Google Scholar]
- He, P.; Hossain, M.U.; Poon, C.S.; Tsang, D.C. Mechanical, durability and environmental aspects of magnesium oxychloride cement boards incorporating waste wood. J. Clean. Prod. 2019, 207, 391–399. [Google Scholar] [CrossRef]
- Wei, L.; Wang, Y.; Yu, J.; Xiao, J.; Xu, S. Feasibility study of strain hardening magnesium oxychloride cement-based composites. Constr. Build. Mater. 2018, 165, 750–760. [Google Scholar] [CrossRef]
- Finkbeiner, M.; Inaba, A.; Tan, R.; Christiansen, K.; Klüppel, H.-J. The new international standards for life cycle assessment: ISO 14040 and ISO 14044. Int. J. Life Cycle Assess. 2006, 11, 80–85. [Google Scholar] [CrossRef]
- Alejandre, F.; Flores-Ales, V.; Villegas, R.; Garcia-Heras, J.; Moron, E. Estimation of Portland cement mortar compressive strength using microcores. Influence of shape and size. Constr. Build. Mater. 2014, 55, 359–364. [Google Scholar] [CrossRef]
- Li, Z.J.; Qiao, F.; Chau, C.K. Recent Development of Magnesium-Based Cements-Magnesium Phosphate Cement and Magnesium Oxychloride Cement. Adv. Sci. Tech. 2010, 69, 21–30. [Google Scholar] [CrossRef]
- Ecoinvent. The Life Cycle Inventory Data; Swiss Centre for Life Cycle Inventories: Duebendorf, Switzerland, 2014. [Google Scholar]
- ETH-ESU. Ökoinventare von Energiesystemen; ETH Technical University of Zürich: Zurich, Switzerland, 1996. [Google Scholar]
- Ruan, S.; Unluer, C. Comparative life cycle assessment of reactive MgO and Portland cement production. J. Clean. Prod. 2016, 137, 258–273. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Shao, S.; Zhang, S. Comparative life cycle assessment of conventional and new fused magnesia production. J. Clean. Prod. 2015, 91, 170–179. [Google Scholar] [CrossRef]
- Spielmann, M.; Bauer, C.; Dones, R.; Tuchschmid, M. Life Cycle Inventories of Transport Services. Int. J. Life Cycle Assess. 2005, 10, 10–94. [Google Scholar] [CrossRef]
- Kellenberger, D.; Althaus, H.-J.; Jungbluth, N.; Künniger, T.; Lehmann, M.; Thalmann, P. Life Cycle Inventories of Building Products; Swiss Centre for Life Cycle Inventories: Duebendorf, Switzerland, 2007. [Google Scholar]
- Josa, A.; Aguado, A.; Heino, A.; Byars, E.; Cardim, A. Comparative analysis of available life cycle inventories of cement in the EU. Cem. Concr. Res. 2004, 34, 1313–1320. [Google Scholar] [CrossRef]
- ATILH. Methodology for Yearly Declaration of Pollutant Emissions in French Cement Industry; Hydraulic binder Industries Union; Association Technique des Liants Hydrauliques: Paris, France, 2003. (In French) [Google Scholar]
- Boesch, M.E.; Hellweg, S. Identifying improvement potentials in cement production with life cycle assessment. Environ. Sci. Technol. 2010, 44, 9143–9149. [Google Scholar] [CrossRef]
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A.; Ventura, A. LCA allocation procedure used as an incitative method for waste recycling: An application to mineral additions in concrete. Resour.Conserv. Recy. 2010, 54, 1231–1240. [Google Scholar] [CrossRef]
- Guardigli, L.; Monari, F.; Bragadin, M.A. Assessing environmental impact of green buildings through LCA methods: Acomparison between reinforced concrete and wood structures in the European context. Proc. Eng. 2011, 21, 1199–1206. [Google Scholar] [CrossRef]
- Ruan, S.; Unluer, C. Comparison of the environmental impacts of reactive magnesia and calcined dolomite and their performance under different curing conditions. J. Mater. Civ. Eng. 2018, 30. [Google Scholar] [CrossRef]
- Goedkoop, M.; Schryver, A.; Oele, M.; Durksz, S.; de Roest, D. Introduction to LCA with SimaPro 7; Pré Consultants: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Hofstetter, P.; Braunschweig, A.; Mettier, T.; Müller-Wenk, R.; Tietje, O. The mixing triangle: Correlation and graphical decision support for LCA–based comparisons. J. Ind. Ecol. 1999, 3, 97–115. [Google Scholar] [CrossRef]
- Sayagh, S.; Ventura, A.; Hoang, T.; Francois, D.; Jullien, A.S. Sensitivity of the LCA allocation procedure for BFS recycled into pavement structures. Resour. Conserv. Recy. 2010, 54, 348–358. [Google Scholar] [CrossRef]
- Sugimoto, K.; Dinnebier, R.E.; Schlecht, T. Structure determination of Mg3 (OH) 5Cl4·H2O (F5 phase) from laboratory powder diffraction data and its impact on the analysis of problematic magnesia floors. Acta Crystallogr. Sect. B Struct. Sci. 2007, 63, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Damineli, B.L.; Kemeid, F.M.; Aguiar, P.S.; John, V.M. Measuring the eco-efficiency of cement use. Cem. Concr. Compos. 2010, 32, 555–562. [Google Scholar] [CrossRef]
- Kastiukas, G.; Zhou, X.; Neyazi, B.; Wan, K.T. Sustainable Calcination of Magnesium Hydroxide for Magnesium Oxychloride Cement Production. J. Mater. Civ. Eng. 2019, 31. [Google Scholar] [CrossRef]
- Damtoft, J.S.; Lukasik, J.; Herfort, D.; Sorrentino, D.; Gartner, E.M. Sustainable development and climate change initiatives. Cem. Concr. Res. 2008, 38, 115–127. [Google Scholar] [CrossRef]
- Kim, J.-K.; Moon, Y.-H.; Eo, S.-H. Compressive strength development of concrete with different curing time and temperature. Cem. Concr. Res. 1998, 28, 1761–1773. [Google Scholar] [CrossRef]

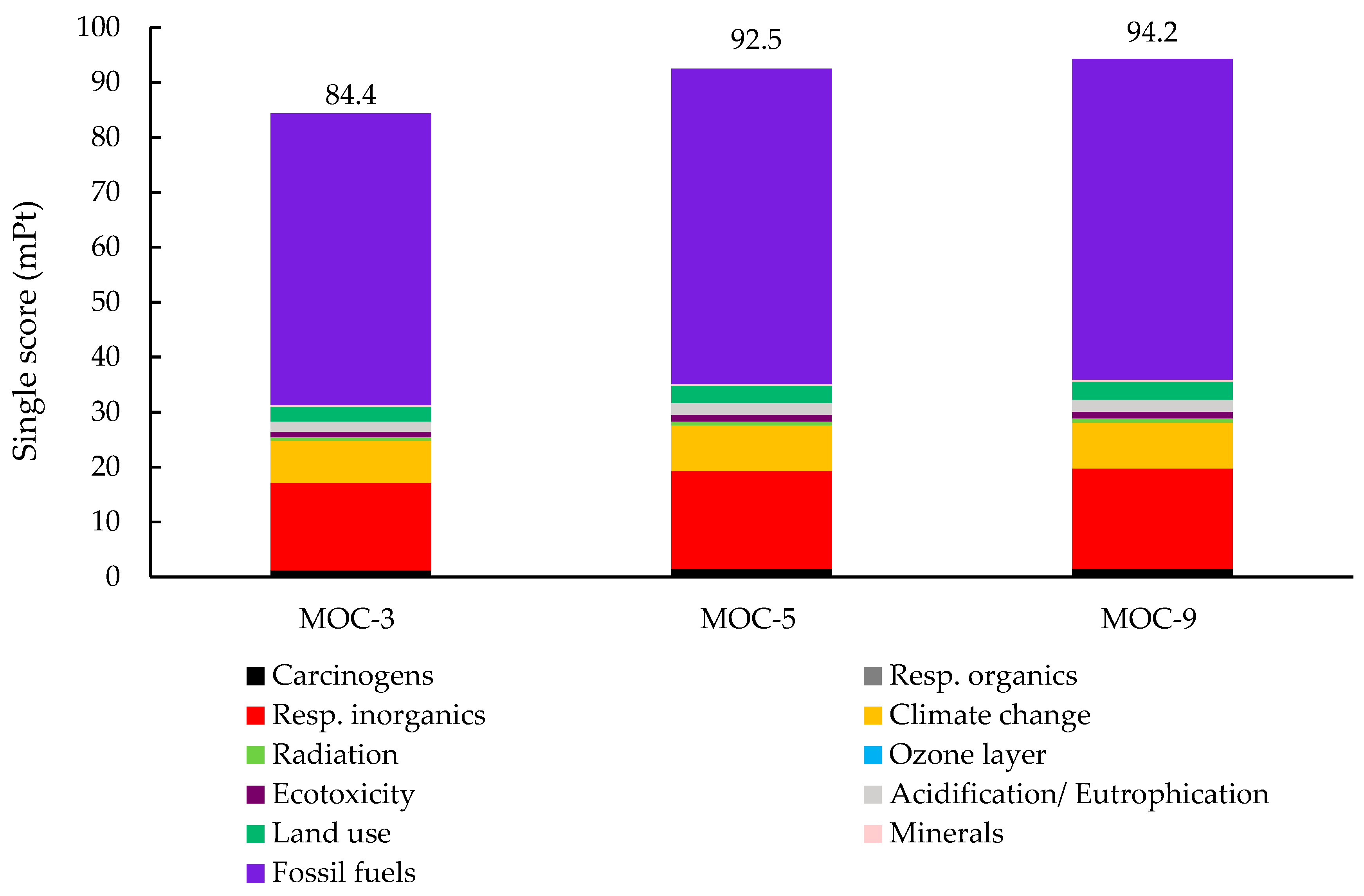
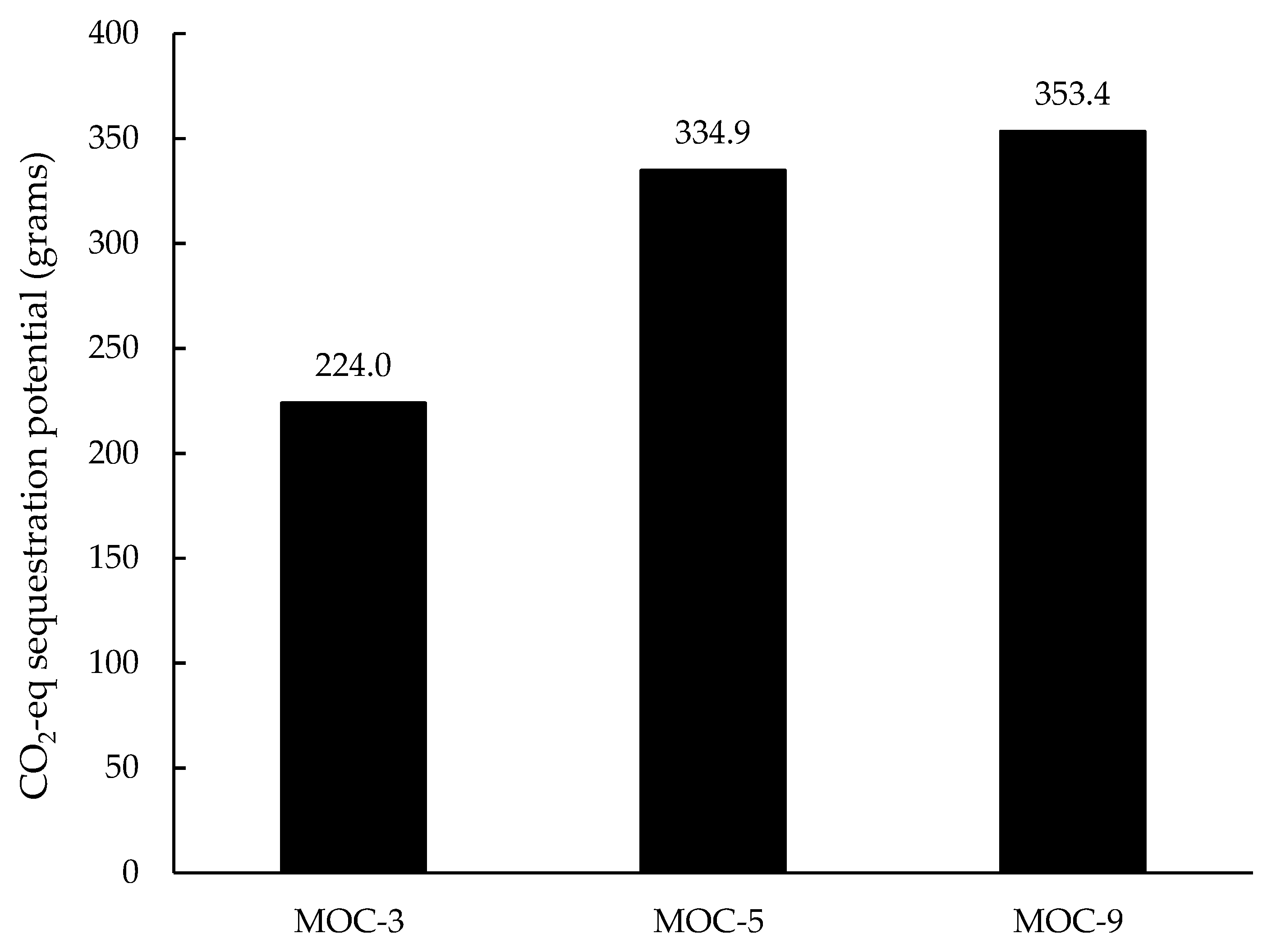


| w/s Ratio | Water (g) | MgO/MgCl2*6H2O Molar Ratio | MgO (g) | MgCl2*6H2O (g) |
|---|---|---|---|---|
| 0.22 | 181.9 | 3 | 305.1 | 513.0 |
| 0.18 | 151.2 | 5 | 422.5 | 426.3 |
| 0.34 | 253.1 | 9 | 475.3 | 269.1 |
| Input/Emissions | Amount |
|---|---|
| Raw Materials | |
| Magnesite (tonne) | 2.17 |
| Water (tonne) | 0.01 |
| Energy | |
| Coal (tonne) | 0.27 |
| Electricity (kWh) | 8.67 |
| Diesel (tonne) | 0.54 |
| Emissions | |
| CO2 (kg) | 1096.54 |
| SO2 (kg) | 4.21 |
| CO (kg) | 0.02 |
| NOx (kg) | 0.01 |
| Water vapour (m3) | 0.01 |
| Magnesite dust (kg) | 0.99 |
| Raw Materials | |
| Limestone (tonne) | 1.32 |
| Clay (tonne) | 0.32 |
| Sand (tonne) | 0.07 |
| Iron ore (tonne) | 0.01 |
| Gypsum (tonne) | 0.05 |
| Water (tonne) | 0.53 |
| Energy | |
| Coal (tonne) | 5.53E-09 |
| Fuel oil (tonne) | 1.53E-09 |
| Electricity (kWh) | 71.92 |
| Petroleum coke (tonne) | 0.1 |
| Diesel (tonne) | 9E-7 |
| Emissions | |
| CO2 (kg) | 832.61 |
| SO2 (kg) | 0.63 |
| CO (kg) | 1.96 |
| NOx (kg) | 1.79 |
| Cement kiln dust (kg) | 0.87 |
| Particulate matter (kg) | 0.03 |
| Input/Emissions | PFA |
|---|---|
| Water (m3) | 0.01 |
| Electricity (kwh) | 6.8 |
| Natural gas (MJ) | 290.0 |
| Fuel oil (kg) | 1.0 |
| SO2 (kg) | 9.1 × 10−5 |
| NOx (kg) | 1.8 × 10−2 |
| Particulate matter (kg) | 3.2 × 10−2 |
| CO (kg) | 9.1 × 10−3 |
| Sample | w/s Ratio | Water (g) | PC (g) | MgO (g) | MgCl2*6H2O (g) | PFA (g) | Aggregates (g) |
|---|---|---|---|---|---|---|---|
| MOC concrete | 0.34 | 80.4 | - | 151.2 | 85.3 | 101.3 | 591.2 |
| PC concrete | 0.34 | 79.3 | 234.2 | - | - | 100.5 | 585.5 |
| Sample | Flexural Strength (MPa) | Compressive Strength (MPa) |
|---|---|---|
| MOC-3 | 38.6 | 71.8 |
| MOC-5 | 19.6 | 93 |
| MOC-9 | 14.2 | 111 |
| Reaction | Main and by Product | Mass Produced (kg) | Allocation by Mass (%) | Market Price a (GBP/kg) | Allocation by Economic Value (%) |
|---|---|---|---|---|---|
| Seawater (alkali base: CaO) | Mg(OH)2 | 1 | 34.3 | 117 | 61.5 |
| CaCl2 | 1.91 | 65.7 | 73.2 | 38.5 | |
| Seawater (alkali base: NaOH) | Mg(OH)2 | 1 | 33.1 | 117 | 66.7 |
| NaCl | 2.01 | 66.9 | 28.9 | 33.3 | |
| Seawater (alkali base: NH4OH) | Mg(OH)2 | 1 | 35.1 | 117 | 56.7 |
| NH4Cl | 1.84 | 64.9 | 48.6 | 43.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kastiukas, G.; Ruan, S.; Unluer, C.; Liang, S.; Zhou, X. Environmental Assessment of Magnesium Oxychloride Cement Samples: A Case Study in Europe. Sustainability 2019, 11, 6957. https://doi.org/10.3390/su11246957
Kastiukas G, Ruan S, Unluer C, Liang S, Zhou X. Environmental Assessment of Magnesium Oxychloride Cement Samples: A Case Study in Europe. Sustainability. 2019; 11(24):6957. https://doi.org/10.3390/su11246957
Chicago/Turabian StyleKastiukas, Gediminas, Shaoqin Ruan, Cise Unluer, Shuang Liang, and Xiangming Zhou. 2019. "Environmental Assessment of Magnesium Oxychloride Cement Samples: A Case Study in Europe" Sustainability 11, no. 24: 6957. https://doi.org/10.3390/su11246957
APA StyleKastiukas, G., Ruan, S., Unluer, C., Liang, S., & Zhou, X. (2019). Environmental Assessment of Magnesium Oxychloride Cement Samples: A Case Study in Europe. Sustainability, 11(24), 6957. https://doi.org/10.3390/su11246957







