Life Cycle Assessment for Bioethanol Production from Oil Palm Frond Juice in an Oil Palm Based Biorefinery
Abstract
:1. Introduction
2. Materials and Methods
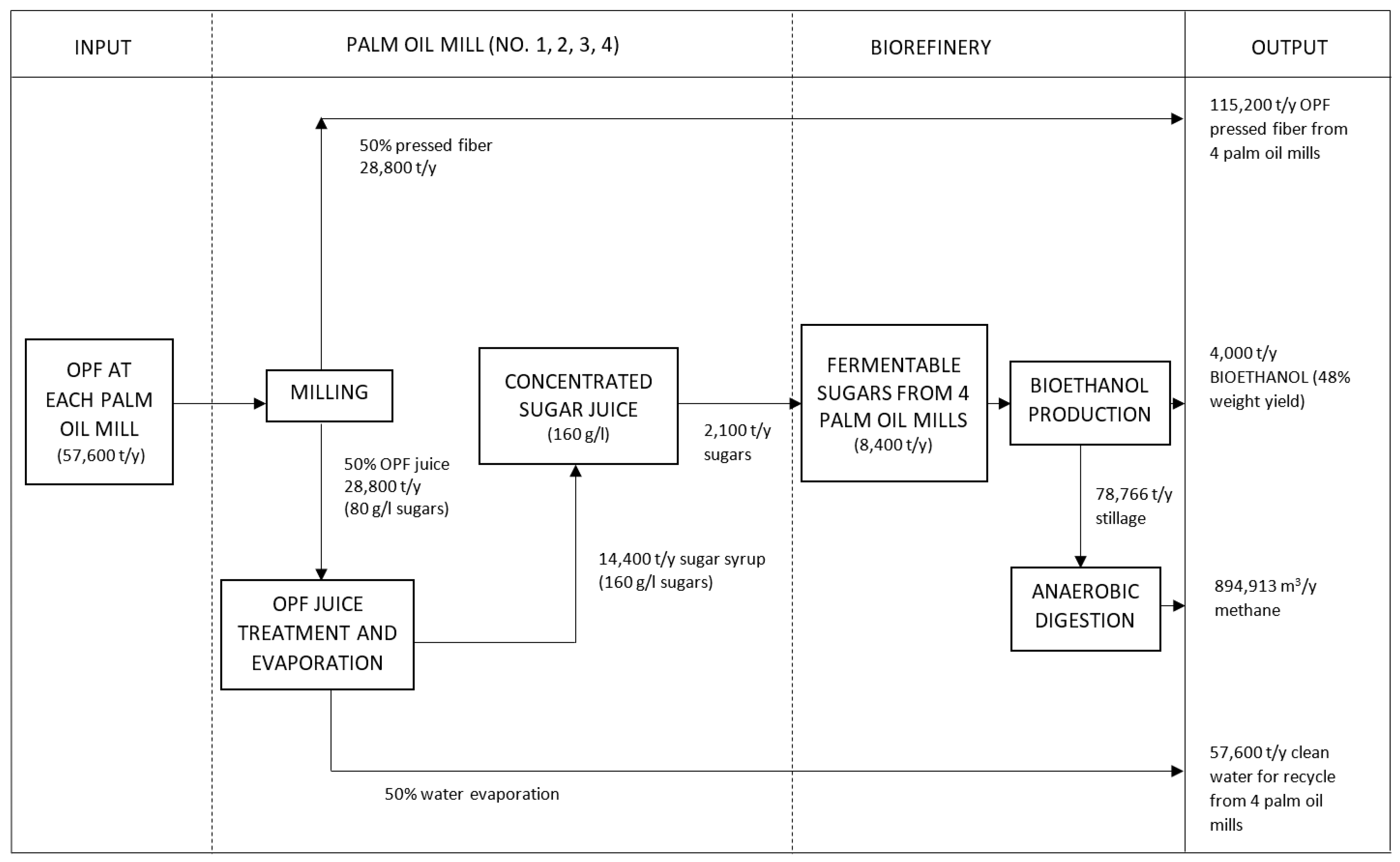
2.1. Process Description
2.1.1. Collection and Transportation of OPF Petioles and Sugar Juice
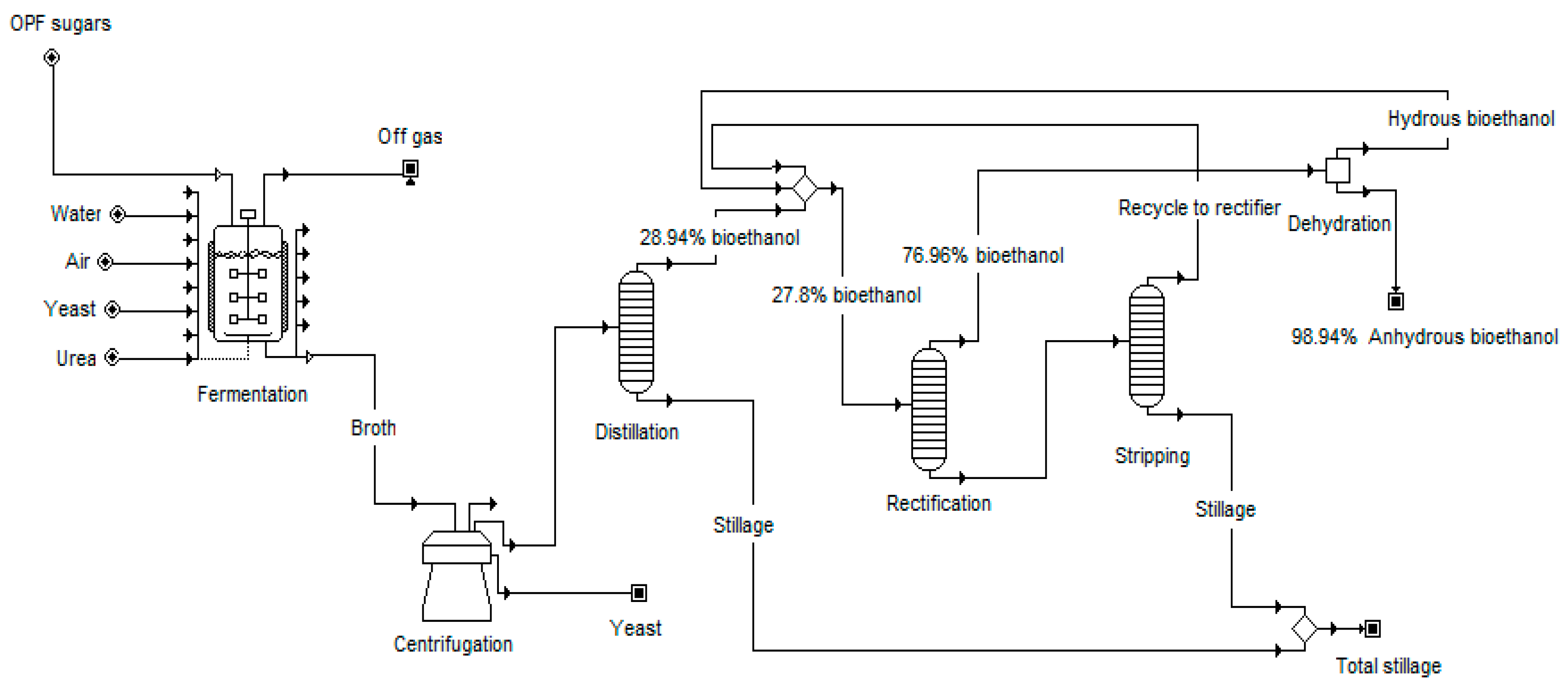
2.1.2. Juice Extraction and Treatment
2.1.3. Ethanol Fermentation and Purification
2.1.4. Waste Management
2.1.5. Energy Consumption
2.2. Life Cycle Assessment (LCA)
2.2.1. Scope of Study and Functional Unit
2.2.2. Inventory Analysis
2.2.3. Assumptions
2.2.4. Characterization Model and Impact Categories
3. Results and Discussion
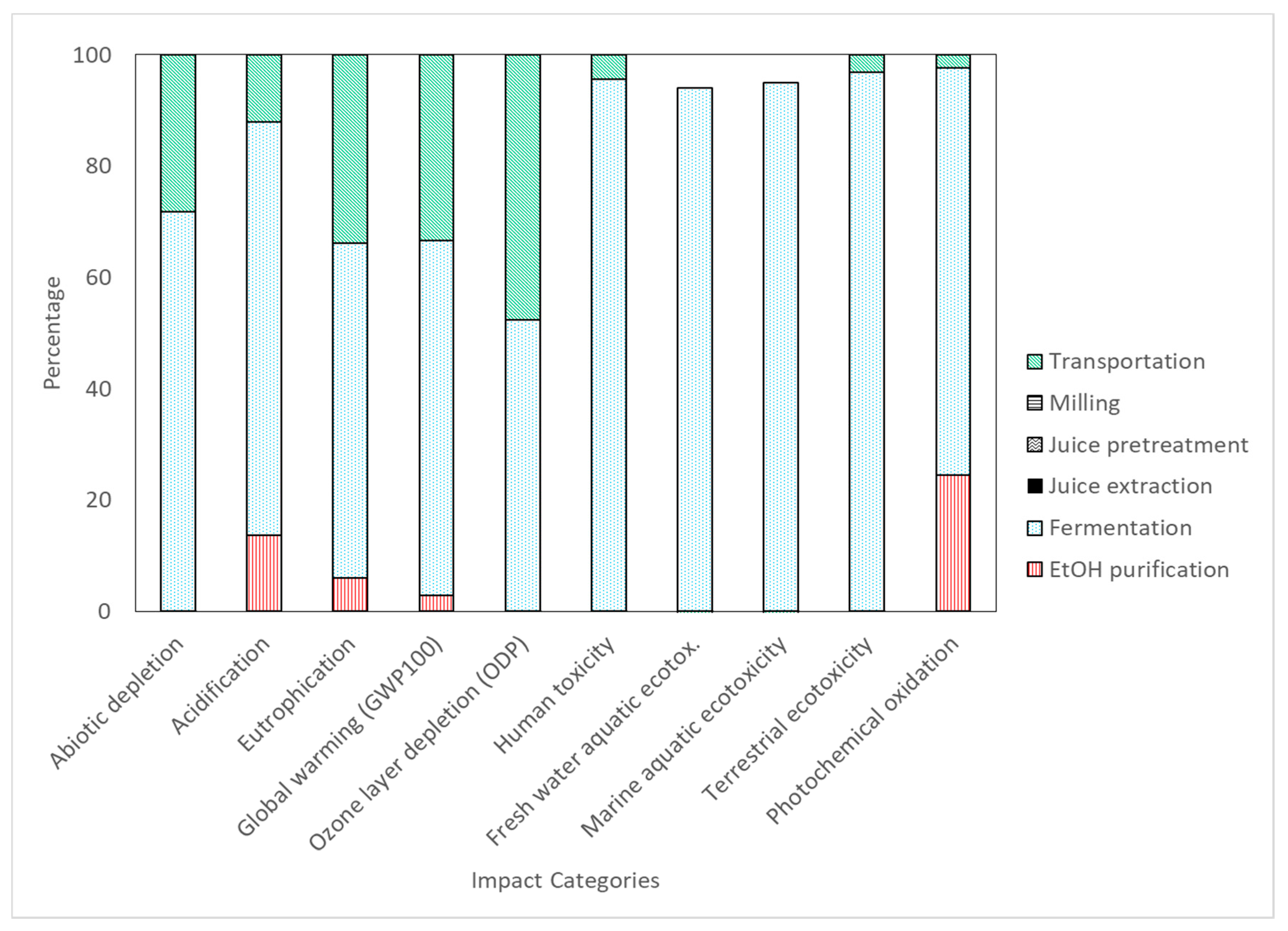
Life Cycle Impact Assessment
- Transportation: 236,700 tonnes of OPF petioles and sugars;
- Milling: 115,200 tonnes of OPF pressed fiber;
- OPF juice extraction: 115,200 tonnes of OPF juice;
- OPF juice treatment: 57,600 tonnes of OPF juice;
- Fermentation: 3984 tonnes of bioethanol;
- Bioethanol purification: 3971 tonnes of bioethanol.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- U.S. Energy Information Administration. International Energy Outlook 2017; Energy Information Administration: Washington, DC, USA, 2017; pp. 1–151.
- Roy, P.; Orikasa, T.; Tokuyasu, K.; Nakamura, N.; Shiina, T. Evaluation of the life cycle of bioethanol produced from rice straws. Bioresour. Technol. 2012, 110, 239–244. [Google Scholar] [CrossRef] [PubMed]
- US Energy Information Administration. International Energy Outlook 2016; Chapter 1: World Energy Demand and Economic Outlook 2016; Energy Information Administration: Washington, DC, USA, 2016; pp. 7–17.
- Morales, M.; Quintero, J.; Conejeros, R.; Aroca, G. Life cycle assessment of lignocellulosic bioethanol: Environmental impacts and energy balance. Renew. Sustain. Energy Rev. 2015, 42, 1349–1361. [Google Scholar] [CrossRef]
- Borrion, A.L.; McManus, M.C.; Hammond, G.P. Environmental life cycle assessment of lignocellulosic conversion to ethanol: A review. Renew. Sustain. Energy Rev. 2012, 16, 4638–4650. [Google Scholar] [CrossRef]
- Pourbafrani, M.; MacLean, H.L.; McKechnie, J.; Saville, B.A. Life cycle greenhouse gas impacts of ethanol, biomethane and limonene production from citrus waste. Environ. Res. Lett. 2013, 8, 015007. [Google Scholar] [CrossRef]
- Loh, S.K. The potential of the Malaysian oil palm biomass as a renewable energy source. Energy Convers. Manag. 2017, 141, 285–298. [Google Scholar] [CrossRef]
- Lee, K.T.; Ofori-Boateng, C. Sustainability of Biofuel Production from Oil Palm Biomass; Springer Science and Business Media LLC: Berlin, Germany, 2013. [Google Scholar]
- Abdullah, S.S.S.; Shirai, Y.; Ali, A.A.M.; Mustapha, M.; Hassan, M.A. Case study: Preliminary assessment of integrated palm biomass biorefinery for bioethanol production utilizing non-food sugars from oil palm frond petiole. Energy Convers. Manag. 2016, 108, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Ofori-Boateng, C.; Lee, K.T. Sono-assisted organosolv/H2O2 pretreatment of oil palm (Elaeis guineensis Jacq.) fronds for recovery of fermentable sugars: Optimization and severity evaluation. Fuel 2014, 115, 170–178. [Google Scholar] [CrossRef]
- Lee, K.T.; Ofori-Boateng, C. Advances in Biofuels; Pogaku, R., Sarbatly, R.H., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Zahari, M.A.K.M.; Ariffin, H.; Mokhtar, M.N.; Salihon, J.; Shirai, Y.; Hassan, M.A. Case study for a palm biomass biorefinery utilizing renewable non-food sugars from oil palm frond for the production of poly(3-hydroxybutyrate) bioplastic. J. Clean. Prod. 2015, 87, 284–290. [Google Scholar] [CrossRef]
- Abdullah, S.S.S.; Shirai, Y.; Bahrin, E.K.; Hassan, M.A. Fresh oil palm frond juice as a renewable, non-food, non-cellulosic and complete medium for direct bioethanol production. Ind. Crop. Prod. 2015, 63, 357–361. [Google Scholar] [CrossRef]
- Zahari, M.A.K.M.; Abdullah, S.S.S.; Roslan, A.M.; Ariffin, H.; Shirai, Y.; Hassan, M.A. Efficient utilization of oil palm frond for bio-based products and biorefinery. J. Clean. Prod. 2014, 65, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Zahari, M.A.K.M.; Zakaria, M.R.; Ariffin, H.; Mokhtar, M.N.; Salihon, J.; Shirai, Y.; Hassan, M.A. Renewable sugars from oil palm frond juice as an alternative novel fermentation feedstock for value-added products. Bioresour. Technol. 2012, 110, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.S.; Tan, H.T.; Lee, K.T. Pretreatment of oil palm frond using hot compressed water: An evaluation of compositional changes and pulp digestibility using severity factors. Bioresour. Technol. 2012, 110, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.R.; Fujimoto, S.; Hirata, S.; Hassan, M.A. Ball Milling Pretreatment of Oil Palm Biomass for Enhancing Enzymatic Hydrolysis. Appl. Biochem. Biotechnol. 2014, 173, 1778–1789. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.R.; Hirata, S.; Hassan, M.A. Hydrothermal pretreatment enhanced enzymatic hydrolysis and glucose production from oil palm biomass. Bioresour. Technol. 2015, 176, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Sabiha-Hanim, S.; Noor, M.A.M.; Rosma, A. Effect of autohydrolysis and enzymatic treatment on oil palm (Elaeis guineensis Jacq.) frond fibres for xylose and xylooligosaccharides production. Bioresour. Technol. 2011, 102, 1234–1239. [Google Scholar] [CrossRef]
- Scown, C.D.; Nazaroff, W.W.; Mishra, U.; Strogen, B.; Lobscheid, A.B.; Masanet, E.; Santero, N.J.; Horvath, A.; Mckone, T.E. Corrigendum: Lifecycle greenhouse gas implications of US national scenarios for cellulosic ethanol production. Environ. Res. Lett. 2012, 7, 019502. [Google Scholar] [CrossRef]
- Ometto, A.R.; Hauschild, M.Z.; Roma, W.N.L. Lifecycle assessment of fuel ethanol from sugarcane in Brazil. Int. J. Life Cycle Assess. 2009, 14, 236–247. [Google Scholar] [CrossRef]
- Muñoz, I.; Flury, K.; Jungbluth, N.; Rigarlsford, G.I.; Canals, L.M.; King, H. Life cycle assessment of bio-based ethanol produced from different agricultural feedstocks. Int. J. Life Cycle Assess. 2014, 19, 109–119. [Google Scholar] [CrossRef]
- Wang, M.; Han, J.; Dunn, J.B.; Cai, H.; Elgowainy, A. Well-to-wheels energy use and greenhouse gas emissions of ethanol from corn, sugarcane and cellulosic biomass for US use. Environ. Res. Lett. 2012, 7, 045905. [Google Scholar] [CrossRef] [Green Version]
- Borrion, A.L.; McManus, M.C.; Hammond, G.P. Environmental life cycle assessment of bioethanol production from wheat straw. Biomass Bioenergy 2012, 47, 9–19. [Google Scholar] [CrossRef]
- Wang, L.; Littlewood, J.; Murphy, R.J. An economic and environmental evaluation for bamboo-derived bioethanol. RSC Adv. 2014, 4, 29604–29611. [Google Scholar] [CrossRef]
- Roslan, A.M. Oil Palm Frond Petiole Conversion into Biosugars and Bioethanol. Ph.D. Thesis, Kyushu Institute of Technology, Kitakyushu, Japan, 2014. [Google Scholar]
- Roslan, A.M.; Zahari, M.A.K.M.; Hassan, M.A.; Shirai, Y. Investigation of oil palm frond properties for use as biomaterials and biofuels. Trop. Agric. Dev. 2014, 58, 26–29. [Google Scholar]
- Dias, M.O.; Ensinas, A.V.; Nebra, S.A.; Filho, R.M.; Rossell, C.E.; Maciel, M.R.W. Production of bioethanol and other bio-based materials from sugarcane bagasse: Integration to conventional bioethanol production process. Chem. Eng. Res. Des. 2009, 87, 1206–1216. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Hu, B. Vinasse from sugarcane ethanol production: Better treatment or better utilization? Front. Energ. Res. 2017, 5, 1–17. [Google Scholar]
- Wilkie, A.C.; Riedesel, K.J.; Owens, J.M. Stillage characterization and anaerobic treatment of ethanol stillage from conventional and cellulosic feedstocks. Biomass Bioenergy 2000, 19, 63–102. [Google Scholar] [CrossRef]
- Sebastião, D.; Gonçalves, M.S.; Marques, S.; Fonseca, C.; Gírio, F.; Oliveira, A.C.; Matos, C.T. Life cycle assessment of advanced bioethanol production from pulp and paper sludge. Bioresour. Technol. 2016, 208, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Krzywonos, M.; Cibis, E.; Miskiewicz, T.; Ryznar-Luty, A. Utilization and biodegradation of starch stillage (distillery wastewater). Electron. J. Biotechnol. 2009, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kaparaju, P.; Serrano, M.; Angelidaki, I. Optimization of biogas production from wheat straw stillage in UASB reactor. Appl. Energy 2010, 87, 3779–3783. [Google Scholar] [CrossRef]
- Parsaee, M.; Kiani, M.K.D.; Karimi, K. A review of biogas production from sugarcane vinasse. Biomass Bioenergy 2019, 122, 117–125. [Google Scholar] [CrossRef]
- Tian, Z.; Mohan, G.R.; Ingram, L.; Pullammanappallil, P. Anaerobic digestion for treatment of stillage from cellulosic bioethanol production. Bioresour. Technol. 2013, 144, 387–395. [Google Scholar] [CrossRef]
- Nasrin, A.; Ravi, N.; Lim, W.; Choo, Y.; Fadzil, A. Assessment of the performance and potential export renewable energy (RE) from typical cogeneration plants used in palm oil mills. J. Eng. Appl. Sci. 2011, 6, 433–439. [Google Scholar]
- Yoshizaki, T.; Shirai, Y.; Hassan, M.A.; Baharuddin, A.S.; Abdullah, N.M.R.; Sulaiman, A.; Busu, Z. Improved economic viability of integrated biogas energy and compost production for sustainable palm oil mill management. J. Clean. Prod. 2013, 44, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ensinas, A.V.; Nebra, S.A.; Lozano, M.A.; Serra, L.M. Analysis of process steam demand reduction and electricity generation in sugar and ethanol production from sugarcane. Energy Convers. Manag. 2007, 48, 2978–2987. [Google Scholar] [CrossRef]
- Renó, M.L.G.; Almazán del Olmo, O.A.; Palacio, J.C.E.; Lora, E.E.S.; Venturini, O.J. Sugarcane biorefineries: Case studies applied to the Brazilian sugar—Alcohol industry. Energy Convers. Manag. 2014, 86, 981–991. [Google Scholar]
- Wang, L.; Littlewood, J.; Murphy, R.J. Environmental sustainability of bioethanol production from wheat straw in the UK. Renew. Sustain. Energy Rev. 2013, 28, 715–725. [Google Scholar] [CrossRef] [Green Version]
- Chau, C.-K.; Leung, T.; Ng, W. A review on Life Cycle Assessment, Life Cycle Energy Assessment and Life Cycle Carbon Emissions Assessment on buildings. Appl. Energy 2015, 143, 395–413. [Google Scholar] [CrossRef]
- Chiew, Y.L.; Shimada, S. Current state and environmental impact assessment for utilizing oil palm empty fruit bunches for fuel, fiber and fertilizer—A case study of Malaysia. Biomass Bioenergy 2013, 51, 109–124. [Google Scholar] [CrossRef]
- Kaparaju, P.; Serrano, M.; Thomsen, A.B.; Kongjan, P.; Angelidaki, I. Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour. Technol. 2009, 100, 2562–2568. [Google Scholar] [CrossRef]
- Kemppainen, A.J.; Shonnard, D. Life cycle assessments for biomass-to-ethanol production from different regional feedstock. Biotechnol. Prog. 2005, 21, 1075–1084. [Google Scholar] [CrossRef]
- Prasad, A.; Sotenko, M.; Blenkinsopp, T.; Coles, S.R. Life cycle assessment of lignocellulosic biomass pretreatment methods in biofuel production. Int. J. Life Cycle Assess. 2016, 21, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Chen, Y.; Xia, X.; Li, J.; Liu, J. Energy efficiency and environmental performance of bioethanol production from sweet sorghum stem based on life cycle analysis. Bioresour. Technol. 2014, 163, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Bensah, E.C.; Mensah, M. Chemical Pretreatment Methods for the Production of Cellulosic Ethanol: Technologies and Innovations. Int. J. Chem. Eng. 2013, 2013, 1–21. [Google Scholar] [CrossRef]

| Description | Value | Reference |
|---|---|---|
| Power demand for OPF preparation and juice extraction | 16 kWh/t OPF | [38] |
| Steam demand for OPF juice evaporation (three multiple effects evaporators) | 86.6 kg/t OPF | [9] |
| Steam demand for sterilization of sugar syrup | 563 kg/t sugar | [9] |
| Steam demand for distillation and dehydration of hydrous bioethanol | 2.55 kg/l bioethanol | [38] |
| Power demand for bioethanol production | 12 kWh/t sugar | [38,39] |
| Process | Electricity Consumption (GWh/year) | Steam Consumption (tonne/year) |
|---|---|---|
| Milling and juice extraction | 0.92 | - |
| Juice pretreatment | - | 4988 |
| Fermentation | 0.10 | 4696 |
| Ethanol purification | - | 13,005 |
| Total | 1.02 | 22,689 |
| Values | References | |
|---|---|---|
| Transportation | ||
| Input | ||
| Trucks, 6 tonne capacity, tkm/year | 720,000 | This study |
| Trucks, 20 tonne capacity, tkm/year | 504,000 | This study |
| Bioethanol production | ||
| Input | ||
| OPF petiole, tonne/year | 230,400 | [9] |
| OPF juice, tonne/year | 115,200 | [9] |
| OPF petiole sugars, tonne/year | 8400 | [9] |
| Urea, tonne/year | 193 | This study |
| Air, tonne/year | 168,401 | This study |
| River water, tonne/year | 83,927 | This study |
| Output | ||
| OPF pressed fiber, tonne/year | 115,200 | [9] |
| Bioethanol, tonne/year | 4000 | This study |
| Methane, m3/year | 894,913 | This study |
| Emissions | ||
| CO2, tonne/year | 4074 | This study |
| N2, tonne/year | 129,266 | This study |
| O2, tonne/year | 39,243 | This study |
| Stillage, tonne/year | 78,766 | This study |
| Water for recycle, tonne/year | 57,600 | [9] |
| Impact Category | Unit | Ethanol Purification | Fermentation | Juice Extraction | Juice Pretreatment | Milling | Transportation |
|---|---|---|---|---|---|---|---|
| Abiotic depletion | kg Sb eq | 5.30E-03 | 1.29E+00 | 0.00E+00 | 0.00E+00 | 0.00E+00 | 5.08E-01 |
| Acidification | kg SO2 eq | 1.83E-01 | 9.89E-01 | 0.00E+00 | 0.00E+00 | 0.00E+00 | 1.61E-01 |
| Eutrophication | kg PO4 eq | 9.53E-03 | 9.37E-02 | 0.00E+00 | 0.00E+00 | 0.00E+00 | 5.29E-02 |
| Global warming (GWP100) | kg CO2 eq | 6.38E+00 | 1.43E+02 | 0.00E+00 | 0.00E+00 | 0.00E+00 | 7.46E+01 |
| Ozone layer depletion | kg CFC–11 eq | 5.00E-08 | 6.00E-06 | 0.00E+00 | 0.00E+00 | 0.00E+00 | 5.50E-06 |
| Human toxicity | kg 1,4–DB eq | 1.28E-01 | 5.78E+01 | 0.00E+00 | 0.00E+00 | 0.00E+00 | 2.61E+00 |
| Fresh water aquatic ecotoxicity. | kg 1,4–DB eq | 3.50E-03 | 6.47E+00 | 0.00E+00 | 0.00E+00 | 0.00E+00 | -4.07E-01 |
| Marine aquatic ecotoxicity | kg 1,4–DB eq | 8.49E+00 | 3.49E+04 | 0.00E+00 | 0.00E+00 | 0.00E+00 | -1.84E+03 |
| Terrestrial ecotoxicity | kg 1,4–DB eq | 2.83E-04 | 1.28E+00 | 0.00E+00 | 0.00E+00 | 0.00E+00 | 4.10E-02 |
| Photochemical oxidation | kg C2H4 eq | 1.28E-02 | 3.83E-02 | 0.00E+00 | 0.00E+00 | 0.00E+00 | 1.21E-03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd YUSOF, S.J.H.; Roslan, A.M.; Ibrahim, K.N.; Syed ABDULLAH, S.S.; Zakaria, M.R.; Hassan, M.A.; Shirai, Y. Life Cycle Assessment for Bioethanol Production from Oil Palm Frond Juice in an Oil Palm Based Biorefinery. Sustainability 2019, 11, 6928. https://doi.org/10.3390/su11246928
Mohd YUSOF SJH, Roslan AM, Ibrahim KN, Syed ABDULLAH SS, Zakaria MR, Hassan MA, Shirai Y. Life Cycle Assessment for Bioethanol Production from Oil Palm Frond Juice in an Oil Palm Based Biorefinery. Sustainability. 2019; 11(24):6928. https://doi.org/10.3390/su11246928
Chicago/Turabian StyleMohd YUSOF, Siti Jamilah Hanim, Ahmad Muhaimin Roslan, Khairul Nadiah Ibrahim, Sharifah Soplah Syed ABDULLAH, Mohd Rafein Zakaria, Mohd Ali Hassan, and Yoshihito Shirai. 2019. "Life Cycle Assessment for Bioethanol Production from Oil Palm Frond Juice in an Oil Palm Based Biorefinery" Sustainability 11, no. 24: 6928. https://doi.org/10.3390/su11246928
APA StyleMohd YUSOF, S. J. H., Roslan, A. M., Ibrahim, K. N., Syed ABDULLAH, S. S., Zakaria, M. R., Hassan, M. A., & Shirai, Y. (2019). Life Cycle Assessment for Bioethanol Production from Oil Palm Frond Juice in an Oil Palm Based Biorefinery. Sustainability, 11(24), 6928. https://doi.org/10.3390/su11246928





