Benefits of Ryegrass on Multicontaminated Soils Part 1: Effects of Fertilizers on Bioavailability and Accumulation of Metals
Abstract
1. Introduction
2. Materials and Methods
2.1. Context of the Study and Soil Analysis
2.2. Soil Amendment and Design of the Incubation Experiment
2.3. Phytoavailability of Metals
2.4. Analytical Techniques and Quality Control
2.5. Statistical Analysis
3. Results and Discussion
3.1. Concentration of Transition, Alkali, and Alkaline Earth Metals and Aluminum in Soils
3.2. Biomass, Concentrations of Transition, Alkali, and Alkaline Earth Metals and Aluminum in the Ryegrass Shoots
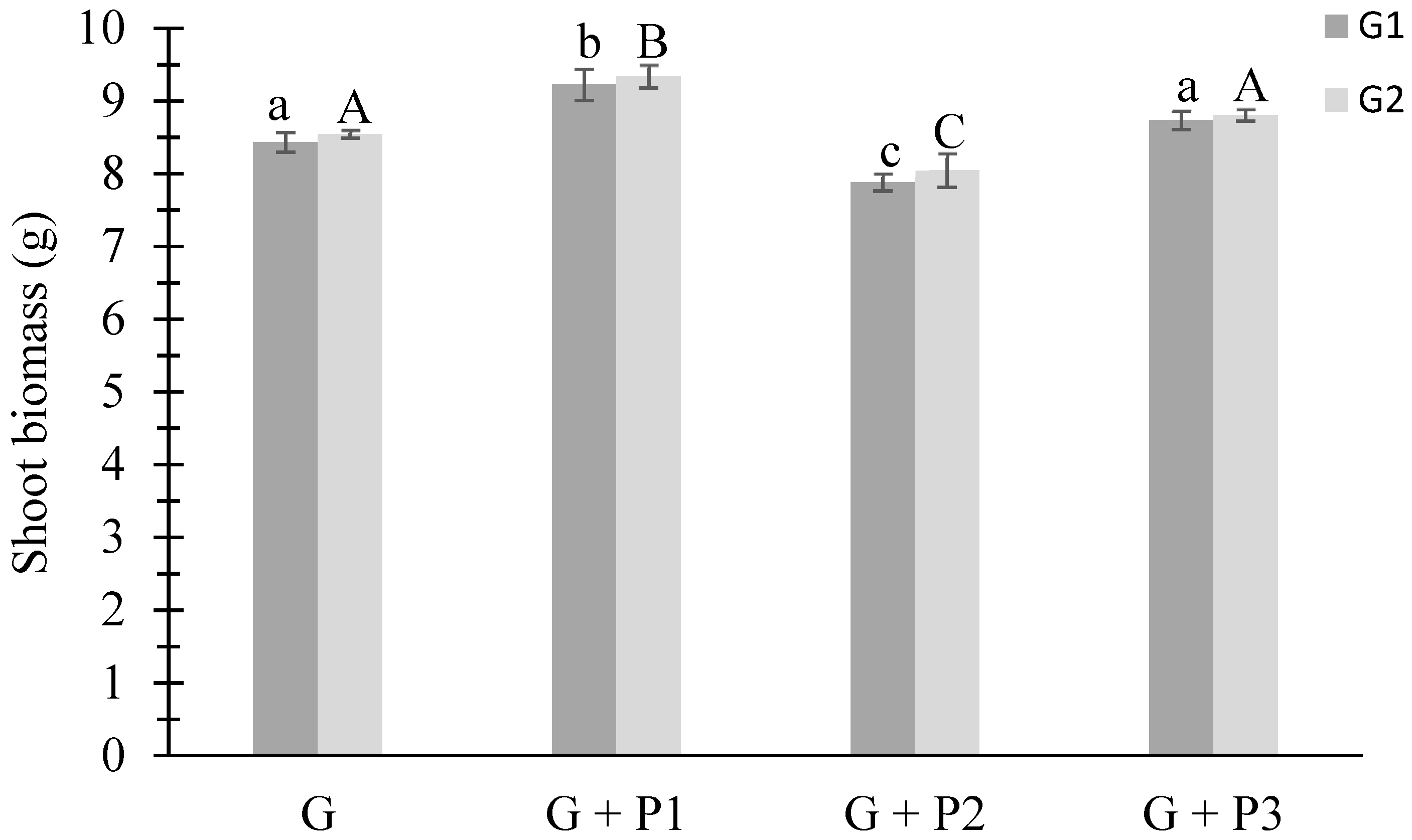
3.2.1. Shoot Biomass of Ryegrass
3.2.2. Concentrations of Transition, Alkali, and Alkaline Earth Metals and Aluminum in the Shoots of Ryegrass
3.3. Concentrations of Transition, Alkali, and Alkaline Earth Metals And Aluminum in the Roots of Ryegrass
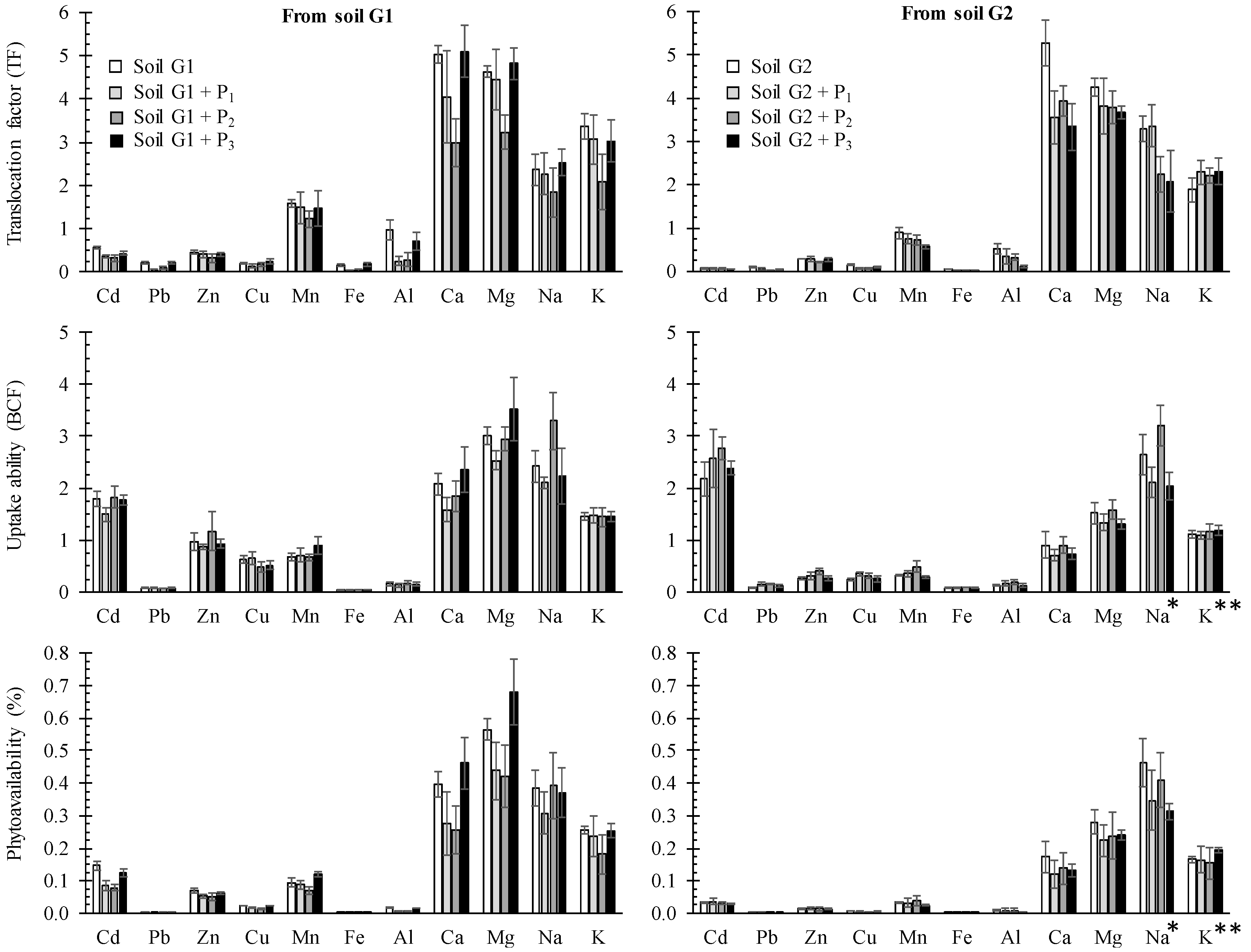
3.4. Evaluation of the Phytoavailability Using a Mixture of Organic Acids
3.5. Phytoavailability of PTEs, Alkali and Alkaline Earth Metals in Unamended and Amended Soils
3.6. Potential Application of the Aerial Parts of Ryegrass
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arienzo, M.; Adamo, P.; Cozzolino, V. The potential of Lolium perenne for revegetation of contaminated soil from a metallurgical site. Sci. Total Environ. 2004, 319, 13–25. [Google Scholar] [CrossRef]
- Peng, F.; Li, S.; Xiao-Hui, S.; Ru-Lai, L.; Cheng, J.; Hai-Yan, Z.; Zeng-Jie, L.; Zhi-Min, L.; Wei, G.; Xu-Dong, H.; et al. Response and accumulation ability of perennial ryegrass to plumbum and cadmium stress. Fresenius Environ. Bull. 2017, 26, 598–606. [Google Scholar]
- Salama, A.K.; Osman, K.A.; Gouda, N.A.R. Remediation of lead and cadmium-contaminated soils. Int. J. Phyt. 2016, 18, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, B.; Singhal, N.; Johnson, A. Amendments and their combined application for enhanced copper, cadmium, lead uptake by Lolium perenne. Plant Soil 2010, 329, 283–294. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Lin, L.J.; Yan, Q.L.; Yang, Y.X.; Zhu, X.M.; Shao, J.R. Effects of EDTA and DTPA on lead and zinc accumulation of ryegrass. J. Environ. Prot. 2011, 2, 932–939. [Google Scholar] [CrossRef][Green Version]
- Kamari, A.; Pulford, I.D.; Hargreaves, J.S.J. Metal accumulation in Lolium perenne and Brassica napus as affected by application of chitosans. Int. J. Phyt. 2012, 14, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, W.; Zhao, Y.; Yang, S.; Wang, F.; Zhang, J.; Yongchang, S. Enhancement of lead phytoremediation by perennial ryegrass (Lolium perenne L.) using agent of Streptomyces pactum Act 12. J. Pet. Environ. Biotechnol. 2016, 7, 269. [Google Scholar] [CrossRef]
- Liang, S.X.; Jin, Y.; Liu, W.; Li, X.; Shen, S.G.; Ding, L. Feasibility of Pb phytoextraction using nano-materials assisted ryegrass: Results of a one-tear field-scale experiment. J. Environ. Manag. 2017, 190, 170–175. [Google Scholar] [CrossRef]
- Black, A.; McLaren, R.G.; Reichman, S.M.; Speir, T.W.; Condron, L.M.; Houliston, G. Metal bioavailability dynamics during a two-year trial using ryegrass (Lolium perenne L.) grown in soils treated with biosolids and metal salts. Soil Res. 2012, 50, 304–311. [Google Scholar] [CrossRef]
- Afegbua, S.L.; Batty, L.C. Effect of single and mixed polycyclic aromatic hydrocarbon contamination on plant biomass yield and PAH dissipation during phytoremediation. Environ. Sci. Pollut. Res. 2018, 25, 18596–18603. [Google Scholar] [CrossRef]
- Radziemska, M.; Bilgin, A.; Vaverková, M.D. Application of mineral-based amendments for enhancing phytostabilization in Lolium perenne L. cultivation. Clean Soil Air Water 2018, 46, 1–10. [Google Scholar] [CrossRef]
- Burges, A.; Alkorta, I.; Epelde, L.; Garbisu, C. From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int. J. Phyt. 2018, 20, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhu, L.; Wang, J. Combined treatment of contaminated soil with a bacterial Stenotrophomonas strain DXZ9 and ryegrass (Lolium perenne) enhances DDT and DDE remediation. Environ. Sci. Pollut. Res. 2018, 25, 31895–31905. [Google Scholar] [CrossRef] [PubMed]
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P.K.S.M. Integrated remediation processes toward heavy metal removal/recovery from various environments—A review. Front. Environ. Sci. 2019, 7, 1–15. [Google Scholar] [CrossRef]
- Han, S.; Li, X.; Amombo, E.; Fu, J.; Xie, Y. Cadmium tolerance of perennial ryegrass induced by Aspergillus aculeatus. Front Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Wang, K.; Huang, H.; Zhu, Z.; Li, T.; He, Z.; Yang, X.; Alva, A. Phytoextraction of metals and rhizoremediation of PAHs in co-contaminated (Lolium perenne) or cator (Rininus Communis). Int. J. Phyt. 2013, 15, 283–298. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.Y.; Lu, Y.C.; Jiang, S.S.; Wu, H.J.; Yang, H. Comprehensive analysis of degradation and accumulation of ametryn in soils and in wheat, maize, ryegrass and alfalfa plants. Ecotoxicol. Environ. Saf. 2017, 140, 264–270. [Google Scholar] [CrossRef]
- Pruvot, C.; Douay, F.; Fourrier, H.; Waterlot, C. Heavy metals in soil, crops and grass as a source of human exposure in the former mining areas. J. Soils Sediments 2006, 6, 215–220. [Google Scholar] [CrossRef]
- Douay, F.; Roussel, H.; Pruvot, C.; Waterlot, C. Impact of a smelter closedown on metal contents of wheat cultivated in the neighbourhood. Environ. Sci. Pollut. Res. 2008, 15, 162–169. [Google Scholar] [CrossRef]
- Augustsson, A.; Uddh-Söderberg, T.; Filipsson, M.; Helmfrid, I.; Berglund, M.; Karlsson, H.; Hogmalm, J.; Karlsson, A.; Alriksson, S. Challenges in assessing the health risks of consuming vegetables in metal-contaminated environments. Environ. Int. 2018, 113, 269–280. [Google Scholar] [CrossRef]
- Osborne, L.R.; Baker, L.L.; Strawn, D.G. Lead immobilization and phosphorous availability in phosphate-amended, mine-contaminated soils. J. Environ. Qual. 2015, 8, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; McBride, M.B.; Li, K.; Li, Z. Bioaccessibility of as and Pb in orchard and urban soils amended with phosphate, Fe oxide and organic matter. Chemosphere 2017, 173, 153–159. [Google Scholar] [CrossRef]
- Li, S.W.; Liu, X.; Sun, H.J.; Li, M.Y.; Zhao, D.; Luo, J.; Li, H.B.; Ma, L.Q. Effect of phosphate amendment on relative bioavailability and bioaccessibility of lead and arsenic in contaminated soils. J. Hazard. Mater. 2017, 339, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Hans, N.; Kumar, S.; Rani, R. A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol. Environ. Saf. 2018, 147, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Bolan, N.; Megharaj, M.; Naidu, R. Comparative value of phosphate sources on the immobilization of lead, and leaching of lead and phosphorous in lead contaminated soils. Sci. Total Environ. 2011, 409, 853–860. [Google Scholar] [CrossRef]
- Tang, X.; Yang, J. Long-term stability and risk assessment of lead in mill waste treated by soluble phosphate. Sci. Total Environ. 2012, 438, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Tekaya, M.; El-Gharbi, S.; Chehab, H.; Attia, F.; Hammami, M.; Mechri, B. Long-term field evaluation of the changes in fruit and olive oil chemical compositions after agronomic application of olive mill wastewater with rock phosphate. Food Chem. 2018, 239, 664–670. [Google Scholar] [CrossRef]
- Hettiarachchi, G.M.; Pierzynski, G.M. Soil lead bioavailability and in situ remediation of lead-contaminated soils: A review. Environ. Prog. 2004, 23, 78–93. [Google Scholar] [CrossRef]
- Park, J.H.; Bolan, N.; Megharaj, M.; Naidu, R. Isolation of phosphate solubilizing bacteria and their potential for lead immobilization in soil. J. Hazard. Mater. 2011, 185, 829–836. [Google Scholar] [CrossRef]
- Bolan, N.S.; Domy, C.A.; Ravi, N. Role of phosphorous in (im)mobilization and bioavailability of heavy metals in the soil plant system. A review. Rev. Environ. Contam. Toxicol. 2003, 177, 1–44. [Google Scholar]
- Sterckeman, T.; Douay, F.; Proix, N.; Fourrier, H.; Perdrix, E. Assessment of the contamination of cultivated soils by eighteen trace elements around smelters in the North of France. Water Air Soil Pollut. 2002, 135, 173–194. [Google Scholar] [CrossRef]
- Pelfrêne, A.; Waterlot, C.; Douay, F. Influence of land use on human bioaccessibility of metals in smelter-impacted soils. Environ. Pollut. 2013, 178, 80–88. [Google Scholar] [CrossRef] [PubMed]
- AFNOR. Soil Quality—Determination of Carbonate Content—Volumetric Method; NF ISO 10693; Association Française de Normalisation: Paris, France, 1995. [Google Scholar]
- AFNOR. Soil Quality—Determination of the Cation Exchange Capacity (CEC) and Extractable Cations; NF X31-130; Association Française de Normalisation: Paris, France, 1999. [Google Scholar]
- AFNOR. Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis); NF ISO 10694; Association Française de Normalisation: Paris, France, 1995. [Google Scholar]
- AFNOR. Soil Quality—Determination of Soluble Phosphorus in Ammonium Oxalate 0.1 M—Joret-Hébert Method; NF X 31-161; Association Française de Normalisation: Paris, France, 1993. [Google Scholar]
- AFNOR. General Requirements for the Competence of Testing and Calibration Laboratories; NF ISO 17025; Association Française de Normalisation: Paris, France, 2005. [Google Scholar]
- USEPA. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils, 2nd ed.; USEPA: Washington, DC, USA, 1996. [Google Scholar]
- Feng, M.H.; Shan, X.Q.; Zhang, S.Z.; Wen, B. Comparison of a rhizosphere-based method with other one-step extraction methods for assessing the bioavailability of soil metals to wheat. Chemosphere 2005, 50, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Y.; Traina, S.J.; Logan, T.L.; Ryan, J.A. In situ lead immobilization by apatite. Environ. Sci. Technol. 1993, 27, 1803–1810. [Google Scholar] [CrossRef]
- Buscaroli, A. An overview of indexes to evaluate terrestrial plants for phytoremediation purposes (Review). Ecol. Indic. 2017, 82, 367–380. [Google Scholar] [CrossRef]
- Cao, X.; Wahbi, A.; Ma, L.; Li, B.; Yang, Y. Immobilization of Zn, Cu, Cu and Pb in contaminated soils using phosphate rock and phosphoric acid. J. Hazard. Mater. 2009, 164, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Waterlot, C.; Douay, F. The problem of arsenic interference in the analysis of Cd to evaluate its extractability in soils contaminated by arsenic. Talanta 2009, 80, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Savio, M.; Cerutti, S.; Martinez, L.D.; Smichowski, P.; Gil, R.A. Study of matrix and spectral interferences in the determination of lead in sediments, sludges and soils by SR-ETAAS using slurry sampling. Talanta 2010, 82, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Waterlot, C.; Bidar, G.; Pruvot, C.; Douay, F. Analysis of cadmium in water extracts from contaminated soils with high arsenic and iron concentration levels. J. Environ. Sci. Eng. 2011, 5, 271–280. [Google Scholar]
- Douay, F.; Pruvot, C.; Roussel, H.; Ciesielski, H.; Fourrier, H.; Proix, N.; Waterlot, C. Contamination of urban soils in an area of Northern France polluted by dust emissions of two smelters. Water Air Soil Pollut. 2008, 188, 247–260. [Google Scholar] [CrossRef]
- Pelfrêne, A.; Waterlot, C.; Mazzuca, M.; Nisse, C.; Cuny, D.; Richard, A.; Denys, S.; Heyman, C.; Roussel, H.; Bidar, G.; et al. Bioaccessibility of trace elements as affected by soil parameters in smelter-contaminated agricultural soils: A statistical modelling approach. Environ. Pollut. 2012, 160, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Pelfrêne, A.; Détriché, S.; Douay, F. Combining spatial distribution with oral bioaccessibility of metals in smelter-impacted soils: Implications for human health risk assessment. Environ. Geochem. Health 2015, 37, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Waterlot, C.; Pruvot, C.; Ciesielski, H.; Douay, F. Effects of a P amendment and the pH of water used for watering on the mobility and phytoavailability of Cd, Pb and Zn in highly contaminated kitchen garden soils. Ecol. Eng. 2011, 37, 1081–1093. [Google Scholar] [CrossRef]
- Chen, S.Y.; Ou, S.F.; Teng, N.C.; Kung, C.M.; Tsai, H.L.; Chu, K.T.; Ou, K.L. Phase transformation on bone cement: Monocalcium phosphate monohydrate into calcium-deficient hydroxyapatite during setting. Ceram. Int. 2013, 39, 2451–2455. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, X.; Xu, Y.; Qin, X.; Huang, Q.; Wang, L.; Sun, Y. Remediation of heavy metal-polluted agricultural soils using clay minerals: A review. Pedosphere 2017, 27, 193–204. [Google Scholar] [CrossRef]
- Ou, J.; Li, H.; Yan, Z.; Zhou, Y.; Bai, L.; Zhang, C.; Wang, W.; Chen, G. In situ immobilisation of toxic metals in soil using Maifan stone and illite/smectite clay. Sci. Rep. 2018, 8, 4618–4627. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Bolan, N.S.; Chung, J.W.; Naidu, R.; Megharaj, M. Environmental monitoring of the role of phosphate compounds in enhancing immobilization and reducing bioavailability of lead in contaminated soils. J. Environ. Monit. 2011, 13, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, L.; Liu, Y.; Qu, Y. Adsorption mechanisms of metal ions on the potassium dihydrogen phosphate (100) surface: A density functional theory-based investigation. J. Colloid Int. Sci. 2018, 522, 256–263. [Google Scholar] [CrossRef]
- Naim Mulana, K.; Fauzjah Ishak, C.; Abu Bakar, R. In-situ immobilization of lead using different source of phosphate amendments for the organic production of misai kucing (Orthosiphon stamineus). Soil Environ. 2017, 36, 20–27. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorous Dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Hodgson, J.F. Chemistry of the micronutrient elements in soils. Adv. Agron. 1963, 15, 119–159. [Google Scholar]
- Haby, V.A.; Russelle, M.P.; Skogley, E.O. Soil Testing for Potassium, Calcium and Magnesium. In Soil Testing and Plant Analysis; Westerman, R.L., Ed.; Soil Science Society of America Inc.: Madison, WI, USA, 1990; pp. 181–227. [Google Scholar]
- Tripathy, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M.; Dubey, N.K. Role of macronutrients in plant growth and acclimation: Recent advances and future prospective. In Improvement of Crops in the Era of Climatic Changes; Ahmad, P., Wani, M.R., AzoozMM, L.-S.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 197–216. [Google Scholar]
- Leigh, R.A.; Wyn Jones, R.G. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Vose, P.B. The cation content of perennial ryegrass Lolium Perenne L. in relation to intraspecific variability and nitrogen/potassium interaction. Plant Soil 1963, 19, 49–64. [Google Scholar] [CrossRef]
- Kidambi, S.P.; Matches, A.G.; Griggs, T.C. Variability for Ca, Mg, K, Cu, Zn and K/(Ca+ Mg) ratio among 3 wheatgrasses and sainfoin on the southern high plains. Rangel. Ecol. Manag. 1989, 42, 316–322. [Google Scholar] [CrossRef]
- Barnum, D.W. Hydrolysis of cations. Formation constants and standard free energies of formation of hydroxy complexes. Inorg. Chem. 1983, 22, 2297–2305. [Google Scholar] [CrossRef]
- Tyler, G. Ionic charge, radius, and potential control root/soil concentration ratios of fifty cationic elements in the organic horizon of a beech (Fagus Sylvatica) forest podzol. Sci. Total Environ. 2004, 329, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Waterlot, C.; Bidar, G.; Pelfrêne, A.; Roussel, H.; Fourrier, H.; Douay, F. Contamination, fractionation and availability of metals in urban soils in the vicinity of former lead and zinc smelters, France. Pedosphere 2013, 23, 143–159. [Google Scholar] [CrossRef]
- Nardi, S.; Concheri, G.; Pizzeghello, D.; Sturaro, A.; Rella, R.; Parvoli, G. Soil organic matter mobilization by root exudates. Chemosphere 2000, 41, 653–658. [Google Scholar] [CrossRef]
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of root-mediated pH changes in the rhizosphere and their response to environmental constraints: A review. Plant Soil 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Marschner, H.; Romheld, V.; Horst, W.J.; Martin, P. Root-induced changes in the rhizosphere: Importance for the mineral nutrition of plants. J. Plant Nutr. Soil Sci. 1986, 149, 441–456. [Google Scholar] [CrossRef]
- Chang, A.C.; Page, A.L.; Koo, B.J. Biogeochemistry of phosphorous, iron, and trace elements as influenced by soil–plant–microbial interactions. In Soil Mineral–Organic Matter–Microorganism Interactions and Ecosystem Health: Ecological Significance of the Interactions Among Clay Minerals, Organic Matter and Soil Biota; Violante, A., Huang, P.M., Bollag, J.M., Gianfreda, L., Eds.; Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 2002; pp. 43–57. [Google Scholar]
- Violante, A.; Huang, P.M.; Bollag, J.M.; Gianfreda, L. Soil Mineral–Organic Matter–Microorganism Interactions and Ecosystem Health: Dynamics, Mobility and Transformations of Pollutants and Nutrients; Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Fan, C.; Zhang, Y. Environmentally friendly remediation of lead/cadmium co-contaminated loess soil in northwestern China using a humificated straw solution. Environ. Sci. Pollut. Res. 2018, 25, 25243–25254. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Ali, S.; Zubair, M.; Saeed, R.; Rizwan, M.; Sallah-Ud-Din, R.; Azam, A.; Ashraf, R.; Ashraf, W. Glutamic acid assisted phyto-management of silver contaminated soils through sunflower; physiological and biochemical response. Environ. Sci. Pollut. Res. 2018, 25, 25300–25400. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L. Organic acids in the rhizosphere—A critical review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Kutrowska, A.; Szelag, M. Low-molecular weight organic acids and peptides involved in the long-distance transport of trace metals. Acta Physiol. Plant. 2014, 36, 1957–1968. [Google Scholar] [CrossRef]
- Li, Y.; Hou, Y.; Song, X.; Sun, L.; Li, S.; Guo, Q.; Hu, X. Effect of pH on the desorption of trace metals by different LMWOAs from a field soil. Adv. Mater. Res. 2015, 1073, 193–196. [Google Scholar] [CrossRef]
- Shan, X.-Q.; Wang, Z.; Wang, W.; Zhang, S.; Ben, W. Labile rhizosphere soil solution fraction for prediction of bioavailability of heavy metals and rare earth elements to plants. Anal. Bioanal. Chem. 2003, 375, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Abedin, J.; Beckett, P.; Spiers, G. An evaluation of extractants for assessment of metal phytoavailability to guide reclamation practices in acidic soilscapes in northern regions. Can. J. Soil Sci. 2012, 92, 253–268. [Google Scholar] [CrossRef]
- Vásquez, S.; Moreno, E.; Carpena, R.O. Bioavailability of metals and as from acidified multicontaminated soils: Use of white lupin to validate several extraction methods. Environ. Geochem. Health 2008, 30, 193–198. [Google Scholar] [CrossRef]
- Pérez-Esteban, J.; Escolástico, C.; Moliner, A.; Masaguer, A.; Ruiz-Fernández, J. Phytostabilization of metals in mine soils using Brassica juncea in combination with organic amendments. Plant Soil 2014, 377, 97–109. [Google Scholar] [CrossRef]
- Cieslinski, G.; Van Rees, K.C.J.; Szmigielska, A.M.; Krishnamurti, G.S.R.; Huang, P.M. Low-molecular-weight organic acids in rhizosphere soils of durum wheat and their effect on cadmium bioaccumulation. Plant Soil 1998, 203, 109–117. [Google Scholar] [CrossRef]
- Soriano-Disla, J.M.; Gómez, I.; Navarro-Pedreno, J. Use of a rhizosphere-based method for the assessment of heavy-metal bioavailability in soils amended with polluted sewage sludge. Commun. Soil Sci. Plant Anal. 2013, 44, 1599–1609. [Google Scholar] [CrossRef]
- Hechelski, M.; Ghinet, A.; Louvel, B.; Dufrénoy, P.; Rigo, B.; Daïch, A.; Waterlot, C. From Conventional Lewis acids to heterogeneous montmorillonite K10, eco-friendly plant-based catalysts used as green Lewis acids. ChemSusChem 2018, 11, 1249–1277. [Google Scholar] [CrossRef] [PubMed]
- Deyris, P.A.; Bert, V.; Diliberto, S.; Boulanger, C.; Petit, E.; Legrand, Y.M.; Grison, C. Biosourced polymetallic catalysis: A surprising and efficient means to promote the Knoevenagel condensation. Front. Chem. 2018, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Deyris, P.A.; Grison, C. Nature, ecology and chemistry: An unusual combination for a new green catalysis, ecocatalysis. Curr. Opin. Green Sustain. Chem. 2018, 10, 6–10. [Google Scholar] [CrossRef]
- Hazotte, C.; Laubie, B.; Rees, F.; Morel, J.L.; Simonnot, M.O. A novel process to recover cadmium and zinc from the hyperaccumulator plant Noccaea caerulescens. Hydrometallurgy 2017, 174, 56–65. [Google Scholar] [CrossRef]
- Vaughan, J.; Riggio, J.; Chen, J.; Peng, H.; Harris, H.H.; Van der Ent, A. Characterisation and hydrometallurgical processing of nickel from tropical agromined bio-ore. Hydrometallurgy 2017, 169, 346–355. [Google Scholar] [CrossRef]
- Guilpain, M.; Laubie, B.; Zhang, X.; Morel, J.L.; Simonnot, M.O. Speciation of nickel extracted from hyperaccumulator plants by water leaching. Hydrometallurgy 2018, 180, 192–200. [Google Scholar] [CrossRef]
- Jakovljević, M.D.; Kostić, N.M.; Antić-Mladenović, S.B. The availability of base elements (Ca, Mg, Na, K) in some important soil types in Serbia, Proceedings for Natural Sciences. Zbornik Matice Srpske Za Prirodne Nauke 2003, 104, 11–21. [Google Scholar] [CrossRef]
- Jungk, A.; Claassen, N. Availability of phosphate and potassium as the result of interactions between root and soil in the rhizosphere. J. Plant Nutr. Soil. Sci. 1986, 149, 411–427. [Google Scholar] [CrossRef]
- Mitsios, I.K.; Rowell, D.L. Plant uptake of exchangeable and non-exchangeable potassium. I. Measurement and modelling for onion roots in a Chalky Boulder clay soil. J. Soil. Sci. 1987, 38, 53–63. [Google Scholar] [CrossRef]
- Treeby, M.; Marschner, H.; Romheld, V. Mobilization of iron and other micronutrient cations from a calcareous soil by plant-borne, microbial, and synthetic metal chelators. Plant Soil 1989, 114, 217–226. [Google Scholar] [CrossRef]
- Naidu, R.; Harter, R.D. Effect of different organic ligands on cadmium sorption by and extractability from soils. Soil Sci. Soc. Am. J. 1998, 62, 644–650. [Google Scholar] [CrossRef]
- Tester, M. Tansley Review No. 21—Plant ion channels: Whole-cell and single channel studies. New Phytol. 1990, 114, 305–340. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, Q.L. Accumulation of Pb, Cu and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Tang, S.; Wang, R.; Ju, X.; Ding, Y.; Tu, S.; Smith, D.L. Effects of elevated on growth, photosynthesis, elemental composition, antioxidant level, and phytochelatin concentration in Lolium multiforum and Lolium perenne under Cd stress. J. Hazard. Mater. 2010, 180, 384–394. [Google Scholar] [CrossRef]
- Sarret, G.; Balesdent, J.; Bouziri, L.; Garnier, J.M.; Marcus, M.A.; Geoffroy, N.; Panfili, F.; Manceau, A. Zn speciation in the organic horizon of a contaminated soil by Micro-X-ray Fluorescence, micro- and power-EXAFS Spectroscopy, and isotopic dilution. Environ. Sci. Technol. 2004, 38, 2792–2801. [Google Scholar] [CrossRef]
- Couder, E.; Mattielli, N.; Drouet, T.; Smolders, E.; Delvaux, B.; Iserentant, A.; Meeus, C.; Maerschalk, C.; Opfergelt, S.; Houben, D. Transpiration flow controls Zn transport in Brassica napus and Lolium multiflorum under toxic levels as evidenced from isotopic fractionation. C. R. Geosci. 2015, 347, 386–396. [Google Scholar] [CrossRef]
- Kříbek, B.; Šípková, A.; Ettler, V.; Mihaljevič, M.; Majer, V.; Knési, I.; Mapanu, B.; Penížek, V.; Vaněk, A.; Sracek, O. Variability of the copper isotopic composition in soil and grass affected by mining and smelting in Tsumeb, Namibia. Chem. Geol. 2018, 493, 121–135. [Google Scholar] [CrossRef]
- Escande, V.; Olszewski, T.K.; Grison, C. Preparation of ecological catalysts derived from Zn hyperaccumulating plants and their catalytic activity in Diels-Alder reaction. C. R. Chim. 2014, 17, 731–737. [Google Scholar] [CrossRef]
- Oustrière, N.; Marchand, L.; Roulet, E.; Mench, M. Rhizofiltration of a Bordeaux mixture effluent in pilot-scale constructed wetland using Arundo donax L. coupled with potential Cu-ecocatalyst production. Ecol. Eng. 2017, 105, 296–305. [Google Scholar] [CrossRef]
| Soil | Clay a | Coarse Silt a | Fine Silt a | Coarse Sand a | Fine Sand a | pH | CaCO3 a | CEC | OM | P2O5 b |
|---|---|---|---|---|---|---|---|---|---|---|
| G1 | 189 | 315 | 190 | 110 | 196 | 7.39 ± 0.03 | 7.2 ± 0.1 | 23.8 ± 1.2 | 71.2 ± 1.3 | 290 ± 2 |
| G2 | 288 | 324 | 196 | 45 | 147 | 7.21 ± 0.02 | 16.2 ± 0.2 | 12.5 ± 1.2 | 95.8 ± 2.0 | 207 ± 2 |
| Element | Cd | Pb | Zn | Al | Ca | Cu | Fe | K | Mg | Mn | Na |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD | 2.1 | 41.0 | 1.5 | 156.0 | 32.2 | 49.0 | 41.9 | 5.0 | 12.0 | 4.1 | 5.0 |
| LOQ | 7.0 | 136.7 | 5.0 | 520.0 | 107.3 | 163.3 | 139.7 | 16.7 | 40.0 | 13.7 | 16.7 |
| Metal | CRM BCR 141 R | ERM® CC141 | PCRM CTA-VTL-2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Certified Value | Obtained Value (n = 3) | Precision (%) | Trueness (%) | Certified Value | Obtained Value (n = 3) | Precision (%) | Trueness (%) | Certified Value | Obtained Value (n = 3) | Precision (%) | Trueness (%) | |
| Al | 32,200 ± 600 a | 32,412 ± 135 | 0.41 | −0.66 | - | - | - | - | 1682 b | 1614 ± 50 | 3.01 | 4.04 |
| Cd | 13.96 ± 0.46 | 13.31 ± 0.09 | 0.67 | −4.66 | 0.25 ± 0.04 | 0.22 ± 0.04 | 18.20 | −13.60 | 1.52 ± 0.17 | 1.50 ± 0.13 | 8.70 | −1.31 |
| Cu | 46.9 ± 1.8 | 44.6 ± 1.6 | 3.59 | −4.90 | 12.4 ± 0.9 | 12.7 ± 0.3 | 2.36 | 0.24 | 18.2 ± 0.9 | 19.8 ± 0.2 | 1.01 | 8.79 |
| Fe | 25,850 ± 400 | 24,955 ± 1605 | 6.43 | −3.46 | - | 11901 ± 78 | 0.66 | - | 1083 ± 33 | 1088 ± 35 | 3.21 | 0.46 |
| Mn | 653 ± 20 | 664 ± 7 | 1.05 | 1.68 | 387 ± 17 | 400 ± 4 | 1.00 | 3.36 | 79.7 ± 2.6 | 83.4 ± 3.5 | 4.20 | 4.60 |
| Pb | 51.3 ± 2.0 | 50.0 ± 1.2 | 2.40 | −2.53 | 32.2 ± 1.4 | 30.6 ± 1.7 | 5.55 | −4.97 | 22.1 ± 1.2 | 21.8 ± 1.2 | 5.50 | −1.35 |
| Zn | 270 ± 8 | 277 ± 1.6 | 0.57 | 2.59 | 50 ± 4 | 47 ± 1 | 2.12 | −6.00 | 43.0 ± 2.1 | 42.9 ± 2.2 | 5.13 | −0.23 |
| Metal | Soil G1 | Soil G1 + P1 | Soil G1 + P2 | Soil G1 + P3 | Soil G2 | Soil G2 + P1 | Soil G2 + P2 | Soil G2 + P3 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd | 17.2 | ± | 1.6 | 17.6 | ± | 0.8 | 17.9 | ± | 0.7 | 16.9 | ± | 1.0 | 21.7 | ± | 1.0 | 21.3 | ± | 1.2 | 21.3 | ± | 2.0 | 21.9 | ± | 1.1 |
| Pb | 503 | ± | 47 | 478 | ± | 50 | 515 | ± | 26 | 537 | ± | 19 | 1656 | ± | 163 | 1573 | ± | 154 | 1607 | ± | 134 | 1748 | ± | 92 |
| Zn | 2459 | ± | 326 | 2274 | ± | 159 | 2221 | ± | 161 | 2359 | ± | 142 | 4651 | ± | 553 | 4512 | ± | 556 | 4162 | ± | 555 | 4821 | ± | 332 |
| Cu | 45 | ± | 1 | 45 | ± | 3 | 46 | ± | 2 | 45 | ± | 1 | 108 | ± | 9 | 105 | ± | 9 | 102 | ± | 9 | 116 | ± | 5 |
| Mn | 404 | ± | 59 | 402 | ± | 41 | 391 | ± | 45 | 390 | ± | 34 | 402 | ± | 24 | 357 | ± | 35 | 371 | ± | 47 | 391 | ± | 14 |
| Fe | 36,565 | ± | 3465 | 35,691 | ± | 1036 | 32,824 | ± | 3825 | 35,753 | ± | 1772 | 32,124 | ± | 1948 | 30,593 | ± | 2131 | 29,694 | ± | 2389 | 31,118 | ± | 776 |
| Al | 2464 | ± | 297 | 2637 | ± | 290 | 2746 | ± | 471 | 2572 | ± | 161 | 2205 | ± | 280 | 2132 | ± | 168 | 2133 | ± | 491 | 2537 | ± | 163 |
| Ca | 7783 | ± | 655 | 8403 | ± | 1177 | 7988 | ± | 1689 | 7226 | ± | 1071 | 26,685 | ± | 7165 | 26,440 | ± | 3807 | 25,352 | ± | 3776 | 26,492 | ± | 3614 |
| Mg | 1789 | ± | 106 | 1767 | ± | 119 | 1682 | ± | 172 | 1568 | ± | 204 | 3434 | ± | 433 | 3332 | ± | 339 | 3129 | ± | 193 | 3269 | ± | 145 |
| Na | 363 | ± | 48 | 356 | ± | 16 | 292 | ± | 14 | 390 | ± | 110 | 382 | ± | 62 | 373 | ± | 65 | 317 | ± | 49 | 390 | ± | 27 |
| K | 2184 | ± | 100 | 2168 | ± | 152 | 2127 | ± | 149 | 2259 | ± | 162 | 2293 | ± | 145 | 2281 | ± | 175 | 2106 | ± | 145 | 2138 | ± | 103 |
| Metal | Soil G1 | Soil G1 + P1 | Soil G1 + P2 | Soil G1 + P3 | Soil G2 | Soil G2 + P1 | Soil G2 + P2 | Soil G2 + P3 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd | 11.0 | ± | 0.1 | 7.1 | ± | 0.3 | 7.7 | ± | 0.9 | 9.0 | ± | 0.2 | 3.3 | ± | 0.0 | 3.6 | ± | 0.1 | 3.5 | ± | 0.7 | 2.8 | ± | 0.1 |
| Pb | 7.0 | ± | 0.5 | 1.4 | ± | 0.8 | 3.8 | ± | 1.0 | 7.2 | ± | 0.6 | 12.7 | ± | 0.6 | 11.3 | ± | 2.2 | 6.8 | ± | 1.2 | 7.1 | ± | 0.6 |
| Zn | 737 | ± | 5 | 566 | ± | 28 | 587 | ± | 11 | 624 | ± | 10 | 273 | ± | 2 | 295 | ± | 13 | 297 | ± | 16 | 271 | ± | 3 |
| Cu | 4.5 | ± | 0.3 | 3.5 | ± | 0.1 | 3.2 | ± | 0.2 | 4.3 | ± | 0.2 | 3.3 | ± | 0.1 | 2.3 | ± | 0.7 | 2.0 | ± | 0.3 | 2.7 | ± | 0.1 |
| Mn | 164 | ± | 1 | 167 | ± | 12 | 144 | ± | 19 | 199 | ± | 3 | 60 | ± | 0 | 53 | ± | 6 | 74 | ± | 9 | 41 | ± | 0 |
| Fe | 184 | ± | 1 | 37 | ± | 2 | 56 | ± | 40 | 210 | ± | 3 | 116 | ± | 1 | 72 | ± | 20 | 24 | ± | 5 | 20 | ± | 2 |
| Al | 172 | ± | 8 | 55 | ± | 23 | 75 | ± | 32 | 152 | ± | 5 | 106 | ± | 7 | 106 | ± | 46 | 125 | ± | 32 | 28 | ± | 1 |
| Ca | 13,390 | ± | 354 | 10,466 | ± | 1340 | 10,642 | ± | 976 | 13,936 | ± | 352 | 19,027 | ± | 6 | 14,408 | ± | 1128 | 17,919 | ± | 398 | 14,656 | ± | 278 |
| Mg | 4416 | ± | 18 | 3632 | ± | 70 | 3761 | ± | 357 | 4485 | ± | 91 | 4162 | ± | 24 | 3502 | ± | 325 | 3903 | ± | 379 | 3343 | ± | 115 |
| Na | 12,105 | ± | 97 | 10306 | ± | 188 | 12,114 | ± | 1117 | 11,793 | ± | 370 | 15,181 | ± | 306 | 11,854 | ± | 1009 | 13,907 | ± | 2556 | 10,404 | ± | 122 |
| K | 24,533 | ± | 129 | 23916 | ± | 269 | 20,392 | ± | 3871 | 24,430 | ± | 523 | 16,625 | ± | 68 | 17,315 | ± | 457 | 16,832 | ± | 1180 | 17,686 | ± | 91 |
| Metal | Soil G1 | Soil G1 + P1 | Soil G1 + P2 | Soil G1 + P3 | Soil G2 | Soil G2 + P1 | Soil G2 + P2 | Soil G2 + P3 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd | 19.7 | ± | 0.9 | 19.2 | ± | 1.9 | 25.0 | ± | 3.4 | 21.1 | ± | 2.2 | 43.7 | ± | 6.3 | 51.0 | ± | 10.7 | 55.4 | ± | 6.1 | 49.6 | ± | 4.6 |
| Pb | 33.9 | ± | 4.2 | 32.4 | ± | 5.6 | 34.3 | ± | 1.6 | 33.9 | ± | 4.6 | 122.4 | ± | 26.9 | 222.8 | ± | 46.7 | 240.1 | ± | 15.1 | 200.7 | ± | 50.2 |
| Zn | 1610 | ± | 157 | 1409 | ± | 207 | 1973 | ± | 640 | 1545 | ± | 148 | 954 | ± | 24 | 1105 | ± | 316 | 1366 | ± | 78 | 1009 | ± | 155 |
| Cu | 23.4 | ± | 3.1 | 25.8 | ± | 7.4 | 19.1 | ± | 4.6 | 19.0 | ± | 4.3 | 21.7 | ± | 2.0 | 34.9 | ± | 2.1 | 29.4 | ± | 3.9 | 26.9 | ± | 5.0 |
| Mn | 104 | ± | 6 | 118 | ± | 30 | 117 | ± | 9 | 146 | ± | 44 | 70 | ± | 13 | 71 | ± | 6 | 105 | ± | 21 | 72 | ± | 6 |
| Fe | 1114 | ± | 190 | 1100 | ± | 167 | 1267 | ± | 138 | 1234 | ± | 248 | 2302 | ± | 137 | 2541 | ± | 170 | 2657 | ± | 47 | 2506 | ± | 180 |
| Al | 185 | ± | 51 | 234 | ± | 81 | 281 | ± | 60 | 232 | ± | 75 | 213 | ± | 52 | 320 | ± | 64 | 381 | ± | 58 | 300 | ± | 76 |
| Ca | 2666 | ± | 71 | 2676 | ± | 440 | 3664 | ± | 782 | 2761 | ± | 318 | 3633 | ± | 368 | 4129 | ± | 650 | 4579 | ± | 421 | 4481 | ± | 660 |
| Mg | 954 | ± | 27 | 833 | ± | 120 | 1179 | ± | 207 | 936 | ± | 75 | 980 | ± | 48 | 947 | ± | 250 | 1033 | ± | 58 | 915 | ± | 57 |
| Na | 5220 | ± | 743 | 4688 | ± | 888 | 6340 | ± | 863 | 4724 | ± | 660 | 4630 | ± | 388 | 3582 | ± | 569 | 6221 | ± | 337 | 5415 | ± | 1458 |
| K | 7317 | ± | 637 | 8011 | ± | 1354 | 10,303 | ± | 2290 | 8239 | ± | 1143 | 8992 | ± | 1207 | 7647 | ± | 1073 | 7672 | ± | 807 | 7778 | ± | 1187 |
| Metal | Soil G1 | Soil G1 + P1 | Soil G1 + P2 | Soil G1 + P3 | Soil G2 | Soil G2 + P1 | Soil G2 + P2 | Soil G2 + P3 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd | 1.6 | ± | 0.0 | 1.5 | ± | 0.1 | 1.6 | ± | 0.1 | 1.6 | ± | 0.0 | 0.2 | ± | 0.0 | 0.2 | ± | 0.1 | 0.2 | ± | 0.0 | 0.2 | ± | 0.0 |
| Pb | 1.3 | ± | 0.1 | 1.3 | ± | 0.1 | 1.3 | ± | 0.1 | 1.4 | ± | 0.1 | 2.2 | ± | 0.1 | 2.2 | ± | 0.1 | 2.1 | ± | 0.1 | 2.2 | ± | 0.2 |
| Zn | 319 | ± | 5 | 291 | ± | 34 | 288 | ± | 45 | 328 | ± | 12 | 65 | ± | 27 | 59 | ± | 19 | 59 | ± | 15 | 61 | ± | 2 |
| Cu | 0.8 | ± | 0.1 | 0.8 | ± | 0.1 | 0.8 | ± | 0.1 | 0.8 | ± | 0.0 | 0.5 | ± | 0.0 | 0.6 | ± | 0.2 | 0.5 | ± | 0.0 | 0.5 | ± | 0.0 |
| Mn | 26 | ± | 1 | 27 | ± | 8 | 24 | ± | 4 | 26 | ± | 2 | 22 | ± | 1 | 22 | ± | 1 | 21 | ± | 2 | 22 | ± | 1 |
| Fe | 115 | ± | 9 | 117 | ± | 27 | 113 | ± | 17 | 108 | ± | 2 | 142 | ± | 13 | 153 | ± | 24 | 131 | ± | 24 | 142 | ± | 23 |
| Ca | 135 | ± | 27 | 153 | ± | 20 | 158 | ± | 23 | 152 | ± | 11 | 214 | ± | 13 | 199 | ± | 14 | 218 | ± | 28 | 198 | ± | 10 |
| Mg | 82 | ± | 5 | 85 | ± | 6 | 80 | ± | 6 | 79 | ± | 1 | 121 | ± | 7 | 121 | ± | 7 | 125 | ± | 4 | 124 | ± | 4 |
| Na | 170 | ± | 3 | 156 | ± | 17 | 165 | ± | 26 | 172 | ± | 1 | 25 | ± | 2 | 28 | ± | 4 | 24 | ± | 2 | 24 | ± | 1 |
| K | 364 | ± | 9 | 363 | ± | 8 | 372 | ± | 7 | 375 | ± | 3 | 256 | ± | 9 | 259 | ± | 4 | 256 | ± | 5 | 251 | ± | 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waterlot, C.; Hechelski, M. Benefits of Ryegrass on Multicontaminated Soils Part 1: Effects of Fertilizers on Bioavailability and Accumulation of Metals. Sustainability 2019, 11, 5093. https://doi.org/10.3390/su11185093
Waterlot C, Hechelski M. Benefits of Ryegrass on Multicontaminated Soils Part 1: Effects of Fertilizers on Bioavailability and Accumulation of Metals. Sustainability. 2019; 11(18):5093. https://doi.org/10.3390/su11185093
Chicago/Turabian StyleWaterlot, Christophe, and Marie Hechelski. 2019. "Benefits of Ryegrass on Multicontaminated Soils Part 1: Effects of Fertilizers on Bioavailability and Accumulation of Metals" Sustainability 11, no. 18: 5093. https://doi.org/10.3390/su11185093
APA StyleWaterlot, C., & Hechelski, M. (2019). Benefits of Ryegrass on Multicontaminated Soils Part 1: Effects of Fertilizers on Bioavailability and Accumulation of Metals. Sustainability, 11(18), 5093. https://doi.org/10.3390/su11185093






