Socioeconomic Impact of Genome Editing on Agricultural Value Chains: The Case of Fungal-Resistant and Coeliac-Safe Wheat
Abstract
1. Introduction
- (1)
- What are the requirements for using genome-edited crops in agricultural value chains?
- (2)
- What are the motives of actors in agricultural value chains to use genome-edited crops?
- (3)
- What are the costs and benefits of using genome-edited crops in agricultural value chains?
- (4)
- What are the economic risks of using genome-edited crops?
- (5)
- What are the potential drivers and barriers for using genome-edited crops?
- (6)
- What are the challenges for the use of genome-edited crops?
2. Conceptual Framing
3. Materials and Methods
3.1. The Case Study
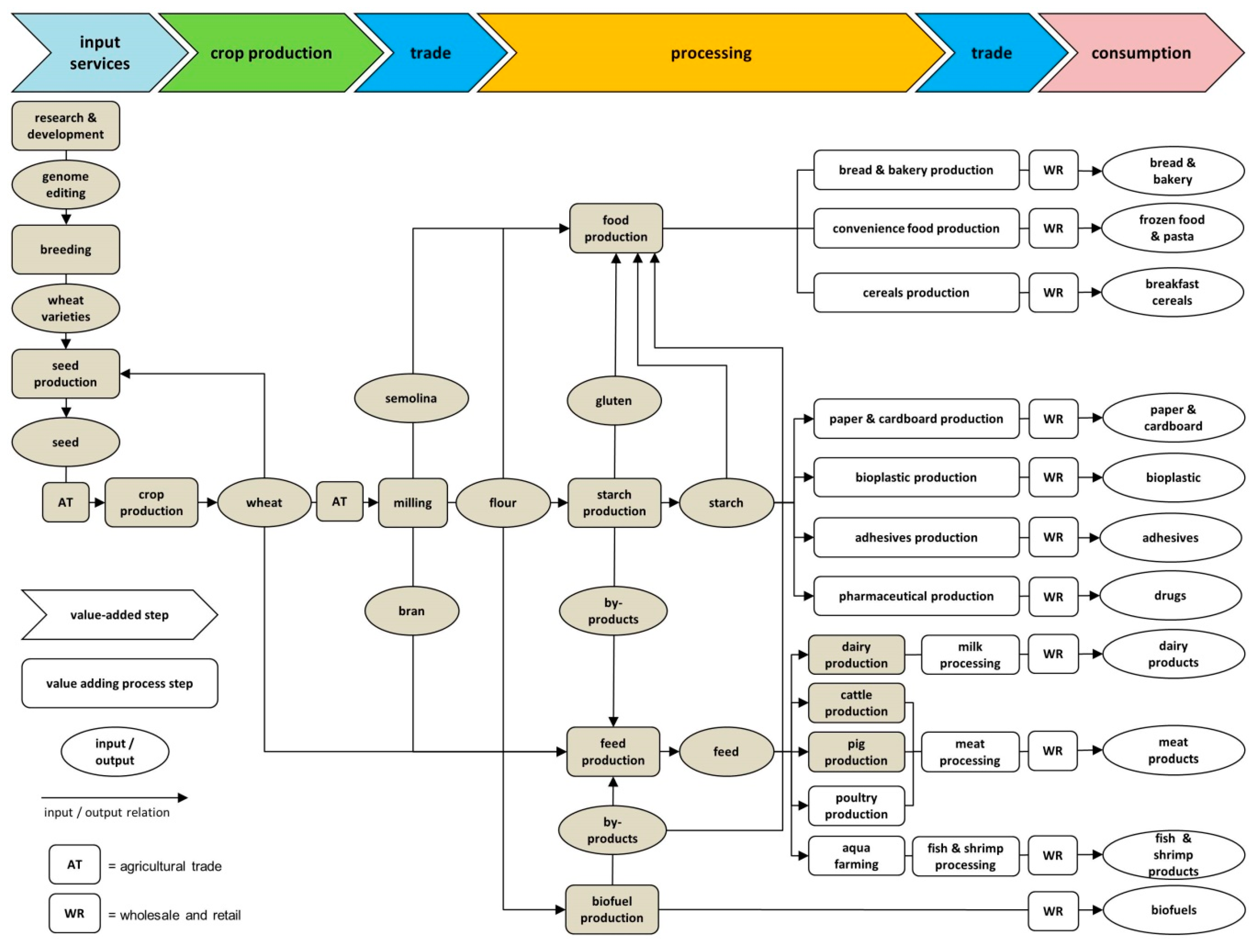
3.1.1. The Value Chains of Wheat
3.1.2. Breeding Goals
3.2. Data Collection
3.3. Data Analysis
3.3.1. Study Focus
3.3.2. Scenarios
3.3.3. Qualitative Content Analysis
4. Results
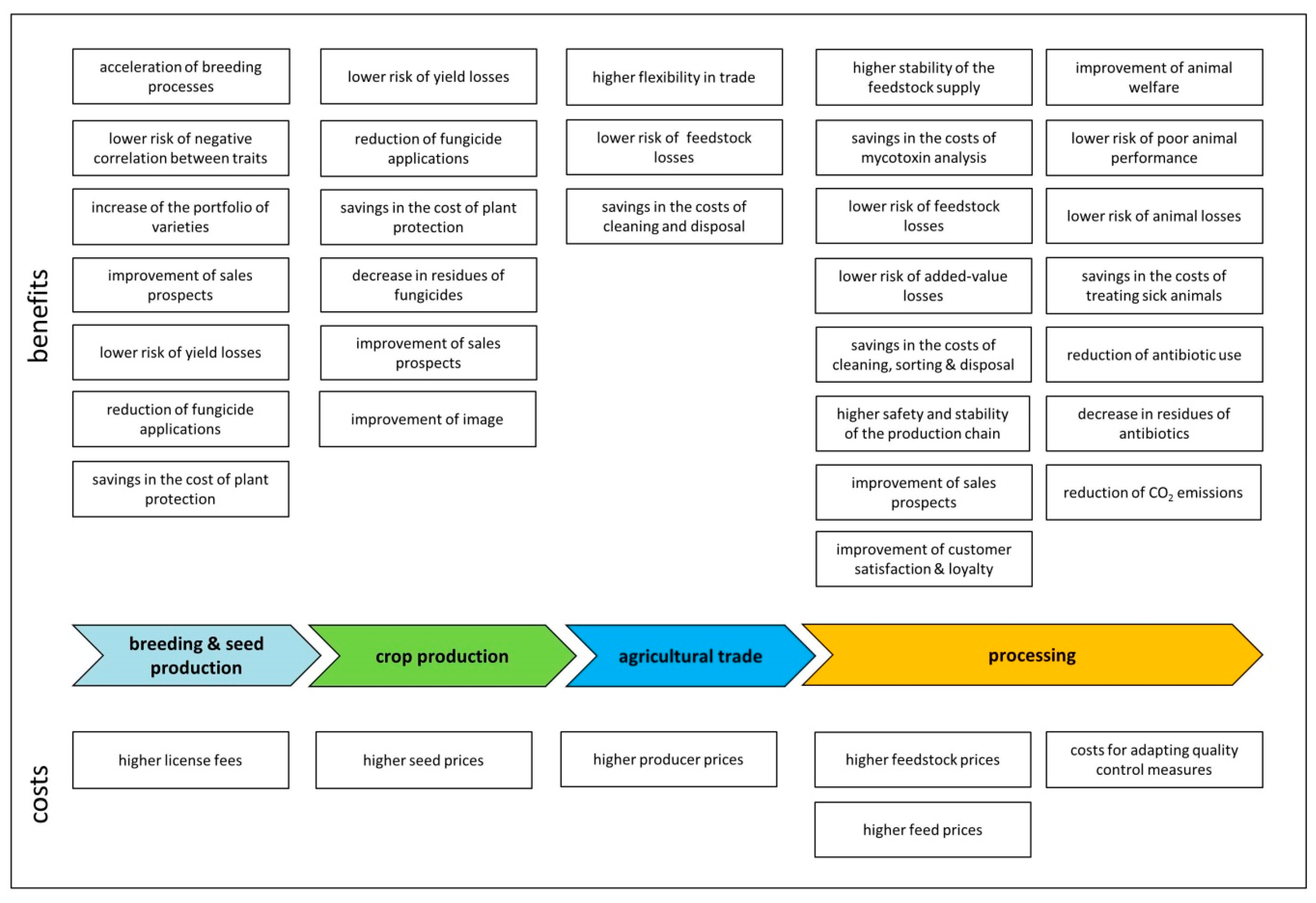
4.1. Fungal-Resistant Wheat
4.1.1. Breeding
4.1.2. Seed Production
4.1.3. Crop Production
4.1.4. Agricultural Trade
4.1.5. Milling
4.1.6. Food Production
4.1.7. Starch Production
4.1.8. Feed Production
4.1.9. Animal Production
4.1.10. Biofuel Production
4.1.11. Overview
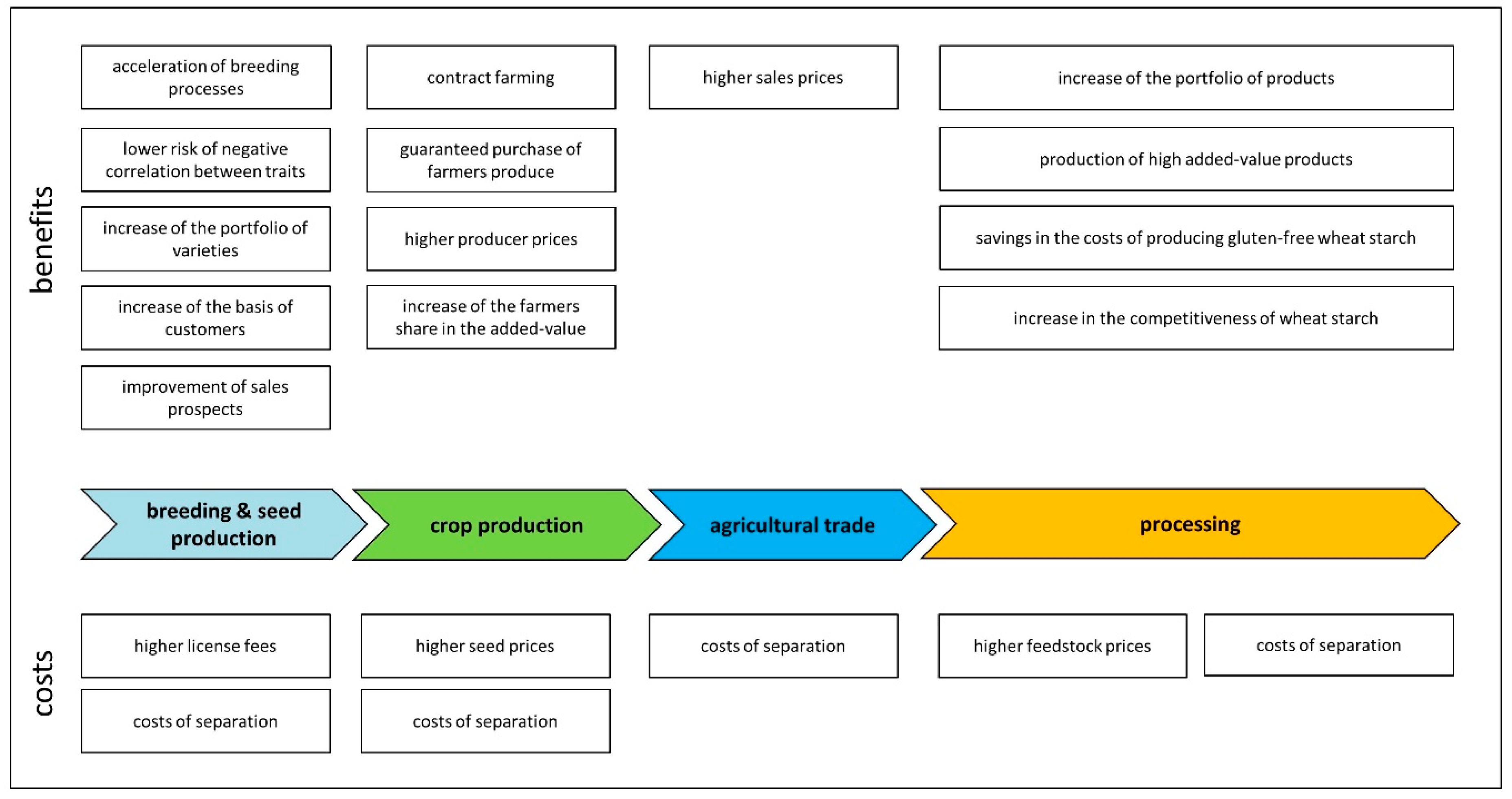
4.2. Coeliac-Safe Wheat
4.2.1. Breeding
4.2.2. Seed Production
4.2.3. Crop Production
4.2.4. Agricultural Trade
4.2.5. Milling
4.2.6. Food Production
4.2.7. Starch Production
4.2.8. Overview
4.3. Drivers and Barriers
4.4. Challenges
5. Discussion
5.1. Empirical Findings
5.2. Lessons Learned and Contribution to the Literature
5.3. Research Design and Future Research Directions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019—Highlights. Available online: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf (accessed on 1 September 2019).
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working Paper; FAO: Rome, Italy, 2012; pp. 1–154. [Google Scholar]
- Ma, X.; Mau, M.; Sharbel, T.F. Genome Editing for Global Food Security. Trends Biotechnol. 2018, 36, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Bujnicki, J.; Dykstra, P.; Wegener, H. New Techniques in Agricultural Biotechnology; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar]
- Carroll, D. Genome Editing: Past, Present, and Future. Yale J. Biol. Med. 2017, 90, 653–659. [Google Scholar]
- Modrzejewski, D.; Hartung, F.; Sprink, T.; Krause, D.; Kohl, C.; Wilhelm, R. What is the available evidence for the range of applications of genome-editing as a new tool for plant trait modification and the potential occurrence of associated off-target effects: A systematic map. Environ. Evid. 2019, 8, 11. [Google Scholar] [CrossRef]
- Metje-Sprink, J.; Menz, J.; Modrzejewski, D.; Sprink, T. DNA-Free Genome Editing: Past, Present and Future. Front. Plant Sci. 2018, 9, 1957. [Google Scholar] [CrossRef]
- Kamburova, V.S.; Nikitina, E.V.; Shermatov, S.E.; Buriev, Z.T.; Kumpatla, S.P.; Emani, C.; Abdurakhmonov, I.Y. Genome Editing in Plants: An Overview of Tools and Applications. Int. J. Agron. 2017, 2017, 7315351. [Google Scholar] [CrossRef]
- Abdallah, N.A.; Prakash, C.S.; McHughen, A.G. Genome editing for crop improvement: Challenges and opportunities. GM Crop. Food 2015, 6, 183–205. [Google Scholar] [CrossRef]
- Georges, F.; Ray, H. Genome editing of crops: A renewed opportunity for food security. GM Crop. Food 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Voytas, D.F.; Gao, C. Precision genome engineering and agriculture: Opportunities and regulatory challenges. PLoS Biol. 2014, 12, e1001877. [Google Scholar] [CrossRef]
- Lassoued, R.; Macall, D.M.; Hesseln, H.; Phillips, P.W.B.; Smyth, S.J. Benefits of genome-edited crops: Expert opinion. Transgenic Res. 2019, 28, 247–256. [Google Scholar] [CrossRef] [PubMed]
- van de Wiel, C.C.M.; Schaart, J.G.; Lotz, L.A.P.; Smulders, M.J.M. New traits in crops produced by genome editing techniques based on deletions. Plant Biotechnol. Rep. 2017, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kanchiswamy, C.N.; Maffei, M.; Malnoy, M.; Velasco, R.; Kim, J.-S. Fine-Tuning Next-Generation Genome Editing Tools. Trends Biotechnol. 2016, 34, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Tee, L.Y.; Wang, X.-G.; Huang, Q.-S.; Yang, S.-H. Off-target Effects in CRISPR/Cas9-mediated Genome Engineering. Mol. Ther. Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef] [PubMed]
- Wikmark, O.-G.; Agapito-Tenfen, S.Z. Current Status of Emerging Technologies for Plant Breeding: Biosafety and Knowledge Gaps of Site Directed Nucleases and Oligonucleo De-Directed Mutagenesis; GenØk Centre for Biosafety: Tromsø, Norway, 2015. [Google Scholar]
- Zhao, H.; Wolt, J.D. Risk associated with off-target plant genome editing and methods for its limitation. Emerg. Top. Life Sci. 2017, 1, 231–240. [Google Scholar] [CrossRef]
- Jaganathan, D.; Ramasamy, K.; Sellamuthu, G.; Jayabalan, S.; Venkataraman, G. CRISPR for Crop Improvement: An Update Review. Front. Plant Sci. 2018, 9, 985. [Google Scholar] [CrossRef]
- Calyxt. First Commercial Sale of Calyxt High Oleic Soybean Oil on the U.S. Market. 2019. Available online: https://calyxt.com/wp-content/uploads/2019/02/20190226_PR-Calyno-Commercialization.pdf (accessed on 11 November 2019).
- Waltz, E. With a free pass, CRISPR-edited plants reach market in record time. Nat. Biotechnol. 2018, 36, 6–7. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture—Animal and Plant Health (USDA–APHIS). Confirmation that a Glycine Max (Soybean) Line Mutagenized Using CRISPRCas9 Is Not a Regulated Article. 2017. Available online: https://www.aphis.usda.gov/biotechnology/downloads/reg_loi/17-219-01_air_response_signed.pdf (accessed on 12 August 2019).
- DuPont Pioneer. DuPont Pioneer Announces Intentions to Commercialize First CRISPR-Cas Product. 2016. Available online: https://www.pioneer.com/home/site/about/news-media/news-releases/template.CONTENT/guid.1DB8FB71-1117-9A56-E0B6-3EA6F85AAE92 (accessed on 12 August 2019).
- U.S. Department of Agriculture–Animal and Plant Health (USDA–APHIS). Confirmation of Regulatory Status of Waxy Corn Developed by CRISPR-Cas Technology. 2016. Available online: https://www.aphis.usda.gov/biotechnology/downloads/reg_loi/15-352-01_air_response_signed.pdf (accessed on 12 August 2019).
- Smyth, S.J. Canadian regulatory perspectives on genome engineered crops. GM Crop. Food 2017, 8, 35–43. [Google Scholar] [CrossRef]
- Scheben, A.; Edwards, D. Bottlenecks for genome-edited crops on the road from lab to farm. Genome Biol. 2018, 19, 178. [Google Scholar] [CrossRef]
- Court of Justice of the European Union. In Proceedings of the Press Release No 111/18, Luxembourg, 25 July 2018.
- Devos, Y.; Demont, M.; Dillen, K.; Reheul, D.; Kaiser, M.; Sanvido, O. Coexistence of genetically modified (GM) and non-GM crops in the European Union. A review. Agron. Sustain. Dev. 2009, 29, 11–30. [Google Scholar] [CrossRef]
- Beckmann, V.; Soregaroli, C.; Wesseler, J. Coexistence Rules and Regulations in the European Union. Am. J. Agric. Econ. 2006, 88, 1193–1199. [Google Scholar] [CrossRef]
- Beckmann, V.; Soregaroli, C.; Wesseler, J. Ex-Ante Regulation and Ex-Post Liability under Uncertainty and Irreversibility: Governing the Coexistence of GM Crops. Economics 2010, 4, 1–33. [Google Scholar]
- Consmuller, N.; Beckmann, V.; Schleyer, C. The Role of Coordination and Cooperation in Early Adoption of GM Crops: The Case of Bt Maize in Brandenburg, Germany. AgBioForum 2009, 12, 47–59. [Google Scholar]
- Bullock, D.S.; Desquilbet, M. The economics of non-GMO segregation and identity preservation. Food Policy 2002, 27, 81–99. [Google Scholar] [CrossRef]
- Dong, W.; Yang, L.; Shen, K.; Kim, B.; Kleter, G.A.; Marvin, H.J.P.; Guo, R.; Liang, W.; Zhang, D. GMDD: A database of GMO detection methods. BMC Bioinform. 2008, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, L.; Keilwagen, J.; Duensing, N.; Dagand, E.; Hartung, F.; Wilhelm, R.; Bendiek, J.; Sprink, T. Detection and Identification of Genome Editing in Plants: Challenges and Opportunities. Front. Plant Sci. 2019, 10, 236. [Google Scholar] [CrossRef]
- Whelan, A.I.; Lema, M.A. Regulatory framework for gene editing and other new breeding techniques (NBTs) in Argentina. GM Crop. Food 2015, 6, 253–265. [Google Scholar] [CrossRef]
- Carroll, D.; Charo, R.A. The societal opportunities and challenges of genome editing. Genome Biol. 2015, 16, 242. [Google Scholar] [CrossRef]
- Hua, K.; Zhang, J.; Botella, J.R.; Ma, C.; Kong, F.; Liu, B.; Zhu, J.-K. Perspectives on the Application of Genome-Editing Technologies in Crop Breeding. Mol. Plant 2019, 12, 1047–1059. [Google Scholar] [CrossRef]
- Schaart, J.G.; van de Wiel, C.C.M.; Lotz, L.A.P.; Smulders, M.J.M. Opportunities for Products of New Plant Breeding Techniques. Trends Plant Sci. 2016, 21, 438–449. [Google Scholar] [CrossRef]
- Sprink, T.; Eriksson, D.; Schiemann, J.; Hartung, F. Regulatory hurdles for genome editing: Process- vs. product-based approaches in different regulatory contexts. Plant Cell Rep. 2016, 35, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Hartung, F.; Schiemann, J. Precise plant breeding using new genome editing techniques: Opportunities, safety and regulation in the EU. Plant J. 2014, 78, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Ricroch, A.E.; Ammann, K.; Kuntz, M. Editing EU legislation to fit plant genome editing: The use of genome editing technologies in plant breeding requires a novel regulatory approach for new plant varieties that involves farmers. EMBO Rep. 2016, 17, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Dabrock, P. Mind the gaps! Towards an ethical framework for genome editing. EMBO Rep. 2018, 19, 197–200. [Google Scholar] [CrossRef]
- Caplan, A.L.; Parent, B.; Shen, M.; Plunkett, C. No time to waste—The ethical challenges created by CRISPR: CRISPR/Cas, being an efficient, simple, and cheap technology to edit the genome of any organism, raises many ethical and regulatory issues beyond the use to manipulate human germ line cells. EMBO Rep. 2015, 16, 1421–1426. [Google Scholar] [CrossRef]
- Rodriguez, E. Ethical Issues in Genome Editing using Crispr/Cas9 System. J. Clin. Res. Bioeth. 2016, 7. [Google Scholar] [CrossRef]
- Ishii, T.; Araki, M. Consumer acceptance of food crops developed by genome editing. Plant Cell Rep. 2016, 7, 1507–1518. [Google Scholar] [CrossRef]
- Araki, M.; Ishii, T. Towards social acceptance of plant breeding by genome editing. Trends Plant Sci. 2015, 20, 145–149. [Google Scholar] [CrossRef]
- Shew, A.M.; Nalley, L.L.; Snell, H.A.; Nayga, R.M.; Dixon, B.L. CRISPR versus GMOs: Public acceptance and valuation. Glob. Food Secur. 2018, 19, 71–80. [Google Scholar] [CrossRef]
- Whelan, A.I.; Lema, M.A. A research program for the socioeconomic impacts of gene editing regulation. GM Crop. Food 2017, 8, 74–83. [Google Scholar] [CrossRef][Green Version]
- Kaplinsky, R.; Morris, M. A Handbook for Value Chain Research. 2001. Available online: http://www.prism.uct.ac.za/papers/vchnov01.pdf (accessed on 1 February 2018).
- Porter, M.E. Competitive Advantage: Creating and Sustaining Superior Performance; Free Press: New York, NY, USA, 1985. [Google Scholar]
- Maaß, O.; Grundmann, P. Added-value from linking the value chains of wastewater treatment, crop production and bioenergy production: A case study on reusing wastewater and sludge in crop production in Braunschweig (Germany). Resour. Conserv. Recycl. 2016, 107, 195–211. [Google Scholar] [CrossRef]
- Maaß, O.; Grundmann, P. Governing Transactions and Interdependences between Linked Value Chains in a Circular Economy: The Case of Wastewater Reuse in Braunschweig. Sustainability 2018, 10, 1125. [Google Scholar] [CrossRef]
- Maaß, O.; Grundmann, P.; Bock und Polach, C. Added-value from innovative value chains by establishing nutrient cycles via struvite. Resour. Conserv. Recycl. 2014, 87, 126–136. [Google Scholar] [CrossRef]
- Mann, S. Socioeconomics of Agriculture; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Boyer, R. The quest for theoretical foundations of socio-economics: Epistemology, methodology or ontology? Socio-Econ. Rev. 2008, 6, 733–746. [Google Scholar] [CrossRef][Green Version]
- Catacora-Vargas, G.; Binimelis, R.; Myhr, A.I.; Wynne, B. Socio-economic research on genetically modified crops: A study of the literature. Agric. Hum. Values 2018, 35, 489–513. [Google Scholar] [CrossRef]
- Yin, R.K. Case Study Research: Design and Methods, 5th ed.; Sage Publishing: Los Angeles, CA, USA; London, UK; New Delhi, India; Singapore; Washington, DC, USA, 2014. [Google Scholar]
- Mietzner, D.; Reger, G. Advantages and disadvantages of scenario approaches for strategic foresight. Int. J. Technol. Intell. Plan. 2005, 1, 220–239. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Kemper, S.; Schaack, D.; Schenck, W. AMI Markt Bilanz Getreide Ölsaaten Futtermittel; Agrarmarkt Informations-Gesellschaft mbH: Bonn, Germany, 2019. [Google Scholar]
- Oerke, E.-C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Lopez, J.A.; Rojas, K.; Swart, J. The economics of foliar fungicide applications in winter wheat in Northeast Texas. Crop Prot. 2015, 67, 35–42. [Google Scholar] [CrossRef][Green Version]
- Luo, Y.; Liu, X.; Li, J. Updating techniques on controlling mycotoxins—A review. Food Control 2018, 89, 123–132. [Google Scholar] [CrossRef]
- Tola, M.; Kebede, B. Occurrence, importance and control of mycotoxins: A review. Cogent Food Agric. 2016, 2, 779. [Google Scholar] [CrossRef]
- Stein, R.A.; Bulboacӑ, A.E. Mycotoxins. In Foodborne Diseases; Elsevier: Amsterdam, The Netherlands, 2017; pp. 407–446. [Google Scholar]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Tech. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Gobbetti, M.; Pontonio, E.; Filannino, P.; Rizzello, C.G.; Angelis, M.; Di Cagno, R. How to improve the gluten-free diet: The state of the art from a food science perspective. Food Res. Int. 2018, 110, 22–32. [Google Scholar] [CrossRef]
- Mustalahti, K.; Catassi, C.; Reunanen, A.; Fabiani, E.; Heier, M.; McMillan, S.; Murray, L.; Metzger, M.-H.; Gasparin, M.; Bravi, E.; et al. The prevalence of celiac disease in Europe: Results of a centralized, international mass screening project. Ann. Med. 2010, 42, 587–595. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–676, quiz 677. [Google Scholar] [CrossRef]
- Kupper, C. Dietary guidelines and implementation for celiac disease. Gastroenterology 2005, 128, 121–127. [Google Scholar] [CrossRef]
- Jouanin, A.; Boyd, L.; Visser, R.G.F.; Smulders, M.J.M. Development of Wheat with Hypoimmunogenic Gluten Obstructed by the Gene Editing Policy in Europe. Front. Plant Sci. 2018, 9, 1523. [Google Scholar] [CrossRef]
- Jouanin, A.; Gilissen, L.J.W.J.; Boyd, L.A.; Cockram, J.; Leigh, F.J.; Wallington, E.J.; van den Broeck, H.C.; van der Meer, I.M.; Schaart, J.G.; Visser, R.G.F.; et al. Food processing and breeding strategies for coeliac-safe and healthy wheat products. Food Res. Int. 2018, 110, 11–21. [Google Scholar] [CrossRef]
- Jnawali, P.; Kumar, V.; Tanwar, B. Celiac disease: Overview and considerations for development of gluten-free foods. Food Sci. Hum. Wellness 2016, 5, 169–176. [Google Scholar] [CrossRef]
- Muñoz, I.V.; Sarrocco, S.; Malfatti, L.; Baroncelli, R.; Vannacci, G. CRISPR-Cas for Fungal Genome Editing: A New Tool for the Management of Plant Diseases. Front. Plant Sci. 2019, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Gil-Humanes, J.; Wang, Y.; Liang, Z.; Shan, Q.; Ozuna, C.V.; Sánchez-León, S.; Baltes, N.J.; Starker, C.; Barro, F.; Gao, C.; et al. High-efficiency gene targeting in hexaploid wheat using DNA replicons and CRISPR/Cas9. Plant J. 2017, 89, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-León, S.; Gil-Humanes, J.; Ozuna, C.V.; Giménez, M.J.; Sousa, C.; Voytas, D.F.; Barro, F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018, 16, 902–910. [Google Scholar] [CrossRef]
- Bogner, A.; Littig, B.; Menz, W. Interviewing Experts; Palgrave Macmillan: Basingstoke, UK, 2009. [Google Scholar]
- McCammon, B. Semi-Structured Interviews. 2019. Available online: http://designresearchtechniques.com/casestudies/semi-structured-interviews/ (accessed on 9 March 2019).
- Mayring, P. Qualitative Content Analysis: Theoretical Foundation, Basic Procedures and Software Solution; Philipp Mayring: Klagenfurt, Austria, 2014. [Google Scholar]
- Schreier, M. Qualitative Content Analysis in Practice; SAGE: Los Angeles, CA, USA; London, UK; New Delhi, India; Singapore; Washington, DC, USA, 2012. [Google Scholar]
- Holsti, O.R. Content Analysis for the Social Sciences and Humanities; Addison-Wesley: Boston, MA, USA, 1969. [Google Scholar]
- Neuendorf, K.A. The Content Analysis Guidebook; Sage Publication: Thousand Oaks, CA, USA, 2002. [Google Scholar]
- Lombard, M.; Snyder-Duch, J.; Bracken, C.C. Content Analysis in Mass Communication: Assessment and Reporting of Intercoder Reliability. Hum. Comm. Res. 2002, 28, 587–604. [Google Scholar] [CrossRef]
- Nili, A.; Tate, M.; Barros, A. A Critical Analysis of Inter-Coder Reliability Methods in Information Systems Research. In Proceedings of the Australasian Conference on Information Systems (ACIS 2017), Hobart, Australia, 3–6 December 2017. [Google Scholar]
- Zachariasova, M.; Dzuman, Z.; Veprikova, Z.; Hajkova, K.; Jiru, M.; Vaclavikova, M.; Zachariasova, A.; Pospichalova, M.; Florian, M.; Hajslova, J. Occurrence of multiple mycotoxins in European feedingstuffs, assessment of dietary intake by farm animals. Anim. Feed Sci. Technol. 2014, 193, 124–140. [Google Scholar] [CrossRef]
- Kucek, L.K.; Veenstra, L.D.; Amnuaycheewa, P.; Sorrells, M.E. A Grounded Guide to Gluten: How Modern Genotypes and Processing Impact Wheat Sensitivity. Compr. Rev. Food Sci. F 2015, 14, 285–302. [Google Scholar] [CrossRef]
- Klocke, B.; Dachbrodt-Saaydeh, S. Use of cultivar resistance in practice—Results of the network reference farms in plant protection in the years 2007 to 2016. Jul. Kühn Arch. 2018, 461, 98–99. [Google Scholar]
- Folcher, L.; Delos, M.; Marengue, E.; Jarry, M.; Weissenberger, A.; Eychenne, N.; Regnault-Roger, C. Lower mycotoxin levels in Bt maize grain. Agron. Sustain. Dev. 2010, 30, 711–719. [Google Scholar] [CrossRef]
- Demont, M.; Devos, Y. Regulating coexistence of GM and non-GM crops without jeopardizing economic incentives. Trends Biotechnol. 2008, 26, 353–358. [Google Scholar] [CrossRef]
- Bundesministerium der Justiz und für Verbraucherschutz. Verordnung über die gute fachliche Praxis bei der Erzeugung gentechnisch veränderter Pflanzen (Gentechnik- Pflanzenerzeugungsverordnung—GenTPflEV); Bundesministerium der Justiz und für Verbraucherschutz: Berlin, Germany, 2008. [Google Scholar]
- Stokstad, E. Biotechnology. Monsanto pulls the plug on genetically modified wheat. Science 2004, 304, 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Queirós, A.; Faria, D.; Almeida, F. Strengths and Limitations of Qualitative and Quantitative Research Methods. Eur. J. Educ. Stud. 2017, 3, 369–387. [Google Scholar] [CrossRef]
- Rahman, M.S. The Advantages and Disadvantages of Using Qualitative and Quantitative Approaches and Methods in Language “Testing and Assessment” Research: A Literature Review. J. Educ. Learn. 2016, 6, 102–112. [Google Scholar] [CrossRef]
- Opdenakker, R. Advantages and Disadvantages of Four Interview Techniques in Qualitative Research. Forum Qual. Soc. Res. 2006, 7, 1–13. [Google Scholar]
| Value-Added Step | Interviewees | Date | Duration | Number of Experts Interviewed |
|---|---|---|---|---|
| Breeding and seed production | Association | 26.02.2018 | 137 min | 1 |
| Company | 15.03.2018 | 124 min | 2 | |
| Association | 23.03.2018 | 106 min | 3 | |
| Association | 17.04.2018 | 130 min | 1 | |
| Crop production | Association | 22.02.2018 | 157 min | 1 |
| Association | 23.06.2018 | 132 min | 3 | |
| Agricultural trade | Association | 11.02.2019 | 69 min | 1 |
| Milling | Association | 28.02.2018 | 110 min | 1 |
| Food production | Association | 04.12.2018 | 144 min | 2 |
| Starch production | Association | 28.02.2018 | 38 min | 1 |
| Company | 08.05.2018 | 156 min | 1 | |
| Company | 14.05.2018 | 125 min | 1 | |
| Feed production | Association | 18.04.2018 | 131 min | 1 |
| Animal production | Association | 26.06.2018 | 163 min | 1 |
| Association | 28.06.2018 | 115 min | 1 | |
| Biofuel production | Company | 29.06.2018 | 106 min | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maaß, O.; Consmüller, N.; Kehlenbeck, H. Socioeconomic Impact of Genome Editing on Agricultural Value Chains: The Case of Fungal-Resistant and Coeliac-Safe Wheat. Sustainability 2019, 11, 6421. https://doi.org/10.3390/su11226421
Maaß O, Consmüller N, Kehlenbeck H. Socioeconomic Impact of Genome Editing on Agricultural Value Chains: The Case of Fungal-Resistant and Coeliac-Safe Wheat. Sustainability. 2019; 11(22):6421. https://doi.org/10.3390/su11226421
Chicago/Turabian StyleMaaß, Oliver, Nicola Consmüller, and Hella Kehlenbeck. 2019. "Socioeconomic Impact of Genome Editing on Agricultural Value Chains: The Case of Fungal-Resistant and Coeliac-Safe Wheat" Sustainability 11, no. 22: 6421. https://doi.org/10.3390/su11226421
APA StyleMaaß, O., Consmüller, N., & Kehlenbeck, H. (2019). Socioeconomic Impact of Genome Editing on Agricultural Value Chains: The Case of Fungal-Resistant and Coeliac-Safe Wheat. Sustainability, 11(22), 6421. https://doi.org/10.3390/su11226421




