Supercapacitor Energy Storage Device Using Biowastes: A Sustainable Approach to Green Energy
Abstract
1. Introduction
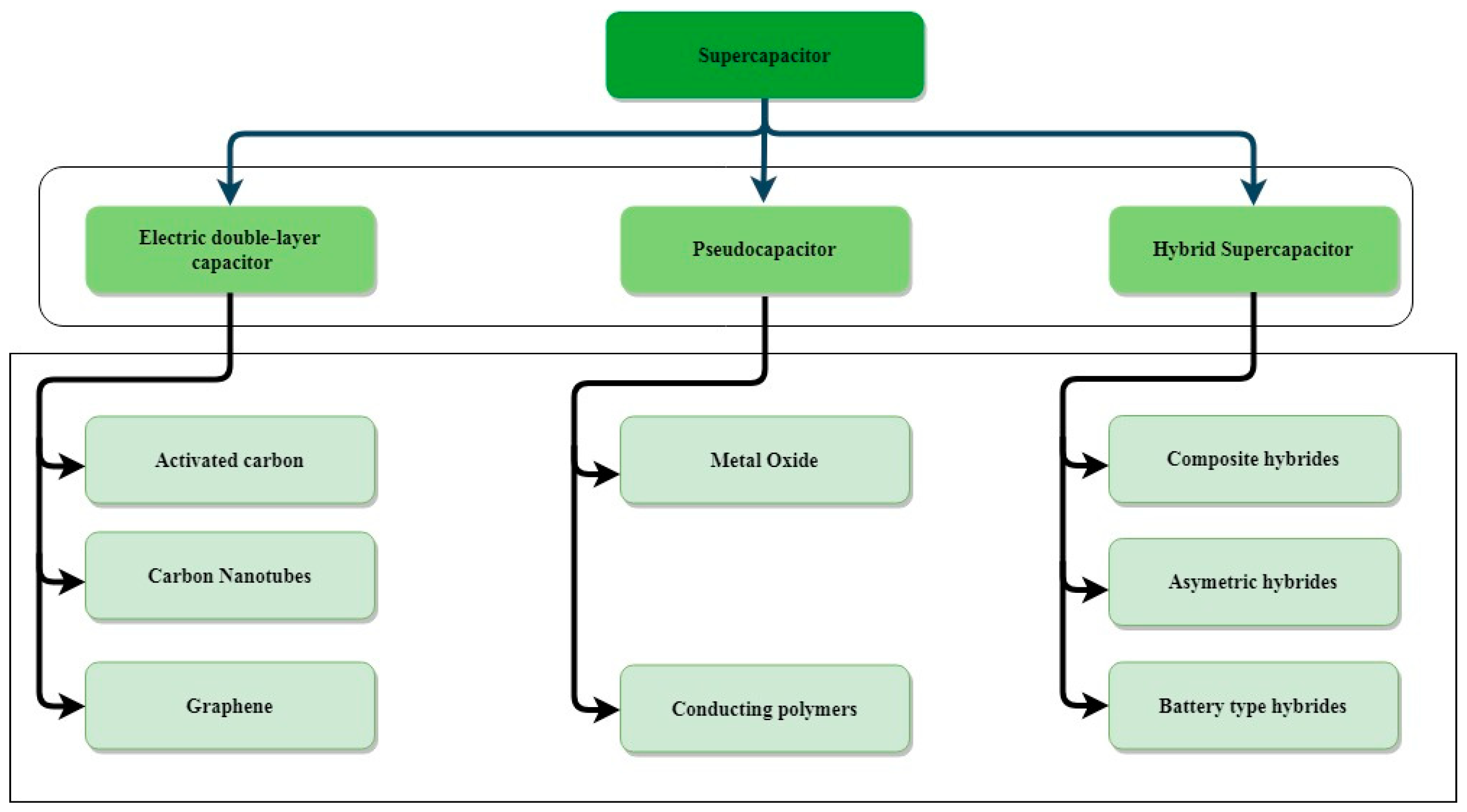
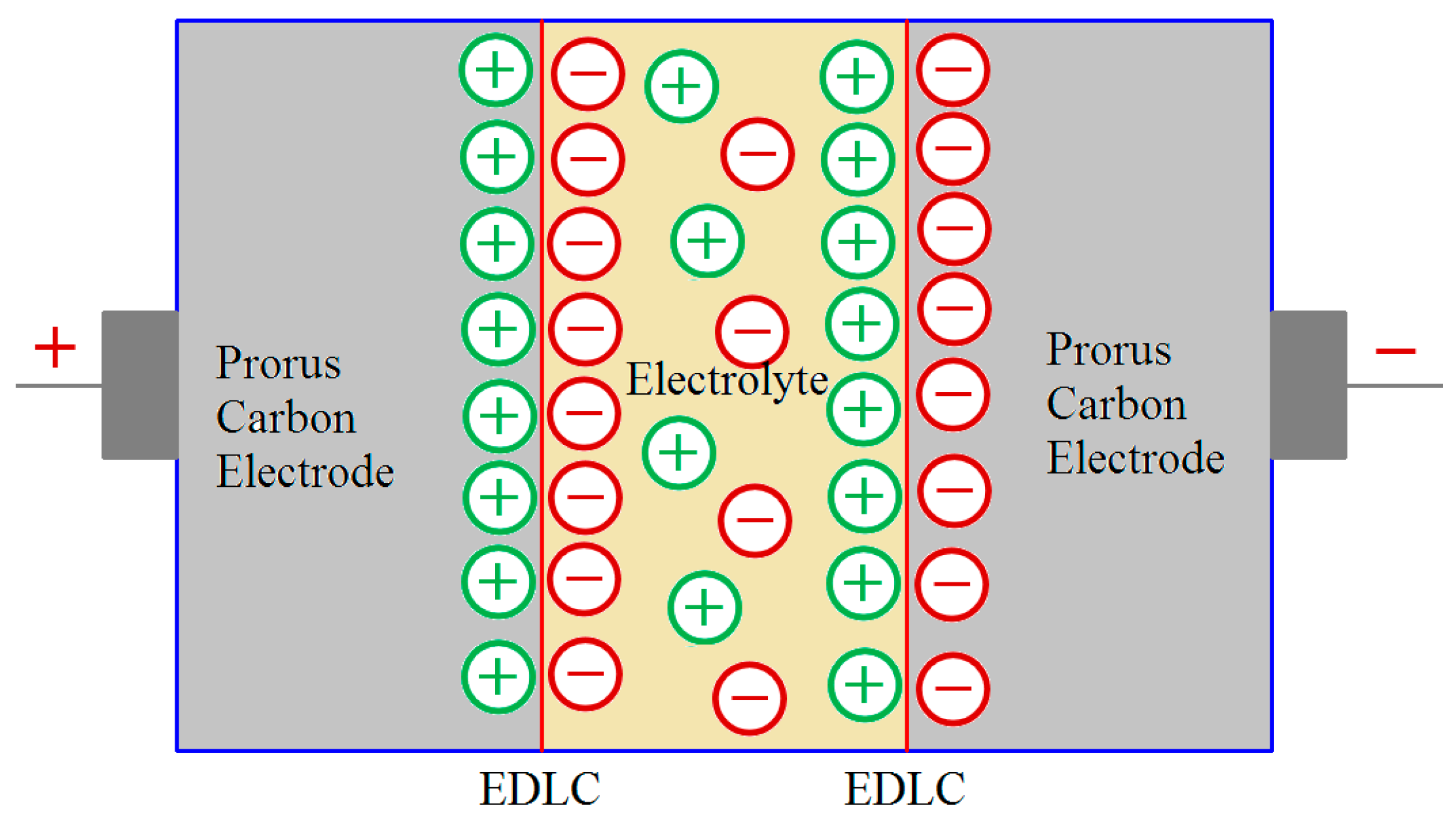
2. Type of Charge-Storage Mechanism
- Limited lifespan due to the usage of an electrolyte.
- Incorrect usage of the capacitor may result in electrolyte leakage.
- These capacitors cannot be used in AC circuits because they have a relatively high internal resistance.
- The temperature ranges at which they can be used is limited because of the organic solvents that may be volatile, toxic and inflammable.
- Enhancing surface area of electrodes.
- Relatively low energy density.
3. Carbon from Bio-Wastes as an Excellent Material for EDLC
4. Summary/Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Singh, S.; Jain, S.; Venkateswaran, P.S.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A sustainable fuel for future of the transport sector. Renew. Sustain. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]
- Hassmann, K.; Kühne, H.-M. Primary energy sources for hydrogen production. Int. J. Hydrogen Energy 1993, 18, 635–640. [Google Scholar] [CrossRef]
- Fera, M.; Macchiaroli, R.; Iannone, R.; Miranda, S.; Riemma, S. Economic evaluation model for the energy Demand Response. Energy 2016, 112, 457–468. [Google Scholar] [CrossRef]
- Gonenc, H.; Scholtens, B. Environmental and Financial Performance of Fossil Fuel Firms: A Closer Inspection of their Interaction. Ecol. Econ. 2017, 132, 307–328. [Google Scholar] [CrossRef]
- Piao, S.; Fang, J.; Ciais, P.; Peylin, P.; Huang, Y.; Sitch, S.; Wang, T. The carbon balance of terrestrial ecosystems in China. Nature 2009, 458, 1009–1013. [Google Scholar] [CrossRef]
- Trancoso, R.; Larsen, J.R.; McVicar, T.R.; Phinn, S.R.; McAlpine, C.A. CO2-vegetation feedbacks and other climate changes implicated in reducing base flow. Geophys. Res. Lett. 2017, 44, 2310–2318. [Google Scholar] [CrossRef]
- Stern, N. What Is the Economics of Climate Change? World Econ. 2006, 7, 1–10. Available online: http://www.eci.ox.ac.uk/~dliverma/articles/Stern%20from%20World%20Economics.pdf (accessed on 12 October 2018).
- Karl, T.R.; Trenberth, K.E. Modern Global Climate Change. Science 2003, 302, 1719–1723. [Google Scholar] [CrossRef]
- Azcárate, C.; Mallor, F.; Mateo, P. Tactical and operational management of wind energy systems with storage using a probabilistic forecast of the energy resource. Renew. Energy 2017, 102, 445–456. [Google Scholar] [CrossRef]
- Gondal, I.A.; Masood, S.A.; Amjad, M. Review of geothermal energy development efforts in Pakistan and way forward. Renew. Sustain. Energy Rev. 2017, 71, 687–696. [Google Scholar] [CrossRef]
- McKone, J.R.; DiSalvo, F.J.; Abruña, H.D. Solar energy conversion, storage, and release using an integrated solar-driven redox flow battery. J. Mater. Chem. A 2017, 5, 5362–5372. [Google Scholar] [CrossRef]
- Holze, R. F. Béguin, E. Frąckowiak (eds): Supercapacitors—Materials, Systems, and Applications. J. Solid State Electrochem. 2015, 19, 1253. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Zhang, Q.; Uchaker, E.; Candelaria, S.L.; Cao, G. Nanomaterials for energy conversion and storage. Chem. Soc. Rev. 2013, 42, 3127–3171. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.-G.; Yang, Z.; Lemmon, J.P.; Imhoff, C.; Graff, G.L.; Li, L.; Hu, J.; Wang, C.; Xiao, J.; et al. Materials Science and Materials Chemistry for Large Scale Electrochemical Energy Storage: From Transportation to Electrical Grid. Adv. Funct. Mater. 2012, 23, 929–946. [Google Scholar] [CrossRef]
- Choi, N.-S.; Chen, Z.; Freunberger, S.A.; Ji, X.; Sun, Y.-K.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges Facing Lithium Batteries and Electrical Double-Layer Capacitors. Angew. Chem. Int. Ed. 2012, 51, 9994–10024. [Google Scholar] [CrossRef]
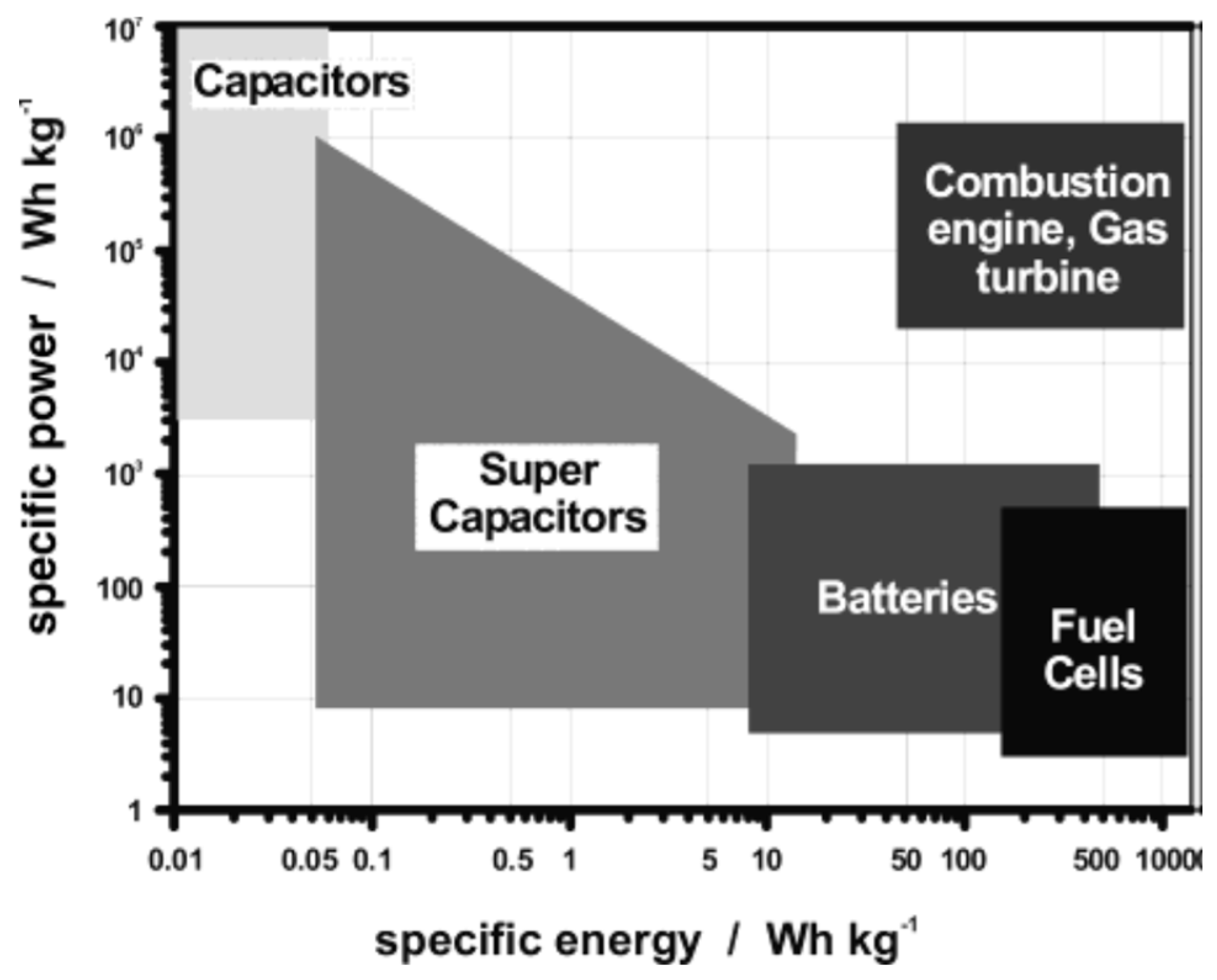
- Lee, S.C.; Jung, W.Y. Analogical Understanding of the Ragone plot and a New Categorization of Energy Devices. Energy Procedia 2016, 88, 526–530. [Google Scholar] [CrossRef]
- Christen, T.; Carlen, M.W. Theory of Ragone plots. J. Power Sources 2000, 91, 210–216. [Google Scholar] [CrossRef]
- Christen, T.; Ohler, C. Optimizing energy storage devices using Ragone plots. J. Power Sources 2002, 110, 107–116. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.C.; Yu, F.; Zhao, X.S.; Wang, H. Boosting the cycling stability of transition metal compounds-based supercapacitors. Energy Storage Mater. 2019, 16, 545–573. [Google Scholar] [CrossRef]
- Miller, E.E.; Hua, Y.; Tezel, F.H. Materials for energy storage: Review of electrode materials and methods of increasing capacitance for supercapacitors. J. Energy Storage 2018, 20, 30–40. [Google Scholar] [CrossRef]
- Song, Z.; Hou, J.; Hofmann, H.; Li, J.; Ouyang, M. Sliding-mode and Lyapunov function-based control for battery/supercapacitor hybrid energy storage system used in electric vehicles. Energy 2017, 122, 601–612. [Google Scholar] [CrossRef]
- Hadjipaschalis, I.; Poullikkas, A.; Efthimiou, V. Overview of current and future energy storage technologies for electric power applications. Renew. Sustain. Energy Rev. 2009, 13, 1513–1522. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon N. Y. 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, S.; Hou, Y.; Hu, C.; Liu, L.; Wu, Y. Electrode materials for aqueous asymmetric supercapacitors. RSC Adv. 2013, 3, 13059–13084. [Google Scholar] [CrossRef]
- Zhang, L.L.; Gu, Y.; Zhao, X.S. Advanced porous carbon electrodes for electrochemical capacitors. J. Mater. Chem. A 2013, 1, 9395–9408. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Supercapacitors: Carbons and Electrolytes for Advanced Supercapacitors (Adv. Mater. 14/2014). Adv. Mater. 2014, 26, 2283. [Google Scholar] [CrossRef]
- Zhao, X.; Sánchez, B.M.; Dobson, P.J.; Grant, P.S. The role of nanomaterials in redox-based supercapacitors for next generation energy storage devices. Nanoscale 2011, 3, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.P.; Gomez-Romero, P. All nanocarbon Li-Ion capacitor with high energy and high power density. Mater. Today Energy 2018, 8, 109–117. [Google Scholar] [CrossRef]
- Divyashree, A.; Hegde, G. Activated carbon nanospheres derived from bio-waste materials for supercapacitor applications—A review. RSC Adv. 2015, 5, 88339–88352. [Google Scholar]
- Fan, Z.; Yan, J.; Wei, T.; Zhi, L.; Ning, G.; Li, T.; Wei, F. Asymmetric Supercapacitors Based on Graphene/MnO2 and Activated Carbon Nanofiber Electrodes with High Power and Energy Density. Adv. Funct. Mater. 2011, 21, 2366–2375. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser Scribing of High-Performance and Flexible Graphene-Based Electrochemical Capacitors. Science 2012, 335, 1326–1330. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-Based Supercapacitor with an Ultrahigh Energy Density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, H.; Wu, X.; Wang, L.; Zhang, A.; Xia, T.; Dong, H.; Li, X.; Zhang, L. Progress of electrochemical capacitor electrode materials: A review. Int. J. Hydrogen Energy 2009, 34, 4889–4899. [Google Scholar] [CrossRef]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. In Nanoscience and Technology; Co-Published with Macmillan Publishers Ltd.: London, UK, 2009; pp. 320–329. ISBN 978-981-4282-68-0. [Google Scholar]
- Peng, C.; Zhang, S.; Zhou, X.; Chen, G.Z. Unequalisation of electrode capacitances for enhanced energy capacity in asymmetrical supercapacitors. Energy Environ. Sci. 2010, 3, 1499–1502. [Google Scholar] [CrossRef]
- Guo, C.X.; Li, C.M. A self-assembled hierarchical nanostructure comprising carbon spheres and graphene nanosheets for enhanced supercapacitor performance. Energy Environ. Sci. 2011, 4, 4504–4507. [Google Scholar] [CrossRef]
- Cheng, Q.; Tang, J.; Ma, J.; Zhang, H.; Shinya, N.; Qin, L.-C. Graphene and carbon nanotube composite electrodes for supercapacitors with ultra-high energy density. Phys. Chem. Chem. Phys. 2011, 13, 17615–17624. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Shi, Y.; Wu, C.; Zhang, Y.; Ma, Y.; Sun, X.; Sun, J.; Zhang, X.; Yuan, C. Monodisperse Metallic NiCoSe2 Hollow Sub-Microspheres: Formation Process, Intrinsic Charge-Storage Mechanism, and Appealing Pseudocapacitance as Highly Conductive Electrode for Electrochemical Supercapacitors. Adv. Funct. Mater. 2018, 28, 1705921. [Google Scholar] [CrossRef]
- Conway, B. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Zhao, Q.; Chen, J.; Luo, F.; Shen, L.; Wang, Y.; Wu, K.; Lu, M. Vertically oriented polyaniline-graphene nanocomposite based on functionalized graphene for supercapacitor electrode. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Khomenko, V.; Frackowiak, E.; Béguin, F. Determination of the specific capacitance of conducting polymer/nanotubes composite electrodes using different cell configurations. Electrochim. Acta 2005, 50, 2499–2506. [Google Scholar] [CrossRef]
- Wang, H.; Pilon, L. Physical interpretation of cyclic voltammetry for measuring electric double layer capacitances. Electrochim. Acta 2012, 64, 130–139. [Google Scholar] [CrossRef]
- Cao, P.; Fan, Y.; Yu, J.; Wang, R.; Song, P.; Xiong, Y. Polypyrrole nanocomposites doped with functional ionic liquids for high performance supercapacitors. New J. Chem. 2018, 42, 3909–3916. [Google Scholar] [CrossRef]
- Zhang, L.; Tsay, K.; Bock, C.; Zhang, J. Ionic liquids as electrolytes for non-aqueous solutions electrochemical supercapacitors in a temperature range of 20 °C–80 °C. J. Power Sources 2016, 324, 615–624. [Google Scholar] [CrossRef]
- Hou, Z.; Lu, H.; Yang, Q.; Zhao, Q.; Liu, J. Micromorphology-controlled synthesis of polypyrrole films by using binary surfactant of Span80/OP10 via interfacial polymerization and their enhanced electrochemical capacitance. Electrochim. Acta 2018, 265, 601–608. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Lee, H.-J.; Park, I.-J.; Choi, S.-R.; Kim, J.-G. Effect of Chloride on Anodic Dissolution of Aluminum in 4 M NaOH Solution for Aluminum-Air Battery. J. Electrochem. Soc. 2017, 164, A549–A554. [Google Scholar] [CrossRef]
- Shang, P.; Zhang, J.; Tang, W.; Xu, Q.; Guo, S. 2D Thin Nanoflakes Assembled on Mesoporous Carbon Nanorods for Enhancing Electrocatalysis and for Improving Asymmetric Supercapacitors. Adv. Funct. Mater. 2016, 26, 7766–7774. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.S. On the Configuration of Supercapacitors for Maximizing Electrochemical Performance. ChemSusChem 2012, 5, 818–841. [Google Scholar] [CrossRef]
- Chapman, D.L. LI. A contribution to the theory of electrocapillarity. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1913, 25, 475–481. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, G.; Sun, M.; Li, X.; Li, C. Fabrication and electrochemical characterization of polyaniline nanorods modified with sulfonated carbon nanotubes for supercapacitor applications. Electrochim. Acta 2011, 56, 1366–1372. [Google Scholar] [CrossRef]
- Laine, J.; Yunes, S. Effect of the preparation method on the pore size distribution of activated carbon from coconut shell. Carbon N. Y. 1992, 30, 601–604. [Google Scholar] [CrossRef]
- Daud, W.M.A.W.; Ali, W.S.W. Comparison on pore development of activated carbon produced from palm shell and coconut shell. Bioresour. Technol. 2004, 93, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yoshio, M.; Thapa, A.K.; Nakamura, H. From symmetric AC/AC to asymmetric AC/graphite, a progress in electrochemical capacitors. J. Power Sources 2007, 169, 375–380. [Google Scholar] [CrossRef]
- Fang, B.; Binder, L. Enhanced surface hydrophobisation for improved performance of carbon aerogel electrochemical capacitor. Electrochim. Acta 2007, 52, 6916–6921. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Zhan, L.; Long, D.; Qiao, W.; Yang, J.; Ling, L. Impedance of carbon aerogel/activated carbon composites as electrodes of electrochemical capacitors in aprotic electrolyte. New Carbon Mater. 2007, 22, 153–158. [Google Scholar] [CrossRef]
- Du, J.; Liu, L.; Yu, Y.; Zhang, L.; Zhang, Y.; Chen, A. Synthesis of nitrogen doped graphene aerogels using solid supported strategy for supercapacitor. Mater. Chem. Phys. 2019, 223, 145–151. [Google Scholar] [CrossRef]
- Zhang, G.; Song, Y.; Zhang, H.; Xu, J.; Duan, H.; Liu, J. Radially Aligned Porous Carbon Nanotube Arrays on Carbon Fibers: A Hierarchical 3D Carbon Nanostructure for High-Performance Capacitive Energy Storage. Adv. Funct. Mater. 2016, 26, 3012–3020. [Google Scholar] [CrossRef]
- Wang, H.; Yoshio, M. Graphite, a suitable positive electrode material for high-energy electrochemical capacitors. Electrochem. Commun. 2006, 8, 1481–1486. [Google Scholar] [CrossRef]
- Gomibuchi, E.; Ichikawa, T.; Kimura, K.; Isobe, S.; Nabeta, K.; Fujii, H. Electrode properties of a double layer capacitor of nano-structured graphite produced by ball milling under a hydrogen atmosphere. Carbon N. Y. 2006, 44, 983–988. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhou, R.; Zhao, X.S. Graphene-based materials as supercapacitor electrodes. J. Mater. Chem. 2010, 20, 5983–5992. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Lu, W. Carbon Nanotube Supercapacitors. In Carbon Nanotubes; Marulanda, J.M., Ed.; IntechOpen: Rijeka, Croatia, 2010; Chapter 29. [Google Scholar]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon Nanotubes—The Route Toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable Thin Film Supercapacitors Using Single-Walled Carbon Nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef]
- Liu, C.; Bard, A.J.; Wudl, F.; Weitz, I.; Heath, J.R. Electrochemical Characterization of Films of Single-Walled Carbon Nanotubes and Their Possible Application in Supercapacitors. Electrochem. Solid-State Lett. 1999, 2, 577–578. [Google Scholar] [CrossRef]
- Honda, Y.; Haramoto, T.; Takeshige, M.; Shiozaki, H.; Kitamura, T.; Ishikawa, M. Aligned MWCNT Sheet Electrodes Prepared by Transfer Methodology Providing High-Power Capacitor Performance. Electrochem. Solid-State Lett. 2007, 10, A106–A110. [Google Scholar] [CrossRef]
- Katakabe, T.; Kaneko, T.; Watanabe, M.; Fukushima, T.; Aida, T. Electric Double-Layer Capacitors Using “Bucky Gels” Consisting of an Ionic Liquid and Carbon Nanotubes. J. Electrochem. Soc. 2005, 152, A1913–A1916. [Google Scholar] [CrossRef]
- Xu, B.; Wu, F.; Chen, S.; Zhang, C.; Cao, G.; Yang, Y. Activated carbon fiber cloths as electrodes for high performance electric double layer capacitors. Electrochim. Acta 2007, 52, 4595–4598. [Google Scholar] [CrossRef]
- Kim, S.-U.; Lee, K.-H. Carbon nanofiber composites for the electrodes of electrochemical capacitors. Chem. Phys. Lett. 2004, 400, 253–257. [Google Scholar] [CrossRef]
- Chen, L.F.; Zhang, X.D.; Liang, H.W.; Kong, M.; Guan, Q.F.; Chen, P.; Wu, Z.Y.; Yu, S.H. Synthesis of nitrogen-doped porous carbon nanofibers as an efficient electrode material for supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Ngoc, B.T.N.; Yang, K.S.; Kojima, M.; Kim, Y.A.; Kim, Y.J.; Endo, M.; Yang, S.C. Self-sustained thin Webs consisting of porous carbon nanofibers for supercapacitors via the electrospinning of polyacrylonitrile solutions containing zinc chloride. Adv. Mater. 2007, 19, 2341–2346. [Google Scholar] [CrossRef]
- Tran, C.; Kalra, V. Fabrication of porous carbon nanofibers with adjustable pore sizes as electrodes for supercapacitors. J. Power Sources 2013, 235, 289–296. [Google Scholar] [CrossRef]
- Barranco, V.; Lillo-Rodenas, M.A.; Linares-Solano, A.; Oya, A.; Pico, F.; Ibañez, J.; Agullo-Rueda, F.; Amarilla, J.M.; Rojo, J.M. Amorphous Carbon Nanofibers and Their Activated Carbon Nanofibers as Supercapacitor Electrodes. J. Phys. Chem. C 2010, 114, 10302–10307. [Google Scholar] [CrossRef]
- Sivakkumar, S.R.; Ko, J.M.; Kim, D.Y.; Kim, B.C.; Wallace, G.G. Performance evaluation of CNT/polypyrrole/MnO2 composite electrodes for electrochemical capacitors. Electrochim. Acta 2007, 52, 7377–7385. [Google Scholar] [CrossRef]
- Honda, K.; Yoshimura, M.; Kawakita, K.; Fujishima, A.; Sakamoto, Y.; Yasui, K.; Nishio, N.; Masuda, H. Electrochemical Characterization of Carbon Nanotube/Nanohoneycomb Diamond Composite Electrodes for a Hybrid Anode of Li-Ion Battery and Super Capacitor. J. Electrochem. Soc. 2004, 151, A532–A541. [Google Scholar] [CrossRef]
- Eikerling, M.; Kornyshev, A.A.; Lust, E. Optimized Structure of Nanoporous Carbon-Based Double-Layer Capacitors. J. Electrochem. Soc. 2005, 152, E24–E33. [Google Scholar] [CrossRef]
- Chen, L.-F.; Lu, Y.; Yu, L.; Lou, X.W. Designed formation of hollow particle-based nitrogen-doped carbon nanofibers for high-performance supercapacitors. Energy Environ. Sci. 2017, 10, 1777–1783. [Google Scholar] [CrossRef]
- Tavasoli, A.; Aslan, M.; Salimi, M.; Balou, S.; Pirbazari, S.M.; Hashemi, H.; Kohansal, K. Influence of the blend nickel/porous hydrothermal carbon and cattle manure hydrochar catalyst on the hydrothermal gasification of cattle manure for H2 production. Energy Convers. Manag. 2018, 173, 15–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.; Xu, Y.; Shen, H.; Kong, X.; Xu, H. Utilization of wheat bran for producing activated carbon with high specific surface area via NaOH activation using industrial furnace. J. Clean. Prod. 2019, 210, 366–375. [Google Scholar] [CrossRef]
- Guardia, L.; Suárez, L.; Querejeta, N.; Pevida, C.; Centeno, T.A. Winery wastes as precursors of sustainable porous carbons for environmental applications. J. Clean. Prod. 2018, 193, 614–624. [Google Scholar] [CrossRef]
- Manna, M.C.; Rahman, M.M.; Naidu, R.; Sahu, A.; Bhattacharjya, S.; Wanjari, R.H.; Patra, A.K.; Chaudhari, S.K.; Majumdar, K.; Khanna, S.S. Chapter Three—Bio-Waste Management in Subtropical Soils of India: Future Challenges and Opportunities in Agriculture. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 152, pp. 87–148. [Google Scholar]
- Benedetti, V.; Patuzzi, F.; Baratieri, M. Characterization of char from biomass gasification and its similarities with activated carbon in adsorption applications. Appl. Energy 2018, 227, 92–99. [Google Scholar] [CrossRef]
- Maharjan, M.; Bhattarai, A.; Ulaganathan, M.; Wai, N.; Oo, M.O.; Wang, J.-Y.; Lim, T.M. High surface area bio-waste based carbon as a superior electrode for vanadium redox flow battery. J. Power Sources 2017, 362, 50–56. [Google Scholar] [CrossRef]
- Azwar, E.; Mahari, W.A.W.; Chuah, J.H.; Vo, D.-V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application—A review. Int. J. Hydrogen Energy 2018, 43, 20811–20821. [Google Scholar] [CrossRef]
- Hill, J.M. Sustainable and/or waste sources for catalysts: Porous carbon development and gasification. Catal. Today 2017, 285, 204–210. [Google Scholar] [CrossRef]
- Veerakumar, P.; Maiyalagan, T.; Raj, B.G.S.; Guruprasad, K.; Jiang, Z.; Lin, K.-C. Paper flower-derived porous carbons with high-capacitance by chemical and physical activation for sustainable applications. Arab. J. Chem. 2018, in press. [Google Scholar] [CrossRef]
- Sathyamoorthi, S.; Phattharasupakun, N.; Sawangphruk, M. Environmentally benign non-fluoro deep eutectic solvent and free-standing rice husk-derived bio-carbon based high-temperature supercapacitors. Electrochim. Acta 2018, 286, 148–157. [Google Scholar] [CrossRef]
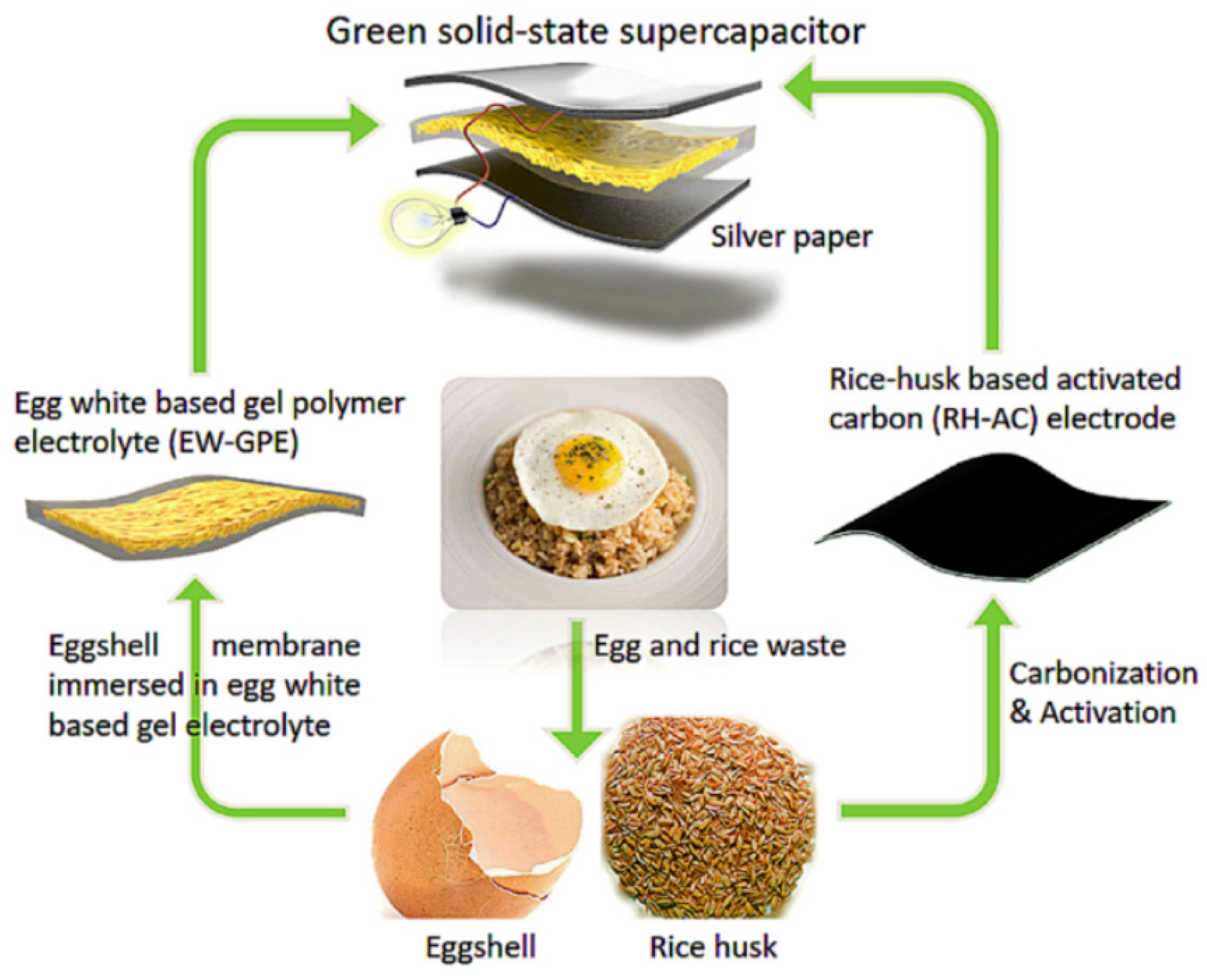
- Na, R.; Wang, X.; Lu, N.; Huo, G.; Lin, H.; Wang, G. Novel egg white gel polymer electrolyte and a green solid-state supercapacitor derived from the egg and rice waste. Electrochim. Acta 2018, 274, 316–325. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Z.; Song, N.; Li, X. High-performance supercapacitors and batteries derived from activated banana-peel with porous structures. Electrochim. Acta 2016, 222, 1257–1266. [Google Scholar] [CrossRef]
- Parveen, N.; Al-Jaafari, A.I.; Han, J.I. Robust cyclic stability and high-rate asymmetric supercapacitor based on orange peel-derived nitrogen-doped porous carbon and intercrossed interlinked urchin-like NiCo2O4@3DNF framework. Electrochim. Acta 2019, 293, 84–96. [Google Scholar] [CrossRef]
- Gong, C.; Wang, X.; Ma, D.; Chen, H.; Zhang, S.; Liao, Z. Microporous carbon from a biological waste-stiff silkworm for capacitive energy storage. Electrochim. Acta 2016, 220, 331–339. [Google Scholar] [CrossRef]
- Misnon, I.I.; Zain, N.K.M.; Aziz, R.A.; Vidyadharan, B.; Jose, R. Electrochemical properties of carbon from oil palm kernel shell for high performance supercapacitors. Electrochim. Acta 2015, 174, 78–86. [Google Scholar] [CrossRef]
- Rawal, S.; Joshi, B.; Kumar, Y. Synthesis and characterization of activated carbon from the biomass of Saccharum bengalense for electrochemical supercapacitors. J. Energy Storage 2018, 20, 418–426. [Google Scholar] [CrossRef]
- Kesavan, T.; Partheeban, T.; Vivekanantha, M.; Kundu, M.; Maduraiveeran, G.; Sasidharan, M. Hierarchical nanoporous activated carbon as potential electrode materials for high performance electrochemical supercapacitor. Microporous Mesoporous Mater. 2019, 274, 236–244. [Google Scholar] [CrossRef]
- Su, X.-L.; Li, S.-H.; Jiang, S.; Peng, Z.-K.; Guan, X.-X.; Zheng, X.-C. Superior capacitive behavior of porous activated carbon tubes derived from biomass waste-cotonier strobili fibers. Adv. Powder Technol. 2018, 29, 2097–2107. [Google Scholar] [CrossRef]
- Nam, H.; Choi, W.; Genuino, D.A.; Capareda, S.C. Development of rice straw activated carbon and its utilizations. J. Environ. Chem. Eng. 2018, 6, 5221–5229. [Google Scholar] [CrossRef]
- Song, M.; Zhou, Y.; Ren, X.; Wan, J.; Du, Y.; Wu, G.; Ma, F. Biowaste-based porous carbon for supercapacitor: The influence of preparation processes on structure and performance. J. Colloid Interface Sci. 2019, 535, 276–286. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, X.; Xu, X.; Zhang, M.; Wang, L.; Zhao, X.; An, Z.; Yao, H.; Gao, J. A novel porous carbon material made from wild rice stem and its application in supercapacitors. Mater. Chem. Phys. 2018, 213, 267–276. [Google Scholar] [CrossRef]
- Genovese, M.; Wu, H.; Virya, A.; Li, J.; Shen, P.; Lian, K. Ultrathin all-solid-state supercapacitor devices based on chitosan activated carbon electrodes and polymer electrolytes. Electrochim. Acta 2018, 273, 392–401. [Google Scholar] [CrossRef]
- Karnan, M.; Subramani, K.; Srividhya, P.K.; Sathish, M. Electrochemical Studies on Corncob Derived Activated Porous Carbon for Supercapacitors Application in Aqueous and Non-aqueous Electrolytes. Electrochim. Acta 2017, 228, 586–596. [Google Scholar] [CrossRef]
- Han, Y.; Shen, N.; Zhang, S.; Li, D.; Li, X. Fish gill-derived activated carbon for supercapacitor application. J. Alloys Compd. 2017, 694, 636–642. [Google Scholar] [CrossRef]
- Mahto, A.; Gupta, R.; Ghara, K.K.; Srivastava, D.N.; Maiti, P.; Kalpana, D.; Rivera, P.-Z.; Meena, R.; Nataraj, S.K. Development of high-performance supercapacitor electrode derived from sugar industry spent wash waste. J. Hazard. Mater. 2017, 340, 189–201. [Google Scholar] [CrossRef] [PubMed]
- González, J.F.; Román, S.; Encinar, J.M.; Martínez, G. Pyrolysis of various biomass residues and char utilization for the production of activated carbons. J. Anal. Appl. Pyrolysis 2009, 85, 134–141. [Google Scholar] [CrossRef]
- Özçimen, D.; Ersoy-Meriçboyu, A. Adsorption of Copper(II) Ions onto Hazelnut Shell and Apricot Stone Activated Carbons. Adsorpt. Sci. Technol. 2010, 28, 327–340. [Google Scholar] [CrossRef]
- Boonpoke, A.; Chiarakorn, S.; Laosiripojana, N.; Towprayoon, S.; Chidthaisong, A. Synthesis of activated carbon and MCM-41 from bagasse and rice husk and their carbon dioxide adsorption capacity. J. Sustain. Environ. 2011, 2, 77–81. [Google Scholar]
- Hirunpraditkoon, S.; Tunthong, N.; Ruangchai, A.; Nuithitikul, K. Adsorption Capacities of Activated Carbons Prepared from Bamboo by KOH Activation. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2011, 5, 477–481. [Google Scholar]
- Zequine, C.; Ranaweera, C.K.; Wang, Z.; Singh, S.; Tripathi, P.; Srivastava, O.N.; Gupta, B.K.; Ramasamy, K.; Kahol, P.K.; Dvornic, P.R.; et al. High Performance and Flexible Supercapacitors based on Carbonized Bamboo Fibers for Wide Temperature Applications. Sci. Rep. 2016, 6, 31704. [Google Scholar] [CrossRef]
- Cruz, G.; Pirilä, M.; Huuhtanen, M.; Carrión, L.; Alvarenga, E.; Keiski, R.L. Production of Activated Carbon from Cocoa (Theobroma cacao) Pod Husk. Civ. Environ. Eng. 2012, 2, 1–6. [Google Scholar] [CrossRef]
- Guo, S.; Peng, J.; Li, W.; Yang, K.; Zhang, L.; Zhang, S.; Xia, H. Effects of CO2 activation on porous structures of coconut shell-based activated carbons. Appl. Surf. Sci. 2009, 255, 8443–8449. [Google Scholar] [CrossRef]
- Li, W.; Yang, K.; Peng, J.; Zhang, L.; Guo, S.; Xia, H. Effects of carbonization temperatures on characteristics of porosity in coconut shell chars and activated carbons derived from carbonized coconut shell chars. Ind. Crops Prod. 2008, 28, 190–198. [Google Scholar] [CrossRef]
- Nahil, M.A.; Williams, P.T. Pore characteristics of activated carbons from the phosphoric acid chemical activation of cotton stalks. Biomass Bioenergy 2012, 37, 142–149. [Google Scholar] [CrossRef]
- Subha, R.; Namasivayam, C. Zinc chloride activated coir pith carbon as low cost adsorbent for removal of 2,4-dichlorophenol: Equilibrium and kinetic studies. Indian J. Chem. Technol. 2009, 16, 471–479. [Google Scholar]
- ThamYee, J.; Arumugam, S.D.; Nur Hidayah, A.L.; Abdullah, A.M.; Latif, P.A. Effect of activation temperature and heating duration on physical characteristics of activated carbon prepared from agriculture waste. Environ. Asia 2010, 3, 143–148. [Google Scholar]
- Demiral, H.; Demiral, İ.; Tümsek, F.; Karabacakoğlu, B. Pore structure of activated carbon prepared from hazelnut bagasse by chemical activation. Surf. Interface Anal. 2008, 40, 616–619. [Google Scholar] [CrossRef]
- Zequine, C.; Ranaweera, C.K.; Wang, Z.; Dvornic, P.R.; Kahol, P.K.; Singh, S.; Tripathi, P.; Srivastava, O.N.; Singh, S.; Gupta, B.K.; et al. High-Performance Flexible Supercapacitors obtained via Recycled Jute: Bio-Waste to Energy Storage Approach. Sci. Rep. 2017, 7, 1174. [Google Scholar] [CrossRef] [PubMed]
- Borhan, A.; Kamil, A.F. Preparation & Characterization of Activated Carbon from Rubber-seed Shell by Chemical Activation. J. Appl. Sci. 2012, 12, 1124–1129. [Google Scholar]
- Girgis, B.S.; Yunis, S.S.; Soliman, A.M. Characteristics of activated carbon from peanut hulls in relation to conditions of preparation. Mater. Lett. 2002, 57, 164–172. [Google Scholar] [CrossRef]
- Lua, A.C.; Yang, T.; Guo, J. Effects of pyrolysis conditions on the properties of activated carbons prepared from pistachio-nut shells. J. Anal. Appl. Pyrolysis 2004, 72, 279–287. [Google Scholar] [CrossRef]
- Devnarain, P.B.; Arnold, D.R.; Davis, S.B. Production of activated carbon from South African sugarcane bagasse. In Proceedings of the 76th Annual Congress of the South African Sugar Technologists’ Association, Mount Edgecombe, South Africa, 30 July–2 August 2002; pp. 477–489. [Google Scholar]
- Bhoyate, S.; Ranaweera, C.K.; Zhang, C.; Morey, T.; Hyatt, M.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Gupta, R.K. Eco-Friendly and High Performance Supercapacitors for Elevated Temperature Applications Using Recycled Tea Leaves. Glob. Chall. 2017, 1, 1700063. [Google Scholar] [CrossRef]
- Bhadusha, N.; Ananthabaskaran, T. Adsorptive removal of methylene blue onto ZnCl2 activated carbon from wood apple outer shell: Kinetics and equilibrium studies. E-J. Chem. 2011, 8, 1696–1707. [Google Scholar] [CrossRef]
- Mi, J.; Wang, X.R.; Fan, R.J.; Qu, W.H.; Li, W.C. Coconut-shell-based porous carbons with a tunable micro/mesopore ratio for high-performance supercapacitors. Energy Fuels 2012, 26, 5321–5329. [Google Scholar] [CrossRef]
- Jain, A.; Tripathi, S.K. Fabrication and characterization of energy storing supercapacitor devices using coconut shell based activated charcoal electrode. Mater. Sci. Eng. B 2014, 183, 54–60. [Google Scholar] [CrossRef]
- Yin, L.; Chen, Y.; Li, D.; Zhao, X.; Hou, B.; Cao, B. 3-Dimensional hierarchical porous activated carbon derived from coconut fibers with high-rate performance for symmetric supercapacitors. Mater. Des. 2016, 111, 44–50. [Google Scholar] [CrossRef]
- Wahid, M.; Puthusseri, D.; Phase, D.; Ogale, S. Enhanced capacitance retention in a supercapacitor made of carbon from sugarcane bagasse by hydrothermal pretreatment. Energy Fuels 2014, 28, 4233–4240. [Google Scholar] [CrossRef]
- Qu, W.-H.; Xu, Y.-Y.; Lu, A.-H.; Zhang, X.-Q.; Li, W.-C. Converting biowaste corncob residue into high value added porous carbon for supercapacitor electrodes. Bioresour. Technol. 2015, 189, 285–291. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Y.; Chen, Y.; Guo, H. Activated biomass carbon made from bamboo as electrode material for supercapacitors. Mater. Res. Bull. 2018, 102, 391–398. [Google Scholar] [CrossRef]
- Yang, C.S.; Jang, Y.S.; Jeong, H.K. Bamboo-based activated carbon for supercapacitor applications. Curr. Appl. Phys. 2014, 14, 1616–1620. [Google Scholar] [CrossRef]
- Kishore, B.; Shanmughasundaram, D.; Penki, T.R.; Munichandraiah, N. Coconut kernel-derived activated carbon as electrode material for electrical double-layer capacitors. J. Appl. Electrochem. 2014, 44, 903–916. [Google Scholar] [CrossRef]
- Yu, K.; Zhu, H.; Qi, H.; Liang, C. High surface area carbon materials derived from corn stalk core as electrode for supercapacitor. Diam. Relat. Mater. 2018, 88, 18–22. [Google Scholar] [CrossRef]
- Cao, W.; Yang, F. Supercapacitors from high fructose corn syrup-derived activated carbons. Mater. Today Energy 2018, 9, 406–415. [Google Scholar] [CrossRef]
- Hong, X.; Hui, K.S.; Zeng, Z.; Hui, K.N.; Zhang, L.; Mo, M.; Li, M. Hierarchical nitrogen-doped porous carbon with high surface area derived from endothelium corneum gigeriae galli for high-performance supercapacitor. Electrochim. Acta 2014, 130, 464–469. [Google Scholar] [CrossRef]
- Fan, H.; Shen, W. Gelatin-Based Microporous Carbon Nanosheets as High Performance Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2016, 4, 1328–1337. [Google Scholar] [CrossRef]
- Li, Y.-T.; Pi, Y.-T.; Lu, L.-M.; Xu, S.-H.; Ren, T.-Z. Hierarchical porous active carbon from fallen leaves by synergy of K2CO3 and their supercapacitor performance. J. Power Sources 2015, 299, 519–528. [Google Scholar] [CrossRef]
- Du, S.; Wang, L.; Fu, X.; Chen, M.; Wang, C. Hierarchical porous carbon microspheres derived from porous starch for use in high-rate electrochemical double-layer capacitors. Bioresour. Technol. 2013, 139, 406–409. [Google Scholar] [CrossRef]
- Rufford, T.E.; Hulicova-Jurcakova, D.; Khosla, K.; Zhu, Z.; Lu, G.Q. Microstructure and electrochemical double-layer capacitance of carbon electrodes prepared by zinc chloride activation of sugar cane bagasse. J. Power Sources 2010, 195, 912–918. [Google Scholar] [CrossRef]
- Liu, J.; Deng, Y.; Li, X.; Wang, L. Promising Nitrogen-Rich Porous Carbons Derived from One-Step Calcium Chloride Activation of Biomass-Based Waste for High Performance Supercapacitors. ACS Sustain. Chem. Eng. 2016, 4, 177–187. [Google Scholar] [CrossRef]
- Peng, C.; Yan, X.B.; Wang, R.T.; Lang, J.W.; Ou, Y.J.; Xue, Q.J. Promising activated carbons derived from waste tea-leaves and their application in high performance supercapacitors electrodes. Electrochim. Acta 2013, 87, 401–408. [Google Scholar] [CrossRef]
- Fu, M.; Chen, W.; Zhu, X.; Yang, B.; Liu, Q. Crab shell derived multi-hierarchical carbon materials as a typical recycling of waste for high performance supercapacitors. Carbon N. Y. 2019, 141, 748–757. [Google Scholar] [CrossRef]
- Ismanto, A.E.; Wang, S.; Soetaredjo, F.E.; Ismadji, S. Preparation of capacitor’s electrode from cassava peel waste. Bioresour. Technol. 2010, 101, 3534–3540. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chen, Y.; Wei, G.; Cao, G.; Zhang, H.; Yang, Y. Activated carbon with high capacitance prepared by NaOH activation for supercapacitors. Mater. Chem. Phys. 2010, 124, 504–509. [Google Scholar] [CrossRef]
- Choy, K.K.H.; Barford, J.P.; McKay, G. Production of activated carbon from bamboo scaffolding waste—Process design, evaluation and sensitivity analysis. Chem. Eng. J. 2005, 109, 147–165. [Google Scholar] [CrossRef]
- Hu, Z.; Srinivasan, M.P. Preparation of high-surface-area activated carbons from coconut shell. Microporous Mesoporous Mater. 1999, 27, 11–18. [Google Scholar] [CrossRef]
- Kumagai, S.; Ishizawa, H.; Toida, Y. Influence of solvent type on dibenzothiophene adsorption onto activated carbon fiber and granular coconut-shell activated carbon. Fuel 2010, 89, 365–371. [Google Scholar] [CrossRef]
- Azevedo, D.C.S.; Araújo, J.C.S.; Bastos-Neto, M.; Torres, A.E.B.; Jaguaribe, E.F.; Cavalcante, C.L. Microporous activated carbon prepared from coconut shells using chemical activation with zinc chloride. Microporous Mesoporous Mater. 2007, 100, 361–364. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined Effect of Nitrogen- and Oxygen-Containing Functional Groups of Microporous Activated Carbon on its Electrochemical Performance in Supercapacitors. Adv. Funct. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Chandra, T.C.; Mirna, M.M.; Sudaryanto, Y.; Ismadji, S. Adsorption of basic dye onto activated carbon prepared from durian shell: Studies of adsorption equilibrium and kinetics. Chem. Eng. J. 2007, 127, 121–129. [Google Scholar] [CrossRef]
- Zhai, D.; Li, B.; Du, H.; Wang, G.; Kang, F. The effect of pre-carbonization of mesophase pitch-based activated carbons on their electrochemical performance for electric double-layer capacitors. J. Solid State Electrochem. 2011, 15, 787–794. [Google Scholar] [CrossRef]
- Endo, M.; Kim, Y.J.; Maeda, T.; Koshiba, K.; Katayam, K.; Dresselhaus, M.S. Morphological effect on the electrochemical behavior of electric double-layer capacitors. J. Mater. Res. 2001, 16, 3402–3410. [Google Scholar] [CrossRef]
- Alonso, A.; Ruiz, V.; Blanco, C.; Santamaría, R.; Granda, M.; Menéndez, R.; de Jager, S.G.E. Activated carbon produced from Sasol-Lurgi gasifier pitch and its application as electrodes in supercapacitors. Carbon N. Y. 2006, 44, 441–446. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Leroux, F.; Béguin, F. A High-Performance Carbon for Supercapacitors Obtained by Carbonization of a Seaweed Biopolymer. Adv. Mater. 2006, 18, 1877–1882. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cadek, M.; Béguin, F. Tuning Carbon Materials for Supercapacitors by Direct Pyrolysis of Seaweeds. Adv. Funct. Mater. 2009, 19, 1032–1039. [Google Scholar] [CrossRef]
- Li, X.; Han, C.; Chen, X.; Shi, C. Preparation and performance of straw based activated carbon for supercapacitor in non-aqueous electrolytes. Microporous Mesoporous Mater. 2010, 131, 303–309. [Google Scholar] [CrossRef]
- Guo, N.; Li, M.; Wang, Y.; Sun, X.; Wang, F.; Yang, R. Soybean Root-Derived Hierarchical Porous Carbon as Electrode Material for High-Performance Supercapacitors in Ionic Liquids. ACS Appl. Mater. Interfaces 2016, 8, 33626–33634. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rafat, M.; Ahmed, A. Nitrogen doped activated carbon derived from orange peel for supercapacitor application. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 35008. [Google Scholar] [CrossRef]
- Gu, X.; Wang, Y.; Lai, C.; Qiu, J.; Li, S.; Hou, Y.; Martens, W.; Mahmood, N.; Zhang, S. Microporous bamboo biochar for lithium-sulfur batteries. Nano Res. 2015, 8, 129–139. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, L.; Qi, P.; Zhu, M.; Wang, G.; Ma, Y.; Guo, X.; Chen, H.; Zhang, B.; Zhao, Z.; et al. Nitrogen-Doped Banana Peel–Derived Porous Carbon Foam as Binder-Free Electrode for Supercapacitors. Nanomaterials 2016, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Taer, E.; Taslim, R.; Aini, Z.; Hartati, S.D.; Mustika, W.S. Activated carbon electrode from banana-peel waste for supercapacitor applications. AIP Conf. Proc. 2017, 1801, 40004. [Google Scholar]
- Yao, Q.; Wang, H.; Wang, C.; Jin, C.; Sun, Q. One Step Construction of Nitrogen–Carbon Derived from Bradyrhizobium japonicum for Supercapacitor Applications with a Soybean Leaf as a Separator. ACS Sustain. Chem. Eng. 2018, 6, 4695–4704. [Google Scholar] [CrossRef]
- Wang, R.; Wang, P.; Yan, X.; Lang, J.; Peng, C.; Xue, Q. Promising Porous Carbon Derived from Celtuce Leaves with Outstanding Supercapacitance and CO2 Capture Performance. ACS Appl. Mater. Interfaces 2012, 4, 5800–5806. [Google Scholar] [CrossRef]
- Jain, A.; Aravindan, V.; Jayaraman, S.; Kumar, P.S.; Balasubramanian, R.; Ramakrishna, S.; Madhavi, S.; Srinivasan, M.P. Activated carbons derived from coconut shells as high energy density cathode material for Li-ion capacitors. Sci. Rep. 2013, 3, 3002. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Leng, C.; Jiang, J.; Bu, Q.; Lin, G.; Lu, X.; Zhu, G. Microporous activated carbons from coconut shells produced by self-activation using the pyrolysis gases produced from them, that have an excellent electric double layer performance. New Carbon Mater. 2017, 32, 451–459. [Google Scholar] [CrossRef]
- Rufford, T.E.; Hulicova-Jurcakova, D.; Zhu, Z.; Lu, G.Q. Nanoporous carbon electrode from waste coffee beans for high performance supercapacitors. Electrochem. Commun. 2008, 10, 1594–1597. [Google Scholar] [CrossRef]
- Huang, C.; Sun, T.; Hulicova-Jurcakova, D. Wide Electrochemical Window of Supercapacitors from Coffee Bean-Derived Phosphorus-Rich Carbons. ChemSusChem 2013, 6, 2330–2339. [Google Scholar] [CrossRef]
- Rufford, T.E.; Hulicova-Jurcakova, D.; Fiset, E.; Zhu, Z.; Lu, G.Q. Double-layer capacitance of waste coffee ground activated carbons in an organic electrolyte. Electrochem. Commun. 2009, 11, 974–977. [Google Scholar] [CrossRef]
- Cheng, P.; Li, T.; Yu, H.; Zhi, L.; Liu, Z.; Lei, Z. Biomass-Derived Carbon Fiber Aerogel as a Binder-Free Electrode for High-Rate Supercapacitors. J. Phys. Chem. C 2016, 120, 2079–2086. [Google Scholar] [CrossRef]
- Biswal, M.; Banerjee, A.; Deo, M.; Ogale, S. From dead leaves to high energy density supercapacitors. Energy Environ. Sci. 2013, 6, 1249–1259. [Google Scholar] [CrossRef]
- Mondal, A.K.; Kretschmer, K.; Zhao, Y.; Liu, H.; Wang, C.; Sun, B.; Wang, G. Nitrogen-Doped Porous Carbon Nanosheets from Eco-Friendly Eucalyptus Leaves as High Performance Electrode Materials for Supercapacitors and Lithium Ion Batteries. Chem. A Eur. J. 2016, 23, 3683–3690. [Google Scholar] [CrossRef]
- Farma, R.; Deraman, M.; Awitdrus, A.; Talib, I.A.; Taer, E.; Basri, N.H.; Manjunatha, J.G.; Ishak, M.M.; Dollah, B.N.M.; Hashmi, S.A. Preparation of highly porous binderless activated carbon electrodes from fibres of oil palm empty fruit bunches for application in supercapacitors. Bioresour. Technol. 2013, 132, 254–261. [Google Scholar] [CrossRef]
- Selvamani, V.; Ravikumar, R.; Suryanarayanan, V.; Velayutham, D.; Gopukumar, S. Garlic peel derived high capacity hierarchical N-doped porous carbon anode for sodium/lithium ion cell. Electrochim. Acta 2016, 190, 337–345. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, K.; Li, S.; Li, M.; Li, J.; Ren, K. Synthesis of garlic skin-derived 3D hierarchical porous carbon for high-performance supercapacitors. Nanoscale 2018, 10, 2427–2437. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Sun, F.; Xu, Y.; Qiu, L.; Liu, C.; Wang, S.; Yan, F. Human hair-derived carbon flakes for electrochemical supercapacitors. Energy Environ. Sci. 2014, 7, 379–386. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Fu, N.; Liu, Y.; Wang, Y.; Zhou, L.; Huang, H. Flexible fiber hybrid supercapacitor with NiCo2O4 nanograss@carbon fiber and bio-waste derived high surface area porous carbon. Electrochim. Acta 2016, 211, 411–419. [Google Scholar] [CrossRef]
- Huang, C.; Puziy, A.M.; Sun, T.; Poddubnaya, O.I.; Suárez-García, F.; Tascón, J.M.D.; Hulicova-Jurcakova, D. Capacitive Behaviours of Phosphorus-Rich Carbons Derived from Lignocelluloses. Electrochim. Acta 2014, 137, 219–227. [Google Scholar] [CrossRef]
- Chang, J.; Gao, Z.; Wang, X.; Wu, D.; Xu, F.; Wang, X.; Guo, Y.; Jiang, K. Activated porous carbon prepared from paulownia flower for high performance supercapacitor electrodes. Electrochim. Acta 2015, 157, 290–298. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmed, A.; Rafat, M. Nitrogen doped activated carbon from pea skin for high performance supercapacitor. Mater. Res. Express 2018, 5, 45508. [Google Scholar] [CrossRef]
- He, X.; Ling, P.; Qiu, J.; Yu, M.; Zhang, X.; Yu, C.; Zheng, M. Efficient preparation of biomass-based mesoporous carbons for supercapacitors with both high energy density and high power density. J. Power Sources 2013, 240, 109–113. [Google Scholar] [CrossRef]
- Goldfarb, J.L.; Dou, G.; Salari, M.; Grinstaff, M.W. Biomass-Based Fuels and Activated Carbon Electrode Materials: An Integrated Approach to Green Energy Systems. ACS Sustain. Chem. Eng. 2017, 5, 3046–3054. [Google Scholar] [CrossRef]
- Xu, J.; Gao, Q.; Zhang, Y.; Tan, Y.; Tian, W.; Zhu, L.; Jiang, L. Preparing two-dimensional microporous carbon from Pistachio nutshell with high areal capacitance as supercapacitor materials. Sci. Rep. 2014, 4, 5545. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, C.-Y.; Chen, M.-M.; Wang, J.; Shi, Z.-Q. Potato starch-based activated carbon spheres as electrode material for electrochemical capacitor. J. Phys. Chem. Solids 2009, 70, 1256–1260. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, C.; Qian, J.; Chen, Z.; Chen, F. Novel 3D porous graphene decorated with Co3O4/CeO2 for high performance supercapacitor power cell. J. Rare Earths 2017, 35, 995–1001. [Google Scholar] [CrossRef]
- Hou, J.; Cao, C.; Ma, X.; Idrees, F.; Xu, B.; Hao, X.; Lin, W. From Rice Bran to High Energy Density Supercapacitors: A New Route to Control Porous Structure of 3D Carbon. Sci. Rep. 2014, 4, 7260. [Google Scholar] [CrossRef] [PubMed]
- Teo, E.Y.L.; Muniandy, L.; Ng, E.-P.; Adam, F.; Mohamed, A.R.; Jose, R.; Chong, K.F. High surface area activated carbon from rice husk as a high performance supercapacitor electrode. Electrochim. Acta 2016, 192, 110–119. [Google Scholar] [CrossRef]
- Hegde, G.; Abdul Manaf, S.A.; Kumar, A.; Ali, G.A.M.; Chong, K.F.; Ngaini, Z.; Sharma, K.V. Biowaste Sago Bark Based Catalyst Free Carbon Nanospheres: Waste to Wealth Approach. ACS Sustain. Chem. Eng. 2015, 3, 2247–2253. [Google Scholar] [CrossRef]
- Xu, G.; Han, J.; Ding, B.; Nie, P.; Pan, J.; Dou, H.; Li, H.; Zhang, X. Biomass-derived porous carbon materials with sulfur and nitrogen dual-doping for energy storage. Green Chem. 2015, 17, 1668–1674. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Fuertes, A.B.; Sevilla, M. From Soybean residue to advanced supercapacitors. Sci. Rep. 2015, 5, 16618. [Google Scholar] [CrossRef] [PubMed]
- Ramasahayam, S.K.; Clark, A.L.; Hicks, Z.; Viswanathan, T. Spent coffee grounds derived P, N co-doped C as electrocatalyst for supercapacitor applications. Electrochim. Acta 2015, 168, 414–422. [Google Scholar] [CrossRef]
- Li, X.; Xing, W.; Zhuo, S.; Zhou, J.; Li, F.; Qiao, S.; Lu, G. Preparation of capacitor’s electrode from sunflower seed shell. Bioresour. Technol. 2011, 102, 1118–1123. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, L.; Liu, Y.; Zhao, G.; Chen, J.Y.; Yu, G. Biobased Nano Porous Active Carbon Fibers for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 15205–15215. [Google Scholar] [CrossRef]

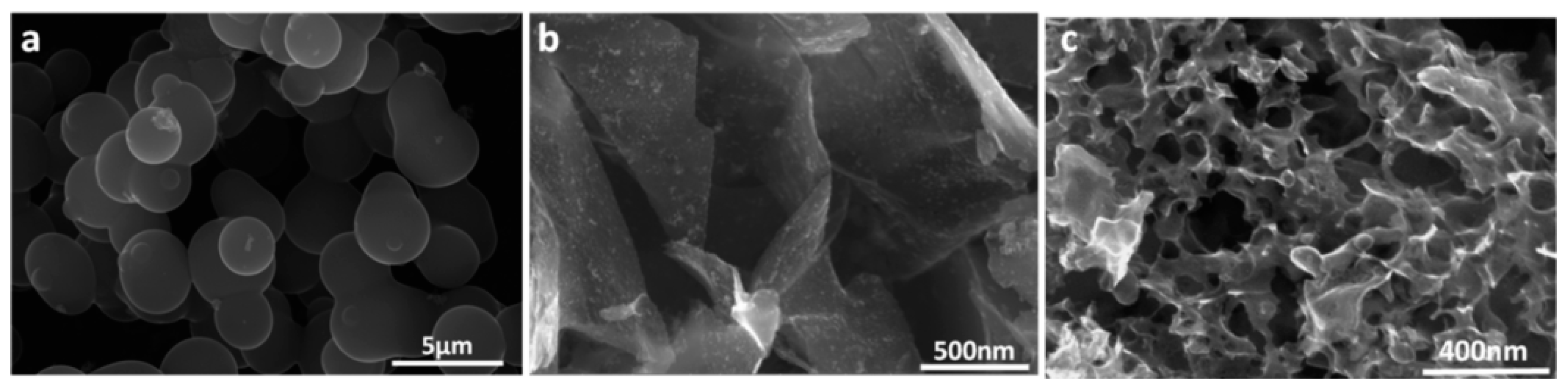
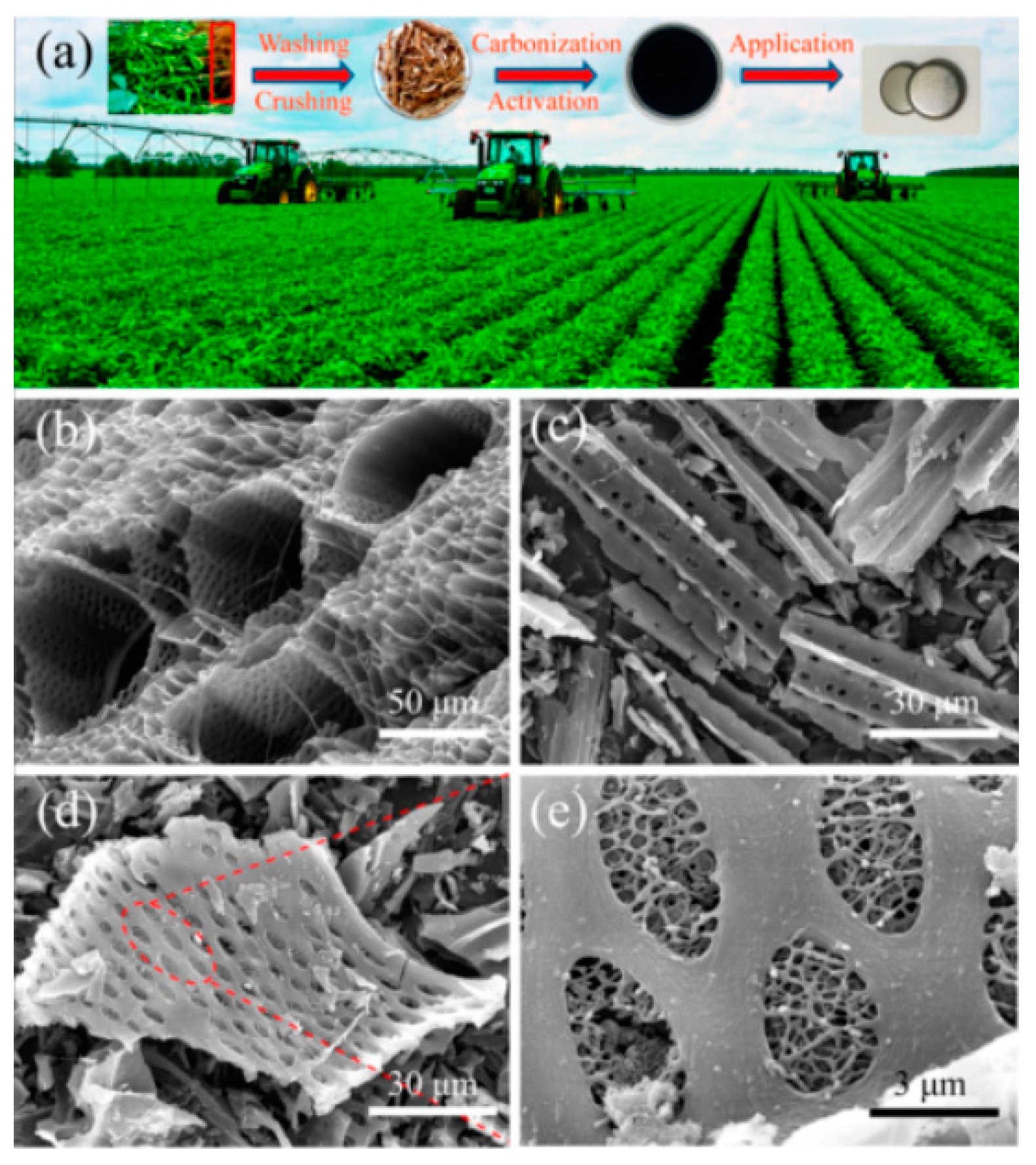
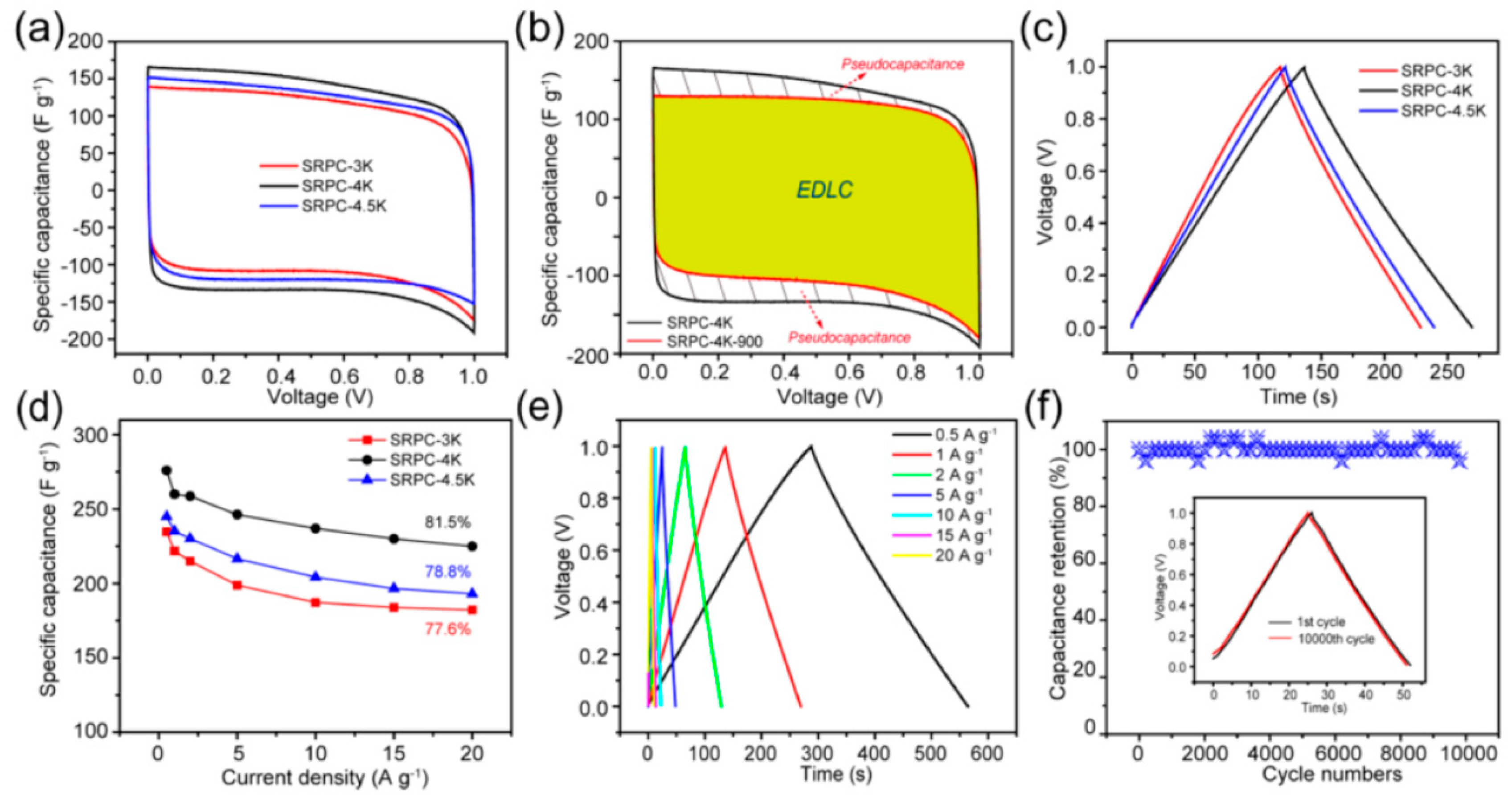
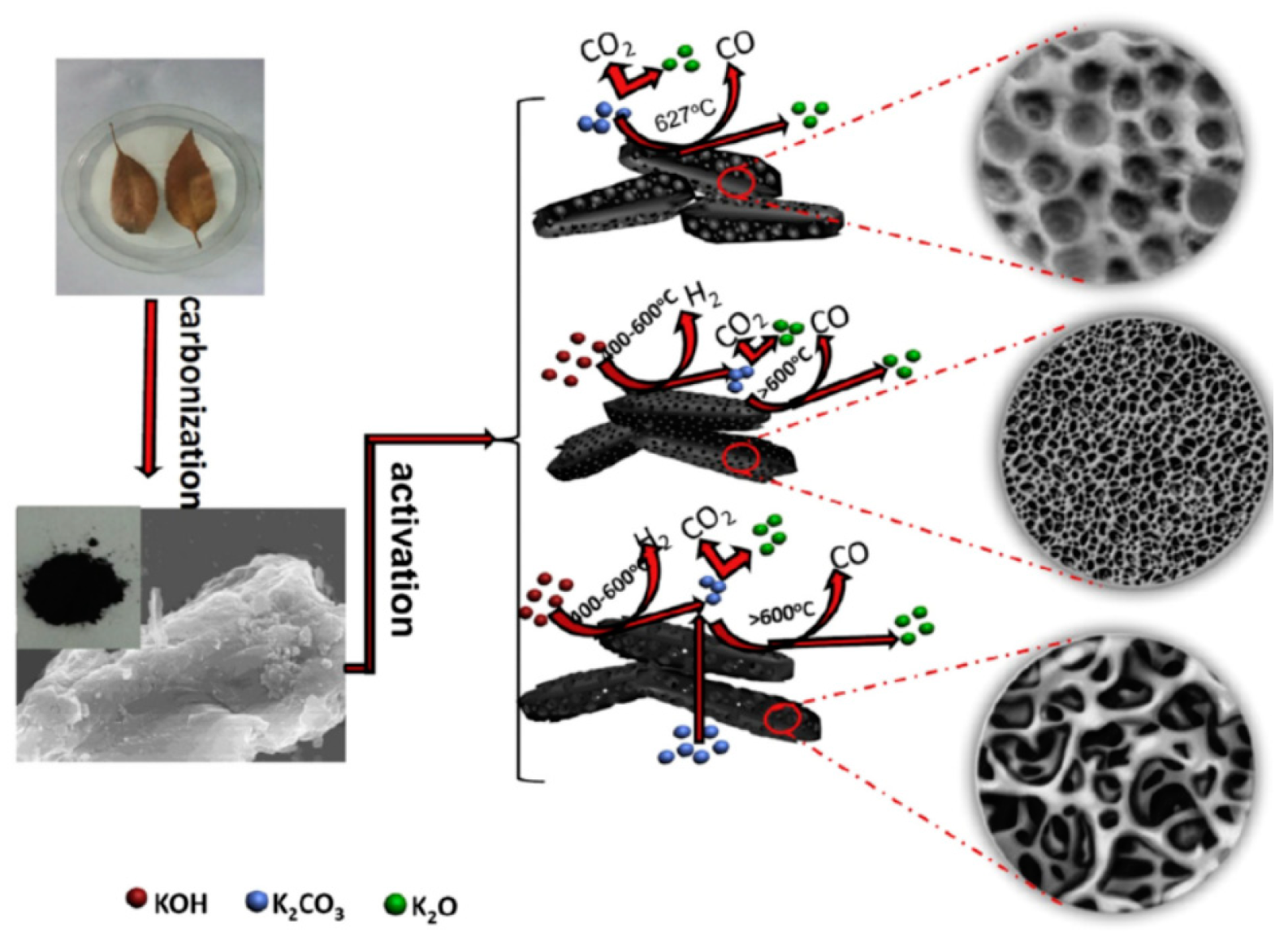
| Bio-Waste | Activation Method | SBET (m2/g) | Ref. |
|---|---|---|---|
| Almond shell | Physical, steam, 850 °C/30 min | 601 | [110] |
| Almond tree pruning | Physical, steam, 850 °C/30 min | 1080 | [110] |
| Apricot stone | Chemical, ZnCl2 | 814 | [111] |
| Bagasse | Chemical, ZnCl2 | 923 | [112] |
| Bamboo | Chemical, KOH | 1533 | [113] |
| Bamboo | Chemical, KOH | 1120 | [114] |
| Cocoa pod husk | Chemical, KOH | 490 | [115] |
| Cocoa pod husk | Chemical, K2CO3 | 615 | [115] |
| Cocoa pod husk | Chemical, ZnCl2 | 780 | [115] |
| Coconut shell | Physical, CO2, 600 °C/2 h | 1700 | [116] |
| Coconut shell | Physical, steam, 1000 °C/120 min | 1926 | [117] |
| Cotton stalk | Chemical, H3PO4 | 1720 | [118] |
| Coir pith | Chemical, ZnCl2 | 910 | [119] |
| Durian shell | Chemical, H3PO4 | 1024 | [120] |
| Hazelnut bagasse | Chemical, KOH | 1642 | [121] |
| Hazelnut bagasse | Chemical, ZnCl2 | 1489 | [121] |
| Hazelnut shell | Chemical, ZnCl2 | 647 | [111] |
| Jute | Chemical, KOH | 1769 | [122] |
| Olive stone | Physical, steam, 850 °C/30 min | 813 | [110] |
| Palm kernel shell | Chemical, KOH | 217 | [123] |
| Peanut hull | Physical, steam, 600 °C/2 h | 253 | [124] |
| Pistacio-nut shell | Physical, CO2, 900 °C/30 min | 778 | [125] |
| Rice husk | Chemical, ZnCl2 | 927 | [112] |
| Sugarcane bagasse | Physical, steam, 900 °C/2 h | 320 | [126] |
| Tea | Chemical, KOH | 2532 | [127] |
| Walnut shell | Physical, steam, 850 °C/30 min | 792 | [110] |
| Wood apple outer shell | Chemical, ZnCl2 | 794 | [128] |
| Biowaste | Process | Material Form | Electrolyte | Electrode Configuration | BET Surface Area (m2/g) | Measurement Protocol | Specific Capacitance (F/g) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Bamboo | carbonization and KOH activation | Activated biomass carbon | 3 M KOH | 3 electrodes | 2221 | 0.5 A/g | 293 | [134] |
| Bamboo | KOH activation | activated carbon | 6 M KOH | 3 electrodes | 3000 | 5 A/g | 300 | [135] |
| Corncob residue | steam activation without pre-carbonization | porous carbon | 6 M KOH | 3 electrodes | 1210 | 1 A/g | 314 | [133] |
| Coconut kernel pulp (Milk free) | KOH activation | activated carbon | 1 M Na2SO4 | 2 electrodes | 1200 | 10 mV/s | 173 | [136] |
| Corn stalk core | KOH activation | activated carbon | - | 3 electrodes | 2350 | 1 A/g | 140 | [137] |
| Corn syrup (High fructose) | Self-Physical | activated carbon | KOH | 2 electrodes | 1473 | 0.2 A/g | 168 | [138] |
| Endothelium corneum Gigeriae galli | carbonized | nitrogen-doped porous carbon | 6 M KOH | 3 electrodes | 2150 | 1 A/g | 198 | [139] |
| Fish gill | carbonization and thermal activation | activated carbon | 6 M KOH | 3 electrodes | 2082 | 2 A/g | 334 | [108] |
| Gelatin | hydrothermal | Porous carbon nanosheets | 6 M KOH | 3 electrodes | 1620 | 50 A/g | 183 | [140] |
| Leaves (Fallen) | activations of (KOH and K2CO3) | porous active carbon | 6 M KOH | 2 electrodes | 1078 | 0.3 A/g | 242 | [141] |
| Starch (Porous) | carbonisation and KOH activation | porous carbon microspheres | 6 M KOH | 2 electrodes | 3251 | 0.05 A/g | 304 | [142] |
| Sugar cane bagasse | chemical activation with ZnCl2 | Activated carbon | 1 M Na2SO4 | 2 electrodes | 1452 | 50 A/g | 300 | [143] |
| Sugar cane bagasse | calcium chloride (CaCl2) activation | Nitrogen-Rich Porous Carbons | 6 M KOH | 2 electrodes | 806 | 30 A/g | 213 | [144] |
| Waste tea-leaves | carbonisation and KOH activation | activated carbons | 2 M KOH | 3 electrodes | 2841 | 1 A/g | 330 | [145] |
| Biowaste | Carbon Content (%) | Ref. |
|---|---|---|
| Apricot shell | 23.2 | [148] |
| Bamboo | 16.60 | [149] |
| Coconut shell | 25–40 | [58,59,150,151,152,153] |
| Durian shell | 23.36 | [154] |
| Palm shell | 18.70 | [59] |
| Pitch | 33.6 | [155,156,157] |
| Seaweed | 16 | [158,159] |
| Sugarcane bagasse | 34.2 | [143] |
| Wheat straw | 37 | [160] |
| Biowaste | Energy Density (Wh/kg) | Power Density (W/kg) | Cycles | Percentage Retention (%) | Ref. |
|---|---|---|---|---|---|
| Bamboo | 3.3 | 2250 | 3000 | 91 | [135] |
| BambooBiochar | - | - | 150 | 95 | [163] |
| Banana-peel | 40.7 | 8400 | 1000 | 88.7 | [96] |
| Banana peel | - | - | 5000 | ~100 | [164] |
| Banana peel waste | 0.75 | 31 | - | - | [165] |
| Bradyrhizobium japonicum with a Soybean Leaf as a Separator | - | - | 8000 | 91 | [166] |
| Celtuce leaves | 2000 | 92.6 | [167] | ||
| Coconut shells | 38.5 | - | >3000 | 93 | [129] |
| Coconut shells | 69 | - | 2000 | 85 | [168] |
| Coconut shell | 3000 | 97.2 | [169] | ||
| Coffee beans | 10–20 | 6000 | >10,000 | - | [170] |
| Coffee Bean | 15 | 75 | 10,000 | 82 | [171] |
| Coffee ground | 34 | 215,000 | - | - | [172] |
| Corncob residue | 5.3–15 | 8276–2827 | 100,000 | 82 | [133] |
| Cotton (natural) | - | - | 20,000 | 97 | [173] |
| Dead Neem leaves (Azadirachta indica) | 55 | 569 | - | - | [174] |
| Eucalyptus tree leaves | - | - | 15,000 | 97.7 | [175] |
| Fibres from oil palm empty fruit bunches | 4.297 | 173 | - | - | [176] |
| Garlic peel | 100 | 95–98 | [177] | ||
| Garlic Skin | 14.65 | 310.67 | 5000 | 94 | [178] |
| Gelatin | 7.43 | 263.5 | 5000 | 92 | [140] |
| Human hair | 29 | 2243 | >20,000 | ~100 | [179] |
| Indian Cake Rusk | 47.1 | 22,644 | 6000 | 95 | [101] |
| Lemon peel | 6.61 | 425.26 | 3000 | 92 | [180] |
| Ligno-cellulosic waste, fruit stones | 13 | 3410 | 20,000 | 99 | [181] |
| Oil palm kernel shell | - | - | 1000 | 95–97 | [99] |
| Orange peel | 23.3 | 2334.3 | - | - | [162] |
| Paulownia flower (PF) | 44.5~22.2 | 247~3781 | 1000 | 93 | [182] |
| Pea skin | 19.6 | 254,000 | 5000 | 75 | [183] |
| Peanut shell and rice husk | 19.3 | 1007 | - | - | [184] |
| Pistachio nutshell | - | - | 4000 | ~100 | [185] |
| Pistachio nutshell | 10–39 | 52,000–286,000 | - | - | [186] |
| Potato starch | - | - | 900 | 86 | [187] |
| Rape flower stems | - | - | 1000 | 96 | [188] |
| Raw rice brans | 70 | 1223 | 10,000 | ~97 | [189] |
| Rice husk | - | - | 10,000 | 97–99 | [94] |
| Rice husk | 5.11 | - | 10,000 | 90 | [190] |
| Sago bark | 5 | 400 | 1700 | 94 | [191] |
| Shells of broad beans | - | - | 3000 | 90 | [192] |
| Soybean residue | 12 | 2000 | 5000–10,000 | 90 | [193] |
| Soybean Root | 100.5 | 63,000 | 10,000 | 98 | [161] |
| Spent coffee grounds | - | - | ~2000 | 98 | [194] |
| Sugarcane bagasse | 5 | 35,000 | 1000 | 90 | [132] |
| Sugar cane bagasse | 5.9 | 10,000 | 5000 | 83 | [143] |
| Sugar industry spent wash waste | - | 414,000 | 1000 | ~100 | [109] |
| Sunflower seed shell | 4.8 | 24,000 | - | - | [195] |
| Waste tea-leaves | - | - | 2000 | 92 | [145] |
| Wood sawdust | 5.7–7.8 | 250–5000 | 10,000 | 94.2 | [196] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensah-Darkwa, K.; Zequine, C.; Kahol, P.K.; Gupta, R.K. Supercapacitor Energy Storage Device Using Biowastes: A Sustainable Approach to Green Energy. Sustainability 2019, 11, 414. https://doi.org/10.3390/su11020414
Mensah-Darkwa K, Zequine C, Kahol PK, Gupta RK. Supercapacitor Energy Storage Device Using Biowastes: A Sustainable Approach to Green Energy. Sustainability. 2019; 11(2):414. https://doi.org/10.3390/su11020414
Chicago/Turabian StyleMensah-Darkwa, Kwadwo, Camila Zequine, Pawan K. Kahol, and Ram K. Gupta. 2019. "Supercapacitor Energy Storage Device Using Biowastes: A Sustainable Approach to Green Energy" Sustainability 11, no. 2: 414. https://doi.org/10.3390/su11020414
APA StyleMensah-Darkwa, K., Zequine, C., Kahol, P. K., & Gupta, R. K. (2019). Supercapacitor Energy Storage Device Using Biowastes: A Sustainable Approach to Green Energy. Sustainability, 11(2), 414. https://doi.org/10.3390/su11020414






