Abstract
With increasing interest in reducing fossil fuel emissions, more and more development is focused on electric mobility. For electric vehicles, the main challenge is the mass of the batteries, which significantly increase the mass of the vehicles and limits their range. One possible concept to solve this is incorporating structural batteries; a structural material that both stores electrical energy and carries mechanical load. The concept envisions constructing the body of an electric vehicle with this material and thus reducing the need for further energy storage. This research is investigating a future structural battery that is incorporated in the roof of an electric vehicle. The structural battery is replacing the original steel roof of the vehicle, and part of the original traction battery. The environmental implications of this structural battery roof are investigated with a life cycle assessment, which shows that a structural battery roof can avoid climate impacts in substantive quantities. The main emissions for the structural battery stem from its production and efforts should be focused there to further improve the environmental benefits of the structural battery. Toxicity is investigated with a novel chemical risk assessment from a life cycle perspective, which shows that two chemicals should be targeted for substitution.
1. Introduction
Electrification is seen as the main answer to the challenges of climate impact in the transport sector. Provided that the electricity can be generated from reasonably carbon lean energy sources, considerable reductions of carbon dioxide equivalents (CO2-eq) emissions from the transport sector are possible [1,2,3]. However, cost and range are still difficult challenges for electric vehicles. One way to increase the vehicle range is by decreasing its mass by incorporating many functions in the same material. A multifunctional material that can carry both mechanical load and store electrical energy is generally referred to as a structural battery. A lot of research has gone into the design and construction of such structural batteries [4,5,6,7], but the actual concept has not yet been demonstrated or validated in an industrial setting.
Life cycle assessment (LCA) and chemical risk assessment (CRA) are well known tools to evaluate cradle-to-grave environmental impacts. Such evaluations are especially fruitful early in the product development in order to help steer the design of both materials and processes [8,9,10,11]. It is understandable that such evaluations then are prospective in nature, since any final material compositions or processes may be different from those investigated. This is technically the purpose of the evaluations in the first place, to predict environmental hotspots in order to modify the design with less environmentally damaging alternatives.
This paper investigates a structural battery with LCA and CRA in order to explore the environmental impact and find such hotspots. The study is based on data from previous research which modelled structural batteries and calculated the potential mass saving of utilizing a structural battery in an electric vehicle [12]. It will be shown that a structural battery roof can avoid climate impacts in substantive quantities and even more so if the amount of energy used in its production can be reduced. The CRA targets two chemicals for substitution.
2. Materials and Methods
2.1. Structural Battery Design
The considered design of a structural battery has similarities to both lithium ion batteries and carbon fibre composites, and are made from alternating layers of negative electrodes, separators, and positive electrodes. Dispersed throughout all layers is a structural battery electrolyte (SBE) to both conduct lithium ions and distribute mechanical load between the carbon fibre electrodes, see Figure 1. The SBE is partly liquid to allow ion conduction, and partly solid to transfer mechanical load [4,6].

Figure 1.
Build-up of a structural battery cell.
The negative electrodes are made from layers of carbon fibres, since carbon fibres are excellent reinforcement materials and it has been proven that they function well as negative electrodes in lithium ion battery cells [7,13,14]. Next, the separators are layers of randomly oriented glass fibres which are used to electrically insulate the two electrodes. The positive electrodes are considered to be based on carbon fibres, which in this case act as reinforcement and current collectors. The carbon fibres in the positive electrode are coated with lithium iron phosphate (LiFePO4) [15], providing electrochemical storage, as well as a binder and carbon black for electrical contact.
Johannisson et al. created an analytical model of a structural battery in order to predict possible mechanical and electrical properties of the material [12]. This study also presented a design case where a roof was made from structural batteries to save mass compared to a separate steel roof and lithium ion traction battery in an electric vehicle. The results were divided into the current state of structural battery research (referred to as initial design), and what can be theoretically reachable with continuous improvements to its manufacturing and design (referred to as target design). The bill of materials (BOM) of these structural batteries are given in Table S1. The structural battery roofs weigh 7.8 kg and 9.2 kg for the initial and target designs and have energy densities of 41 Wh/kg and 121 Wh/kg, respectively. Given a steel roof weighing 18.6 kg, the initial design can save 10.8 kg of mass in the vehicle and the target design can save 9.4 kg. But they can also save between 2.2 and 12.9 kg of traction battery mass, making the total mass savings even greater (see Table S3).
2.2. Life Cycle Assessment
The function of a structural battery car roof is twofold; it needs to function as a car roof and as an electrical energy storage unit for the lifetime of the vehicle. Thus, the reference flow in the study was one structural battery car roof, i.e., most results were measured against this reference flow, see Figure 2. Note, however, that this reference flow comes in two versions, depicting the current development status version and the targeted realistic future development. Results are mainly given as vehicle life cycle environmental impacts. End of life recycling was included, and it was assumed that recycled materials could replace virgin material input. If a quality deterioration of the recycled material compared to the virgin material was evident, this was considered in the model.
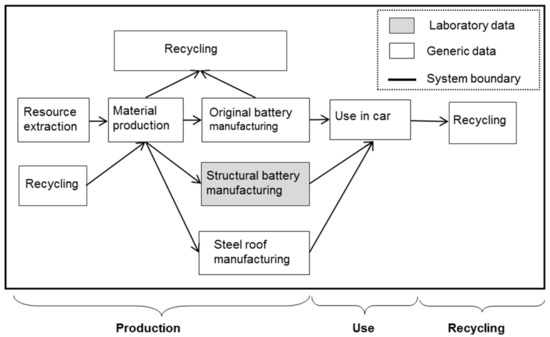
Figure 2.
Life cycle assessment (LCA) system boundary when replacing a steel roof and traction battery with a structural battery in an electric vehicle.
To cover a whole life cycle, the production of the batteries (the structural as well as the original), the use of the batteries in the car and the recycling stages must be included. The production phase models were based on respective BOMs. The use of the structural battery in the car was modelled by considering the electricity needed to carry the mass of the structural battery in relation to the mass of the replaced original battery and steel roof.
The system boundary for the study was such that the vehicle itself was not present in the system, only the original roof, the structural battery roof, and the original battery (see Figure 2). In essence, the study will calculate the change in environmental impact when replacing a steel roof and an equivalent part of the original traction battery with a structural battery roof in a vehicle. Material production and manufacturing of the original battery cells, the structural battery roof, and the steel roof were included, as well as the benefits of the mass reduction during the use phase and the implications for recycling. The life cycle impacts for the alternative systems (structural battery roof system and steel roof system) will thus be compared.
The environmental impact categories climate impact (kilogram CO2-eq), photochemical ozone formation (kilogram nitrogen oxide equivalents, NOx-eq), and resource depletion (gram antimony equivalents, Sb-eq) were used as they are able to assess trade-offs between environmental impacts in different life cycle stages of vehicles. Toxicity has not been evaluated quantitively with LCA. Instead a qualitative assessment has been made, see the section on CRA methodology. The reason for not including toxicity in the LCA was that earlier studies have shown that current methods for toxicity evaluation have considerable inadequacies related to metals and lithium in particular. There is a lack of data concerning lithium emissions and other battery related chemicals during the life cycle, and a lack of characterisation factors to translate such emissions into toxic impacts [16].
2.2.1. Electricity
In the base case, average Western European electricity was used for the use phase as well as the production phase. For sensitivity analysis Swedish average electricity was used for both use and/or production phases. Note that the Western European mix is considered as the present world average concerning climate footprint (414–428 g CO2-eq/kWh). The lower value was associated with high voltage used for production, while the higher value was applied for the battery use phase. The Swedish mix (at 42–48 g CO2-eq/kWh) can be used as an indication to evaluate the effect of future European energy targets [17]. The data sets used in the calculations are given in Table S2.
2.2.2. Production of Original Traction Battery and Steel Roof
Modelling of the batteries to be replaced by the structural battery was based on a model of a 24 kWh Lithium Nickel Manganese Cobalt oxide (NMC) batteries for a Nissan LEAF and on a model of an 85 kWh Lithium Nickel Cobalt Aluminium oxide (NCA) battery used in a Tesla model S. The characteristics of these batteries and the respective BOMs are shown in Tables S3, S4 and S5. The reason for examining two battery types with different sizes was to study the sensitivity of the result of these parameters and thereby increase the possibility to draw robust conclusions. Steel roof production was based on Ecoinvent data for unalloyed steel and hot-rolling along with data from an automobile parts manufacturer using 1.3 kWh electricity per kg for forming.
2.2.3. Production of the Structural Battery
The structural battery was modelled as battery cells with an external glass fibre casing. It will eventually need some battery management system (BMS) and some additional casing/packaging. Any extra devices for cooling a structural battery will not be required. Since there was no BMS for the structural battery, no BMS of the NMC and NCA batteries was considered replaced. The BMS was therefore removed from the NMC and NCA battery models. The weights of the resulting batteries without BMS are given in Table S3. Also, the structural battery has a casing included and natural cooling, it was therefore assumed that it could replace the modified batteries in proportion to its capacity. For an example of the resulting mass savings, see Table S3.
The negative electrode consisted of carbon fibres impregnated with SBE. The separator consisted of glass fibre impregnated with SBE. The upstream production of carbon and glass fibre was modelled with data from Das [18] and from Ecoinvent 3.5 [19] respectively. No losses were assumed.
The positive electrode was made of carbon fibres coated with LiFePO4, Polyvinylidenfluoride (PVDF) binder, and carbon black. The coating process was an electrophoretic deposition (EPD) as presented by Hagberg et al. [15] and used a bath of acetone, iodine, and a surfactant, Triton X-100. The EPD process, including ultrasonication, needed 1 kJ electricity per gram of coated fibre. The bath of acetone, iodine, and a surfactant was assumed to be reused in the same way as other solvents are reused in cell production. According to Dunn et al. [20], about 99.5% of the solvent is recovered and can be reused; the rest is lost during production and combusted and must be replaced. LiFePO4 was made from synthesising LiCO3, Fe2O3, and (NH4)2HPO4. Quantities were decided by molar calculation and the ingredients found in the Ecoinvent 3.5 database. In addition, the process needed 3 kJ electric energy per gram of LiFePO4 for two temperature increases [21].
Production of the chemicals in the SBE was modelled in the LCA with data from Ecoinvent 3.5 [19]. The toxicity of each of the chemicals has also been investigated in a chemical risk assessment. LCA datasets for the main SBE constituents, bisphenol A dimethacrylate, and ethylene carbonate, making up 78% of the SBE, were found in the Ecoinvent database. The remaining SBE ingredients were modelled as average organic or inorganic chemicals, see Table S6.
In general, energy requirements for lithium cell manufacturing and battery assembly can vary largely, mainly depending on (1) which share of the manufacturing/assembly steps require dry room/clean room conditions and (2) plant throughput. Estimations and measurements vary between 1 MJ/kg battery to 400 MJ/kg battery of primary energy [22]. Kim et al. [1] reported 120 MJ/kg of primary energy for the production of the Ford Focus BEV battery. As the analysis required energy carriers, this study assumes 11.7 kWh electricity and 8.8 kWh gas per kg structural battery (corresponding to 150 MJ primary energy according to Kim et al. [1]), earlier reported by Zackrisson et al. [21] and also used for modelling the NMC and NCA batteries in this study. Real life energy requirements for manufacturing structural batteries will eventually depend on how much of the production will have to be done under clean room conditions.
2.2.4. Use Phase
The use phase of an EV battery model normally includes electricity losses and losses related to carrying the mass of the battery. This way of modelling the use phase of a car battery has been used in other LCAs [23]. The electricity losses were not quantified since they are the same in both cases.
The influence of the battery mass was modelled using an energy reduction value (ERV) of 0.69 kWh/(100 kg × 100 km) for the worldwide harmonised light-duty vehicles test procedure (WLTC) [24] as base case. The WLTC, in general, gives higher energy use and therefore also higher energy reductions for savings in mass compared to the new European driving cycle (NEDC), though both test procedures have been criticised as showing lower energy requirements and thus reductions than real life conditions. The NEDC’s ERV value was 0.65 kWh/(100 kg × 100 km). The 18.6 kg steel roof was the reference from which the energy savings were calculated. In addition, the transport of the battery from the car manufacturer to the user was included in the use phase. For all considerations regarding transports, see Section 2.2.4 in the Supplementary material.
2.2.5. Recycling
A 300 km recycling transport was assumed for all parts: structural battery, steel roof, and the original traction battery. This represents a transport between the car scrap yard and fragmentation/recycling plants [25].
For the steel roof, 95% of the raw material was assumed to be recycled in accordance with the end-of-life vehicle directive [26] and replacing input steel quality, resulting in a net life cycle climate impact of 1.9 kg CO2-eq per kg of steel roof.
For the structural battery, only the fibres will sustain the assumed pyrolysis recycling process. Furthermore, the recycled reinforcement fibres are believed to have significantly poorer properties than virgin fibres. The recycled carbon fibre was assumed to avoid 50% of the burdens of virgin carbon fibre [27] because it sells at 50% of virgin carbon fibre’s price [28]. The same assumption was made for glass fibres. The 95% recycling rate from the end-of-life vehicle directive was assumed to apply also to the structural battery.
The recycling of the original batteries was modelled using default data from the Product Environmental Footprint Category Rules for High Specific Energy Rechargeable Batteries [29] for the cell constituents, combined with data from Ellingsen et al. [30] for the rest of the battery pack. Aluminium and steel give considerable climate avoidance contributions (from the rest-of-pack), however, while it entails 15 kg CO2-eq to manufacture 1 kg NMC battery, the recycling avoids 1.2 kg CO2-eq per kg NMC battery. The corresponding figures for the steel roof were 3 kg CO2-eq/kg for the manufacturing of which 1.1 kg CO2-eq/kg was avoided by its recycling, and for the structural battery roof at target level 19 kg CO2-eq/kg for the manufacturing of which 4 kg CO2-eq/kg was avoided by its recycling. See Figure S1 in the Supplementary material.
2.3. Chemical Risk Assessment from a Life Cycle Perspective
The CRA has been inspired by different assessment methods and tools in the open literature [31,32,33,34] and herein adapted to a life cycle perspective. Chemicals and process materials have been evaluated for hazards in the different life cycle phases. Hazards have also been associated with risk for exposure so the chemical risk for the user/worker can be assessed. Physical-chemical properties have thus been used as well as hazard statements according to the Classification, Labelling, and Packaging (CLP) directive [35].
A four step CRA procedure was followed to qualitatively assess chemical hazards and legal risks:
- Collection of safety data sheets (SDS), retrieved from the chemical suppliers.
- Identification of hazard statements of the chemical mixture as well as the individual components from the SDS, according to CLP. In addition, the chemical abstract service (CAS) registry number for classified individual components are listed.
- In order to identify any legal requirements, restrictions, or unharmonized classifications, the CAS numbers are investigated through the European Chemical Agency (ECHA) database [36].
- Evaluation of risk in each life cycle phase according to criteria in Table S7.
Initially, work exposure limits and safety measures were included in the risk assessment matrix. The carcinogenic, mutagenic, and reproduction toxic (CMR), persistent, bio-accumulative, and toxic (PBT), and very persistent and very bio-accumulative (vPvB) properties are related to properties of high concern in existing European chemical legislation, i.e., registration, evaluation, and authorisation of chemicals (REACH) [37], and persistent organic pollutants (POP) regulation [38]. In the evaluation, other properties of lower concern have also been included, such as skin sensitising, respiratory and oral toxicity (see Table S8 for an example of data collected for the CRA).
The assessment was made in the different life cycle phases as well as an overall assessment. Assessment results were condensed and presented as low, medium, and high risk, visualised with green, yellow, and red. Properties that have been evaluated are the chemicals’ intrinsic properties according to the hazard statement. See Table S7 for the evaluation criteria of risk and compliance that have been used for the assessment.
From a life cycle perspective, the risk relates to intrinsic properties (hazards) and exposure in three different life cycle phases of the battery (production, use, and end of life). The assessment of risks relates to the physical-chemical state and potential transformation. Thus, chemical transformations as a result of chemical reactions during production have been considered. In one life cycle phase, the chemicals may have one exposure potential and hazard, but in another phase the particular chemical is virtually non-existent due to the chemical transformation. For instance, the monomer Bisphenol A dimethacrylate which is present in the manufacturing phase but is then polymerised and not present in the following phases.
3. Results and Discussion
3.1. Life Cycle Assessment
Results are presented as the net difference in life cycle environmental impacts when replacing a steel roof and an equivalent part of the original battery with a structural battery roof. To obtain the net difference, the environmental impact of the reference steel roof life cycle is subtracted from the structural battery roof life cycle. If the resulting measure is negative, it means an avoided impact due to the structural battery roof. See Figure S1 for a graphical description of the net life cycle impacts.
The reason for examining two battery types with different sizes, two very different electricity mixes, and two different levels of technology development is to study the sensitivity of the result to these parameters and thereby increase the possibility of drawing robust conclusions. This follows the recommendations of Arvidsson et al. [8] for prospective LCAs to model emerging technologies using predictive scenarios.
It should be noted that assembly energy is very environmentally dominant for production of normal lithium ion batteries [1] and that the energy to produce carbon fibre is very environmentally dominant in production of normal carbon fibre reinforced plastic [18].
The net life cycle climate impacts are shown in Figure 3 for all the different cases of structural battery design, electricity mixes, and vehicle size. For all the cases with European electricity, the structural battery roof avoided climate impact. As expected, the climate avoidance was larger at the target level of structural battery development. The climate avoidance was also larger in the small EV compared to the large EV. This is mainly due to that the NMC battery in the small vehicle has a lower energy density (81 Wh/kg) than the NCA battery in the large vehicle (138 Wh/kg). Only at the initial level of structural battery development in combination with Swedish electricity for propulsion did the structural battery lead to increased climate impact. This is mainly because the use phase savings are much smaller in Sweden.
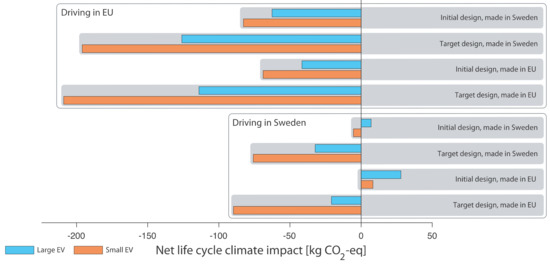
Figure 3.
Net life cycle climate impacts of structural battery roof, all cases.
The net life cycle ozone formation impacts are shown in Figure 4, where it can be seen that using a structural battery roof avoids ozone formation in all examined cases. However, photochemical ozone formation impacts show a different pattern compared to climate impacts in that the largest savings from using a structural battery roof are for the large EV. The reason is not the size of the EV but the production of the NCA cathode which has relatively large NOx-eq emissions [39].
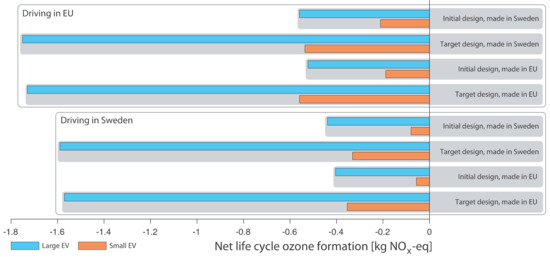
Figure 4.
Net life cycle ozone formation of structural battery roof, all cases.
The results for abiotic depletion are shown in Figure 5. It can be seen that using a structural battery roof avoids abiotic depletion in all examined cases. Abiotic depletion showed the largest savings for the small EV, as for climate impact, but it is the replaced battery that dominated savings, similar to ozone formation. The scientific base for calculating abiotic resource depletion is not very solid [16]. There is a lack of universal consensus on methodology [40,41]. Resource depletion was included in the study for reasons of completeness but not further discussed here. It should be pointed out that, as measured, a structural battery solution resulted in less resource depletion in all the 16 cases included in the sensitivity calculations (see Figure 5).
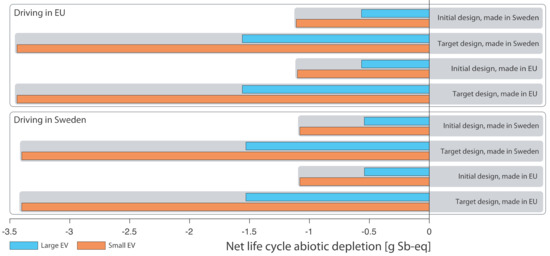
Figure 5.
Net life cycle abiotic depletion of structural battery roof, all cases.
In summary, the structural battery in the small EV gave larger climate and abiotic depletion reductions than in the larger EV, while the structural battery in the large EV gave larger ozone formation reductions. However, the difference was more due to different battery chemistries and energy efficiency in the large EV (NCA, 138 Wh/kg) and the small EV (NMC, 81 Wh/kg) than the size of the vehicles. In all cases above, the structural battery outperformed the steel roof when European average electricity was used for manufacturing and during use of the battery, i.e., base case conditions. Since European average electricity is considered close to global average electricity and the electricity mix is the largest cause of results variability [3], it is concluded that from a global perspective, a structural battery roof applied in a small or a large EV gives less climate impact, ozone formation, and abiotic depletion than a steel roof. Furthermore, a structural battery at the target level gives more environmental impact avoidance than a structural battery at the initial level.
Environmental Impact of Structural Battery Production
Figure 6 shows the most environmentally dominant processes/materials for producing a structural battery.
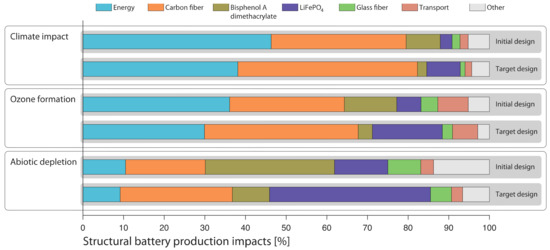
Figure 6.
Source of environmental impacts for production of structural battery. European average electricity for production.
Climate impact for production of a structural battery roof at the initial level and target level is dominated by the assembly energy, the carbon fibre (most of which is manufacturing energy), and Bisphenol A dimethacrylate in the SBE and LiFePO4 (see Figure 6). Since there are many more lithium coated carbon fibres and much less electrolyte in the target level structural battery roof, the relative climate impact changes for these constituents, i.e., carbon fibre and LiFePO4, increase at the expense of Bisphenol A dimethacrylate in the SBE. While the amount of assembly energy actually is larger at the target than initial level, its relative share of the climate impact decreases at target level due to the increase of lithium coated carbon fibres.
The ozone formation stems mainly from assembly energy, carbon fibre, Bisphenol A dimethacrylate in the SBE, LiFePO4, transports from raw material producers to cell manufacturing, and glass fibre, i.e., more or less the same dominance pattern as for the climate impact (see Figure 6). Carbon fibre and LiFePO4 increase at the expense of Bisphenol A dimethacrylate in the SBE and the relative share of energy when moving from the initial to target level, just as for climate impacts.
Abiotic depletion at the initial level stems mainly from Bisphenol A dimethacrylate in the SBE, the carbon fibre, LiFePO4, assembly energy, and glass fibre (see Figure 6). Just as for climate impacts and ozone formation, carbon fibre and LiFePO4 increase at the target level and Bisphenol A dimethacrylate in the SBE decreases.
3.2. LCA Results in Perspective
Is saving 209 kg CO2-eq when replacing a steel roof with a structural battery roof in a small EV, or 114 kg for the same replacement in a large EV (see Figure 3), a lot or hardly anything? To put these numbers in perspective, they can be distributed on a 200,000 km service life and compared with, for example, the indirect propulsion emissions of small and large vehicles respectively. Saving 209 kg CO2-eq in a small EV life cycle is equal to saving 1.3% of propulsion impacts when the vehicle is driven with West European electricity mix.
Another way of understanding the effects of a structural battery is to calculate how much extra range a structural battery car roof could supply assuming the same traction battery. With knowledge of vehicle electricity consumption, battery size, depth of discharge, weight saving, and energy reduction value, the extra range for the small vehicle is calculated to 5 km or 5% extra range (see Supplementary material Section 3.2 for further description). This 5% extra range is probably worth quite a lot more than about 1% less indirect climate impact. However, gaining range means keeping the original battery intact, so the climate impact avoidance would be less than 1% for the target structural battery design. But it would not be zero, since the battery roof is less heavy than the steel roof. Exchanging ten times more steel components with structural batteries would give ten times more range, i.e., exchanging 92 kg in the small EV would give 50 km, or 50%, more range. For the large EV with the NCA battery, the numbers are 4.5 km extra range, equivalent to 1.6% extra range for the 9.2 kg substitution.
3.3. Chemical Risk Assessment from a Life Cycle Perspective
Chemical risk has been evaluated with a novel CRA methodology to properly consider input chemicals for compliance and hazard. Previous tools for CRA address hazard and risk, but seldom consider life cycle phases. Another novel characteristic is the assessment for future legal risks and chemical legal compliance. For easy to perceive decision support, colour coding has been used. The chemicals included in the BOM have been evaluated and the results are summarised in Figure 7. Green marks indicate no/low risk, yellow indicates medium risk, and red indicates high risk which is recommended for substitution.
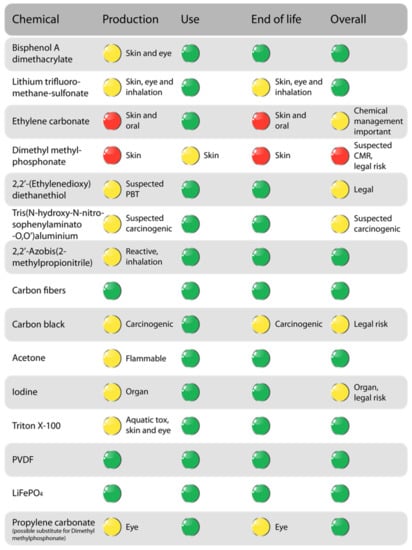
Figure 7.
Results of chemical risk assessment of the structural battery. For a green risk level, the chemicals have no hazard statements of high concern or low exposure of chemicals with properties of low concern such as skin irritants. At a yellow risk level, chemicals with hazard statements of lower concern but with higher exposure are included. A red risk level includes chemicals with properties of high concern such as carcinogenic, mutagenic, and reproduction toxic (CMR), persistent, bio-accumulative, and toxic (PBT), and/or very persistent and very bio-accumulative (vPvB). A red level also includes legal risks, both according to existing chemical regulations and possible coming regulations. For more details, see Figure S2.
The chemical risk assessment method suggested in this study has been iteratively developed, thus some initial aspects were excluded from the final version: protective measures, exposure routes, and work exposure limits were originally included but excluded in the final assessment since these aspects are covered by other included parameters (see Tables S9 and S10). Since the scope does not include upstream processes, possible production routes that include risk chemicals are omitted. This may be the case for production of PVDF that can include hazardous perfluoroalkyl substances (PFAS) and other similar risks connected to precursors and reactants.
Most chemicals used in the production of structural batteries are evaluated as low risk for production, use, and end of life-handling, thus resulting in low overall risk related to compliance and hazard. The main result is that dimethyl methylphosphonate and possibly ethylene carbonate should be investigated for substitution. Propylene carbonate is suggested as a substitute for dimethyl methylphosphonate. This chemical has been assessed with the same method and is also presented in Figure 7.
4. Conclusions
Making an LCA and CRA early in the product development is especially fruitful to help steer the design of both materials and processes. Naturally, such assessments are prospective in nature, since any final material compositions or processes may be different from those investigated. Which is also the desired outcome of the LCA and CRA—to find environmental hotspots in order to modify the design with more environmentally friendly alternatives.
The LCA results show that, with global average conditions/electricity, replacing a steel part and lithium ion traction battery in an electric vehicle with a structural battery can avoid climate impact, ozone formation, and abiotic depletion in substantive quantities. The major climate impact for the production of a structural battery roof at the initial level stems from the assembly energy (45%), carbon fibre (32%), Bisphenol A dimethacrylate in the SBE (10%), and LiFePO4 (3%). Ozone formation and abiotic depletion impacts for production of a structural battery roof show more or less the same pattern. Thus, improvement efforts should be focused on saving energy and/or improving the electricity mix for both cell production and carbon fibre production, and possibly finding alternatives to Bisphenol A dimethacrylate. The CRA suggests that substitution of dimethyl methylphosphonate should be considered due to suspected CMR properties and possible future legal risks. Also, ethylene carbonate is suggested as a candidate for substitution since it is toxic for dermal and oral contact and good safety measures are needed. Propylene carbonate has already been suggested as a substitute for dimethyl methylphosphonate and the assessment shows that it is a good substitute, significantly lowering the risk.
A natural expansion of the LCA presented in this research would be to involve substitution of more automotive parts in other materials, e.g., aluminium and plastic composites. Also, further analysis on the recycling of the structural battery material would be useful, both for more understanding of its impact and possible modification of the design and production to facilitate recycling and the reuse of materials with less quality loss.
Supplementary Materials
The following are available online at https://www.mdpi.com/2071-1050/11/20/5679/s1, Table S1: Bill of materials for structural battery cells at initial and target levels, Table S2: Electricity mixes used in the study and their climate impact, Table S3: 24 kWh NMC battery and 85 kWh NCA battery, Table S4: BOM-list for 33 Ah LEAF original NMC cell (from AESC), Table S5: BOM-list for 3.1 Ah Tesla original NCA cell (NCR18650A from Panasonic), Table S6: Life cycle model for structural battery electrolyte, Table S7: Risk evaluation matrix including assessment criteria for evaluation of risk based on hazard and exposure, and suggested actions for the different identified risks, Table S8: Example of data collected for the chemical risk assessment, including name, CAS-RN, hazard statement and selected physical-chemical properties, Table S9: The information sources that have been used for assessing the chemical risks, Table S10: Information sources and aspects which were originally included in the chemical risk assessment but were excluded from the final assessment, Table S11: Physical hazard codes according to CLP, Table S12: Health hazard codes according to CLP, Table S13: Environmental hazard codes according to CLP, Figure S1: Net life cycle climate impacts of structural battery roof at target level in a small EV compared to steel roof, European electricity for production and propulsion. Processes contributing less than 15% not shown, Figure S2: Chemical risk assessment for chemicals in the structural battery. The table includes hazard information and assessment results for the different life cycle phases.
Author Contributions
Conceptualization, M.Z., C.J., W.J. and D.Z.; Data curation, C.J., W.J. and K.F.; Formal analysis, M.Z., C.J. and K.F.; Funding acquisition, C.J., K.F. and D.Z.; Investigation, M.Z., C.J., W.J. and K.F.; Methodology, M.Z., C.J., W.J. and K.F.; Project administration, C.J. and D.Z.; Resources, M.Z., C.J., W.J. and K.F.; Supervision, C.J. and D.Z.; Visualization, W.J.; Writing – original draft, M.Z.; Writing – review & editing, M.Z., C.J., W.J., K.F., S.P., D.Z. and G.L.
Funding
This research was funded by the XPRES initiative, the Swedish Research Council, projects 2017-03898 and 621-2014-4577, the strategic innovation program LIGHTer (funding provided by Vinnova, the Swedish Energy Agency and Formas), H2020 Clean Sky II project no. 738085 and by the Air Force Office of Scientific Research under award number FA9550-17-1-0244.
Acknowledgments
The LCA and CRA have been carried out by RISE IVF in close cooperation with the structural battery research group (Kombatt) at the Department of Aeronautical and Vehicle Engineering and Department of Chemical Engineering, KTH Royal Institute of Technology. The results of the LCA and CRA was communicated to the structural battery research group in an idea generation workshop in order to make the most possible use of the LCA results. It resulted in 35 ideas aiming at improving the environmental performance of a structural battery.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kim, H.C.; Wallington, T.J.; Arsenault, R.; Bae, C.; Ahn, S.; Lee, J. Cradle-to-Gate Emissions from a Commercial Electric Vehicle Li-Ion Battery: A Comparative Analysis. Environ. Sci. Technol. 2016, 50, 7715–7722. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, L.A.; Singh, B.; Strømman, A.H. The size and range effect: lifecycle greenhouse gas emissions of electric vehicles. Environ. Res. Lett. 2016, 11, 054010. [Google Scholar] [CrossRef]
- Cox, B.; Mutel, C.L.; Bauer, C.; Beltran, A.M.; Van Vuuren, D.P. Uncertain Environmental Footprint of Current and Future Battery Electric Vehicles. Environ. Sci. Technol. 2018, 52, 4989–4995. [Google Scholar] [CrossRef] [PubMed]
- South, J.T.; Carter, R.H.; Snyder, J.F.; Hilton, C.D.; O’Brien, D.J.; Wetzel, E.D. Multifunctional Power-Generating and Energy-Storing Structural Composites for U.S. Army Applications. MRS Proc. 2004, 851, NN4.6. Available online: https://www.cambridge.org/core/journals/mrs-online-proceedings-library-archive/article/multifunctional-powergenerating-and-energystoring-structural-composites-for-us-army-applications/EE493315B815801CD4F5BB1BB97AB64A (accessed on 11 October 2019). [CrossRef]
- Snyder, J.F.; Gienger, E.B.; Wetzel, E.D.; Xu, K.; Curtis, P.T. Multifunctional Structural Composite Batteries. In Proceedings of the Society for the Advancement of Materiel and Process Engineering (SAMPE) 2006 Fall Technical Conference, Dallas, TX, USA, 6–9 November 2010. [Google Scholar]
- Wong, E.L.; Baechle, D.M.; Xu, K.; Carter, R.H.; Snyder, J.F.; Wetzel, E.D. Design and Processing of Structural Composite Batteries. In Proceedings of the Society of Advancement of Material and Process Engineering, Baltimore, MD, USA, 3–7 June 2007. [Google Scholar]
- Kjell, M.H.; Jacques, E.; Zenkert, D.; Behm, M.; Lindbergh, G. PAN-Based Carbon Fiber Negative Electrodes for Structural Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, A1455. [Google Scholar] [CrossRef]
- Arvidsson, R.; Janssen, M.; Nordelöf, A. Environmental Assessment of Emerging Technologies Recommendations for Prospective LCA. J. Ind. Ecol. 2017, 22, 1286–1294. [Google Scholar] [CrossRef]
- Nielsen, P.H.; Wenzel, H. Integration of environmental aspects in product development: A Stepwise Procedure Based on Quantitative Life Cycle Assessment. J. Clean. Prod. 2002, 10, 247–257. [Google Scholar] [CrossRef]
- Simon, M.; Poole, S.; Sweatman, A.; Evans, S.; Bhamra, T.; McAloone, T. Environmental Priorities in Strategic Product Development. Bus Strateg. Environ. 2000, 9, 367–377. [Google Scholar] [CrossRef]
- Zackrisson, M. Product Orientation of Environmental Work: Barriers & Incentives; Degree in Licentiate of Engineering, Department of Machine Design, Royal Institute of Technology; KTH: Stockholm, Sweden, 2009; Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:kth:diva-10585 (accessed on 11 October 2019).
- Johannisson, W.; Zenkert, D.; Lindbergh, G. Model of a structural battery and its potential for system level mass savings. Multifunct. Mater. 2019, 2, 035002. [Google Scholar] [CrossRef]
- Jacques, E.; Kjell, M.H.; Zenkert, D.; Lindbergh, G.; Behm, M.; Willgert, M. Impact of electrochemical cycling on the tensile properties of carbon fibres for structural lithium-ion composite batteries. Compos. Sci. Technol. 2012, 72, 792–798. [Google Scholar] [CrossRef]
- Jacques, E.H.; Kjell, M.; Zenkert, D.; Lindbergh, G. The effect of lithium-intercalation on the mechanical properties of carbon fibres. Carbon N. Y. 2014, 68, 725–733. [Google Scholar] [CrossRef]
- Hagberg, J.; Maples, H.A.; Alvim, K.S.P.; Xu, J.; Johannisson, W.; Bismarck, A.; Zenkert, D.; Lindbergh, G. Lithium iron phosphate coated carbon fiber electrodes for structural lithium ion batteries. Compos. Sci. Technol. 2018, 162, 235–243. [Google Scholar] [CrossRef]
- Zackrisson, M.; Fransson, K.; Hildenbrand, J.; Lampic, G.; O’Dwyer, C. Life cycle assessment of lithium-air battery cells. J. Clean. Prod. 2016, 135, 299–311. [Google Scholar] [CrossRef]
- European Commission. Energy Roadmap 2050; Publications Office of the European Union: Luxembourg, 2012. [Google Scholar]
- Das, S. Life cycle assessment of carbon fiber-reinforced polymer composites. Int. J. Life Cycle Assess. 2011, 16, 268–282. [Google Scholar] [CrossRef]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Sullivan, J.; Wang, M.Q. Impact of recycling on cradle-to-gate energy consumption and greenhouse gas emissions of automotive lithium-ion batteries. Environ. Sci. Technol. 2012, 46, 12704–12710. [Google Scholar] [CrossRef]
- Zackrisson, M.; Avellán, L.; Orlenius, J. Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles-Critical issues. J. Clean. Prod. 2010, 18, 1517–1527. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Kelly, J.C.; James, C.; Gallagher, K.G. The significance of Li-ion batteries in electric vehicle life-cycle energy and emissions and recycling’s role in its reduction. Energy Environ. Sci. 2014, 8, 158–168. [Google Scholar] [CrossRef]
- Matheys, J.; Van Autenboer, W.; Van Mierlo, J. Subat: Sustainable Batteries. Work Package 5: Overall Assessment; Final Public Report; Vrije Universiteit Brussels-ETEC: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Forell, A.; Busa, A.; Wilbert, G. Advanced High Volume Affordable Lightweighting for Future Electric Vehicles; Thinkstep AG: Wolfsburg, Germany, 2016. [Google Scholar]
- Cullbrand, F.K.; Fråne, A.; Jensen, C. Utökad demontering av personbilar; IVL Svenska Miljöinstitutet: Stockholm, Sweden, 2015. [Google Scholar]
- Ec. Directive 200053EC of the European Parliament and of the Council on end-of Life Vehicles. Off. J. Eur. Communities 2000, 1–15. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32000L0053 (accessed on 11 October 2019).
- Zackrisson, M.; Jönsson, C.; Olsson, E. Life Cycle Assessment and Life Cycle Cost of Waste Management—Plastic Cable Waste. Adv. Chem. Eng. Sci. 2014, 4, 221–232. [Google Scholar] [CrossRef]
- Hagnell, M.K.; Åkermo, M. The economic and mechanical potential of closed loop material usage and recycling of fibre-reinforced composite materials. J. Clean. Prod. 2019, 223, 957–968. [Google Scholar] [CrossRef]
- RECHARGE. PEFCR—Product Environmental Footprint Category Rules for High Specific Energy Rechargeable Batteries for Mobile Applications; The Advanced Rechargeable & Lithium Batteries Association: Brussels, Belgium, 2018. [Google Scholar]
- Ellingsen, L.A.; Majeau-bettez, G.; Singh, B.; Srivastava, A.K.; Valøen, L.O.; Strømman, A.H. Life Cycle Assessment of a Lithium-Ion Battery Vehicle Pack. J. Ind. Ecol. 2013, 18, 113–124. [Google Scholar] [CrossRef]
- Askham, C.; Gade, A.L.; Hanssen, O.J. Linking chemical risk information with life cycle assessment in product development. J. Clean. Prod. 2013, 51, 196–204. [Google Scholar] [CrossRef]
- Barberio, G.; Scalbi, S.; Buttol, P.; Masoni, P.; Righi, S. Combining life cycle assessment and qualitative risk assessment: The case study of alumina nanofluid production. Sci. Total Environ. 2014, 496, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Harmon, J.P.; Otter, R. Green Chemistry and the Search for New Plasticizers. ACS Sustain. Chem. Eng. 2018, 6, 2078–2085. [Google Scholar] [CrossRef]
- Jacobs, M.M.; Malloy, T.F.; Tickner, J.A.; Edwards, S. Alternatives assessment frameworks: Research needs for the informed substitution of hazardous chemicals. Environ. Health Perspect. 2016, 124, 265–280. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC); Publications Office of the European Union: Luxembourg, 2008. [Google Scholar]
- ECHA. European Chemicals Agency, Chemical Database. 2019. Available online: https://echa.europa.eu/sv/home (accessed on 27 may 2019).
- European Commission. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH); Publications Office of the European Union: Luxembourg, 2006. [Google Scholar]
- EU; European Commission. Regulation (EC) No 850/2004 of the European Parliament and of the Council of 29 April 2004 on Persistent Organic Pollutants and Amending Directive 79/117/EEC; Publications Office of the European Union: Luxembourg, 2004. [Google Scholar]
- Bauer, C. Ökobilanz Von Lithium-Ionen Batterien; Paul Scherrer Institut: Villigen, Switzerland, 2010. [Google Scholar]
- Peters, J.; Weil, M. A Critical Assessment of the Resource Depletion Potential of Current and Future Lithium-Ion Batteries. Resources 2016, 5, 46. [Google Scholar] [CrossRef]
- Klinglmair, M.; Sala, S.; Brandão, M. Assessing resource depletion in LCA: A review of methods and methodological issues. Int. J. Life Cycle Assess. 2014, 19, 580–592. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).