Morpho-Agronomic Characterisation of Runner Bean (Phaseolus coccineus L.) from South-Eastern Europe
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Morpho-Agronomic Characterisation
2.3. Statistical Analysis
3. Results
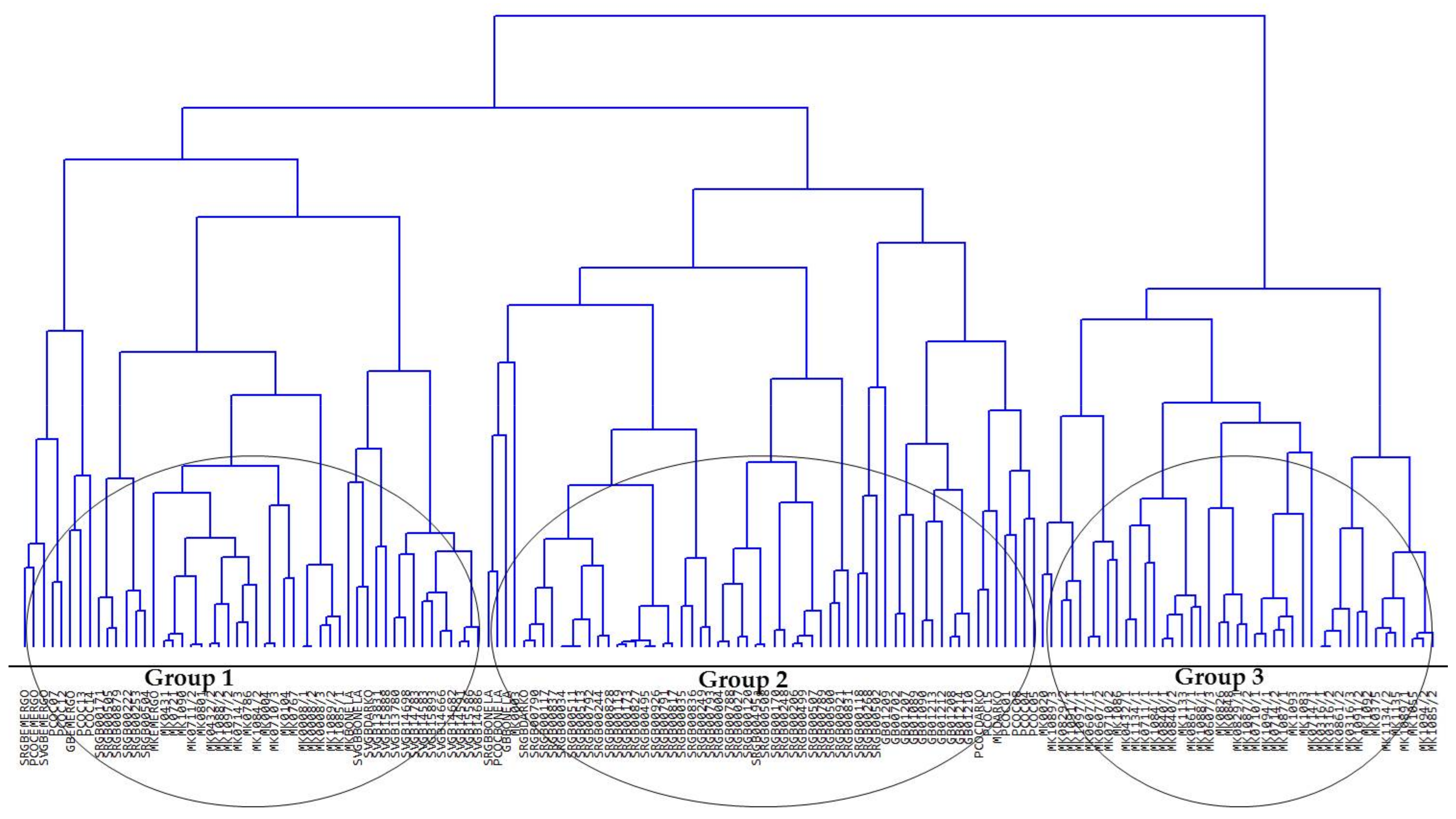
3.1. General Variability of the Runner-Beans from South-Eastern Europe
3.2. Comparison of Runner-Bean Variability among the South-Eastern European Countries
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schwember, A.R.; Carrasco, B.; Gepts, P. Unraveling agronomic and genetic aspects of runner bean (Phaseolus coccineus L.). Field Crops Res. 2017, 206, 86–94. [Google Scholar] [CrossRef]
- Guerra-García, A.; Suárez-Atilano, M.; Mastretta-Yanes, A.; Delgado-Salinas, A.; Piñero, D. Domestication genomics of the open-pollinated scarlet runner bean (Phaseolus coccineus L.). Front. Plant Sci. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Rodino, A.P.; Lema, M.; Pérez-Barbeito, M.; Santalla, M.; De Ron, A.M. Assessment of runner bean (Phaseolus coccineus L.) germplasm for tolerance to low temperature during early seedling growth. Euphytica 2007, 155, 63–70. [Google Scholar] [CrossRef]
- Gioia, T.; Logozzo, G.; Attene, G.; Bellucci, E.; Benedettelli, S.; Negri, V.; Papa, R.; Zeuli, P.S. Evidence for introduction bottleneck and extensive inter-gene pool (Mesoamerica×Andes) hybridization in the European common bean (Phaseolus vulgaris L.) germplasm. PLoS ONE 2013, 8, e75974. [Google Scholar] [CrossRef]
- Santalla, M.; Monteagudo, A.B.; González, A.M.; De Ron, A.M. Agronomical and quality traits of runner bean germplasm and implications for breeding. Euphytica 2004, 135, 205–215. [Google Scholar] [CrossRef]
- Spataro, G.; Tiranti, B.; Arcaleni, P.; Belluci, E.; Attene, G.; Papa, R.; Spagnoletti Zeuli, P.; Negri, V. Genetic diversity and structure of a worldwide collection of Phaseolus coccineus L. Theor. Appl. Genet. 2011, 122, 1281–1291. [Google Scholar] [CrossRef]
- Mercati, F.; Catarcione, G.; Paolacci, A.R.; Abenavolo, M.R.; Sunseri, F.; Ciaffi, M. Genetic diversity and population structure of an Italian landrace of runner bean (Phaseolus coccineus L.): Inferences for its safeguard and on-farm conservation. Genetica 2015, 143, 473–485. [Google Scholar] [CrossRef]
- Rodriguez Monica, M.; Rau, D.; Angioi, S.A.; Bellucci, E.; Bitocchi, E.; Nanni, L.; Knüpffer, H.; Negri, V.; Papa, R.; Attene, G. European Phaseolus coccineus L. landraces: Population structure and adaptation, as revealed by cpSSRs and phenotypic analyses. PLoS ONE 2013, 8, e57337. [Google Scholar] [CrossRef]
- Zeven, A.C.; Mohamed, H.H.; Waninge, J.; Veurink, H. Phenotypic variation within a Hungarian landrace of runner bean (Phaseolus coccineus L.). Euphytica 1993, 68, 155–166. [Google Scholar] [CrossRef]
- Westphal, E. Phaseolus L. Pulses in Ethiopia, Their Taxonomy and Agricultural Significance; Centre for Agricultural Publishing and Documentation (PUDOC): Wageningen, The Netherlands, 1974; pp. 129–176. [Google Scholar]
- Palmero, D.; Iglesias, C.; De Cara, M.; Tello, J.C.; Camacho, F. Diversity and health traits of local landraces of runner bean (Phaseolus coccineus L.) from Spain. J. Food Agric. Environ. 2011, 9, 290–295. [Google Scholar]
- Vasić, M.A.; Tepić, A.N.; Mihailović, V.M.; Mikić, A.M.; Gvozdanović-Varga, J.M.; Šumić, Z.M.; Todorović, V.J. Phytic acid content in different dry bean and faba bean landraces and cultivars. Rom. Agric. Res. 2012, 29, 79–85. [Google Scholar]
- Khoury, C.; Laliberté, B.; Guarino, L. Trends in ex-situ conservation of plant genetic resources: A review of global crop and regional conservation strategies. Genet. Resour. Crop Evol. 2010, 57, 625–639. [Google Scholar] [CrossRef]
- Sinkovič, L.; Pipan, B.; Sinkovič, E.; Meglič, V. Morphological seed characterization of common (Phaseolus vulgaris L.) and runner (Phaseolus coccineus L.) bean germplasm: A Slovenian gene bank example. Biomed. Res. Int. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Hadžić, A.; Ćota, J.; Sarić, E.; Hodžić, I.; Salman, N.; Ćota, J. Energy and nutritional value of raw grains of domestic bean varieties. Agroznanje 2013, 14, 51–58. [Google Scholar] [CrossRef][Green Version]
- Ai, Y.; Jin, Y.; Kelly, J.D.; Ng, P.K. Composition, functional properties, starch digestibility and cookie-baking performance of dry bean powders from 25 Michigan-grown varieties. Cereal Chem. 2017, 94, 400–408. [Google Scholar] [CrossRef]
- Akinterinwa, A.; Osemeahon, S.A.; Akinsola, A.F.; Reuben, U. Physicochemical and pasting characterization of carboxymethylated scarlet runner bean (Phaseolus coccineus) starch. J. Agric. Food Technol. 2014, 4, 13–20. [Google Scholar]
- Audu, S.S.; Aremu, M.O. Effect of processing on chemical composition of red kidney bean (Phaseolus vulgaris L.) flour. Pak. J. Nutr. 2011, 10, 1069–1075. [Google Scholar] [CrossRef]
- Olaleke, A.M.; Olorunfemi, O.; Akintayo, T.E. Compositional evaluation of cowpea (Vigna unguiculata) and scarlet runner bean (Phaseolus coccineus) varieties grown in Nigeria. J. Food Agric. Environ. 2006, 4, 39–43. [Google Scholar]
- Boczkowska, M.; Bulińska-Radomska, Z.; Nowosielski, J. AFLP analysis of genetic diversity in five accessions of Polish runner bean (Phaseolus coccineus L.). Genet. Resour. Crop Evol. 2012, 59, 473–478. [Google Scholar] [CrossRef][Green Version]
- CPVO Phaseolus coccineus L. Protocol for Tests on Distinctness, Uniformity and Stability Tests: Runner Bean. 2007; pp. 1–21. Available online: https://cpvo.europa.eu/sites/default/files/documents/phaseolus_coccineus_1.pdf (accessed on 4 November 2019).
- CPVO Phaseolus vulgaris L. Protocol for Tests on Distinctness, Uniformity and Stability: French Bean. 2013; pp. 1–34. Available online: https://cpvo.europa.eu/sites/default/files/documents/phaseolus_vulgaris.pdf (accessed on 4 November 2019).
- International Board for Plant Genetic Resources. Descriptors for Phaseolus vulgaris; Crop Genetic Resources Centre, Plant Production and Protection Division: Rome, Italy, 1982; pp. 1–32. [Google Scholar]
- De La Cuadra, C.; De Ron, A.M.; Schachl, R. Handbook on Evaluation of Phaseolus Germplasm; Mission Biologica de Galicia (CSIC): Salcedo, Spain, 2001; pp. 1–84. [Google Scholar]
- Descriptor list for Phaseleus. European Cooperative Programme for Plant Genetic Resources (ECPGR). 2014; pp. 1–7. Available online: https://www.genbank.at/en/ecpgr-phaseolus/database-descriptors/phaseolus-descriptor.html (accessed on 28 August 2019).
- De Ron, A.M.; Bebeli, P.J.; Negri, V.; Patto, M.C.V.; Revilla, P. Warm season grain legume landraces from the south of Europe for germplasm conservation and genetic improvement. Front. Plant Sci. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Bitocchi, E.; Rau, D.; Bellucci, E.; Rodriguez, M.; Murgia, M.L.; Gioia, T.; Santo, D.; Nanni, L.; Attene, G.; Papa, R. Beans (Phaseolus ssp.) as a model for understanding crop evolution. Front. Plant Sci. 2017, 8, 1–21. [Google Scholar] [CrossRef]
- Rodiño, A.P.; Santalla, M.; Montero, I.; Casquero, P.A.; De Ron, A.M. Diversity of common bean (Phaseolus vulgaris L.) germplasm from Portugal. Genet. Resour. Crop Evol. 2001, 48, 409–417. [Google Scholar] [CrossRef]
- Zeven, A.C.; Waninge, J.; Van Hintum, T.; Singh, S.P. Phenotypic variation in a core collection of common bean (Phaseolus vulgaris L.) in The Netherlands. Euphytica 1999, 109, 93–106. [Google Scholar] [CrossRef]
- Geng, Q.H.; Wang, L.F.; Wu, J.; Wang, S.M. QTL mapping for seed size and shape in common bean. Acta Agron. Sin. 2017, 43, 1140–1160. [Google Scholar] [CrossRef]
- Park, S.O.; Coyne, D.P.; Jung, G.; Skroch, P.W.; Arnaud-Santana, E.; Steadman, J.R.; Ariyarathne, H.M.; Nienhuis, J. Mapping of QTL for seed size and shape traits in common bean. J. Am. Soc. Hortic. Sci. 2000, 125, 466–475. [Google Scholar] [CrossRef]
- Pérez-Vega, E.; Pañeda, A.; Rodríguez-Suárez, C.; Campa, A.; Giraldez, R.; Ferreira, J.J. Mapping of QTLs for morpho-agronomic and seed quality traits in a RIL population of common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2010, 120, 1367–1380. [Google Scholar] [CrossRef]
- Yuste-Lisbona, F.J.; González, A.M.; Capel, C.; García-Alcázar, M.; Capel, J.; De Ron, A.M.; Lozano, R.; Santalla, M. Genetic analysis of single-locus and epistatic QTLs for seed traits in an adapted × nuña RIL population of common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2014, 127, 897–912. [Google Scholar] [CrossRef]
- Giurcă, D.M. Morphological and phenological differences between the two species of the Phaseolus genus (Phaseolus vulgaris and Phaseolus coccineus). Cercet. Agron. Mold. 2009, 42, 39–45. [Google Scholar]
- Šuštar-Vozlič, J.; Maras, M.; Javornik, B.; Meglič, V. Genetic diversity and origin of Slovene common bean (Phaseolus vulgaris L.) germplasm as revealed by AFLP markers and phaseolin analysis. J. Am. Soc. Hortic. Sci. 2006, 131, 242–249. [Google Scholar] [CrossRef]
- Maras, M.; Šuštar-Vozlič, J.; Kainz, W.; Meglič, V. Genetic diversity and dissemination pathways of common bean in central Europe. J. Am. Soc. Hortic. Sci. 2013, 138, 297–305. [Google Scholar] [CrossRef]
- Maras, M.; Pipan, B.; Šuštar-Vozlič, J.; Todorović, V.; Đurić, G.; Vasić, M.; Kratovalieva, S.; Ibusovska, A.; Matotan, Z.; Čupić, T.; et al. Examination of genetic diversity of common bean from the Western Balkans. J. Am. Soc. Hortic. Sci. 2015, 140, 308–316. [Google Scholar] [CrossRef]
- Maras, M.; Ibusoska, A.; Kratovalieva, S.; Agić, R.; Šuštar-Vozlič, J.; Meglič, V. Genetic diversity of common bean accessions from former Yugoslav Republic of Macedonia as revealed by molecular and morphological markers. Genetika 2016, 48, 719–742. [Google Scholar] [CrossRef]
- Vasić, M.; Mihailović, V.; Mikić, A.; Mladenović, D.; Gvozdanović Varga, J.; Jovićević, D.; Nikolić, Z. Genetic resources of edible grain legumes in Serbia. IV Balkan Symposium on vegetables and potatoes, 9.–12. September 2008, Plovdiv, Bulgaria. Acta Hortic. ISHS 2009, 830, 715–718. [Google Scholar] [CrossRef]
- Savić, A.; Brdar-Jokanović, M.; Dimitrijević, M.; Petrović, S.; Zdravković, M.; Živanov, D.; Vasić, M. Genetic diversity of common bean (Phaseolus vulgaris L.) breeding collection in Serbia. Genetika 2019, 51, 1–15. [Google Scholar] [CrossRef]
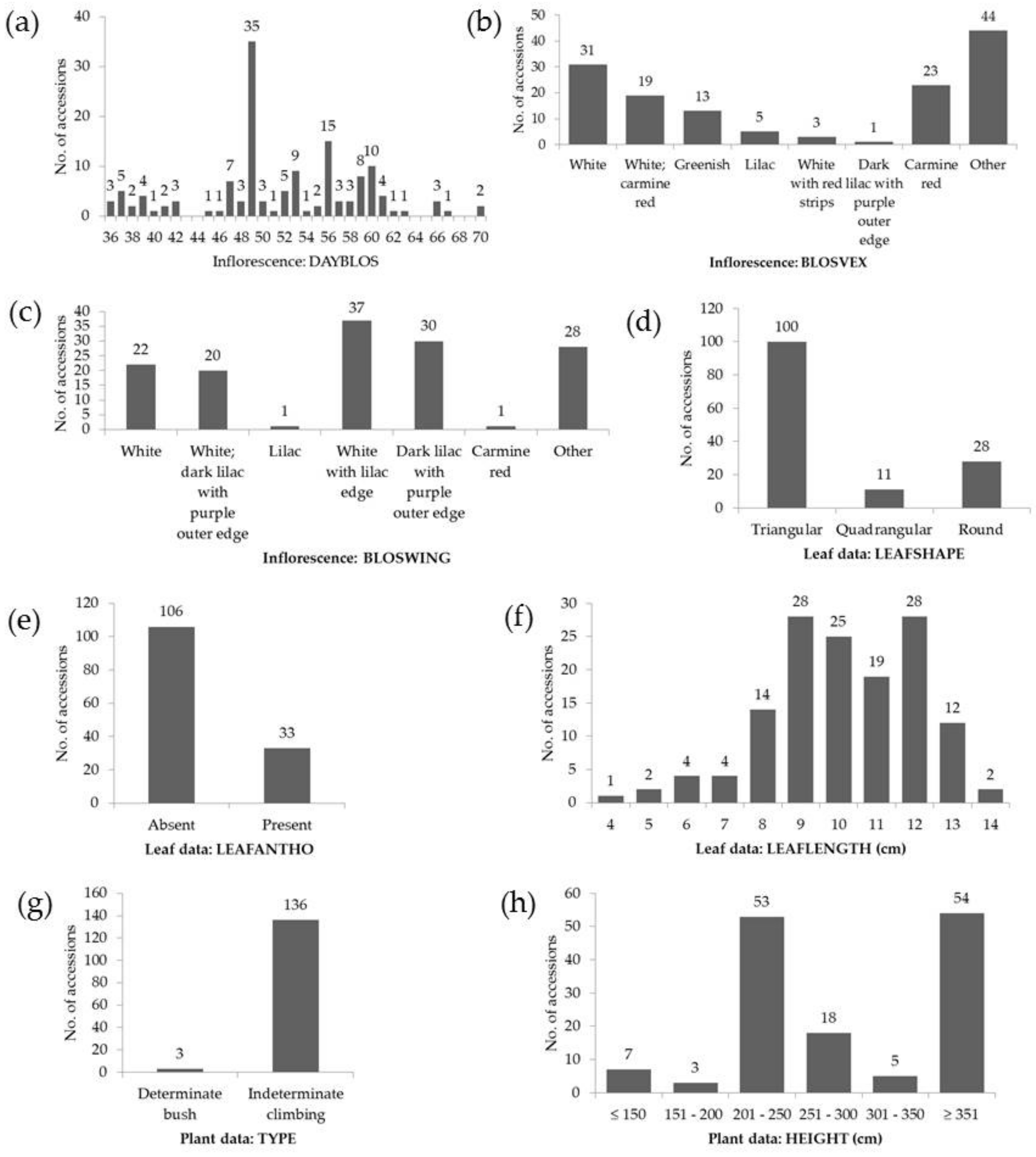
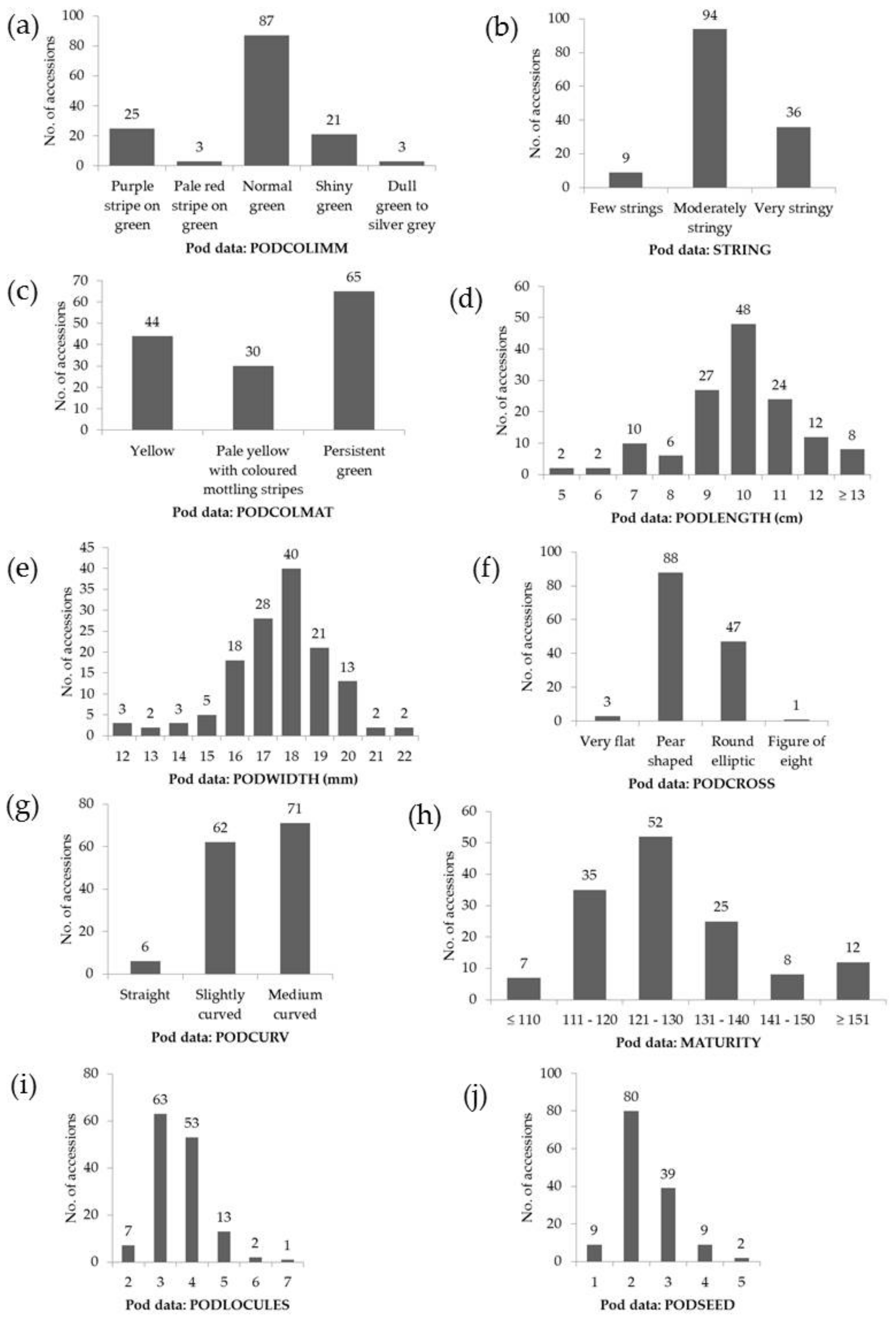
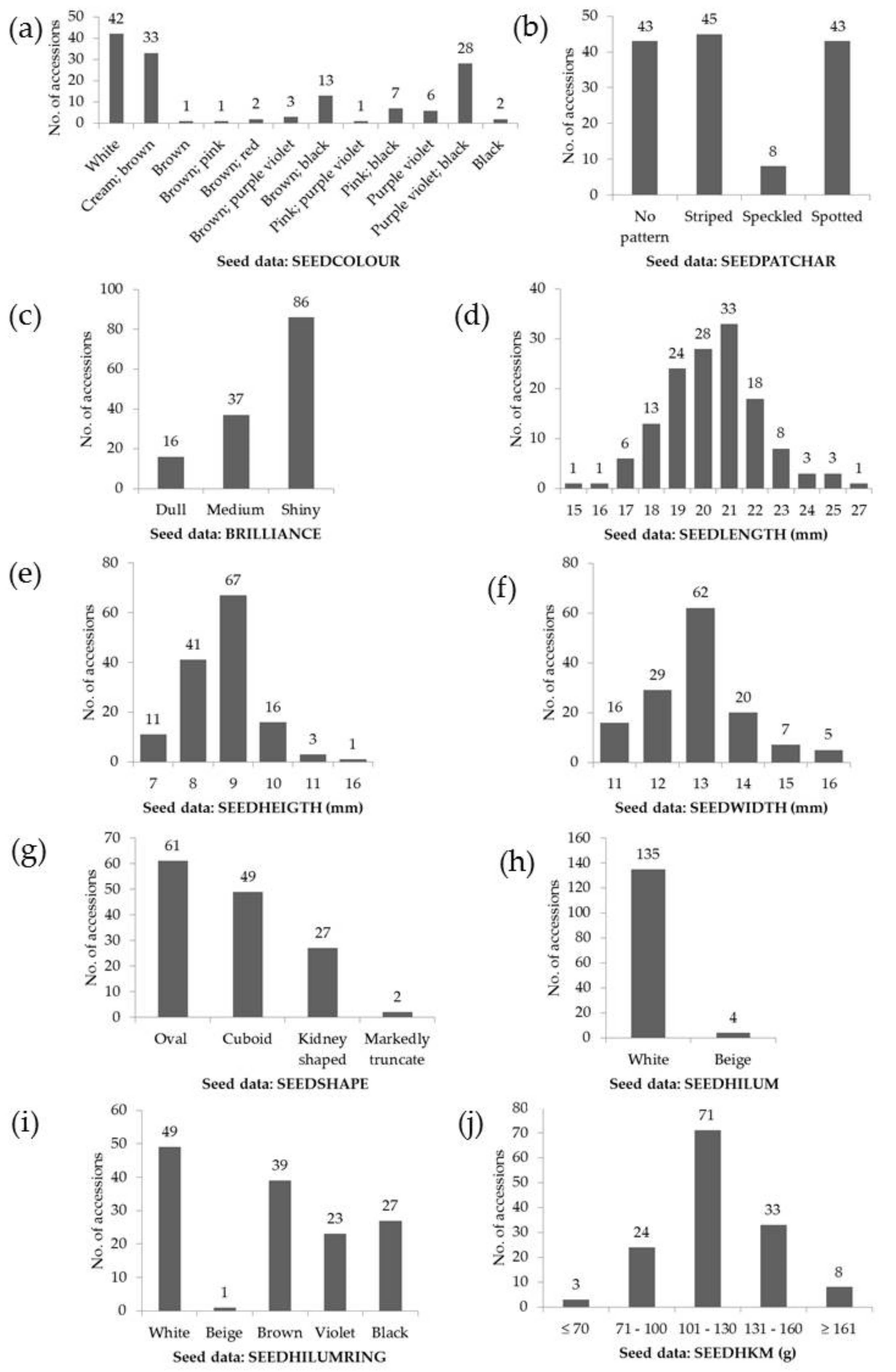
| Trait Assessed | Code | Units | Range | Mean ± SD | CV (%) |
|---|---|---|---|---|---|
| Inflorescences | DAYSBLOS | days | 36–70 | 52 ± 8 | 15.1 |
| Leaves | LEAFLENGTH | cm | 4.0–14.0 | 10.1 ± 2.0 | 19.8 |
| Plants | HEIGHT | cm | 70.0–500.0 | 326.0 ± 138.0 | 42.5 |
| Pods | PODLENGTH | cm | 4.5–18.5 | 10.3 ± 2.3 | 22.4 |
| PODWIDTH | mm | 11.9–22.9 | 17.4 ± 2.0 | 11.3 | |
| MATURITY | days | 101–170 | 129 ± 14 | 10.9 | |
| PODLOCULES | n | 2–7 | 4 ± 1 | 26.4 | |
| PODSEED | n | 1–6 | 3 ± 1 | 36.9 | |
| Seeds | SEEDLENGTH | mm | 15.5–27.2 | 20.6 ± 2.0 | 9.6 |
| SEEDHEIGTH | mm | 6.7–15.5 | 8.8 ± 1.0 | 11.5 | |
| SEEDWIDTH | mm | 10.6–16.5 | 13.0 ± 1.2 | 9.0 | |
| SEEDHKM | g | 61.9–203.3 | 120.0 ± 25.2 | 21.0 |
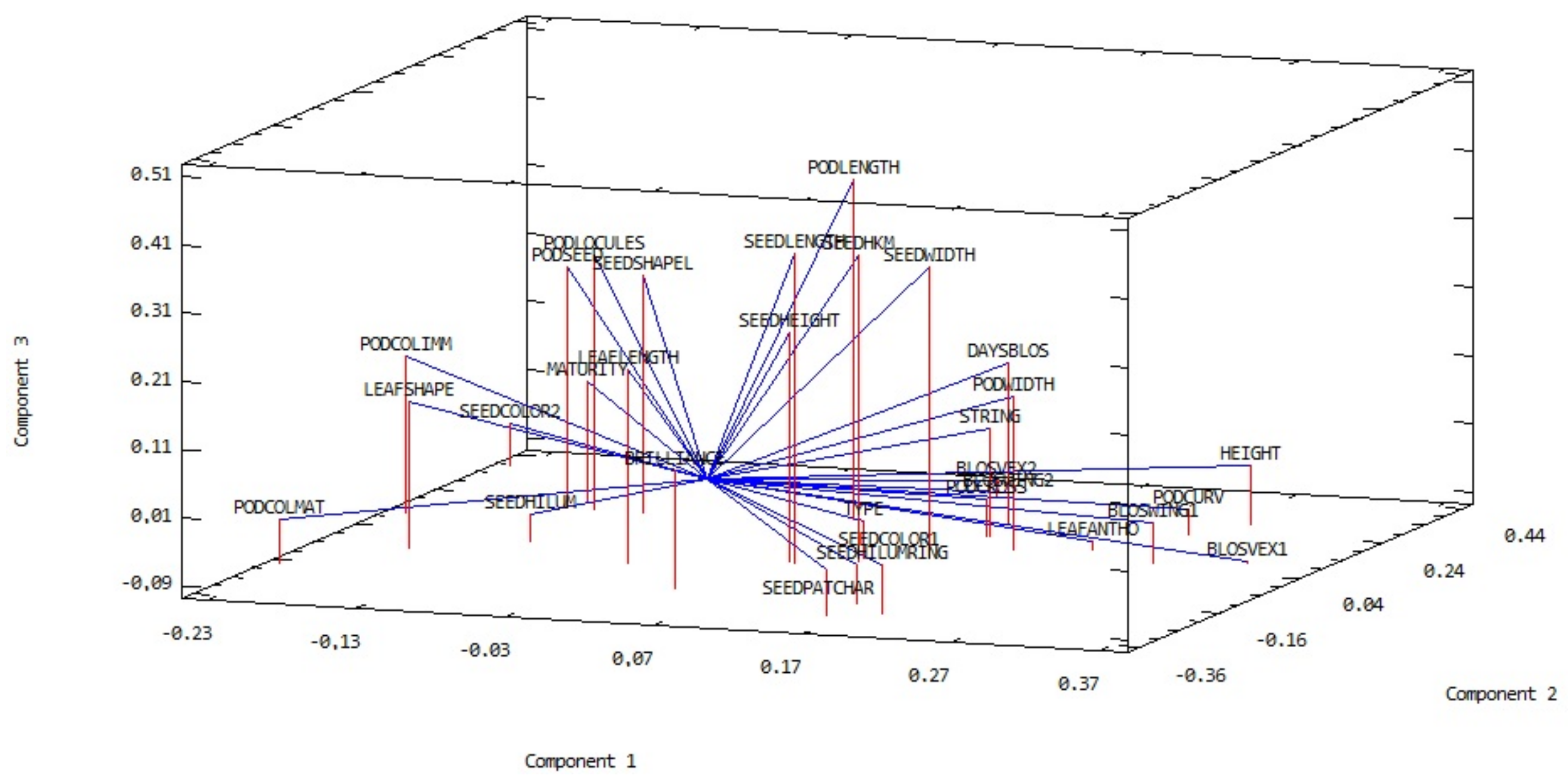
| Trait | Trait | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | ||
| 1 | DAYSBLOS | ||||||||||||||||||||||||||||||
| 2 | BLOSVEX1 | 0.25 b | |||||||||||||||||||||||||||||
| 3 | BLOSVEX2 | 0.17 a | 0.26 b | ||||||||||||||||||||||||||||
| 4 | BLOSWING1 | 0.26 c | 0.75 c | 0.20 a | |||||||||||||||||||||||||||
| 5 | BLOSWING2 | 0.15 | 0.27 c | 0.85 c | 0.21 b | ||||||||||||||||||||||||||
| 6 | LEAFSHAPE | −0.08 | −0.39 c | −0.09 | −0.28 c | −0.17 a | |||||||||||||||||||||||||
| 7 | LEAFANTHO | 0.24 b | 0.62 c | 0.21 a | 0.39 c | 0.21 b | −0.26 b | ||||||||||||||||||||||||
| 8 | LEAFLENGTH | 0.19 a | −0.10 | −0.29 c | −0.14 | −0.32 c | 0.06 | −0.13 | |||||||||||||||||||||||
| 9 | TYPE | 0.27 c | 0.12 | −0.07 | 0.10 | −0.07 | 0.03 | 0.10 | 0.22 b | ||||||||||||||||||||||
| 10 | HEIGHT | 0.48 c | 0.62 c | 0.31 c | 0.47 c | 0.32 c | −0.40 c | 0.39 c | 0.00 | 0.19 a | |||||||||||||||||||||
| 11 | PODCOLIMM | −0.12 | −0.60 c | −0.11 | −0.31 c | −0.11 | 0.22 b | −0.33 c | −0.02 | −0.14 | −0.38 c | ||||||||||||||||||||
| 12 | STRING | 0.07 | 0.37 c | 0.15 | 0.20 a | 0.16 | −0.14 | 0.28 c | −0.03 | 0.01 | 0.35 c | −0.08 | |||||||||||||||||||
| 13 | PODCOLMAT | −0.18 a | −0.43 c | −0.32 c | −0.39 c | −0.33 c | 0.34 c | −0.27 b | 0.21 b | −0.01 | −0.69 c | −0.10 | −0.42 c | ||||||||||||||||||
| 14 | PODLENGTH | 0.23 b | 0.11 | 0.09 | 0.17 a | 0.09 | −0.01 | 0.03 | 0.19 a | −0.06 | 0.21 b | 0.07 | 0.18a | −0.25 b | |||||||||||||||||
| 15 | PODWIDTH | 0.18 a | 0.34 c | 0.02 | 0.26 b | 0.03 | −0.11 | 0.11 | 0.10 | 0.19 a | 0.42 c | −0.13 | 0.20 a | −0.45 c | 0.18 a | ||||||||||||||||
| 16 | PODCROSS | 0.38 c | 0.31 c | 0.08 | 0.35 c | 0.12 | −0.19 a | 0.29c | −0.02 | 0.25 b | 0.30 c | −0.30 c | −0.01 | −0.07 | 0.14 | 0.05 | |||||||||||||||
| 17 | PODCURV | 0.35 c | 0.51 c | 0.25 b | 0.37 c | 0.26 b | −0.35 c | 0.33 c | −0.07 | 0.32 c | 0.65 c | −0.31 c | 0.31c | −0.53 c | 0.09 | 0.32 c | 0.34 c | ||||||||||||||
| 18 | MATURITY | 0.34 c | -0.25 b | 0.13 | −0.14 | 0.13 | 0.26 b | −0.09 | −0.22 b | −0.06 | −0.17 a | 0.26 b | −0.09 | 0.18 a | 0.25 | −0.21 a | 0.02 | −0.22 b | |||||||||||||
| 19 | PODLOCULES | 0.08 | -0.15 | −0.02 | −0.05 | −0.02 | 0.13 | −0.12 | 0.21 b | −0.17 a | −0.14 | 0.21 b | 0.07 | 0.04 | 0.69 c | −0.08 | 0.04 | −0.23 b | 0.08 | ||||||||||||
| 20 | PODSEED | −0.02 | -0.19 a | 0.05 | −0.11 | 0.04 | 0.20 a | −0.16 | −0.05 | −0.22 b | −0.23 b | 0.18 a | 0.01 | 0.07 | 0.52 c | −0.09 | −0.01 | −0.22 b | 0.25 b | 0.71 c | |||||||||||
| 21 | SEEDCOLOR1 | 0.00 | 0.33 c | −0.05 | 0.51 c | −0.03 | 0.03 | 0.25 b | −0.11 | 0.09 | −0.02 | −0.17 a | 0.09 | 0.04 | −0.07 | 0.02 | 0.08 | 0.04 | −0.09 | −0.13 | −0.14 | ||||||||||
| 22 | SEEDCOLOR2 | −0.12 | -0.45 c | 0.13 | −0.31 c | 0.10 | −0.02 | −0.26 b | −0.22 b | −0.24 b | −0.13 | 0.34 c | −0.11 | −0.02 | 0.01 | −0.13 | −0.16 a | −0.11 | 0.20 a | 0.16 a | 0.14 | −0.47 c | |||||||||
| 23 | SEEDPATCHAR | −0.08 | 0.33 c | −0.07 | 0.36 c | −0.04 | 0.07 | 0.20 a | −0.03 | 0.15 | −0.08 | −0.09 | 0.08 | 0.04 | −0.13 | 0.11 | 0.02 | 0.02 | −0.16 a | −0.20a | −0.14 | 0.65 c | −0.78 c | ||||||||
| 24 | BRILLIANCE | −0.19 a | 0.07 | −0.21 a | 0.12 | −0.22 b | 0.04 | −0.08 | 0.24 b | −0.08 | −0.05 | 0.06 | 0.14 | −0.07 | 0.13 | 0.10 | −0.14 | −0.10 | −0.42 c | 0.15 | 0.02 | 0.30 c | −0.32 c | 0.40 c | |||||||
| 25 | SEEDLENGTH | 0.08 | 0.03 | −0.04 | 0.09 | −0.06 | 0.12 | 0.09 | 0.09 | 0.06 | 0.01 | 0.00 | 0.07 | 0.05 | 0.29 c | 0.13 | −0.02 | 0.01 | 0.09 | −0.12 | −0.01 | 0.18 a | −0.19 a | 0.16 a | −0.01 | ||||||
| 26 | SEEDHEIGHT | 0.15 | 0.05 | −0.09 | −0.01 | −0.09 | 0.01 | 0.29 | 0.27 | 0.45 | 0.87 | −0.15 | 0.05 | 0.07 | 0.15 | 0.09 | 0.12 | 0.12 | 0.03 | −0.01 | −0.06 | 0.04 | −0.21 b | 0.08 | 0.07 | 0.36 c | |||||
| 27 | SEEDWIDTH | 0.25 b | 0.19 a | 0.02 | 0.17 a | 0.00 | 0.00 | 0.11 | 0.10 | 0.15 | 0.28c | −0.08 | 0.11 | −0.14 | 0.19 a | 0.39 c | −0.02 | 0.17 a | 0.09 | −0.19 a | −0.11 | 0.09 | −0.19 a | 0.14 | −0.06 | 0.73 c | 0.42 c | ||||
| 28 | SEEDSHAPE | 0.08 | −0.17 a | 0.07 | −0.05 | 0.09 | 0.09 | −0.02 | 0.00 | −0.16 | −0.10 | 0.34c | 0.02 | −0.07 | 0.32 c | −0.07 | −0.13 | −0.15 | 0.23 b | 0.25 b | 0.34c | −0.04 | 0.17 a | −0.07 | 0.01 | 0.22 b | −0.09 | 0.17 a | |||
| 29 | SEEDHILUM | −0.11 | −0.11 | −0.19 a | −0.09 | −0.18 a | 0.09 | −0.09 | 0.05 | −0.20 a | −0.14 | 0.00 | −0.07 | 0.16 | −0.14 | −0.18 a | −0.18 a | −0.22 b | −0.02 | −0.08 | −0.09 | −0.12 | 0.13 | −0.11 | 0.06 | −0.04 | −0.01 | −0.10 | −0.08 | ||
| 30 | SEEDHILUMRING | −0.05 | 0.36 c | −0.11 | 0.42c | −0.05 | 0.00 | 0.18 a | 0.06 | 0.12 | 0.04 | −0.26 b | 0.08 | −0.01 | 0.00 | 0.20 a | 0.08 | 0.14 | −0.30c | −0.13 | −0.14 | 0.72 c | −0.64 c | 0.71 c | 0.37 c | 0.14 | 0.06 | 0.08 | −0.16 | −0.14 | |
| 31 | SEEDHKM | 0.14 | 0.09 | −0.03 | 0.10 | −0.05 | −0.05 | 0.03 | 0.17 a | −0.09 | 0.14 | −0.06 | 0.15 | −0.11 | 0.31 c | 0.29 c | −0.03 | 0.15 | −0.15 | 0.00 | −0.03 | 0.07 | −0.16 | 0.08 | 0.11 | 0.60 c | 0.44b | 0.62 c | 0.04 | 0.07 | 0.15 |
| Country of Origin | Number of Accessions | Variability (%) | F-Ratio |
|---|---|---|---|
| Slovenia | 44 | 44.96 *** | 58.19 |
| Bosnia and Herzegovina | 10 | 3.01 *** | 366.65 |
| Serbia | 9 | 26.28 *** | 31.89 |
| North Macedonia | 64 | 9.64 *** | 639.44 |
| Romania | 12 | 9.10 *** | 144.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinkovič, L.; Pipan, B.; Vasić, M.; Antić, M.; Todorović, V.; Ivanovska, S.; Brezeanu, C.; Šuštar-Vozlič, J.; Meglič, V. Morpho-Agronomic Characterisation of Runner Bean (Phaseolus coccineus L.) from South-Eastern Europe. Sustainability 2019, 11, 6165. https://doi.org/10.3390/su11216165
Sinkovič L, Pipan B, Vasić M, Antić M, Todorović V, Ivanovska S, Brezeanu C, Šuštar-Vozlič J, Meglič V. Morpho-Agronomic Characterisation of Runner Bean (Phaseolus coccineus L.) from South-Eastern Europe. Sustainability. 2019; 11(21):6165. https://doi.org/10.3390/su11216165
Chicago/Turabian StyleSinkovič, Lovro, Barbara Pipan, Mirjana Vasić, Marina Antić, Vida Todorović, Sonja Ivanovska, Creola Brezeanu, Jelka Šuštar-Vozlič, and Vladimir Meglič. 2019. "Morpho-Agronomic Characterisation of Runner Bean (Phaseolus coccineus L.) from South-Eastern Europe" Sustainability 11, no. 21: 6165. https://doi.org/10.3390/su11216165
APA StyleSinkovič, L., Pipan, B., Vasić, M., Antić, M., Todorović, V., Ivanovska, S., Brezeanu, C., Šuštar-Vozlič, J., & Meglič, V. (2019). Morpho-Agronomic Characterisation of Runner Bean (Phaseolus coccineus L.) from South-Eastern Europe. Sustainability, 11(21), 6165. https://doi.org/10.3390/su11216165









