Cadmium Hyperaccumulation and Translocation in Impatiens Glandulifera: From Foe to Friend?
Abstract
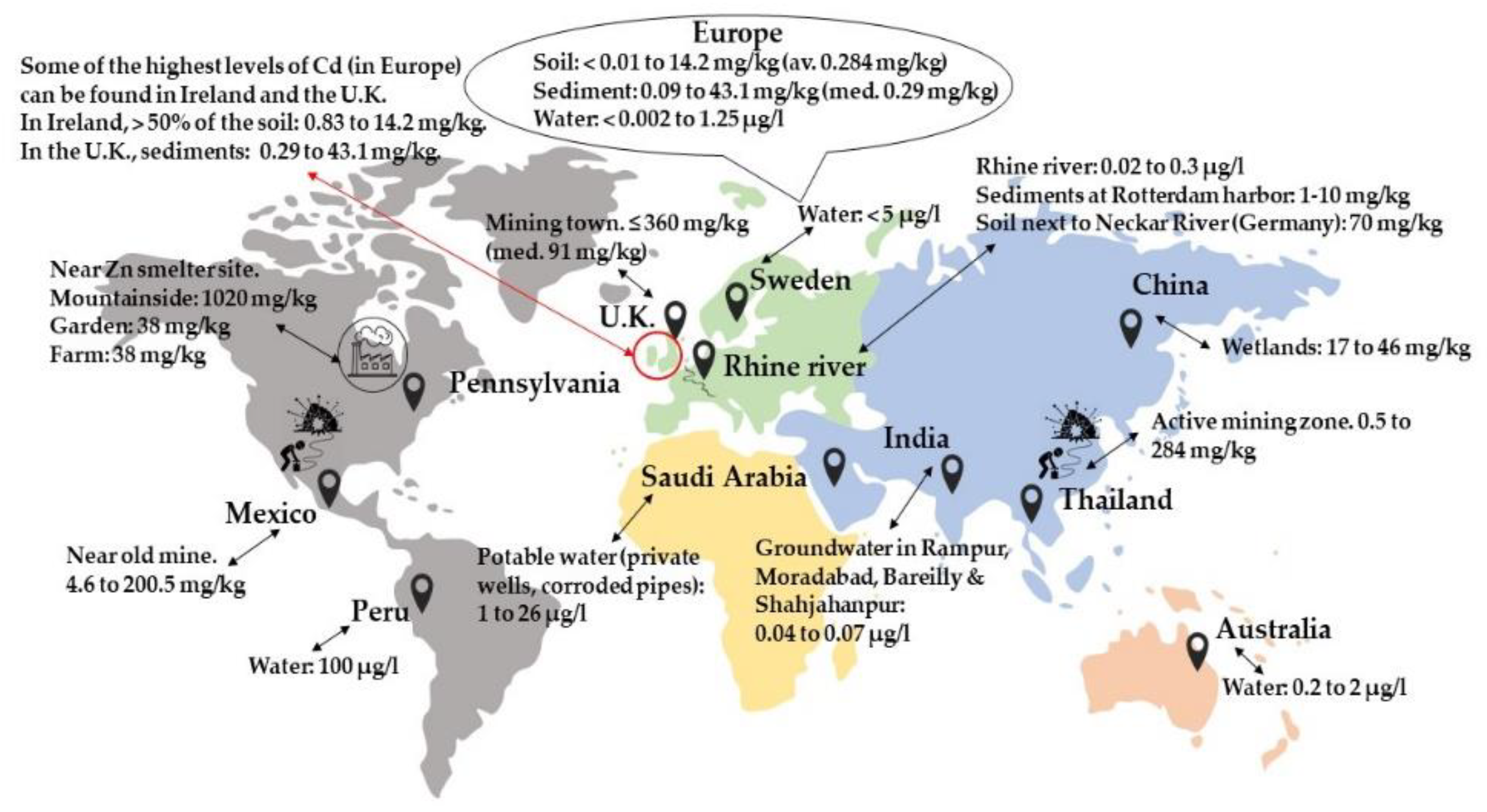
:1. Introduction
2. Materials and Methods
2.1. Localization of Cadmium in I. glandulifera
2.2. Soil Characteristics
2.3. Cadmium Trials
2.4. Cadmium Analysis
2.5. Effect of Cadmium on Germination
2.5.1. In Vitro Seed Germination
2.5.2. Pollen Germination
2.6. Statistical Analysis
3. Results and Discussion
3.1. Localization of Cadmium
3.2. Cadmium Trials
3.2.1. Soil Characteristics
3.2.2. Plant Growth
3.2.3. Cadmium Accumulation
3.2.4. Bioconcentration Factor and Translocation Factor
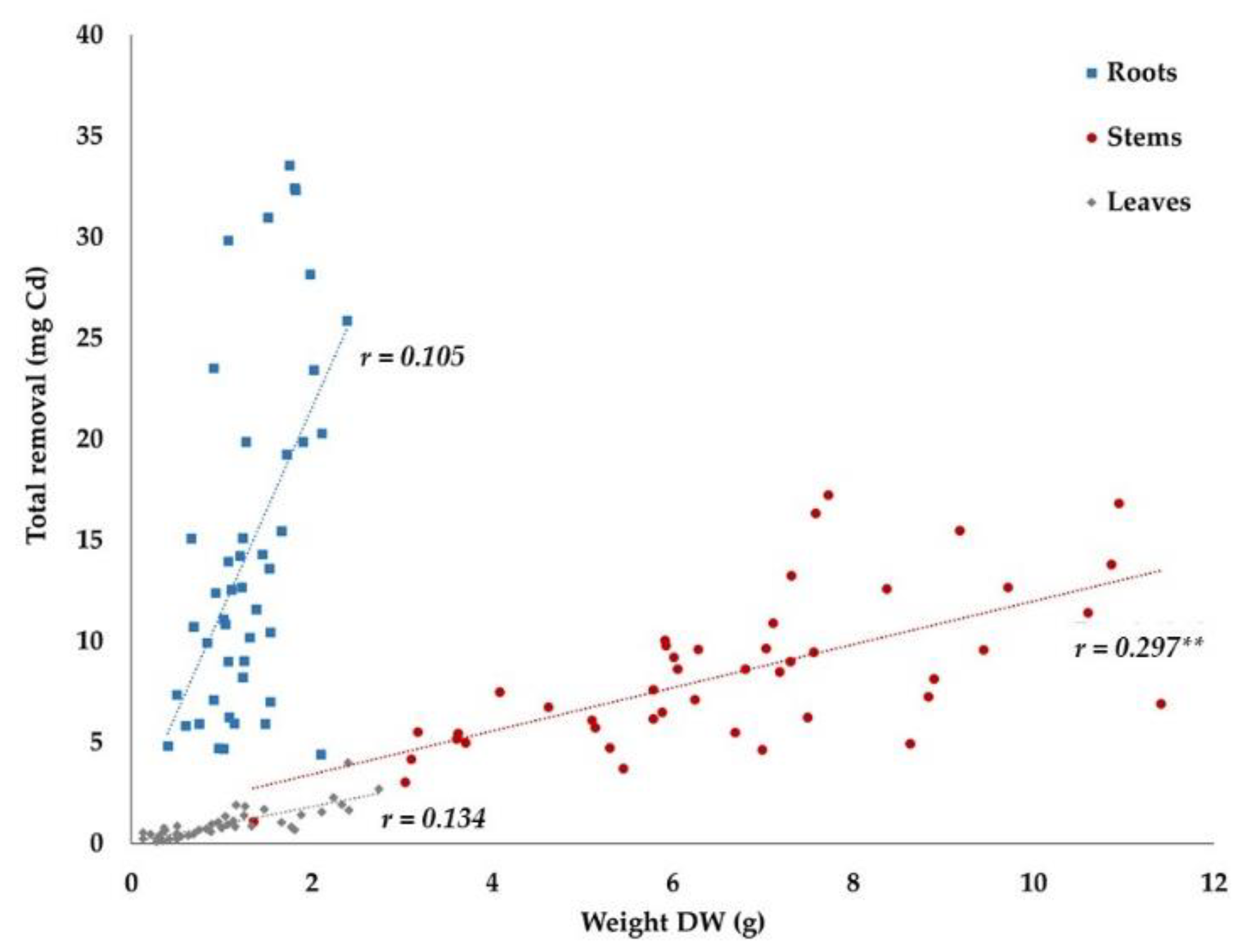
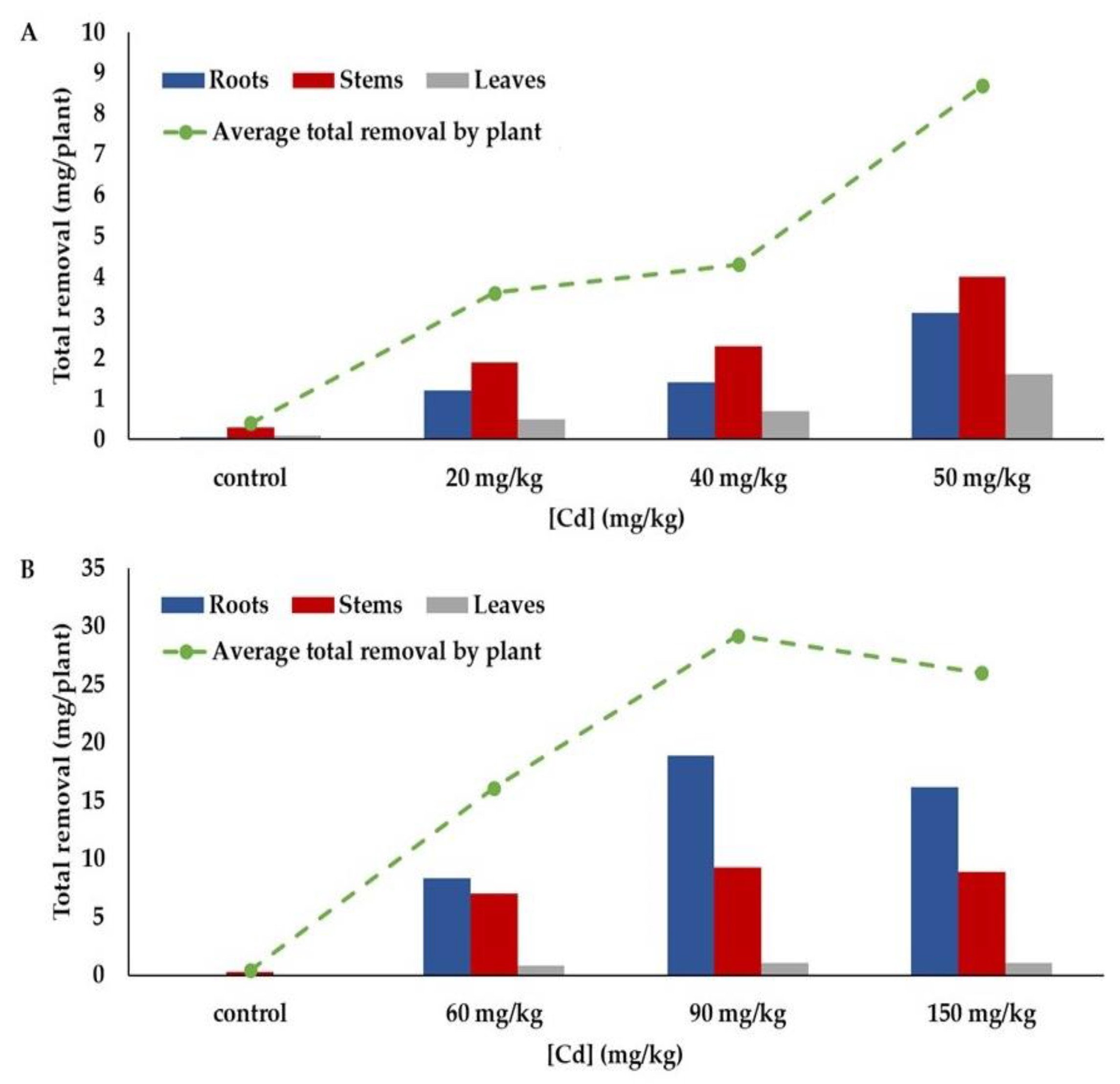
3.2.5. Total Removal
3.3. Germination
3.3.1. In Vitro Seed Germination
3.3.2. Pollen Germination
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Yang, Y.; Li, C.; Ni, X.; Ma, W.; Wei, H. Assessing soil metal levels in an industrial environment of northwest china and the phytoremediation potential of its native plants. Sustainability 2018, 10, 2686. [Google Scholar] [CrossRef]
- Henson, T.M.; Cory, W.; Rutter, M.T. Extensive Variation in Cadmium Tolerance and Accumulation among Populations of Chamaecrista fasciculate. PLoS ONE 2013, 8, e63200. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-T.; Baker, A.J.M.; Ye, Z.-H.; Wang, H.-B.; Shu, W.-S. Phytoextraction of Cd-contaminated soils: Current status and future challenges. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2113–2152. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario Delgado-Caballero, M.; Alarcón-Herrera, M.T.; Valles-Aragón, M.C.; Melgoza-Castilo, A.; Ojeda-Barrios, D.L.; Leyva-Chávez, A. Germination of Bouteloua dactyloides and Cynodon dactylon in a multi-polluted soil. Sustainability 2017, 9, 81. [Google Scholar] [CrossRef]
- Science Communication Unit, University of the West of England, Bristol. Science for Environment Policy In-depth Report: Soil Contamination: Impacts on Human Health. Report produced for the European Commission DG Environment, September 2013. Available online: https://ec.europa.eu/search/?queryText=Soil+Contamination%3A+Impacts+on+Human+Health&query_source=ENVSCPOL&filterSource=ENVSCPOL&swlang=en&more_options_language=en&more_options_f_formats=&more_options_date= (accessed on 12 September 2019).
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals- Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.-T.; Peng, Y.-H. Response of pollen germination and tube growth to cadmium with special reference to low concentration exposure. Ecotoxicol. Environ. Saf. 2001, 48, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.L.; Chaney, R.L.; Angle, J.S.; Baker, A.J.M. Phytoremediation potential of Thlaspi caerulescens and bladder campion for zinc- and cadmium-contaminated soil. J. Environ. Qual. 1994, 23, 1151–1157. [Google Scholar] [CrossRef]
- Pan, J.; Plant, J.A.; Voulvoulis, N. Cadmium levels in Europe: Implications for human health. Environ. Geochem. Health 2010, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.W.; Pongsakul, P.; Saiyasitpanich, D.; Klinphoklap, S. Elevated levels of cadmium and zinc in paddy soils and elevated levels of cadmium in rice grain downstream of a zinc mineralized area in Thailand: Implication for public health. Environ. Geochem Health 2005, 27, 501–511. [Google Scholar] [CrossRef]
- World Health Organisation, Geneva. Cadmium in Drinking-Water. Background Document for Development of WHO Guidelines for Drinking-Water Quality. 2011. Available online: https://www.who.int/water_sanitation_health/dwq/chemicals/cadmium.pdf (accessed on 12 September 2019).
- World Health Organisation, Geneva. Cadmium-Environmental Aspects. 1992. Environmental Health Criteria 135. Available online: http://www.inchem.org/documents/ehc/ehc/ehc134.htm (accessed on 12 September 2019).
- Idrees, N.; Tabassum, B.; Abd_Allah, E.F.; Hashem, A.; Sarah, R.; Hashim, M. Groundwater contamination with cadmium concentrations in some West U.P. Regions, India. Saudi J. Biol. Sci. 2018, 25, 1365–1368. [Google Scholar] [CrossRef]
- Deng, H.; Ye, Z.H.; Wong, M.H. Accumulation of lead, zinc, copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environ. Pollut. 2004, 132, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Hua, S.; Shamsi, I.H.; Jilani, G.; Li, Y.; Jiang, L. Cadmium-induced stress on the seed germination and seedling growth of Brassica napus L., and its alleviation through exogenous plant growth regulators. Plant Growth Regul. 2009, 58, 47–59. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S.P. A review on phytoremediation of heavy metals and utilization of it’s by it’s products. Asian J. Energ. Environ. 2005, 6, 214–231. [Google Scholar]
- Lai, H.-Y. Subcellular distribution and chemical forms of cadmium in Impatiens walleriana in relation to it phytoextraction potential. Chemosphere 2015, 138, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation technology: Hyper-accumulation metals in plants. Water Air Soil Pollut. 2007, 184, 105–126. [Google Scholar] [CrossRef]
- Dixit, R.; Wasiullah; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Wei, J.-L.; Lai, H.-Y.; Chen, Z.-S. Chelator effects on bioconcentration and translocation of cadmium by hyperaccumulators, Tagetes patula and Impatiens walleriana. Ecotoxicol. Environ. Saf. 2012, 84, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Baker, A.J.M.; McGrath, S.P.; Reeves, R.D.; Smith, J.A.C. Metal hyperaccumulator plants: A review of the ecology and physiology of a biological resource for phytoremediation of polluted soils. In Phytoremediation of Contaminated Soil and Water; Bañuelos, T.N., Ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 85–107. [Google Scholar]
- Brooks, R.R.; Lee, J.; Reeves, R.D.; Jaffrre, T. Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J. Geochem. Explor. 1977, 7, 49–57. [Google Scholar] [CrossRef]
- Liu, J.-N.; Zhou, Q.-X.; Sun, T.; Ma, L.Q.; Wang, S. Growth responses of three ornamental plants to Cd-Pb stress and their metal accumulation characteristics. J. Hazard. Mater. 2008, 151, 261–267. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Q.; Liu, S.; Zhao, Y.; Long, Y.; Pan, Y. Screening ornamental plants to identify potential Cd hyperaccumulators for bioremediation. Ecotoxicol. Environ. Saf. 2018, 162, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Yang, Q.W.; Wang, H.B.; Shu, W.S. Phytoextraction of heavy metals by eight plant species in the field. Water Air Soil Pollut. 2007, 184, 235–242. [Google Scholar] [CrossRef]
- Zacchini, M.; Pietrini, F.; Mugnozza, G.S.; Iori, V.; Pietrosanti, L.; Massacci, A. Metal tolerance, accumulation and translocation in poplar and willow clones treated with cadmium in hydroponics. Water Air Soil Pollut. 2009, 197, 23–24. [Google Scholar] [CrossRef]
- Akhter, M.F.; Omelon, C.R.; Gordon, R.A.; Moser, D.; Macfie, S. Chemical speciation of cadmium in the roots of barley and lettuce. Environ. Exper. Bot. 2014, 43, 10–196. [Google Scholar] [CrossRef]
- Lai, H.-Y. Effects of leaf area and transpiration rate on accumulation and compartmentalization of cadmium in Impatiens walleriana. Water Air Soil Pollut. 2014, 226, 2246. [Google Scholar] [CrossRef]
- Boominathan, R.; Doran, P.M. Cadmium tolerance and antioxidative defences in hairy roots of the cadmium hyperaccumulator, Thlaspi caerulescens. Biotechnol. Bioeng. 2002, 83, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.-Y.; Cai, M.-C. Effects of extended growth periods on subcellular distribution, chemical forms, and the translocation of cadmium in Impatiens walleriana. Int. J. Phytoremediation. 2016, 18, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Lai, H.-Y.; Chen, Z.-S. Bioavailability assessment and accumulation by five garden flower species grown in artificially cadmium-contaminated soils. Int. J. Phytoremediation 2010, 12, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Beerling, D.J.; Perrins, J.M. Impatiens glandulifera Royle (Impatiens roylei Walp.). J. Ecol. 1993, 81, 367–382. [Google Scholar] [CrossRef]
- Clements, D.R.; Feenstra, K.R.; Jones, K.; Staniforth, R. The biology of invasive alien plants in Canada. 9. Impatiens glandulifera Royle. Can. J. Plant Sci. 2008, 88, 403–417. [Google Scholar] [CrossRef]
- Skálová, H.; Havlíčková, V.; Pyšek, P. Seedling traits, plasticity and local differentiation as strategies of invasive species of Impatiens in central Europe. Ann. Bot. 2012, 110, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; With, K.A.; Baughman, S.; Cabin, R.J.; Cohen, J.E.; Ellstrand, N.C.; et al. The population biology of invasive species. Annu. Rev. Ecol. Syst. 2001, 32, 305–332. [Google Scholar] [CrossRef]
- Vervoort, A.; Cawoy, V.; Jacquemart, A.-L. Comparative reproductive biology in co-occurring invasive and non-invasive Impatiens species. Int. J. Plant Sci. 2011, 172, 366–377. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P. What is the impact of Impatiens glandulifera on species diversity of invaded riparian vegetation? Biol. Conserv. 2006, 132, 143–152. [Google Scholar] [CrossRef]
- Tanner, R.A.; Gange, A.C. The impact of two non-native plant species on native flora performance: Potential implications for habitat restoration. Plant. Ecol. 2013, 214, 423–434. [Google Scholar] [CrossRef]
- Eid, E.M.; Shaltout, K.H.; Moghanm, F.S.; Youssef, M.S.G.; El-Mohsnawy, E.; Haroun, S.A. Bioaccumulation and translocation of nine heavy metals by Eichhornia crassipes in Nile Delta, Egypt: Perspectives for phytoremediation. Int. J. Phytoremediation 2019, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.M.; Abd-Elmabod, S.K.; El-ashry, S.M.; Soliman, W.S.; El-Tayeh, N.; Castillo, J.M. Capability of the invasive tree Proposis glandulosa Torr. to remediate soil treated with sewage sludge. Sustainability 2019, 11, 2711. [Google Scholar] [CrossRef]
- Padley, V.C. Invasive species based efficient green technology for phytoremediation of fly ash deposits. J. Geochem. Explor. 2013, 123, 13–18. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Snyder, W.C. Nutrition of strawberry plant under controlled conditions. J. Am. Soc Hortic Sci. 1933, 30, 288–294. [Google Scholar]
- Wang, A.S.; Angle, J.S.; Chaney, R.L.; Delorme, T.A.; Reeves, R.D. Soil pH effects on uptake of Cd and Zn by Thlaspi caerulescens. Plant Soil 2006, 281, 325–337. [Google Scholar] [CrossRef]
- Lombi, E.; Zhao, F.J.; Dunham, S.J.; McGrath, S.P. Phytoremediation of heavy metal-contaminated soils: Natural hyperaccumulation versus chemically enhanced phytoextraction. J. Environ. Qual. 2001, 30, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Clabeaux, B.L.; Navarro, D.A.G.; Aga, D.S.; Bisson, M.A. Cd tolerance and accumulation in the aquatic macrophyte, Chara australis: Potential use for charophytes in phytoremediation. Environ. Sci. Technol. 2011, 45, 5332–5338. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.A.; Boersma, L. A unifying quantitative analysis of soil texture. SSSA 1984, 48, 142–147. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis, Part 3, Chemical Methods, 1st ed.; Page, D.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis. J. Plant Nutr. Soil Sci. 1958, 85, 251–252. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- HACH, Laton Total Nitrogen Cuvette Test 20–100 mg/L TNb, 25 Tests. Available online: https://ie.hach.com/asset-get.download.jsa?id=25593604089 (accessed on 12 September 2019).
- HACH, Phosphorus, Reactive (Orthophosphate) Molybdovanadate Method 8114, Test ‘N Tube™ Vials. Available online: https://ie.hach.com/asset-get.download.jsa?id=7639983833 (accessed on 12 September 2019).
- HACH, Potassium, Tetraphenylborate Method 8049, Spectrophotometer, Powder Pillows. Available online: https://ie.hach.com/asset-get.download.jsa?id=7639983843 (accessed on 12 September 2019).
- Perglová, I.; Pergl, J.; Skálová, H.; Moravcová, L.; Jarošík, V.; Pyšek, P. Differences in germination and seedling establishment of alien and native Impatiens species. Preslia 2009, 81, 357–375. [Google Scholar]
- Hussein, N. In vitro manipulation of Impatiens glandulifera pollen for transporting extracellular substances to the embryo sac. Curr. Res. J. Biol. Sci. 2014, 6, 66–70. [Google Scholar] [CrossRef]
- Peterson, R.; Slovin, J.P.; Chen, C. A simplified method for differential staining of aborted and non-aborted pollen grains. Int. J. Plant Biol. 2010, 1, 66–69. [Google Scholar] [CrossRef]
- Milner, M.J.; Kochian, L.V. Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann. Bot. 2008, 102, 3–13. [Google Scholar] [CrossRef]
- Selvam, A.; Wong, J.W.C. Phytochelatin systhesis and cadmium uptake of Brassica napus. Environ. Technol. 2008, 29, 765–773. [Google Scholar] [CrossRef]
- Alaboudi, K.A.; Ahmed, B.; Brodie, G. Phytoremediation of Pb and Cd contaminated soils by using sunflower (Helianthus annuus) plant. AOAS 2018, 63, 123–127. [Google Scholar] [CrossRef]
- Epelde, L.; Becerril, J.M.; Kowalchuk, G.A.; Deng, Y.; Zhou, J.; Garbisu, C. Impact of metal pollution and Thlaspi caerulescens growth soil microbial communities. Appl. Environ. Microbiol. 2010, 76, 7843–7853. [Google Scholar] [CrossRef] [PubMed]
- Mani, D.; Sharma, B.; Kumar, C. Phytoaccumulation, interaction, toxicity and remediation of cadmium from Helianthus annuus L. (sunflower). Bull. Environ. Contam. Toxicol. 2007, 79, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, F.; Zacchini, M.; Iori, V.; Pietrosanti, L.; Bianconi, D.; Massacci, A. Screening of poplar clones for cadmium phytoremediation using photosynthesis, biomass and cadmium content analyses. Int. J. Phytoremediation 2010, 12, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.; Kaushal, J. Role of phytoremediation in reducing cadmium toxicity in soil and water. J. Toxicol. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Alkorta, I.; Hernández-Allica, J.; Becerril, J.M.; Amezaga, I.; Albizu, I.; Garbisu, C. Recent findings on the photoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead and arsenic. Rev. Environ. Sci. Biotechnol. 2004, 3, 71–90. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements—A review of their distribution, ecology and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Sun, R.; Sun, Q.; Wang, R.; Cao, L. Cadmium accumulation and main rhizosphere characteristics of seven French marigold (Tagetes patula L.) cultivars. Int. J. Phytoremediation 2018, 20, 1171–1178. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Zayed, A.M.; Qian, J.-H.; de Souza, M.; Terry, N. Phytoaccumulation of trace elements by wetland plants: II. Water hyacinth. J. Environ. Qual. 1999, 28, 339–344. [Google Scholar] [CrossRef]
- Zhao, F.J.; Lombi, E.; McGrath, S.P. Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant Soil 2003, 249, 37–43. [Google Scholar] [CrossRef]
- Pant, P.; Allen, M.; Tansel, B. Mercury Uptake and Translocation in Impatiens walleriana plants grown in the contaminated soil from oak ridge. Int. J. Phytoremediation 2010, 13, 168–176. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Wang, L.; Liu, W. Cadmium tolerance and accumulation characteristics of Bidens pilosa L. as a potential Cd-hyperaccumulator. J. Hazard. Mater. 2009, 161, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant. Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Shrestha, P.; Bellitürk, K.; Görres, J.H. Phytoremediation of heavy metal-contaminated soil by switchgrass: A comparative study utilizing different composts and coir fiber on pollution remediation, plant productivity, and nutrient leaching. IJERPH 2019, 16, 1261. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Noriharu, A.E.; Murakami, M.; Wagatsuma, T. Is Brassica juncea a suitable plant for phytoremediation of cadmium in soils with moderately low cadmium contamination?—Possibility of using other plant species for Cd-phytoextraction. J. Soil Sci. Plant Nutr. 2006, 52, 32–42. [Google Scholar] [CrossRef]
- Ruckli, R.; Rusterholz, H.-P.; Baur, B. Invasion of Impatiens glandulifera affects terrestrial gastropods by altering microclimate. Acta Oecol. 2013, 47, 16–23. [Google Scholar] [CrossRef]
- Ruckli, R.; Rusterholz, H.-P.; Baur, B. Invasion of an annual exotic plant into deciduous forests suppresses arbuscular mycorrhiza symbiosis and reduces performance of sycamore maple saplings. Forest Ecol. Manag. 2014, 318, 285–293. [Google Scholar] [CrossRef]
- Nirola, R.; Megharaj, M.; Palanisami, T.; Aryal, R.; Venkateswarlu, K.; Naidu, R. Evaluation of metal uptake factors of native trees colonizing an abandoned copper mine—A quest for phytostabilization. J. Sustain. Min. 2015, 14, 115–123. [Google Scholar] [CrossRef]
- Conti, M.E.; Botrè, F. Honeybees and their products as potential bioindicators of heavy metals contamination. Environ. Monit. Assess. 2001, 69, 267–282. [Google Scholar] [CrossRef]
- Tanner, R.A. A review on the potential for the biological control of the invasive weed, Impatiens glandulifera in Europe. In Plant Invasions: Human Perception, Ecological Impacts and Management; Tokarska-Guzik, T., Brock, J.H., Brundu, G., Child, L., Daehler, C.C., Pyšek, P., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2008; pp. 343–354. [Google Scholar]
- Seo, B.-H.; Kim, H.S.; Kuppusamy, S.; Kim, K.-H.; Kim, K.-R. Enhanced nitrogen and phosphorus removal by woody plants with deep-planting technique for the potential environmental management of carcass burial sites. Sustainability 2017, 9, 155. [Google Scholar] [CrossRef]



| Soil Properties | Result |
|---|---|
| Texture | Silt Loam |
| Sand | 320 mg/kg |
| Silt | 640 mg/kg |
| Clay | 40 mg/kg |
| pH (soil:water = 1:1) | 5.8 |
| Organic Carbon (%) | 2.7 mg/kg |
| Total Nitrogen | 74 mg/kg |
| Reactive Phosphorus | 39.3 mg/kg |
| Potassium | 29.4 mg/kg |
| Cation Exchange Capacity | 21.7 meq/100 g |
| Cd concentration | 0.66 mg/kg |
| Cadmium Treatments | Cadmium Concentration (mg/kg) | Biomass (g) | TR (mg/plant) | TF * | BCF ** | |
|---|---|---|---|---|---|---|
| Control | Roots | 30 ±11.1 a | 1.42 ± 0.6 a | 0.05 ± 0.03 a | ||
| Stems | 32.7 ± 11.3a | 8.4 ± 7 a | 0.3 ± 1.7 a | |||
| Leaves | 46.2 ±32.7 a | 2.5 ± 1 a | 0.1 ± 0.1 a | |||
| Total | 10.2 ± 8.8 a | 0.4 ± 0.2 a | 2.6 | 8.3 | ||
| 20 mg/kg | Roots | 822 ± 465 b | 1.5 ± 0.6 a | 1.2 ± 0.8 b | ||
| Stems | 277 ± 102 b | 7.5 ± 3.4 a | 1.9 ± 0.8 b | |||
| Leaves | 208 ± 75 b | 2.5 ± 0.7 a | 0.5 ± 0.3 b | |||
| Total | 11.5 ± 4.1 a | 3.6 ± 1.5 b | 0.7 | 64 | ||
| 40 mg/kg | Roots | 1030 ± 375 b | 1.3 ± 0.6 a | 1.4 ± 0.7 b | ||
| Stems | 470 ± 200 c | 4.8 ± 2.5 a | 2.3 ± 1.1 bc | |||
| Leaves | 325 ± 213 b | 2.1 ± 0.7 a | 0.7 ± 0.4 b | |||
| Total | 8.2 ± 3.5 a | 4.3 ± 1.8 b | 0.8 | 45 | ||
| 50 mg/kg | Roots | 868 ± 158 b | 1.7 ± 0.8 a | 3.1 ± 5 b | ||
| Stems | 426 ± 225 c | 10.9 ± 9.3 a | 4 ± 3.4 c | |||
| Leaves | 193 ± 35 ab | 2.4 ± 1.1 a | 1.6 ± 3.1 b | |||
| Total | 15 ± 10 a | 8.7 ± 7.8 c | 1.2 | 54 |
| Cadmium Treatments | Cadmium Concentration (mg/kg) | Biomass (g) | TR (mg/plant) | TF * | BCF ** | |
|---|---|---|---|---|---|---|
| Control | Roots | 57.2 ± 19.5 a | 1.2 ± 0.49 a | 0.07 ± 0.03 a | ||
| Stems | 44.6 ± 13.8 a | 5.1 ± 2.5 a | 0.3 ± 4.7 a | |||
| Leaves | 55.2 ± 19.7 a | 0.9 ± 0.7 a | 0.06 ± 0.06 a | |||
| Flowers | 0 a | 0.23 ± 0.2 a | 0 a | |||
| Total | 8.2 ± 3.8 a | 0.4 ± 0.2 a | 1.7 | 11.9 | ||
| 60 mg/kg | Roots | 7089 ± 3098 b | 1.2 ± 0.3 a | 8.3 ± 3.3 b | ||
| Stems | 1099 ± 447 b | 7 ± 2 b | 7 ± 2.7 b | |||
| Leaves | 915 ± 357 b | 0.9 ± 0.6 a | 0.8 ± 0.5 b | |||
| Flowers | 98 ± 40 b | 0.2 ± 2 a | 0.07 ± 0.04 b | |||
| Total | 9.2 ± 2.7 a | 16.1 ± 5 b | 0.3 | 206 | ||
| 90 mg/kg | Roots | 14,361 ± 6164 c | 1.3 ± 0.5 a | 18.9 ± 9.5 c | ||
| Stems | 1537 ± 426 c | 6.7 ± 1.8 ab | 9.3 ± 3.6 c | |||
| Leaves | 825 ± 297 b | 1.2 ± 0.8 a | 1.01 ± 0.7 b | |||
| Flowers | 129 ± 36 b | 0.32 ± 0.3 a | 0.21 ± 0.09 c | |||
| Total | 9.3 ± 2.5 a | 29.2 ± 11.9 c | 0.2 | 236 | ||
| 150 mg/kg | Roots | 13,000 ± 4717 c | 1.3 ± 0.5 a | 16.2 ± 8.1 c | ||
| Stems | 1562 ± 572 c | 6.2 ± 3 ab | 8.9 ± 4.6 bc | |||
| Leaves | 1052 ± 416 b | 0.9 ± 0.7 a | 1.05 ± 1.04 b | |||
| Flowers | 136 ± 88 b | 0.26 ± 0.2 a | 0.13 ± 0.1 bc | |||
| Total | 8.4 ± 4 a | 26 ± 11.9 c | 0.2 | 134 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coakley, S.; Cahill, G.; Enright, A.-M.; O’Rourke, B.; Petti, C. Cadmium Hyperaccumulation and Translocation in Impatiens Glandulifera: From Foe to Friend? Sustainability 2019, 11, 5018. https://doi.org/10.3390/su11185018
Coakley S, Cahill G, Enright A-M, O’Rourke B, Petti C. Cadmium Hyperaccumulation and Translocation in Impatiens Glandulifera: From Foe to Friend? Sustainability. 2019; 11(18):5018. https://doi.org/10.3390/su11185018
Chicago/Turabian StyleCoakley, Stephanie, Gary Cahill, Anne-Marie Enright, Brian O’Rourke, and Carloalberto Petti. 2019. "Cadmium Hyperaccumulation and Translocation in Impatiens Glandulifera: From Foe to Friend?" Sustainability 11, no. 18: 5018. https://doi.org/10.3390/su11185018
APA StyleCoakley, S., Cahill, G., Enright, A.-M., O’Rourke, B., & Petti, C. (2019). Cadmium Hyperaccumulation and Translocation in Impatiens Glandulifera: From Foe to Friend? Sustainability, 11(18), 5018. https://doi.org/10.3390/su11185018






