Soil Microbiota of Dystric Cambisol in the High Tatra Mountains (Slovakia) after Windthrow
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Soil Sampling
2.2. Basic Chemical Analyses
2.3. DNA Extraction and PCR Amplification
2.4. High-Throughput Sequencing
2.5. Bioinformatic Evaluation
2.6. Isolation and Cultivation of Soil Filamentous Fungi Including Heat-Resistant Species
3. Results and Discussion
3.1. Soil Characteristics
3.2. High-Throughput Sequencing Analysis of Bacterial 16S rRNA Gene
3.3. High-Throughput Sequencing Analysis of Fungal ITS Fragment
3.3.1. The Most Abundant Fungal Taxa in All Investigated Samples
3.3.2. The Significant Abundance of Fungal Taxa on the NEX Locality
3.3.3. The Significant Abundance of Fungal Taxa on the FIR Locality
3.3.4. The Significant Abundance of Fungal Taxa on the EXT and REF Localities
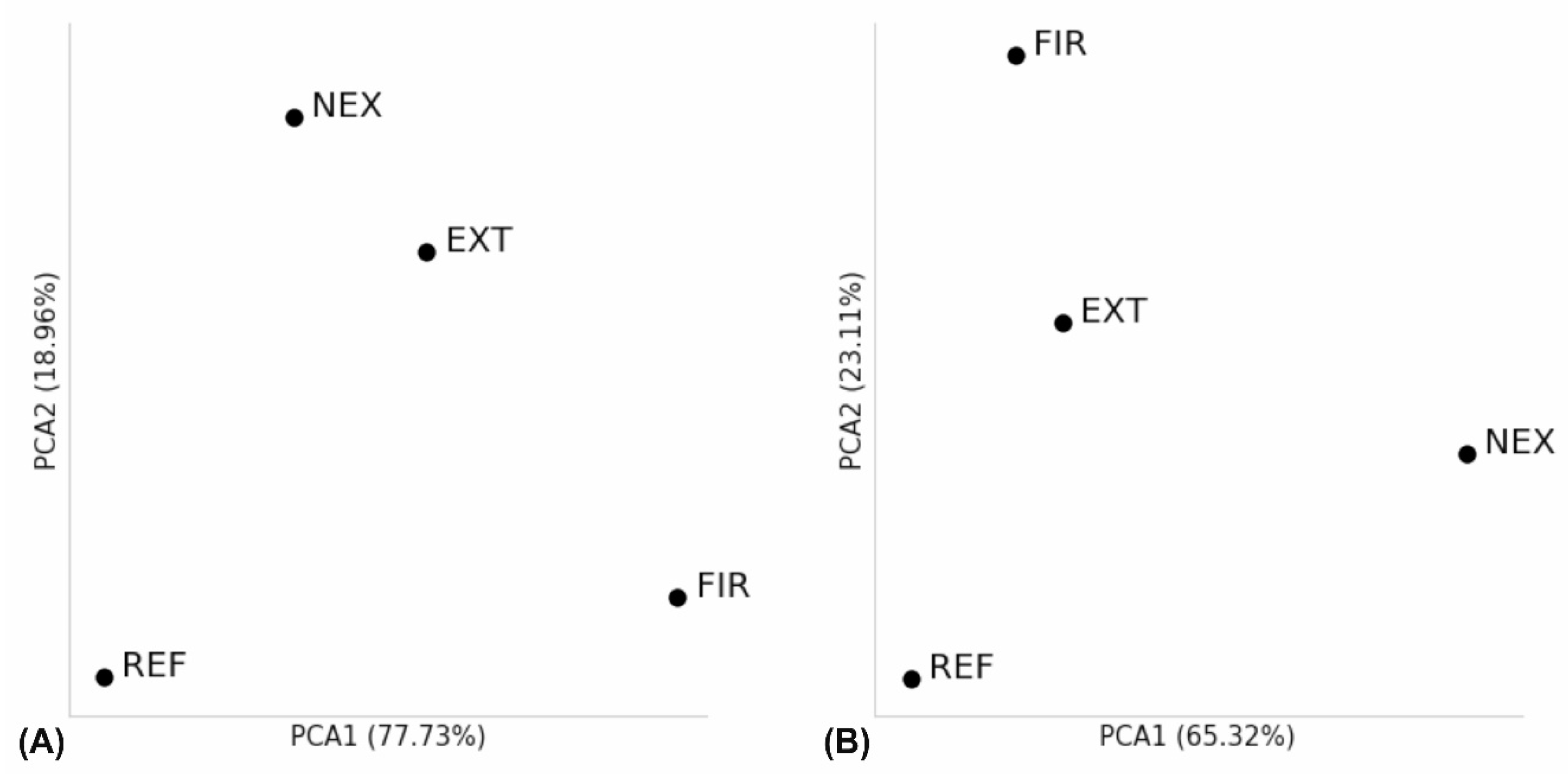
3.4. Alpha and Beta Diversity Analysis
3.5. Cultivable Soil Filamentous Fungi
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Perry, D.A.; Oren, R.; Hart, C. Forest Ecosystems; Johns Hopkins University Press: Baltimore, MD, USA, 2008. [Google Scholar]
- Emerton, L.; Bishop, J.; Thomas, L. Sustainable Financing of Protected Areas: A Global Review of Challenges and Options; IUCN: Gland, Switzerland; Cambridge, UK, 2006; 97p. [Google Scholar]
- Füssel, H.M.; Klein, R.J.T. Climate change vulnerability assessments: An evolution of conceptual thinking. Clim. Chang. 2006, 75, 301–329. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolstro, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Goetz, S.J.; Bond-Lamberty, B.; Law, B.E.; Hicke, J.A.; Huang, C.; Houghton, R.A. Observations and assessment of forest carbon dynamics following disturbance in North America. J. Geophys. Res. Biogeosci. 2012, 117, 1–17. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W.; Blennow, K. Simulating wind disturbance impacts on forest landscapes: Tree-level heterogeneity matters. Environ. Model. Softw. 2014, 51, 1–11. [Google Scholar] [CrossRef]
- European Environment Agency. European Forest Ecosystems State and Trends; EEA Report No 5/2016; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- San-Miguel-Ayanz, J.; Durrant, T.; Boca, R.; Libertà, G.; Branco, A.; de Rigo, D.; Ferrari, D.; Maianti Vivancos, T.A.; Costa, H.; Lana, F.; et al. Forest Fires in Europe, Middle East and North Africa 2017; Joint Research Centre—Technical Reports; European Commission: Ispra, Italy, 2017; 142p. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; González-Vila, J.F.; Almendros, G.; Knicker, H. The effect of fire on soil organic matter—A review. Environ. Int. 2004, 30, 855–870. [Google Scholar] [CrossRef]
- Rousk, J.; Bengtson, P. Microbial regulation of global biogeochemical cycles. Front. Microbiol. 2014, 5, 103. [Google Scholar] [CrossRef]
- Hartmann, A.; Kobler, J.; Kralik, M.; Dirnböck, T.; Humer, F.; Weiler, M. Model-aided quantification of dissolved carbon and nitrogen release after windthrow disturbance in an Austrian karst system. Biogeoscience 2016, 13, 159–174. [Google Scholar] [CrossRef]
- Kramer, K.; Brang, P.; Bachofen, H.; Bugmann, H.; Wohlgemuth, T. Site factors are more important than salvage loggingfor tree regeneration after wind disturbance in Central European forests. For. Ecol. Manag. 2014, 331, 116–128. [Google Scholar] [CrossRef]
- Logue, J.B.; Findlay, S.E.G.; Comte, J. Editorial: Microbial Responses to Environmental Changes. Front. Microbiol. 2015, 6, 1364. [Google Scholar] [CrossRef]
- Falt’an, V.; Bánovský, M.; Blažek, M. Evaluation of land cover changes after extraordinary windstorm by using the land cover metrics: A case study on the High Tatras foothill. Geografie 2011, 116, 156–171. [Google Scholar]
- Fleisher, P.; Koreň, M.; Škvarenina, J.; Kunca, V. Risk Assessment of the Tatra Mountains Forest. In Bioclimatology and Natural Hazards; Fleisher, P.; Koreň, M.; Škvarenina, J.; Kunca, V. Springer: Berlin/Heidelberg, Germany, 2009; pp. 145–154. [Google Scholar]
- Jonášová, M.; Vávrová, E.; Cudlín, P. Western Carpathian mountain spruce forest after a windthrow: Natural regeneration in cleared and uncleared areas. For. Ecol. Manag. 2010, 259, 1127–1134. [Google Scholar] [CrossRef]
- Fleisher, P.; Homolová, Z. Tatra Mts. As the objects for long-term ecological research of natural disturbances. Životné Prostredie 2016, 50, 40–43. (In Slovak) [Google Scholar]
- Fleisher, P.; Homolová, Z. Long-term research on ecological conditions in the larch-spruce forests in High Tatras after natural disturbances. Lesn. Čas. For. J. 2012, 57, 237–250. (In Slovak) [Google Scholar]
- Fleischer, P.; Pichler, V.; Fleischer, P., Jr.; Holko, L.; Gőmőryová, E.; Mális, F.; Cudlín, P.; Holeksa, J.; Michalová, Z.; Homolová, Z.; et al. Forest ecosystem services affected by natural disturbances, climate and land-scape changes in the Tatra Mountains. Clim. Res. 2017, 73, 57–71. [Google Scholar] [CrossRef]
- Šimkovic, I.; Dlapa, P.; Šimonovičová, A.; Ziegler, W. Water repellency of mountain forest soils in relation to impact of the katabatic windstorm and subsequent management practices. Pol. J. Environ. Stud. 2009, 18, 443–454. [Google Scholar]
- Gáfriková, J.; Hanajík, P.; Zvarík, M. Determination of organic fractions and enzymatic activity in forest spruce soil of Tatra National Park. Ekológia Bratisl. 2018, 37, 328–337. [Google Scholar]
- Gőmőryová, E.; Fleischer, P.; Gömöry, D. Soil microbial community responses to windthrow disturbance in Tatry National Park (Slovakia) during the period 2006–2013. Lesn. Cas. For. J. 2014, 60, 137–142. [Google Scholar] [CrossRef]
- Hanajík, P.; Gáfriková, J.; Zvarík, M. Dehydrogenase activity in topsoil at windthrow plots in Tatra national Park. Cent. Eur. J. 2017, 63, 91–96. [Google Scholar] [CrossRef]
- Homolová, Z.; Kyselová, Z.; Šoltés, R. Dynamics of vegetation on calamity areas in plant community of Lariceto-Piceetum. Stud. Tatra Natl. Park 2015, 11, 183–191. (In Slovak) [Google Scholar]
- Zielonka, T.; Holeksa, J.; Fleischer, P.; Kapusta, P. A tree-ring reconstruction of wind disturbances in a forest of the Slovakian Tatra Mountains, Western Carpathians. J. Veg. Sci. 2010, 21, 31–42. [Google Scholar] [CrossRef]
- Urbanovičová, V.; Miklisová, D.; Kováč, L. The effect of windthrow, wild fire, and management practices on epigeic Collembola in windthrown forest stands of the High Tatra Mts. (Slovakia). Biológia 2013, 68, 941–949. [Google Scholar] [CrossRef]
- Mitchell, S.J. Wind as a natural disturbance agent in forests: A synthesis. Forestry 2013, 86, 147–157. [Google Scholar] [CrossRef]
- Hrivnáková, K.; Makovníková, J.; Barančí;ková, G.; Bezák, P.; Bezáková, Z.; Dodok, R.; Grečo, V.; Chĺpik, J.; Kobza, J.; Lištjak, M.; et al. Unified Working Procedures of Soil Analyses; Výskumný ústav pôdoznalectva a ochrany pôdy (Research Institute of Pedology and Soil Protection): Bratislava, Slovak, 2011; 136p. (In Slovak) [Google Scholar]
- Zbíral, J.; Honsa, I. Soil Analysis; Ústřední kontrolní a zkušební ústav zemědelský, Národní referenční laboratoř (Central Control and Analytical Agroinstitute, National Reference Laboratory): Brno, Czech, 2010; 230p. (In Czech) [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A quality control tool for high throughput sequence data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 12 December 2018).
- Xu, H.; Luo, X.; Qian, J.; Pang, X.; Song, J.; Qian, G.; Chen, J.; Chen, S. FastUniq: A fast de novo duplicates removal tool for paired short reads. PLoS ONE 2012, 7, e52249. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, 590–596. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Hartmann, M.; Eriksson, K.M.; Pal, C.; Thorell, K.; Larsson, D.G.J.; Nilsson, R.H. METAXA2: Improved identification and taxonomic classification of small and large subunit rRNA in metagenomic data. Mol. Ecol. Resour. 2015, 15, 1403–1414. [Google Scholar] [CrossRef]
- Miller, C.S.; Baker, B.J.; Thomas, B.C.; Singer, S.W.; Banfield, J.F. EMIRGE: Reconstruction of full-length ribosomal genes from microbial community short read sequencing data. Genome Biol. 2011, 12, 44. [Google Scholar] [CrossRef]
- Kõljalg, U.; Larsson, K.H.; Abarenkov, K.; Nilsson, R.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; et al. UNITE: A database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol. 2005, 166, 1063–1068. [Google Scholar] [CrossRef]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C.; Filtenborg, O. Introduction to Food-Borne Fungi, 5th ed.; Centraalbureau voor Schimmelcultures: Baarn, The Netherlands, 1996. [Google Scholar]
- Pitt, J.I. The Genus Penicillium and Its Teleomorphic States Eupenicillium and Talaromyces; Academic Press: London, UK, 1979. [Google Scholar]
- De Hoog, G.S.; Guarro, J.; Gené, J.; Figueras, M.J. Atlas of Clinical Fungi; Centraalbureau voor Schimmelcultures: Baarn and Delft, The Netherlands; Universitat Rovira i Virgili: Reus, Spain, 2000. [Google Scholar]
- Samson, R.A.; Frisvad, J.C. Penicillium Subgenus Penicillium: New Taxonomic Schemes and Mycotoxins and Other Extrolites; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2004. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi, 2nd ed.; taxonomically revised by Walter Gams; IHW–Verlag: Eching, Germany, 2007. [Google Scholar]
- Hubka, V. Taxonomic revision of Eurotium and transfer of species to Aspergillus. Mycologia 2013, 105, 912–937. [Google Scholar] [CrossRef] [PubMed]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the Penicillium. Study Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.H. Evolution and measurement of species diversity. TAXON 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Ramírez, J.S.; Hoyos, V.C.; Guido Plaza, T. Phytosociology of weeds associated with rice crops in the department of Tolima, Colombia. Agron. Colomb. 2015, 33, 64–73. [Google Scholar] [CrossRef]
- WRB—World Reference Base for Soil Resources. A Framework for International Classification, Corelation and Communication; FAO-ISRIC-ISSS: Rome, Italy, 2006. [Google Scholar]
- Fernandéz-Delgado Juárez, M.; Gómez-Brandón, M.; Knapp, A.; Stöhr, D.; Insam, H. Chemical and microbiological properties of alpine forest soils: Effects of pelletizatized ashes in a short-term trial. For. Ecol. Manag. 2015, 357, 42–49. [Google Scholar] [CrossRef]
- Santin, C.; Doerr, S.H. Fere effect on soils: The human dimension. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150171. [Google Scholar] [CrossRef] [PubMed]
- Zavala, L.M.; De Celis, R.; Jordán, A. How wildfires affect soil properties. A brief review. CIG 2014, 40, 311–331. [Google Scholar]
- Bogunovic, I.; Kisic, I.; Jurisic, A. Influence of wildfire and fire suppression by seawater on soil properties. Appl. Ecol. Environ. Res. 2015, 13, 1157–1169. [Google Scholar] [CrossRef]
- Heydari, M.; Rostamy, A.; Naajafi, F.; Dey, D.C. Effect of fire severity on physical and biochemical soil properties in Zagros oak (Quercus brantii Lindt.) forests in Iran. J. For. Res 2017, 28, 95–104. [Google Scholar] [CrossRef]
- Xue, L.; Qiujing, L.; Hongyue, C. Effects of a wildfire on selected physical, chemical and biochemical soil properties in a Pinus massoniana forest in south China. Forests 2014, 5, 2947–2966. [Google Scholar] [CrossRef]
- Hanajík, P.; Šimonovičová, A.; Piecková, E.; Jesenák, K. Monitoring of pedochemical and microbiological changes at the selected localities in the High Tatras after windstorm calamity Nov 2004, 19. Štúdie o Tatranskom národnom parku (Stud. Tatra Natl. Park) 2009, 9, 42, 199–206. [Google Scholar]
- Šimonovičová, A.; Nováková, A.; Pangallo, D.; Hnátová, V. The occurrence of heat-resistant species of Trichophaea abundans in different types of soil in Slovakia and Czech Republic. Biológia 2014, 69, 168–172. [Google Scholar] [CrossRef]
- Berry, A.M.; Barabote, R.D.; Normand, P. The Family Acidothermaceae. In The Prokaryotes, 4th ed.; Rosenberg, E., De Long, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 13–19. [Google Scholar]
- Mohagheighi, A.; Grohmann, K.; Himmel, M.; Leighton, L.; Updegraff, D.M. Isolation and Characterization of Acidothermus cellulolyticus gem nov.; sp. nov.; a new genus of thermophilic, acidophilic, cellulolytic bacteria. Int. J. Syst. Bacteriol. 1986, 36, 435–443. [Google Scholar] [CrossRef]
- Barbarote, R.D.; Xie, G.; Leu, D.H.; Normand, P.; Nesculea, A.; Daubin, V.; Médigue, C.; Adney, W.S.; Xu, X.C.; Lapidus, A.; et al. Complete genome of the cellulolytic thermophile Acidothermus cellulolyticus 11B provides insights into its ecophysiological and evolutionary adaptations. Genome Res. 2009, 19, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Mikita-Barbatoa, R.A.; Kelly, J.J.; Tate, R.L. Wildfire effects on the properties and microbial community structure of organic horizon soils in the New Jersey Pinelands. Soil Biol. Biochem. 2015, 86, 67–76. [Google Scholar] [CrossRef]
- Leys, N.M.; Ryngaert, A.; Bastiaens, L.; Wattiau, P.; Top, E.M.; Verstraete, W.; Springael, D. Occurrence and community composition of fast-growing Mycobacterium in soils contaminated with polycyclic aromatic hydrocarbons. FEMS Microbiol. Ecol. 2005, 51, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Díaz, J.F.; Fernández-González, A.J.; Villadas, P.J.; Robles, A.B.; Toro, N. Metagenomic assessment of the potential microbial nitrogen pathways in the rhizosphere of a Mediterranean forest after a wildfire. Microb. Ecol. 2015, 69, 895–904. [Google Scholar] [CrossRef]
- Bárcenas-Moreno, G.; García-Orenes, F.; Mataix-Solera, J.; Mataix-Beneyto, J.; Bååth, E. Soil microbial recolonisation after a fire in a Mediterranean forest. Biol. Fert. Soils 2011, 47, 261–272. [Google Scholar] [CrossRef]
- Yeager, C.M.; Northup, D.E.; Grow, C.C.; Barns, S.M.; Kuske, C.R. Changes in nitrogen-fixing and ammonia-oxidizing bacterial communities in soil of a mixed conifer forest after wildfire. Appl. Environ. Microbiol. 2005, 71, 2713–2722. [Google Scholar] [CrossRef]
- Wang, N.F.; Zhang, T.; Zhang, F.; Wang, E.T.; He, J.F.; Ding, H.; Zhang, B.T.; Liu, J.; Ran, X.B.; Zang, J.Y. Diversity and structure of soil bacterial communities in the Fildes Region (maritime Antarctica) as revealed by 454 pyrosequencing. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Famintzin, A. Eine neue Bacterienform: Nevskia ramosa. Bull. Acad. Sci. St Petersburgh New Ser. 1892, 2, 481–486. [Google Scholar]
- Leandro, T.; França, L.; Nobre, M.F.; Schumann, P.; Rosselló-Móra, R.; da Costa, M.S. Nevskia aquatilis sp. nov. and Nevskia persephonica sp. nov.; isolated from a mineral water aquifer and the emended description of the genus Nevskia. Syst. Appl. Microbiol 2012, 35, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Weon, H.Y.; Kim, B.; Son, J.A.; Song, M.H.; Kwon, S.W.; Go, S.J.; Stackebrandt, E. Nevskia soli sp. nov.; isolated from soil cultivated with Korean ginseng. Int. J. Syst. Evol. Microbiol 2008, 1188, 578–580. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.J.; Weon, H.; Kim, Y.S.; Park, I.C.; Son, J.A.; Kwon, S.W. Nevskia terrae sp. nov.; isolated from soil. Int. J. Syst. Evol. Microbiol 2011, 61, 1226–1229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Green, S.J.; Prakash, O.; Jasrotia, P.; Overholt, W.A.; Cardenas, E.; Hubbard, D.; Tiedje, J.M.; Watson, D.B.; Schadt, C.W.; Brooks, S.C.; et al. Denitrifying bacteria from the genus Rhodanobacter dominate bacterial communities in the highly contaminated subsurface of a nuclear legacy waste site. Appl. Environ. Microbiol. 2011, 78, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Rosling, A.; Cox, F.; Cruz-Martinez, K.; Ihrmark, K.; Grelet, G.A.; Lindahl, B.D.; Menkis, A.; James, T.Y. Archaeorhizomycetes: Unearthing an ancient class of ubiquitous soil fungi. Science 2011, 333, 876–879. [Google Scholar] [CrossRef]
- Choma, M.; Bárta, J.; Šantrůčková, H.; Urich, T. Low abundance of Archaeorhizomycetes among fungi in soil metatranscriptomes. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Vaartaja, O. Pythium and Mortierella in soils of Ontario forest nurseries. Can. J. Microbiol. 1968, 14, 265–269. [Google Scholar] [CrossRef]
- Wagner, L.; Stielow, B.; Hoffmann, K.; Petkovits, T.; Papp, T.; Vágvölgyi, C.; de Hoog, G.S.; Verkley, G.; Voigt, K. A comprehensive molecular phylogeny of the Mortierellales (Mortierellomycotina) based on nuclear ribosomal DNA. Persoonia 2013, 30, 77–93. [Google Scholar] [CrossRef]
- Štursová, M.; Šnajdr, J.; Cajthaml, T.; Bárta, J.; Šantrůčková, H.; Baldrian, P. When the forest dies: The response of forest soil fungi to a bark beetle-induced tree dieback. ISME J. 2014, 8, 1920–1931. [Google Scholar] [CrossRef]
- Rayner, A.D.M.; Todd, N.K. Population and community structure and dynamics of Fungi in decaying wood. Adv. Bot. Res. 1980, 7, 333–420. [Google Scholar] [CrossRef]
- González, A.E.; Martínez, A.T.; Almendros, G.; Grinbergs, J. A study of yeasts during the delignification and fungal transformation of wood into cattle feed in Chilean rain forest. Antonie Van Leeuwenhoek 1989, 55, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.; González, A. Candida santjacobensis and Candida ancudensis two new species of yeasts isolated from decayed wood in the evergreen rainy Valdivian forest of Southern Chile. Mycopathologia 1984, 88, 105–109. [Google Scholar] [CrossRef]
- Yurkov, A. Yeasts in Forest Soils. In Yeasts in natural ecosystems: Diversity; Buzzini, P., Lachance, M.A., Yurkov, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 87–116. [Google Scholar]
- Buée, M.; Reich, M.; Murat, C.; Morin, E.; Nilsson, R.H.; Uroz, S.; Martin, F. 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytopathol. 2009, 184, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, S.M.; Jones, M.D.; Bradfield, G.E.; Gillespie, M.; Durall, D.M. Effects of clear-cut logging on the diversity and persistence of ectomycorrhizae at a subalpine forest. Can. J. For. Res. 1999, 29, 124–134. [Google Scholar] [CrossRef]
- Hartmann, M.; Howes, C.G.; Van Insberghe, D.; Yu, H.; Bachar, D.; Christen, R.; Nilsson, R.H.; Hallam, S.J.; Mohn, W.W. Significant and persistent impact of timber harvesting on soil microbial communities in Northern coniferous forests. ISME J. 2012, 6, 2199–2218. [Google Scholar] [CrossRef]
- Štursová, M.; Žifčáková, L.; Leigh, M.B.; Burgess, R.; Baldrian, P. Cellulose utilization in forest litter and soil: Identification of bacterial and fungal decomposers. FEMS Microbiol. Ecol. 2012, 80, 735–746. [Google Scholar] [CrossRef]
- Voříšková, J.; Brabcová, V.; Cajthaml, T.; Baldrian, P. Seasonal dynamics of fungal communities in a temperate oak forest soil. New Phytol. 2013, 201, 269–278. [Google Scholar] [CrossRef]
- Azaz, A.D.; Pekel, O. Comparison of soil fungi in burnt and unburnt forest soils in the vinicity of Kargicak (Alanya, Turkey). Turk. J. Bot. 2002, 26, 409–416. [Google Scholar]
- Widden, P.; Parkinson, D. The effects of a forest fire on soil microfungi. Soil Biol. Biochem. 1975, 7, 125–138. [Google Scholar] [CrossRef]
- Geml, J.; Taylor, D.L. Biodiversity and molecular ecology of Russula and Lactarius in Alaska based on soil and sporocarp DNA sequences. Scr. Bot. Belg. 2013, 51, 132–145. [Google Scholar]
- Early, R. Pathogen control in primary production: Crop foods. In Foodborne Pathogens Hazards, Risk Analysis and Control, 2nd ed.; Blackburn, C.W., McClure, P.J., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead: Sawston, UK, 2009; pp. 205–279. [Google Scholar]
- Šimonovičová, A. Biodiversity of Microscopic Fungi in Soil Types of Slovakia, 1st ed.; Comenius University in Bratislava: Bratislava, Slovakia, 2013. [Google Scholar]
- Zhang, J.; Man, B.; Fu, B.; Liu, L.; Han, C. The diversity of soil culturable fungi in the three alpine shrub grassland of eastern Qilian Mountains. Front. Earth Sci. 2013, 7, 76–84. [Google Scholar] [CrossRef]
- Leckie, S.E. Methods of microbial community profiling and their application to forest soils. For. Ecol. Manag. 2005, 220, 88–106. [Google Scholar] [CrossRef]
- Grishkan, I. Influence of wildfire on diversity of culturable soil microfungal communities in the Mount Carmel forest, Israel. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2014, 150, 1–10. [Google Scholar] [CrossRef]
- Gőmőryová, E.; Fleischer, P.; Pichler, V.; Homolák, M.; Here, R.; Gőmőry, D. Soil microorganisms at the windthrow plots: The effect of post-disturbance management and the time since disturbance. iForest 2017, 10, 515–521. [Google Scholar] [CrossRef]
- Reazin, C.; Morris, S.; Smith, J.E.; Cowan, A.D.; Jumpponen, A. Fires of differing intensities rapidly select distinct soil fungal communities in a Northwest US ponderosa pine forest ecosystem. For. Ecol. Manag. 2016, 377, 118–127. [Google Scholar] [CrossRef]
- Claridge, A.W.; Trappe, J.M.; Hansen, K. Do fungi have a role as soil stabilizers and remediators after forest fire? For. Ecol. Manag. 2009, 257, 1063–1069. [Google Scholar] [CrossRef]
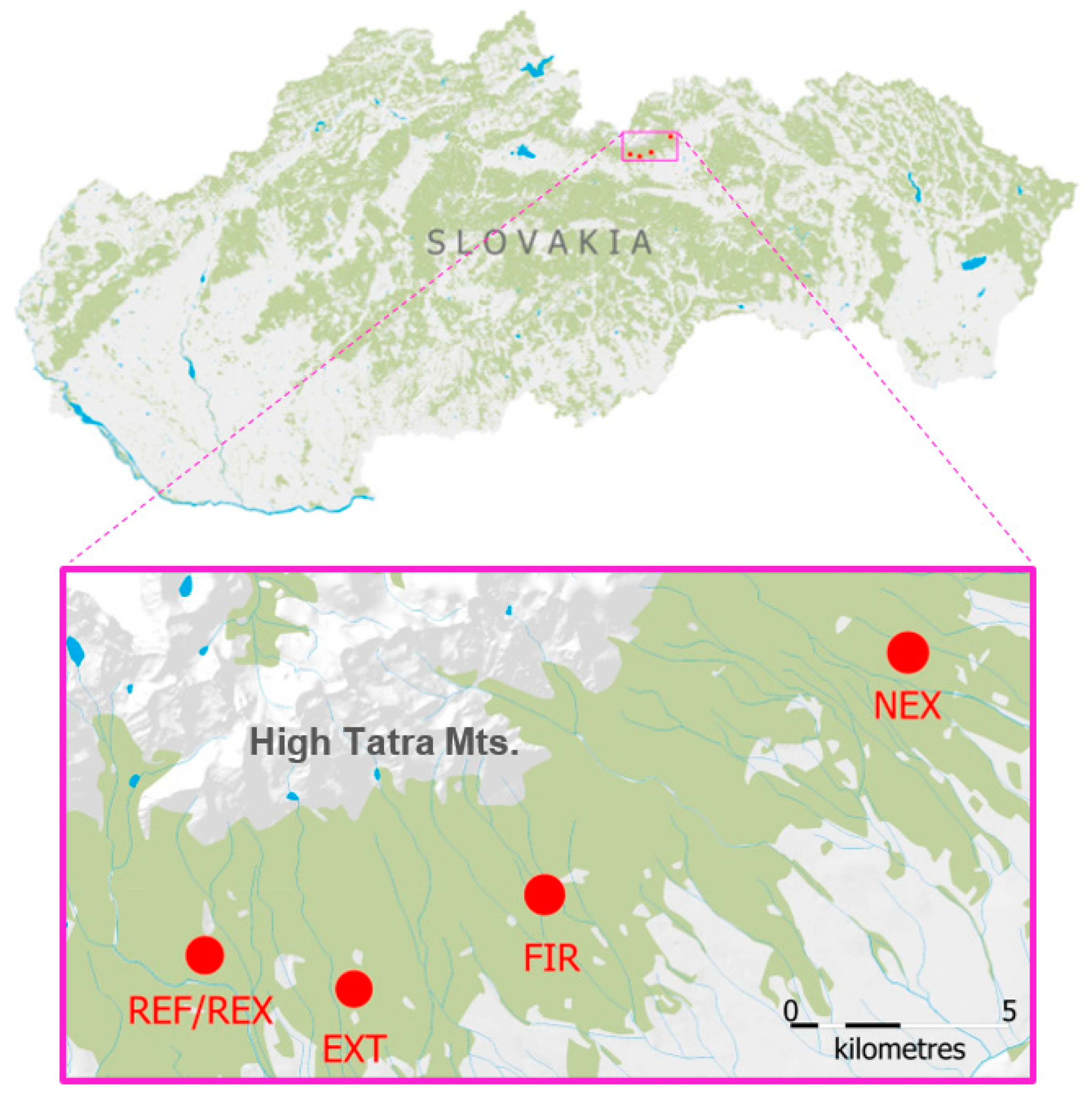
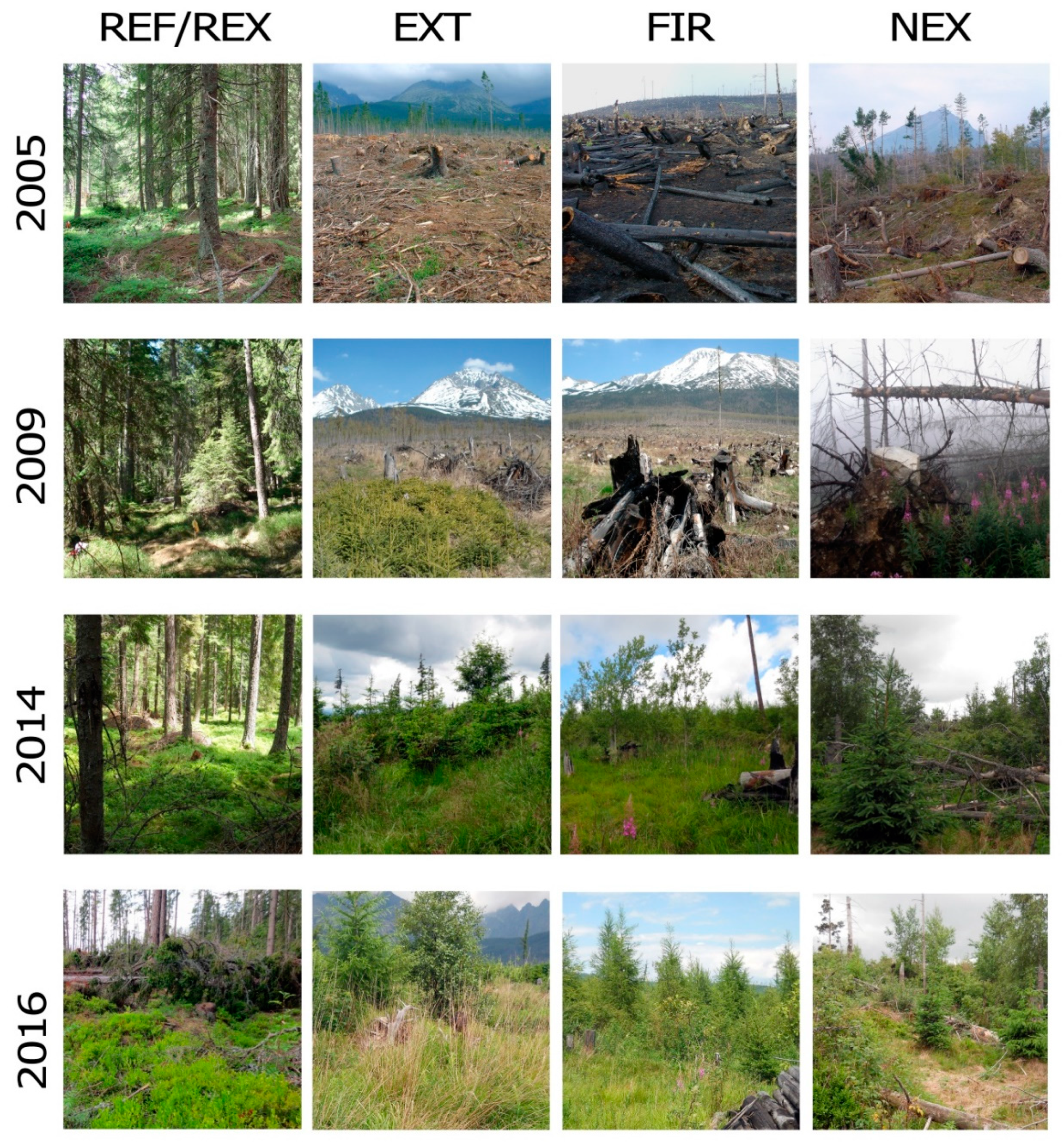

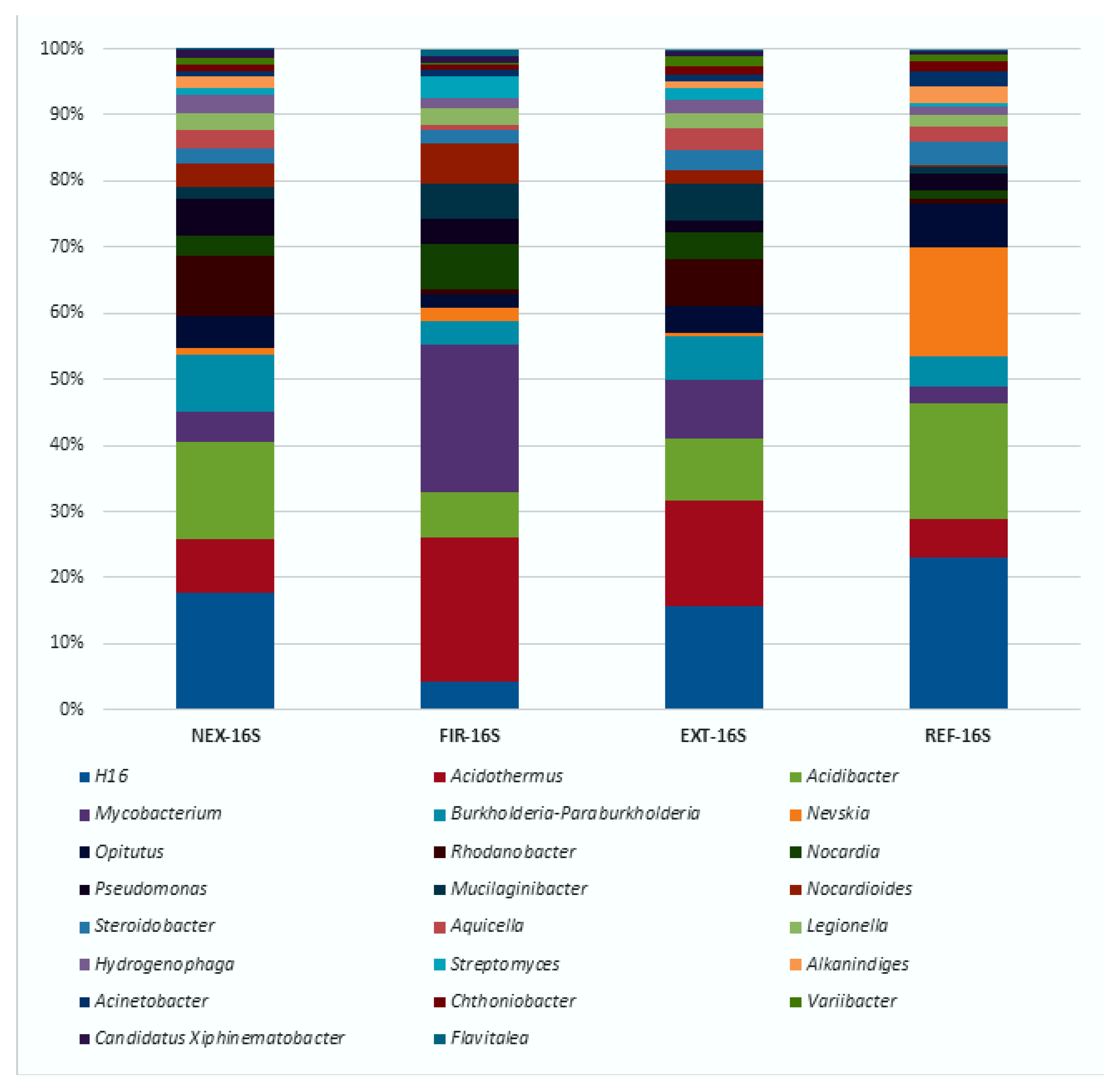
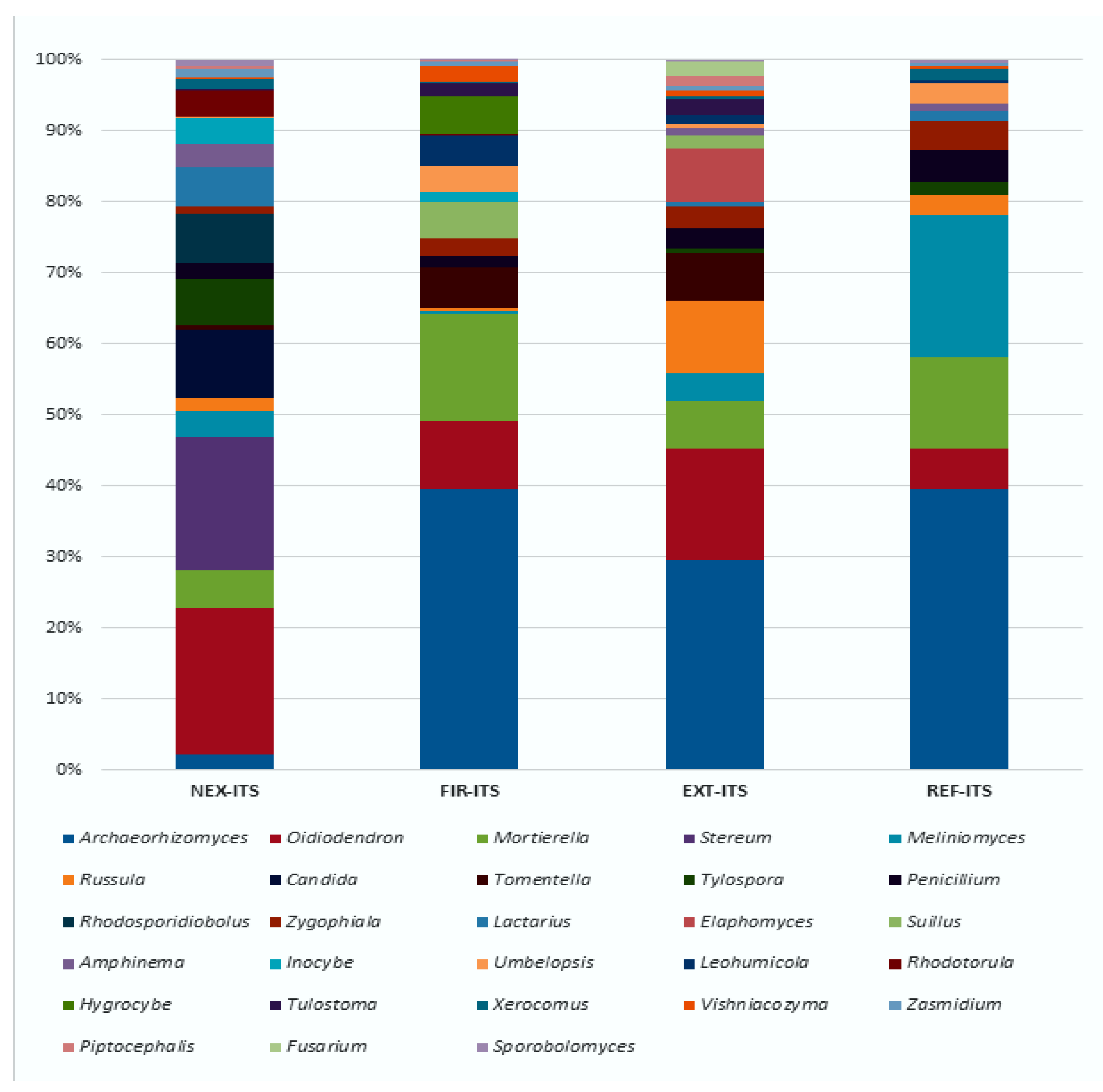
| Monitored Localities | REF | EXT | NEX | FIR |
|---|---|---|---|---|
| GPS localization | N49°07′17.5″ E20°06′16.4″ | N49°07′11.9″ E20°09′47.6″ | N49°09′36.3″ E20°15′08.8″ | N49°07′49.7″ E20°11′57.3″ |
| altitude (a.s.l.) | 1100–1250 | 1040–1260 | 1050–1150 | 1000–1200 |
| soil type | Dystric Cambisol | Dystric Cambisol | Dystric Cambisol | Dystric Cambisol |
| geology | moraine mindel-riss | moraine donau-mindel | moraine wurm | polygenetic moraine d-m |
| community structure (before the windthrow) | Lariceto-Picetum | Lariceto-Picetum | Lariceto-Picetum | Lariceto–Picetum |
| management (after the windthrow) | comparative intact forest | extracted wood mass | non-extracted wood mass | burnt forest |
| pH H2O (2005) | 4.2 | 4.3 | 3.9 | 4.8 |
| pH H2O (2016) | 3.7 | 3.9 | 4.4 | 4.0 |
| % of Cox (2005) | 6.4 | 4.8 | 7.6 | 4.2 |
| % of Cox (2016) | 7.7 | 11.4 | 10.4 | 10.3 |
| % of Ntot (2005) | 0.7 | 0.5 | 0.7 | 0.6 |
| % of Ntot (2016) | 0.4 | 0.5 | 0.5 | 0.5 |
| Basic Nutrients | Monitored Localities | |||
|---|---|---|---|---|
| REF | EXT | NEX | FIR | |
| KMII mg/kg | 88.1 | 106 | 94.1 | 132 |
| MgMII mg/kg | 26.5 | 79 | 47 | 104 |
| CaMII mg/kg | 103 | 461 | 320 | 820 |
| KMIII mg/kg | 150 | 156 | 147 | 183 |
| MgMIII mg/kg | 27.9 | 77.7 | 44.8 | 98.2 |
| CaMIII mg/kg | 103 | 458 | 310 | 801 |
| Alpha Diversity Analysis | EXT | FIR | NEX | REF |
|---|---|---|---|---|
| 16S bacteria (genus degree) | 0.945 | 0.920 | 0.943 | 0.907 |
| ITS fungi (genus degree) | 0.944 | 0.934 | 0.946 | 0.905 |
| Genera/Species/Sequence Similarity | Localities | |||
|---|---|---|---|---|
| REF | EXT | NEX | FIR | |
| Absidia sp. | −/− | +/+ | −/− | −/− |
| Achaetomium sp. | −/− | −/− | +/+ | +/+ |
| Acremonium murorum | −/− | +/+ | −/− | −/− |
| Alternaria alternata | +/+ | +/+ | −/− | +/+ |
| Aspergillus fischeri | +/− | +/− | +/− | +/− |
| Aspergillus fischeri as * Neosartorya fischeri | + | + | − | + |
| Aspergillus fischeri var. glaber as EF669938 * Neosartorya glabra 100% | −/+ | −/+ | −/− | −/+ |
| Aspergillus thermomutatus as Neosartorya pseudofischeri | −/− | −/− | −/− | −/+ |
| Botryotrichum piluliferum | −/− | −/− | +/+ | −/− |
| FJ527879 Chaetomium sp. 99.8% | −/− | −/− | −/− | −/+ |
| * Chaetomium atrobrunneum | −/− | −/− | −/− | −/+ |
| Chaetomium funicola | −/− | −/− | −/− | −/+ |
| JN601442 Cladosporium 100% | +/+ | −/− | +/+ | −/− |
| LN834375 Cladosporium halotolerans 100% | −/− | −/− | −/+ | −/− |
| KC113297 Cladosporium sphaeospermum 100% | −/+ | −/+ | −/+ | −/− |
| Clonostachys rosea f. rosea | −/− | −/+ | −/− | −/− |
| Dichobotrys abundans as DQ220449 Trichophaea abundans 100% | −/− | −/− | −/− | −/+ |
| * Eupenicillium pinetorum | −/− | −/− | −/+ | −/− |
| AF033389 Geosmithia argillacea 92% | −/+ | −/− | −/− | −/− |
| Gliocladium sp. | −/− | −/− | +/+ | −/− |
| LC076682 Hamigera striata 100% | −/+ | −/+ | −/+ | −/+ |
| * Lasiodipodia theobromae | −/− | −/− | −/− | −/+ |
| Lecanicillium muscarium | −/− | −/+ | −/− | −/− |
| Mortierella alpina | −/− | −/+ | −/− | −/− |
| Mortierella bainieri | +/+ | +/+ | +/+ | −/− |
| Mucor sp. | +/+ | −/− | +/+ | +/+ |
| Mucor hiemalis f. hiemalis | −/+ | −/+ | −/+ | −/− |
| Myceliophtora thermophila | −/− | +/+ | +/+ | −/− |
| * Myxotrichum deflexum | −/+ | −/− | −/− | −/− |
| Paecilomyces sp. | +/+ | −/− | −/− | +/+ |
| Penicillium sp. | +/+ | +/+ | +/+ | +/+ |
| Penicillium abidjanum as * Eupenicillium abidjanum | +/+ | −/− | +/+ | +/+ |
| Penicillium alutaceum as * Eupenicillium alutaceum | −/− | −/− | −/+ | −/+ |
| Penicillium baarnense as * Eupenicillium baarnense | −/− | −/+ | −/− | −/− |
| Penicillium brefeldianum as * Eupenicillium brefeldianum | −/− | −/+ | −/− | −/− |
| KT200263 Penicillium chrysogenum var. chrasogenum 100% | −/+ | −/+ | −/+ | −/− |
| Penicillium expansum | −/− | −/− | −/− | +/− |
| Penicillium funiculosum | −/− | −/− | −/− | −/+ |
| DQ682590 Penicillium glabrum 99.8% | −/− | +/+ | +/+ | +/+ |
| Penicillium inusitatum as * Eupenicillium inusitatum | −/− | −/− | −/− | −/+ |
| Penicillium indonesiae as * Eupenicillium erubescens | −/− | −/+ | −/− | −/− |
| Penicillium indonesiae as * Eupenicillium ludwigii | −/− | −/− | −/− | −/+ |
| * Penicillium janthinellum | −/− | +/+ | −/− | −/− |
| Penicillium lapidosum as * Eupenicillium lapidosum | +/+ | −/− | −/− | +/+ |
| Penicillium melaforme as * Eupenicillium melaforme | −/− | −/− | −/− | −/+ |
| Penicillium osmophilum as * Eupenicillium osmophilum | −/− | −/− | −/− | −/+ |
| KM871190 Penicillium oxalicum 100% | −/+ | −/− | −/− | −/− |
| Penicillium parvum as * Eupenicillium parvum | −/− | −/− | −/− | −/+ |
| Penicillium raistrickii | −/− | +/+ | −/− | −/− |
| * Penicillium sclerotigenum | −/− | −/+ | −/+ | −/− |
| * Phialophora bubakii | −/− | −/− | −/− | −/+ |
| * Phoma sp. | −/− | −/+ | −/− | −/− |
| Rhizopus stolonifer var. stolonifer | +/+ | −/− | −/− | −/− |
| Scopulariopsis sp. | −/− | −/− | −/− | −/+ |
| * Scopulariopsis asperula | −/+ | −/− | −/− | −/− |
| * Staphylotricum coccosporum | −/− | −/− | −/− | −/+ |
| * Talaromyces flavus | +/+ | +/+ | −/− | +/+ |
| * Talaromyces ohiensis | −/− | +/+ | −/− | −/− |
| GU092968 Talaromyces striatus 100% | +/+ | +/+ | +/+ | +/+ |
| * Talaromyces thermophilus | −/+ | −/− | −/− | −/− |
| * Talaromyces trachyspermus | −/+ | −/− | −/− | −/− |
| * Talaromyces wortmanii | −/− | −/− | −/− | +/+ |
| Trichoderma harzianum | −/− | −/− | +/+ | +/+ |
| Trichoderma koningii | +/+ | +/+ | +/+ | +/+ |
| DQ845431 Trichoderma viride aggregate 100% | +/+ | +/+ | +/+ | +/+ |
| Verticillium lecani | −/− | +/+ | −/− | −/− |
| Zygorhynchus heterogamus | +/+ | +/+ | +/+ | +/+ |
| Zygorhynchus moelleri | +/+ | +/+ | +/+ | +/+ |
| REF | EXT | NEX | FIR | |
|---|---|---|---|---|
| REF | - | SS = 53.3 SJ = 36.4 | SS = 53.8 SJ = 36.8 | SS = 50.0 SJ = 33.3 |
| EXT | SS = 53.3 SJ = 36.4 | - | SS = 39.3 SJ = 24.4 | SS = 20.6 SJ = 11.5 |
| NEX | SS = 53.8 SJ = 36.8 | SS = 39.3 SJ = 24.4 | - | SS = 43.3 SJ = 27.2 |
| FIR | SS = 50.0 SJ = 33.3 | SS = 20.6 SJ = 11.5 | SS = 43.3 SJ = 27.2 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimonovičová, A.; Kraková, L.; Piecková, E.; Planý, M.; Globanová, M.; Pauditšová, E.; Šoltys, K.; Budiš, J.; Szemes, T.; Gáfriková, J.; et al. Soil Microbiota of Dystric Cambisol in the High Tatra Mountains (Slovakia) after Windthrow. Sustainability 2019, 11, 6851. https://doi.org/10.3390/su11236851
Šimonovičová A, Kraková L, Piecková E, Planý M, Globanová M, Pauditšová E, Šoltys K, Budiš J, Szemes T, Gáfriková J, et al. Soil Microbiota of Dystric Cambisol in the High Tatra Mountains (Slovakia) after Windthrow. Sustainability. 2019; 11(23):6851. https://doi.org/10.3390/su11236851
Chicago/Turabian StyleŠimonovičová, Alexandra, Lucia Kraková, Elena Piecková, Matej Planý, Mária Globanová, Eva Pauditšová, Katarína Šoltys, Jaroslav Budiš, Tomáš Szemes, Jana Gáfriková, and et al. 2019. "Soil Microbiota of Dystric Cambisol in the High Tatra Mountains (Slovakia) after Windthrow" Sustainability 11, no. 23: 6851. https://doi.org/10.3390/su11236851
APA StyleŠimonovičová, A., Kraková, L., Piecková, E., Planý, M., Globanová, M., Pauditšová, E., Šoltys, K., Budiš, J., Szemes, T., Gáfriková, J., & Pangallo, D. (2019). Soil Microbiota of Dystric Cambisol in the High Tatra Mountains (Slovakia) after Windthrow. Sustainability, 11(23), 6851. https://doi.org/10.3390/su11236851






