Field Margin Vegetation in Tropical African Bean Systems Harbours Diverse Natural Enemies for Biological Pest Control in Adjacent Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Insects Sampling in the Field Margin and at Different Distances into the Bean Field
2.3. The Assessment of Predominant Field Margin Plants in Each Elevation Zone
2.4. Fluorescent Dye Experiment to Monitor the Movement of the Natural Enemies and Pests
2.5. Field Predation Experiment
2.6. Field Parasitism Experiment
2.7. Data Analysis
3. Results
3.1. Sampled Insects in the Three Coloured Pan Traps across the Three Zones
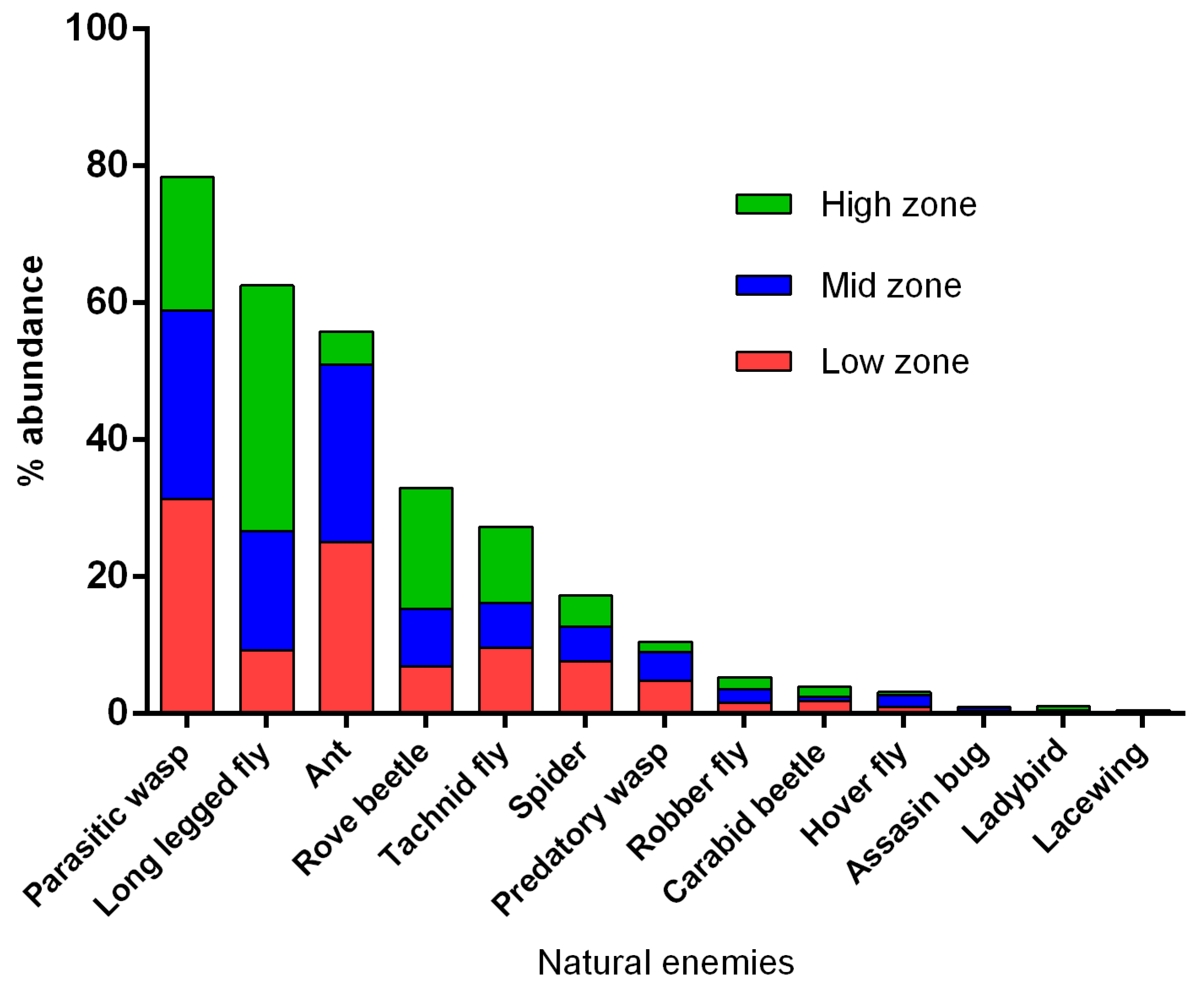
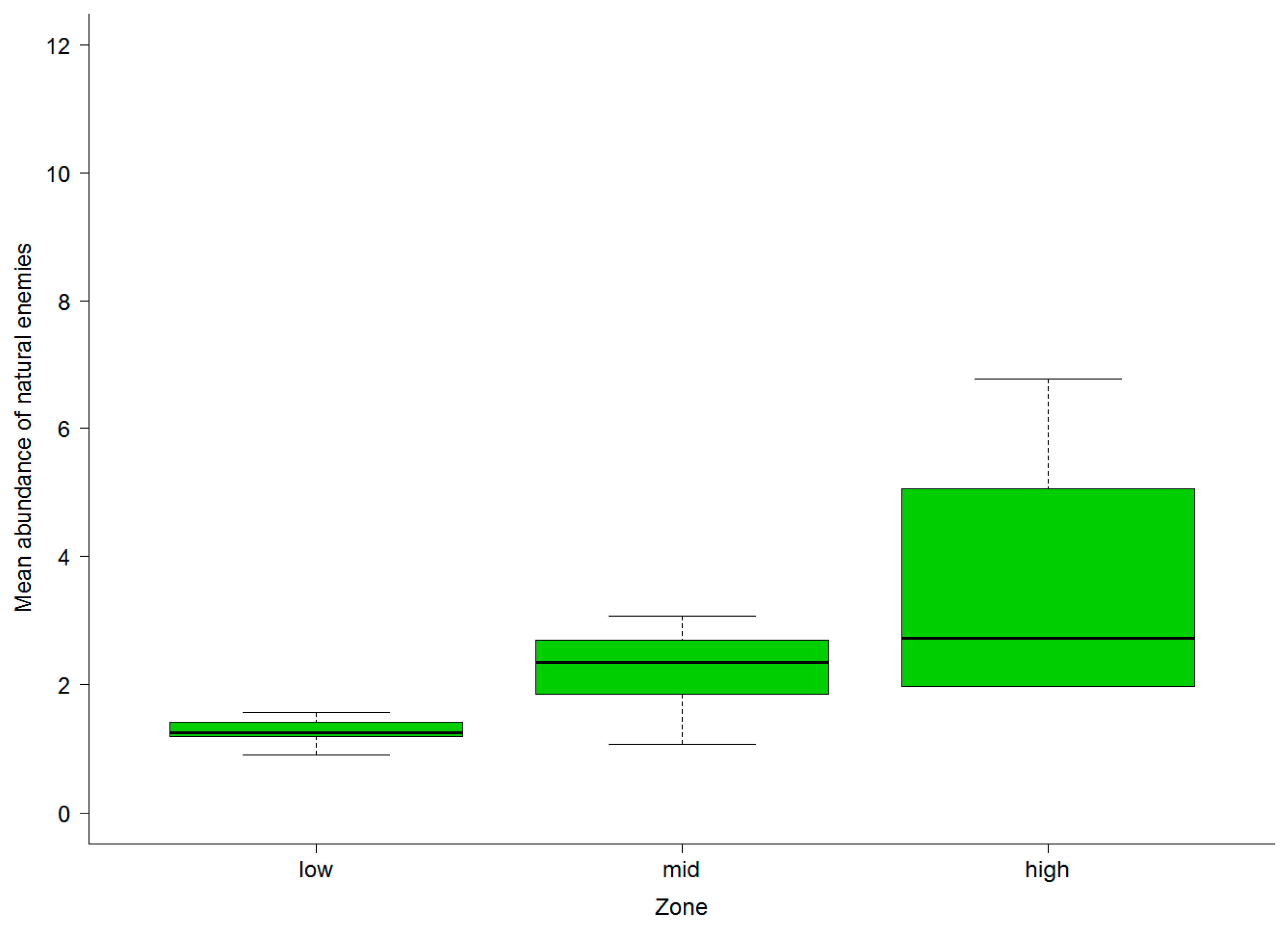
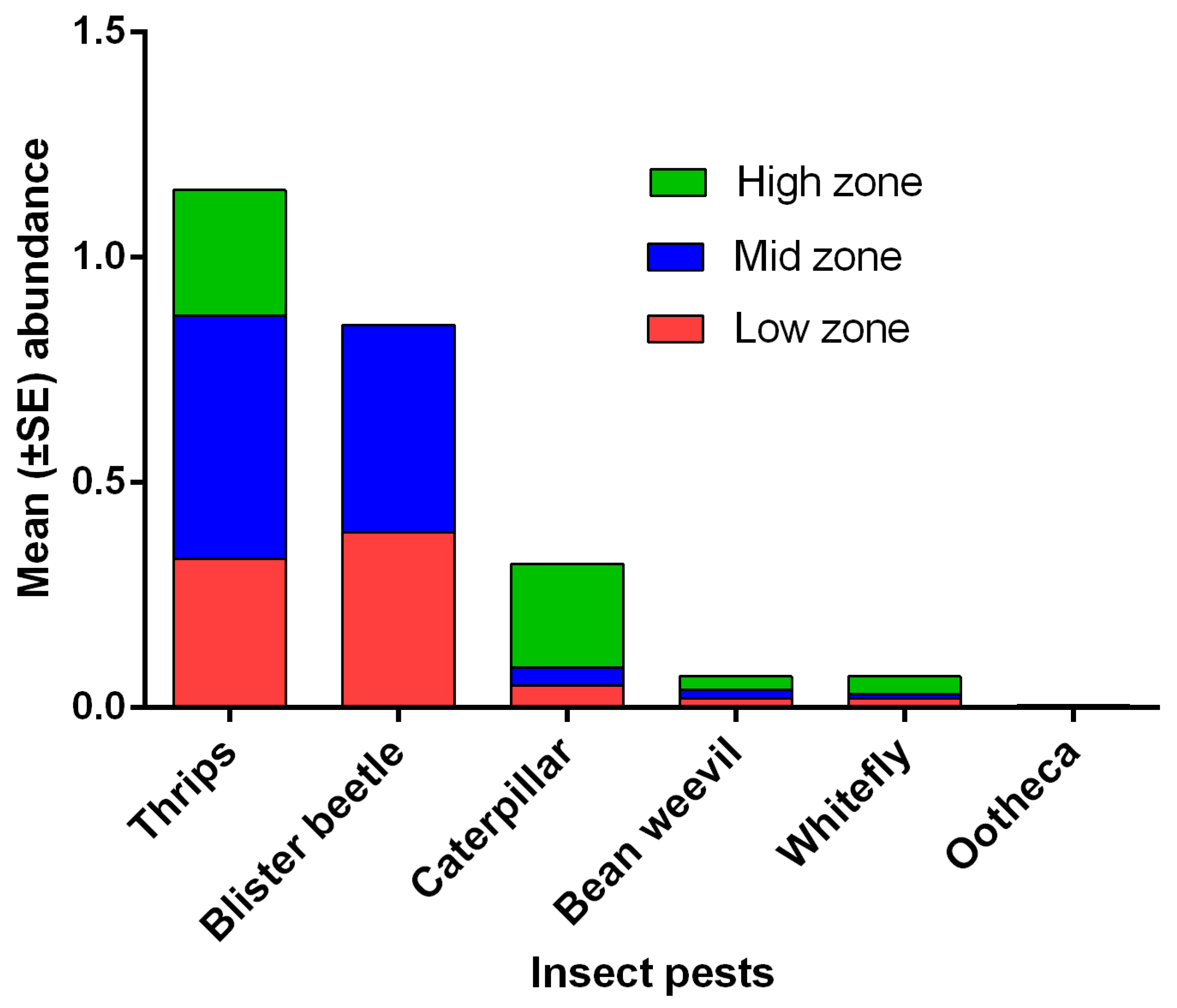
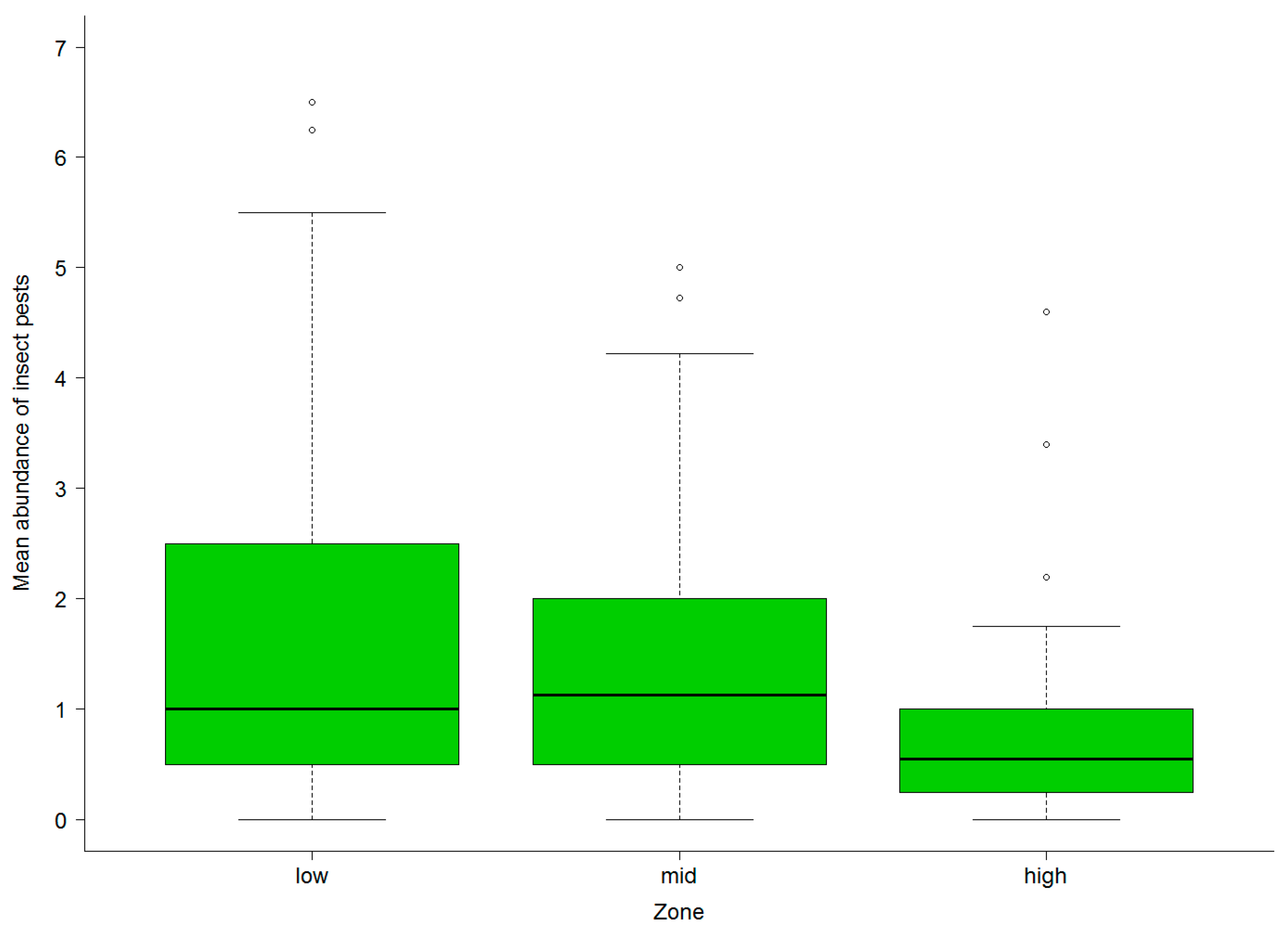
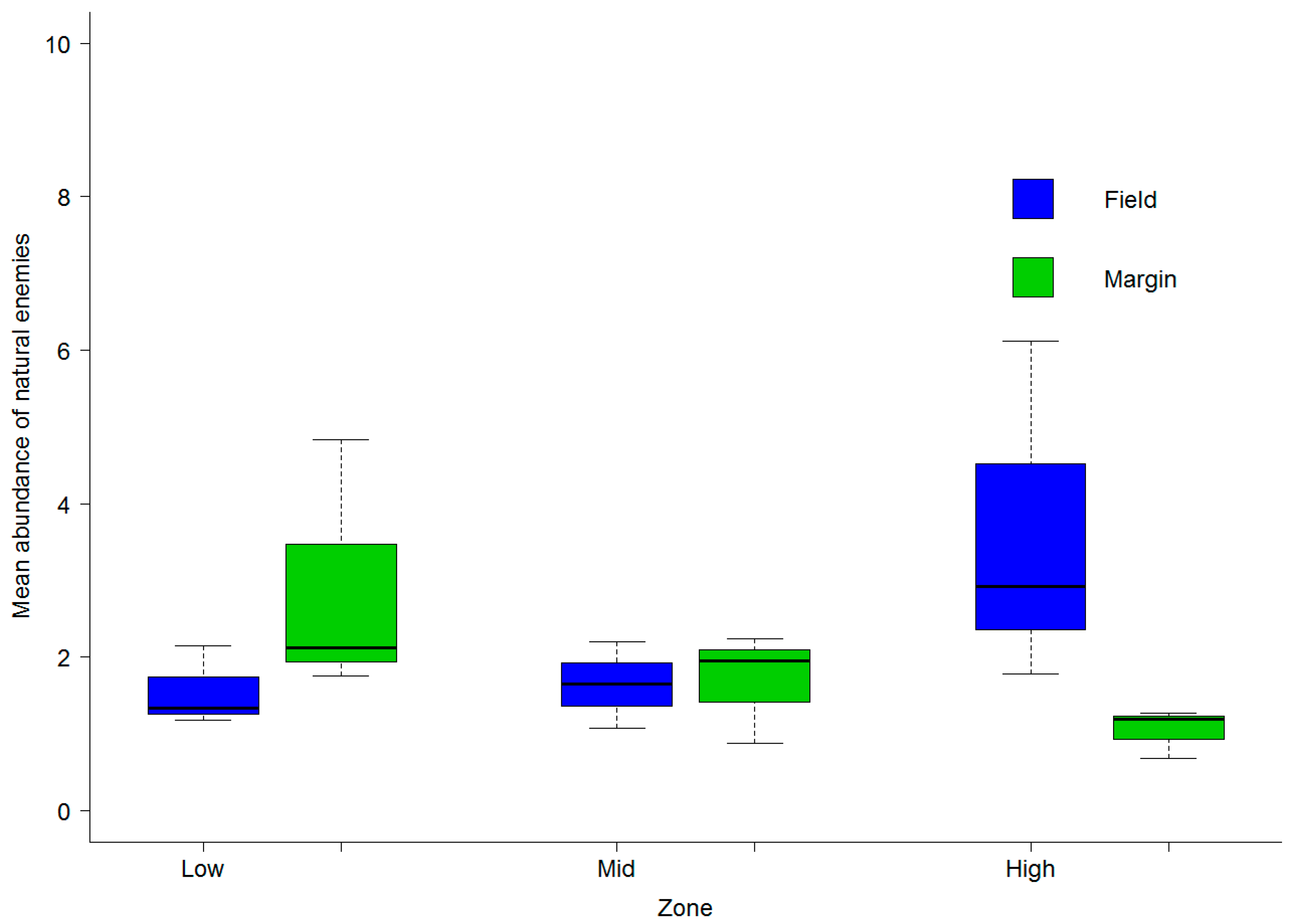
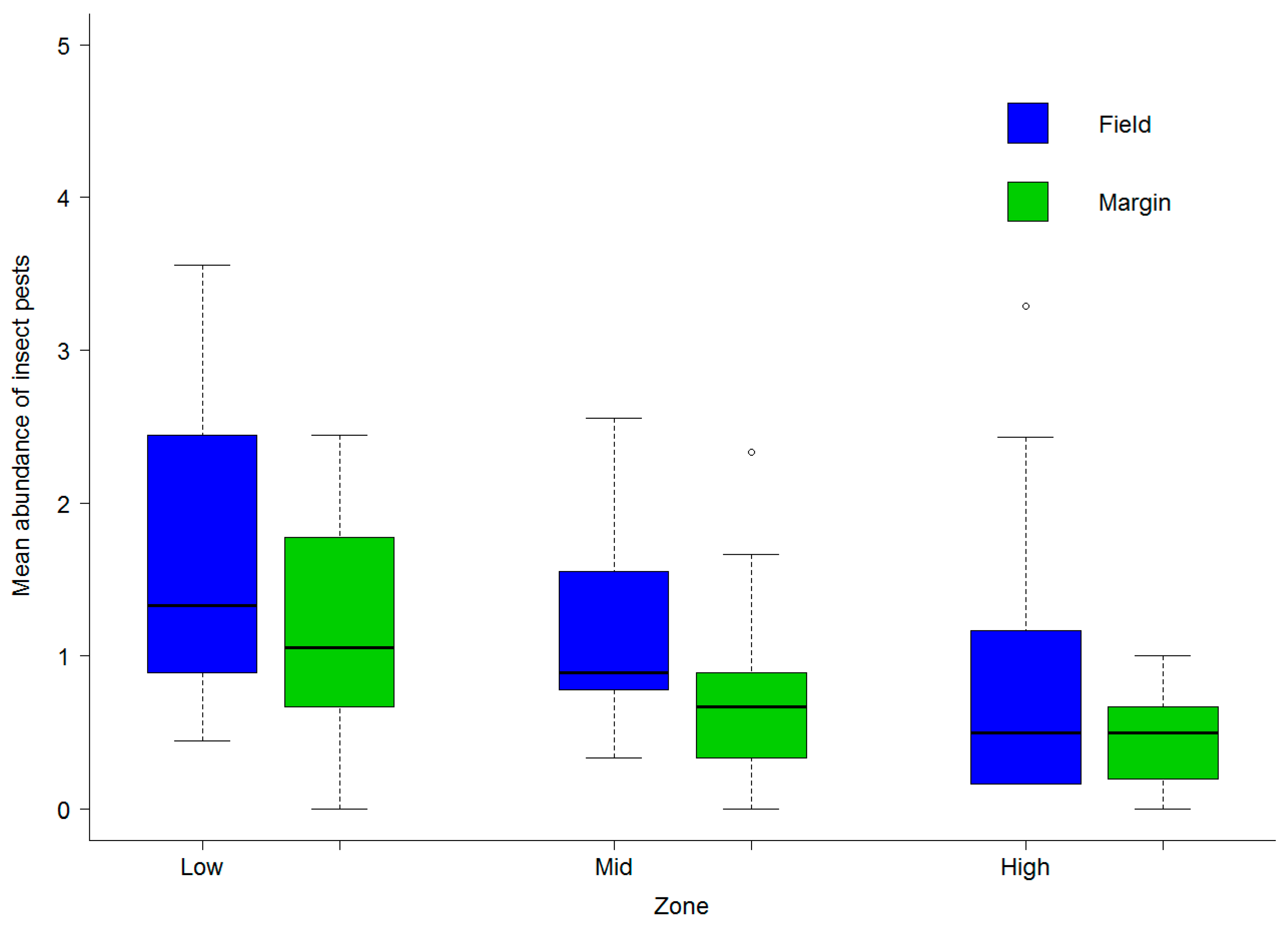
3.2. Abundance of Natural Enemies and Insect Pests in Field Margins and Crops
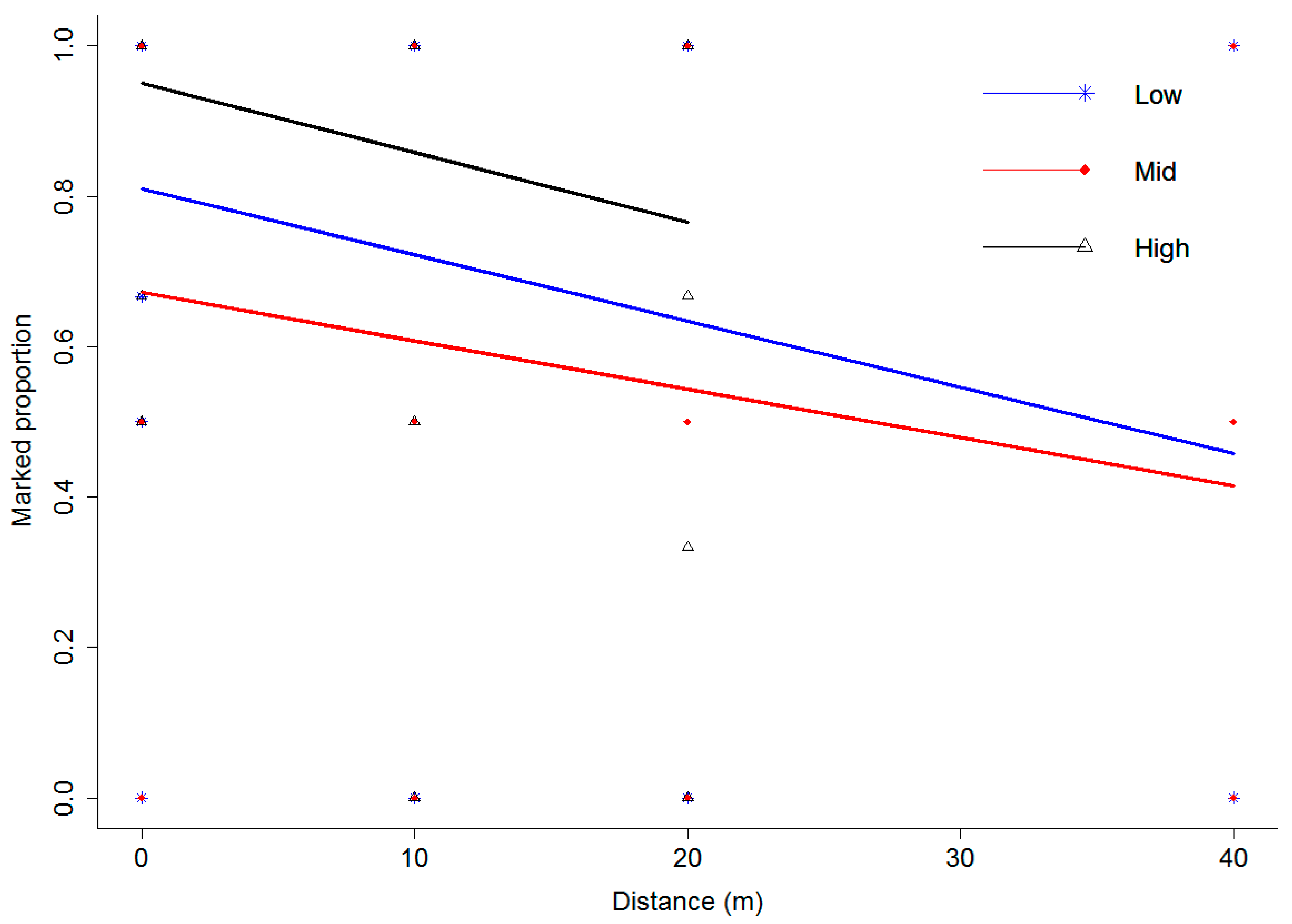
3.3. Spatial Variation of the Sampled Insects in the Bean Field
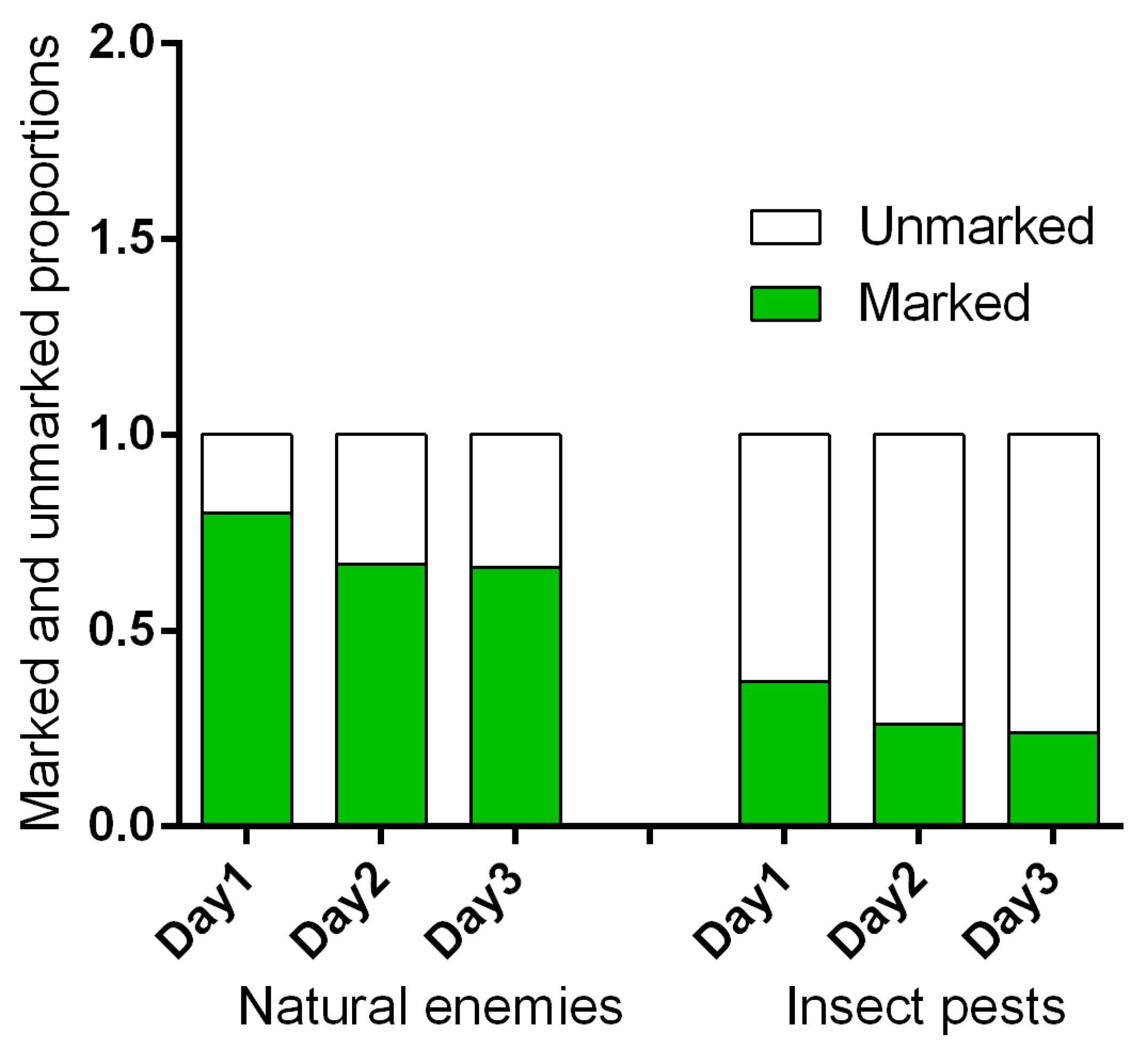
3.4. Fluorescent Dye Experiment
3.4.1. Movement of the Natural Enemies from the Field Margin to the Bean Crop
3.4.2. Movement of the Insect Pests from the Field Margin to the Bean Crop
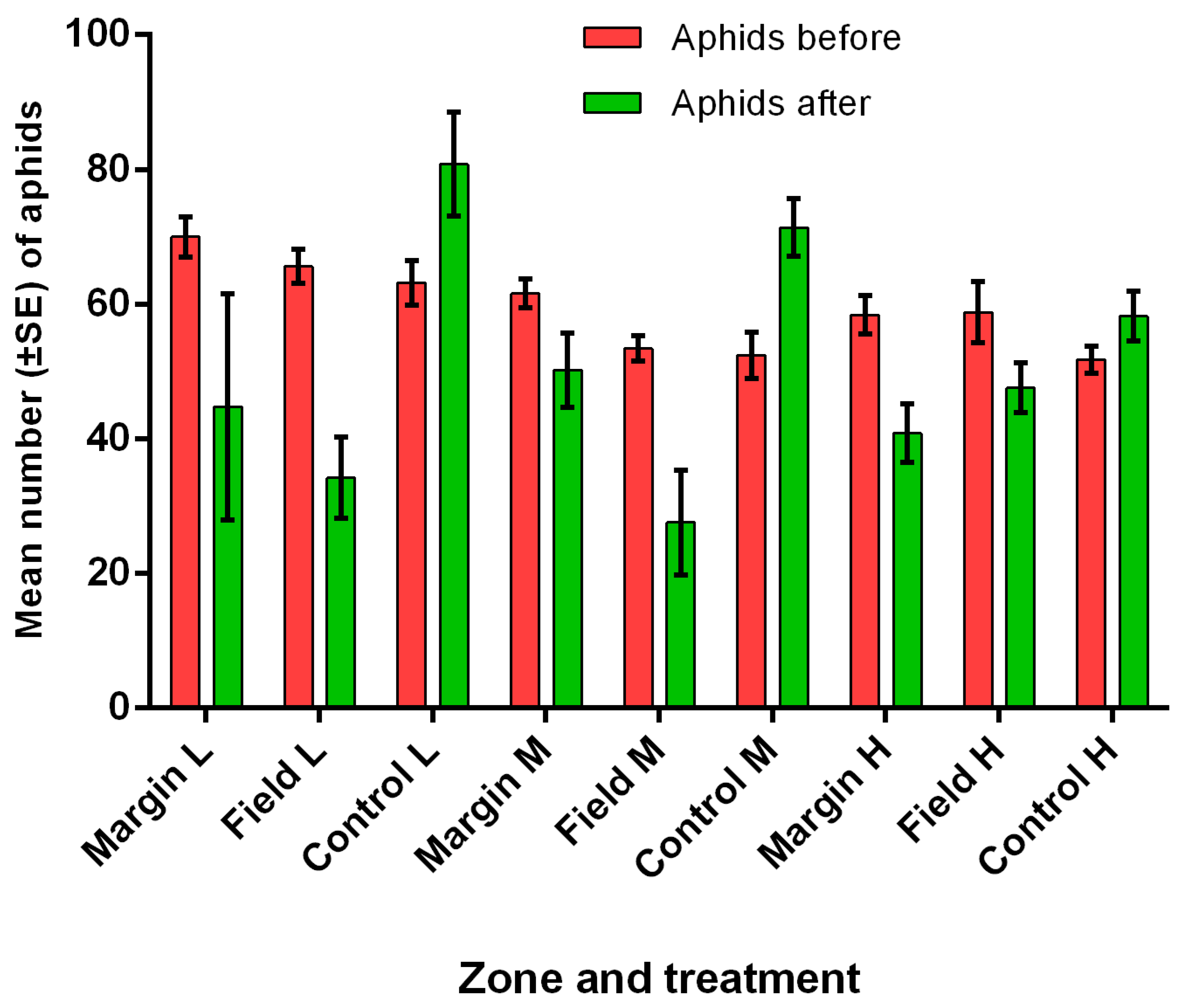
3.5. Field Predation and Parasitism
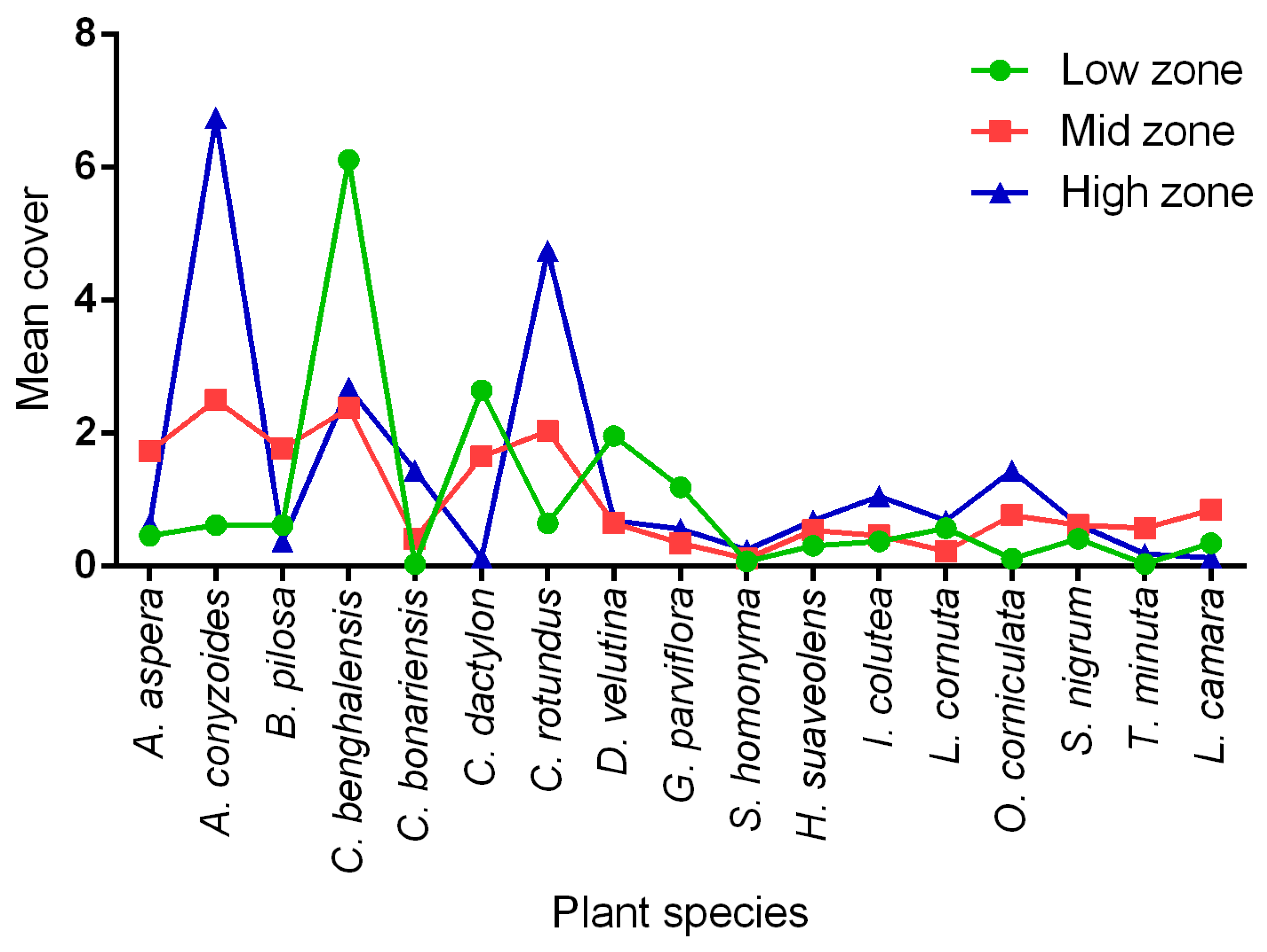
3.6. Predominant Field Margin Plants in Each Elevation Zone
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gurr, G.M.; Lu, Z.; Zheng, X.; Xu, H.; Zhu, P.; Chen, G.; Yao, X.; Cheng, J.; Zhu, Z.; Catindig, J.L.; et al. Multi-country evidence that crop diversification promotes ecological intensification of agriculture. Nat. Plants 2016, 2, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, R.; Wright, M.G. Effects of interplanting flowering plants on the biological control of corn earworm (Lepidoptera: Noctuidae) and thrips (Thysanoptera: Thripidae) in sweet corn. J. Econ. Entomol. 2016, 109, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Schellhorn, N.A.; Macfadyen, S.; Bianchi, F.J.J.A.; Williams, D.G.; Zalucki, M. Managing ecosystem services in broadacre landscapes: What are the appropriate spatial scales? Aust. J. Exp Agrc. 2008, 48, 1549–1559. [Google Scholar] [CrossRef]
- Werling, B.P.; Gratton, C. Influence of field margins and landscape context on ground beetle diversity in Wisconsin (USA) potato fields. Agrc. Ecosyst. Environ. 2008, 128, 104–108. [Google Scholar] [CrossRef]
- Eilenberg, J.; Hajek, A.; Lomer, C. Suggestions for unifying the terminology in biological control. BioControl 2001, 46, 387–400. [Google Scholar] [CrossRef]
- Anderson, A.; Carnus, T.; Helden, A.J.; Sheridan, H.; Purvis, G. The influence of conservation field margins in intensively managed grazing land on communities of five arthropod trophic groups. Insect Conserv. Diver. 2013, 6, 201–211. [Google Scholar] [CrossRef]
- Heimoana, V.; Pilkington, L.J.; Raman, A.; Mitchell, A.; Nicol, H.I.; Johnson, A.C.; Gurr, G.M. Integrating spatially explicit molecular and ecological methods to explore the significance of non-crop vegetation to predators of brassica pests. Agrc. Ecosyst. Environ. 2017, 239, 12–19. [Google Scholar] [CrossRef]
- Inclán, D.J.; Dainese, M.; Cerretti, P.; Paniccia, D.; Marini, L. Spillover of tachinids and hoverflies from different field margins. Basic Appl. Ecol. 2016, 17, 33–42. [Google Scholar] [CrossRef]
- Fusser, M.S.; Holland, J.M.; Jeanneret, P.; Pfister, S.C.; Entling, M.H.; Schirmel, J. Interactive effects of local and landscape factors on farmland carabids. Agrc. For. Entomol. 2018, 20, 549–557. [Google Scholar] [CrossRef]
- Amaral, D.S.S.L.; Venzon, M.; dos Santos, H.H.; Sujii, E.R.; Schmidt, J.M.; Harwood, J.D. Non-crop plant communities conserve spider populations in chili pepper agro ecosystems. BioControl 2016, 103, 69–77. [Google Scholar] [CrossRef]
- Gardiner, M.M.; Landis, D.A.; Gratton, C.; DiFonzo, C.D.; O’Neal, M.; Chacon, J.M.; Wayo, M.T.; Schmidt, N.P.; Mueller, E.E.; Heimpel, G.E. Landscape diversity enhances biological control of an introduced crop pest in the northcentral USA. Ecol. Appl. 2009, 19, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity–Ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Veres, A.; Petit, S.; Conord, C.; Lavigne, C. Does landscape composition affect pest abundance and their control by natural enemies? A review. Agrc. Ecosyst. Environ. 2013, 166, 110–117. [Google Scholar] [CrossRef]
- González, E.; Salvo, A.; Defagó, M.T.; Valladares, G. A moveable feast: Insects moving at the forest-crop interface are affected by crop phenology and the mount of forest in the landscape. PLoS ONE 2016, 11, e0158836. [Google Scholar] [CrossRef]
- Benton, T.G.; Bryant, D.M.; Cole, L.; Crick, H.Q.P. Linking agricultural practice to insect and bird populations: A historical study over three decades. J. Appl. Ecol. 2002, 39, 673–687. [Google Scholar] [CrossRef]
- Thies, C.; Roschewitz, I.; Tscharntke, T. The landscape context of cereal aphid–parasitoid interactions. Proc. R Soc. B Biol. Sci. 2005, 272, 203–210. [Google Scholar] [CrossRef]
- Griffiths, G.J.K.; Holland, J.M.; Bailey, A.; Thomas, M.B. Efficacy and economics of shelter habitats for conservation biological control. Biol. Control 2008, 45, 200–209. [Google Scholar] [CrossRef]
- Lee, J.C.; Menalled, F.B.; Landis, D.A. Refuge habitats modify impact of insecticide disturbance on carabid beetle communities. J. Appl. Ecol. 2001, 38, 472–483. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C.; Frisvold, G.B. Economic value of biological control in Integrated Pest Management of managed plant systems. Ann. Rev. Entomol. 2015, 60, 621–645. [Google Scholar] [CrossRef]
- Boetzl, F.A.; Krimmer, E.; Krauss, J.; Steffan-Dewenter, I. Agri-environmental schemes promote ground-dwelling predators in adjacent oilseed rape fields: Diversity, species traits and distance-decay functions. J. Appl. Ecol. 2019, 56, 10–20. [Google Scholar] [CrossRef]
- Macfadyen, S.; Hopkinson, J.; Parry, H.; Neave, M.J.; Bianchi, F.J.J.A.; Zalucki, M.P.; Schellhorn, N.A. Early-season movement dynamics of phytophagous pest and natural enemies across a native vegetation-crop ecotone. Agrc. Ecosyst. Environ. 2015, 200, 110–118. [Google Scholar] [CrossRef]
- Brosius, T.R.; Higley, L.G.; Hunt, T.E. Population dynamics of soybean aphid and biotic mortality at the edge of its range. J. Econ. Entomol. 2007, 100, 1268–1275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pfab, F.; Stacconi, M.V.R.; Anfora, G.; Grassi, A.; Walton, V.; Pugliese, A. Optimized timing of parasitoid release: A mathematical model for biological control of Drosophila Suzukii. Theor. Ecol. 2018, 11, 489–501. [Google Scholar] [CrossRef]
- González, E.; Salvo, A.; Valladares, G. Sharing enemies: Evidence of forest contribution to natural enemy communities in crops, at different spatial scales. Insect Conserv. Diver. 2015, 8, 359–366. [Google Scholar] [CrossRef]
- Tindo, M.; Hanna, R.; Goergen, G.; Zapfack, L.; Tata-Hangy, K.; Attey, A. Host plants of Stictococcus vayssierei Richard (Stictococcidae) in non-crop vegetation in the Congo Basin and implications for developing scale management options. Int. J. Pest. Manag. 2009, 55, 339–345. [Google Scholar] [CrossRef]
- Arnó, J.; Solà, M.; Riudavets, J.; Gabarra, R. Population dynamics, non-crop hosts, and fruit susceptibility of Drosophila suzukii in Northeast Spain. J. Pest. Sci. 2016, 89, 713–723. [Google Scholar] [CrossRef]
- Kenis, M.; Tonina, L.; Eschen, R.; van der Sluis, B.; Sancassani, M.; Mori, N.; Haye, T.; Helsen, H. Non-crop plants used as hosts by Drosophila suzukii in Europe. J. Pest. Sci. 2016, 89, 735–748. [Google Scholar] [CrossRef]
- Ohno, S.; Miyagi, A.; Ganaha-Kikumura, T.; Gotoh, T.; Kijima, K.; Ooishi, T.; Moromizato, C.; Haraguchi, D.; Yonamine, K.; Uezato, T. Non-crop host plants of Tetranychus spider mites (Acari: Tetranychidae) in the field in Okinawa, Japan: Determination of possible sources of pest species and inference on the cause of peculiar mite fauna on crops. Appl. Entomol. Zool. 2010, 45, 465–475. [Google Scholar] [CrossRef][Green Version]
- Tscharntke, T.; Karp, D.S.; Chaplin-kramer, R.; Batary, P.; Declerck, F.; Gratton, C.; Hunt, L.; Ives, A.; Jonsson, M.; Larsen, A.; et al. When natural habitat fails to enhance biological pest control–five hypotheses. Biol. Conserv. 2016, 204B, 449–458. [Google Scholar] [CrossRef]
- Balzan, M.V.; Bocci, G.; Moonen, A.C. Landscape complexity and field margin vegetation diversity enhance natural enemies and reduce herbivory by Lepidoptera pests on tomato crop. BioControl 2016, 61, 141–154. [Google Scholar] [CrossRef]
- Fusser, M.S.; Pfister, S.C.; Entling, M.H.; Schirmel, J. Effects of landscape composition on carabids and slugs in herbaceous and woody field margins. Agrc. Ecosyst. Environ. 2016, 226, 79–87. [Google Scholar] [CrossRef]
- Sorribas, J.; González, S.; Domínguez-Gento, A.; Vercher, R. Abundance, movements and biodiversity of flying predatory insects in crop and non-crop agro ecosystems. Agron. Sustain. Dev. 2016, 36, 34. [Google Scholar] [CrossRef]
- Ribeiro, A.L.; Gontijo, L.M. Alyssum flowers promote biological control of collard pests. BioControl 2017, 62, 185–196. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Collatz, J.; Dubsky, V.; Entling, M.H.; Najar-Rodriguez, A.J.; Jacot, K. Tailored flower strips promote natural enemy biodiversity and pest control in potato crops. J. Appl. Ecol. 2016, 53, 1169–1176. [Google Scholar] [CrossRef]
- Bryant, A.; Coudron, T.; Brainard, D.; Szendrei, Z. Cover crop mulches influence biological control of the imported cabbageworm (Pieris rapae L., Lepidoptera: Pieridae) in cabbage. Biol. Control 2014, 73, 75–83. [Google Scholar] [CrossRef]
- Laubertie, E.A.; Wratten, S.D.; Sedcole, J.R. The role of odour and visual cues in the pan-trap catching of hoverflies (Diptera: Syrphidae). Ann. Appl. Biol. 2006, 148, 173–178. [Google Scholar] [CrossRef]
- Abrahamczyk, S.; Steudel, B.; Kessler, M. Sampling Hymenoptera along a precipitation gradient in tropical forests: The effectiveness of different coloured pan traps. Entomol. Exp. Appl. 2010, 137, 262–268. [Google Scholar] [CrossRef]
- Perović, D.J.; Gurr, G.M.; Raman, A.; Nicol, H.I. Effect of landscape composition and arrangement on biological control agents in a simplified agricultural system: A cost-distance approach. Biol. Control 2010, 52, 263–270. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing (Version 3.4.2); R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; SpringerVerlag: New York, NY, USA, 2002. [Google Scholar]
- Atakan, E. Influence of weedy field margins on abundance patterns of the predatory bugs Orius spp. and their prey, the western flower thrips (Frankliniella occidentalis), on faba bean. Phytoparasitica 2010, 38, 313–325. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Ann. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Wäckers, F.L. Effects of flower attractiveness and nectar availability in field margins on biological control by parasitoids. BioControl 2008, 46, 400–408. [Google Scholar] [CrossRef]
- Bäckman, J.P.C.; Tiainen, J. Habitat quality of field margins in a Finnish farmland area for bumblebees (Hymenoptera: Bombus and Psithyrus). Agrc. Ecosyst. Environ. 2002, 89, 53–68. [Google Scholar] [CrossRef]
- Alomar, O.; Gabarra, R.; González, O.; Arnó, J. Selection of insectary plants for ecological infrastructure in Mediterranean vegetable crops. IOBC/WPRS Bull. 2006, 29, 5–8. [Google Scholar]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. 2006, 273, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.S.; Venzon, M.; Duarte, M.V.; Sousa, F.F.; Pallini, A.; Harwood, J.D. Non-crop vegetation associated with chili pepper agroecosystems promote the abundance and survival of aphid predators. Biol. Control 2013, 64, 338–346. [Google Scholar] [CrossRef]
- Lin, S.H.; Chang, S.Y.; Chen, S.H. The study of bee-collected pollen loads in Nantou, Taiwan. Taiwania 1993, 38, 117–134. [Google Scholar] [CrossRef]
- Ngongolo, K.; Mtoka, S.; Mafuwe, K.; Mahulu, A. Floral visitors of the Ageratum conyzoides in Amani Nature Reserve, Tanzania. Int. J. Dev. Sustain. 2014, 3, 1060–1065. [Google Scholar]
- Cruz, W.P.; Sarmento, R.A.; Teodoro, A.V.; Neto, M.P.; Ignacio, M. Driving factors of the communities of phytophagous and predatory mites in a physic nut plantation and spontaneous plants associated. Exp. Appl. Acarol. 2013, 60, 509–519. [Google Scholar] [CrossRef][Green Version]
- Sadof, C.S.; Linkimer, M.; Hidalgo, E.; Casanoves, F.; Gibson, K.; Benjamin, T.J. Effects of weed cover composition on insect pest and natural enemy abundance in a field of Dracaena marginata (Asparagales: Asparagaceae) in Costa Rica. Environ. Entomol. 2014, 43, 320–327. [Google Scholar] [CrossRef]
- Sigsgaard, L.; Betzer, C.; Naulin, C.; Eilenberg, J.; Enkegaard, A.; Kristensen, K.; Michaud, J.P. The effect of floral resources on parasitoid and host longevity: Prospects for conservation biological control in strawberries. J. Insect Sci. 2013, 13, 1–17. [Google Scholar] [CrossRef]
- Bischoff, A.; Pollier, A.; Lamarre, E.; Salvadori, O.; Cortesero, A.M.; Le Ralec, A.; Jaloux, B. Effects of spontaneous field margin vegetation and surrounding landscape on Brassica oleracea crop herbivory. Agrc. Ecosyst. Environ. 2016, 223, 135–143. [Google Scholar] [CrossRef]
- Denisow, B.; Wrzesien, M. The importance of field-margin location for maintenance of food niches for pollinators. J. Apic. Sci. 2015, 59, 27–37. [Google Scholar] [CrossRef]
- Timler, D.; Michalscheck, M.; Klapwijk, C.; Mashingaidze, N.; Ollenburger, M.; Falconnier, G.; Kuivanen, K.; Descheemaeker, K.; Groot, J. Characterization of Farming Systems in Africa RISING Intervention Sites in Malawi, Tanzania, Ghana and Mali. 2014. Available online: https://cgspace.cgiar.org/handle/10568/42331 (accessed on 6 August 2019).
- Rouabah, A.; Villerd, J.; Amiaud, B.; Plantureux, S.; Lasserre-Joulin, F. Response of carabid beetles diversity and size distribution to the vegetation structure within differently managed field margins. Agrc. Ecosyst. Environ. 2015, 200, 21–32. [Google Scholar] [CrossRef]
- Fischer, C.; Schlinkert, H.; Ludwig, M.; Holzschuh, A.; Gallé, R.; Tscharntke, T.; Batáry, P. The impact of hedge-forest connectivity and microhabitat conditions on spider and carabid beetle assemblages in agricultural landscapes. J. Insect Conserv. 2013, 17, 1027–1038. [Google Scholar] [CrossRef]
- Parajulee, M.N.; Phillips, T.W.; Hogg, D.B. Functional response of Lyctocoris campestris (F.) adults: Effects of predator sex, prey type, and experimental habitat. Biol. Control 1994, 4, 80–87. [Google Scholar] [CrossRef]
- Diepenbrock, L.M.; Swoboda-Bhattarai, K.A.; Burrack, H.J. Ovipositional preference, fidelity, and fitness of Drosophila suzukii in a co-occurring crop and non-crop host system. J. Pest. Sci. 2016, 89, 761–769. [Google Scholar] [CrossRef]
| Zone | Number of Farms (N) | Mean Farm Length (m) | Mean Farm Width (m) | Mean Farm Size (m2) |
|---|---|---|---|---|
| Low | 8 | 73.8 ± 2.79 a | 55.1 ± 6.68 a | 4167.5 ± 648.85 a |
| Mid | 8 | 71.0 ± 3.69 a | 56.6 ± 4.98 a | 4116.4 ± 568.37 a |
| High | 8 | 38.5 ± 2.78 b | 29.5 ± 1.09 b | 1132.9 ± 79.82 b |
| ANOVA, F value | 39.534 *** | 9.828 *** | 12.068 *** | |
| Natural Enemies | Total Sampled | Marked Insects after Spray | Total Marked | ||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |||
| Parasitoid wasps | 65 (27.5%) | 19 | 17 | 13 | 49 (75.4%) |
| Predatory wasps | 51 (21.6%) | 10 | 19 | 11 | 40 (78.4%) |
| Assassin bug | 28 (11.9%) | 9 | 8 | 6 | 23 (82.1%) |
| Hover fly | 19 (8.1%) | 5 | 3 | 4 | 12 (63.2%) |
| Spider | 19 (8.1%) | 3 | 3 | 6 | 12 (63.2%) |
| Tachinid fly | 12 (5.1%) | 4 | 6 | 2 | 12 (100%) |
| Carabid beetle | 10 (4.2%) | 3 | 1 | 1 | 5 (50%) |
| Lady beetle | 10 (4.2%) | 3 | 0 | 2 | 5 (50%) |
| Long legged fly | 7 (3.0%) | 1 | 1 | 1 | 3 (42.3%) |
| Robber fly | 5 (2.1%) | 1 | 3 | 1 | 5 (100%) |
| Dragonfly | 4 (1.7%) | 0 | 0 | 0 | 0 (0%) |
| Rove beetle | 3 (1.3%) | 0 | 0 | 1 | 1 (33.3%) |
| Ants | 3 (1.3%) | 0 | 0 | 0 | 0 (0%) |
| Dependent Variable | Independent Variable | Sum of Squares | Mean Squares | Df | F Value | p Value |
|---|---|---|---|---|---|---|
| Marked proportions of natural enemies | Zone | 2.594 | 1.297 | 2178 | 8.398 | <0.001 |
| Distance | 3.309 | 1.103 | 3178 | 7.144 | <0.001 | |
| Farm size | 1.242 | 1.241 | 1178 | 8.040 | 0.005 | |
| Day | 0.870 | 0.435 | 2178 | 2.816 | 0.063 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkenda, P.A.; Ndakidemi, P.A.; Stevenson, P.C.; Arnold, S.E.J.; Belmain, S.R.; Chidege, M.; Gurr, G.M. Field Margin Vegetation in Tropical African Bean Systems Harbours Diverse Natural Enemies for Biological Pest Control in Adjacent Crops. Sustainability 2019, 11, 6399. https://doi.org/10.3390/su11226399
Mkenda PA, Ndakidemi PA, Stevenson PC, Arnold SEJ, Belmain SR, Chidege M, Gurr GM. Field Margin Vegetation in Tropical African Bean Systems Harbours Diverse Natural Enemies for Biological Pest Control in Adjacent Crops. Sustainability. 2019; 11(22):6399. https://doi.org/10.3390/su11226399
Chicago/Turabian StyleMkenda, Prisila A., Patrick A. Ndakidemi, Philip C. Stevenson, Sarah E. J. Arnold, Steven R. Belmain, Maneno Chidege, and Geoff M. Gurr. 2019. "Field Margin Vegetation in Tropical African Bean Systems Harbours Diverse Natural Enemies for Biological Pest Control in Adjacent Crops" Sustainability 11, no. 22: 6399. https://doi.org/10.3390/su11226399
APA StyleMkenda, P. A., Ndakidemi, P. A., Stevenson, P. C., Arnold, S. E. J., Belmain, S. R., Chidege, M., & Gurr, G. M. (2019). Field Margin Vegetation in Tropical African Bean Systems Harbours Diverse Natural Enemies for Biological Pest Control in Adjacent Crops. Sustainability, 11(22), 6399. https://doi.org/10.3390/su11226399








