Comparative Investigation of Total, Recoverable and Bioavailable Fractions of Sediment Metals and Metalloids in the Lagos Harbour and Lagoon System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Design
2.2. Collection of Sediment Samples
2.3. Total Metals and Metalloids Analysis Using X-ray Fluorescence (XRF) Spectrometry
2.4. Recoverable Metals and Metalloids (Aqua Regia—Inductively Coupled Plasma Optical Emission Spectrometry, ICP-OES) Analysis
2.5. Determination of Bioavailable Metals and Metalloids (1M Hydrochloric Acid Inductively Coupled Plasma Mass Spectrometry, HCl–ICP-MS)
2.6. Spatial Distribution Analysis of the Heavy Metals
2.7. Statistical Analysis of Results
3. Results
3.1. Total Metal and Metalloid Concentrations for Dry Season 1 (DS1), Wet Season (WS) and DS2
3.2. Recoverable Concentrations for DS1, WS and DS2
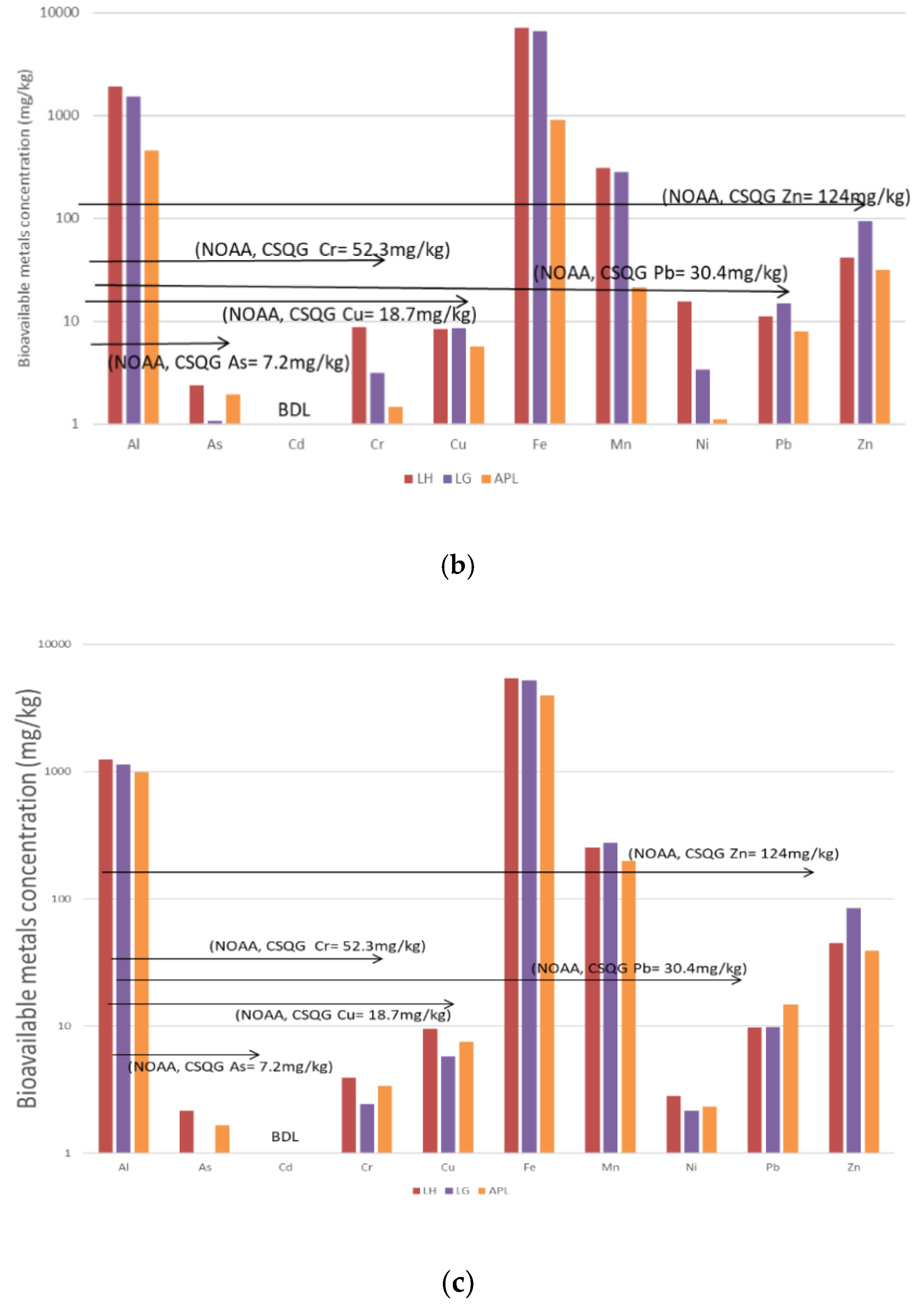
3.3. Bioavailable Concentrations for DS1, WS and DS2
3.4. Summary of the Polluting Activities and Relative Sediment Metal Concentrations
3.5. Geochemical Contour and Spatial Maps of Metal Distributions in Lagos Harbour (LH), Lagos Lagoon (LG) and Apese Lagoon (APL)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sarkar, S.K.; Frančišković-Bilinski, S.; Bhattacharya, A.; Saha, M.; Bilinski, H. Levels of elements in the surficial estuarine sediments of the Hugli River, northeast India and their environmental implications. Environ. Int. 2004, 30, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Wardhani, E.; Roosmini, D.; Notodarmojo, S. Status of heavy metal in sediment of Saguling Lake, West Java. In Proceedings of the IOP Conference Series: Earth and Environmental Science—The First International Symposium on Green Technology for Value Chains, Tangerang, Indonesia, 3–5 October 2016; IOP Publishing: Bristol, UK, 2017; Volume 60, p. 012035. [Google Scholar]
- Swarnalatha, K.; Letha, J.; Ayoob, S. Ecological risk assessment of a tropical lake system. J. Urban Environ. Eng. 2013, 7, 323–329. [Google Scholar] [CrossRef]
- Davutluoglu, O.I.; Seckin, G.; Ersu, C.B.; Yilmaz, T.; Sari, B. Assessment of metal pollution in water and surface sediments of the Seyhan River, Turkey, using different indexes. Clean Soil Air Water 2011, 9, 185–194. [Google Scholar] [CrossRef]
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017. [Google Scholar] [CrossRef] [PubMed]
- Muduli, P.R.; Vardhan, K.V.; Ganguly, D.; Abhilash, K.R.; Balasubramanian, T. Heavy metal contamination and risk assessment in the marine environment of Arabian Sea, along the southwest coast of India. Am. J. Chem. 2012, 2, 191–208. [Google Scholar]
- Clark, R.B. Marine Pollution, 5th ed.; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Valavanidis, A.; Vlachogianni, T. Metal Pollution in Ecosystems. Ecotoxicology Studies and Risk Assessment in the Marine Environment. Available online: https://www.researchgate.net/profile/Athanasios_Valavanidis/publication/236623174_Metal_Pollution_in_Ecosystems_Ecotoxicology_Studies_and_Risk_Assessment_in_the_Marine_Environment/links/00b4952fba8bb82bbd000000/Metal-Pollution-in-Ecosystems-Ecotoxicology-Studies-and-Risk-Assessment-in-the-Marine-Environment.pdf (accessed on 6 August 2019).
- Filipkowska, A.; Lubecki, L.; Kowalewska, G. Polycyclic aromatic hydrocarbon analysis in different matrices of the marine environment. Anal. Chim. Acta 2005, 547, 243–254. [Google Scholar] [CrossRef]
- Knox, A.S.; Rinklebe, J.; Paller, M.H. Heavy metals in sediments and remediation technologies. In Proceedings of the 17th International Conference on Heavy Metals in the Environment, Guiyang, China, 22–26 September 2014. [Google Scholar]
- Knox, A.S.; Paller, M.H. Contaminants in sediments-remediation and management. In Proceedings of the E3S Web of Conferences—16th International Conference on Heavy Metals in the Environment, Rome, Italy, 23–27 September 2012; EDP Sciences: Les Ulis, France, 2017; Volume 1, p. 02003. [Google Scholar]
- Idris, A.M.; Eltayeb, M.A.; Potgieter-Vermaak, S.S.; Van Grieken, R.; Potgieter, J.H. Assessment of heavy metals pollution in Sudanese harbours along the Red Sea Coast. Microchem. J. 2007, 87, 104–112. [Google Scholar] [CrossRef]
- Okoro, H.K.; Fatoki, O.S.; Adekola, F.A.; Ximba, B.J.; Snyman, R.G. A review of sequential extraction procedures for heavy metals speciation in soil and sediments. Sci. Rep. 2012. [Google Scholar] [CrossRef]
- Baeyens, W.; Monteny, F.; Leermakers, M.; Bouillon, S. Evalution of sequential extractions on dry and wet sediments. Anal. Bioanal. Chem. 2003, 376, 890–901. [Google Scholar] [CrossRef]
- Wepener, V.; Vermeulen, L.A. A note on the concentrations and bioavailability of selected metals in sediments of Richards Bay Harbour, South Africa. Water SA 2005, 31, 589–596. [Google Scholar] [CrossRef]
- Khalid, M.; Mohamadein, L.I.; Saad, E.M.; Reda, F.; Mahmoud, S.A. Assessment of Heavy Metals Pollution Using Sediments and Bivalve Brachidontes variabilis as Bioindicator in the Gulf of Suez, Egypt. Int. J. Mar. Sci. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Ajao, E.A. Review of the State of Pollution of the Lagos Lagoon. NIOMR Technical Paper No. 106; Nigerian Institute for Oceanography and Marine Research: Lagos, Nigeria, 1996. [Google Scholar]
- Oyewo, E.O. Industrial Sources and Distribution of Heavy Metals in Lagos Lagoon and their Biological Effects on Estuarine Animals. PhD Thesis, University of Lagos, Lagos, Nigeria, 1998. [Google Scholar]
- Markert, B.; Friese, K. Trace Elements: Their Distribution and Effects in the Environment; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Otitoloju, A.K. Joint Action Toxicity of Heavy Metals and their Bioaccumulation by Benthic Animals of the Lagos Lagoon. Ph.D. Thesis, University of Lagos, Lagos, Nigeria, 2002. [Google Scholar]
- Don-Pedro, K.N.; Oyewo, E.O.; Otitoloju, A.A. Trend of heavy metal concentrations in Lagos lagoon ecosystem, Nigeria. West Afr. J. Appl. Ecol. 2004, 5, 103–114. [Google Scholar] [CrossRef]
- Amaeze, N.H.; Egonmwan, R.I.; Jolaoso, A.F.; Otitoloju, A.A. Coastal environmental pollution and fish species diversity in Lagos Lagoon, Nigeria. Int. J. Environ. Prot. 2012, 2, 8–16. [Google Scholar]
- Alo, B.; Olayinka, K.; Oyeyiola, A.; Oluseyi, T.; Alani, R.; Abayomi, A. Studies and transactions on pollution assessment of the Lagos Lagoon System, Nigeria. In Land/Ocean Interactions in the Coastal Zone of West and Central Africa; Springer: Cham, Switzerland, 2014; pp. 65–76. [Google Scholar]
- Kafilat Adebola, B.A.; Joseph Kayode, S.; Adebayo Akeem, O. Integrated assessment of the heavy metal pollution status and potential ecological risk in the Lagos Lagoon, South West, Nigeria. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 377–397. [Google Scholar] [CrossRef]
- Ajagbe, F.E.; Osibona, A.O.; Otitoloju, A.A. Diversity of the Edible Fishes of the Lagos Lagoon, Nigeria and the Public Health Concerns Based on their Lead (Pb) Content. Int. J. Fish. Aquac. 2012, 4, 55–62. [Google Scholar] [CrossRef]
- Olatunji, A.S.; Abimbola, A.F. Geochemical evaluation of the Lagos Lagoon sediments and water. World Appl. Sci. J. 2010, 9, 178–193. [Google Scholar]
- Babatunde, E.E.; Samuel, O.B. Comparative study of mercury accumulation in some brackish water fishes in a tropical lagoon and its adjacent creek in south western Nigeria. Afr. J. Environ. Sci. Technol. 2009, 3, 180–185. [Google Scholar]
- Williams, A.B.; Edobor-Osoh, A. Assessment of trace metal levels in fish species of Lagos Lagoon. Assess. Trace Met. Levels Fish Species Lagos Lagoon 2013, 2, 1–5. [Google Scholar] [CrossRef]
- NWRI (National Water Research Institute). Canada Centre for Inland Waters National Laboratory for Environmental Testing Certified Reference, Materials & Quality Assurances Services (Version 5.7); NWRI: Burlington, ON, Canada, 2006. [Google Scholar]
- Snape, I.; Scouller, R.C.; Stark, S.C.; Stark, J.; Riddle, M.J.; Gore, D.B. Characterisation of the dilute HCl extraction method for the identification of metal contamination in Antarctic marine sediments. Chemosphere 2004, 57, 491–504. [Google Scholar] [CrossRef]
- Townsend, A.T.; Palmer, A.S.; Stark, S.C.; Samson, C.; Scouller, R.C.; Snape, I. Trace metal characterisation of marine sediment reference materials MESS-3 and PACS-2 in dilute HCl extracts. Mar. Pollut. Bull. 2007, 54, 236–239. [Google Scholar] [CrossRef]
- Okoye, B.C.; Afolabi, O.A.; Ajao, E.A. Heavy metals in the Lagos Lagoon sediments. Int. J. Environ. Stud. 1991, 37, 35–41. [Google Scholar] [CrossRef]
- Otitoloju, A.A. Evaluation of the joint-action toxicity of binary mixtures of heavy metals against the mangrove periwinkle Tympanotonus fuscatus var. radula (L.). Ecotoxicol. Environ. Saf. 2002, 53, 404–415. [Google Scholar] [CrossRef]
- Otitoloju, A.A.; Don-Pedro, K.N. Establishment of the toxicity ranking order of heavy metals and sensitibity scale of benthic animals inhabiting the Lagos lagoon. West Afr. J. Appl. Ecol. 2002, 3, 31–41. [Google Scholar] [CrossRef]
- Sharifuzzaman, S.M.; Rahman, H.; Ashekuzzaman, S.M.; Islam, M.M.; Chowdhury, S.R.; Hossain, M.S. Heavy metals accumulation in coastal sediments. In Environmental Remediation Technologies for Metal-Contaminated Soils; Springer: Tokyo, Japan, 2016; pp. 21–42. [Google Scholar]
- Qiu, Y.W. Bioaccumulation of heavy metals both in wild and mariculture food chains in Daya Bay, South China. Estuar. Coast. Shelf Sci. 2015, 163, 7–14. [Google Scholar] [CrossRef]
- Helaluddin, A.B.; Khalid, R.S.; Alaama, M.; Abbas, S.A. Main analytical techniques used for elemental analysis in various matrices. Trop. J. Pharm. Res. 2016, 15, 427–434. [Google Scholar] [CrossRef]
- Baysal, A.; Ozbek, N.; Akman, S. Determination of trace metals in waste water and their removal processes. In Waste-Water Treatment Technologies and Recent Analytical Developments; Einschlag, F.S.G., Carlos, L., Eds.; Intech Open: London, UK, 2013; pp. 145–171. [Google Scholar]
- Pyle, S.M.; Nocerino, J.M.; Deming, S.N.; Palasota, J.A.; Palasota, J.M.; Miller, E.L.; Hillman, D.C.; Kuharic, C.A.; Cole, W.H.; Fitzpatrick, P.M.; et al. Comparison of AAS, ICP-AES, PSA, and XRF in determining lead and cadmium in soil. Environ. Sci. Technol. 1995, 30, 204–213. [Google Scholar] [CrossRef]
- Rasdi, F.; Bakar, N.; Mohamad, S. A comparative study of selected trace element content in Malay and Chinese traditional herbal medicine (THM) using an inductively coupled plasma-mass spectrometer (ICP-MS). Int. J. Mol. Sci. 2013, 14, 3078–3093. [Google Scholar] [CrossRef]
- Santos-Echeandía, J.; Prego, R.; Cobelo-García, A. Influence of the heavy fuel spill from the Prestige tanker wreckage in the overlying seawater column levels of copper, nickel and vanadium (NE Atlantic Ocean). J. Mar. Syst. 2008, 72, 350–357. [Google Scholar] [CrossRef]
- Manousakas, M.; Papaefthymiou, H.; Diapouli, E.; Migliori, A.; Karydas, A.G.; Bogdanovic-Radovic, I.; Eleftheriadis, K. Assessment of PM2.5 sources and their corresponding level of uncertainty in a coastal urban area using EPA PMF 5.0 enhanced diagnostics. Sci. Total Environ. 2017, 574, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Solomon, F. Impacts of copper on aquatic ecosystems and human health. Environ. Communities 2009, 15, 25–29. [Google Scholar]
- Srinivasan, M.; Swain, G.W. Managing the use of copper-based antifouling paints. Environ. Manag. 2007, 39, 423–441. [Google Scholar] [CrossRef]
- Abiodun, O.A.; Oyeleke, P.O. Analysis and seasonal distribution of some heavy metals in sediment of Lagos lagoon using environmental pollution indices. Phys. Sci. Int. J. 2016, 10. [Google Scholar] [CrossRef]
- Kersten, M.; Garbe-Schönberg, C.D.; Thomsen, S.; Anagnostou, C.; Sioulas, A. Source apportionment of Pb pollution in the coastal waters of Elefsis Bay, Greece. Environ. Sci. Technol. 1997, 31, 1295–1301. [Google Scholar] [CrossRef]
- Amaeze, N.H.; Adeyemi, R.O.; Adebesin, A.O. Oxidative stress, heats shock protein and histopathological effects in the gills of African catfish, Clarias gariepinus induced by bridge runoffs. Environ. Monit. Assess. 2015, 187, 172. [Google Scholar] [CrossRef]

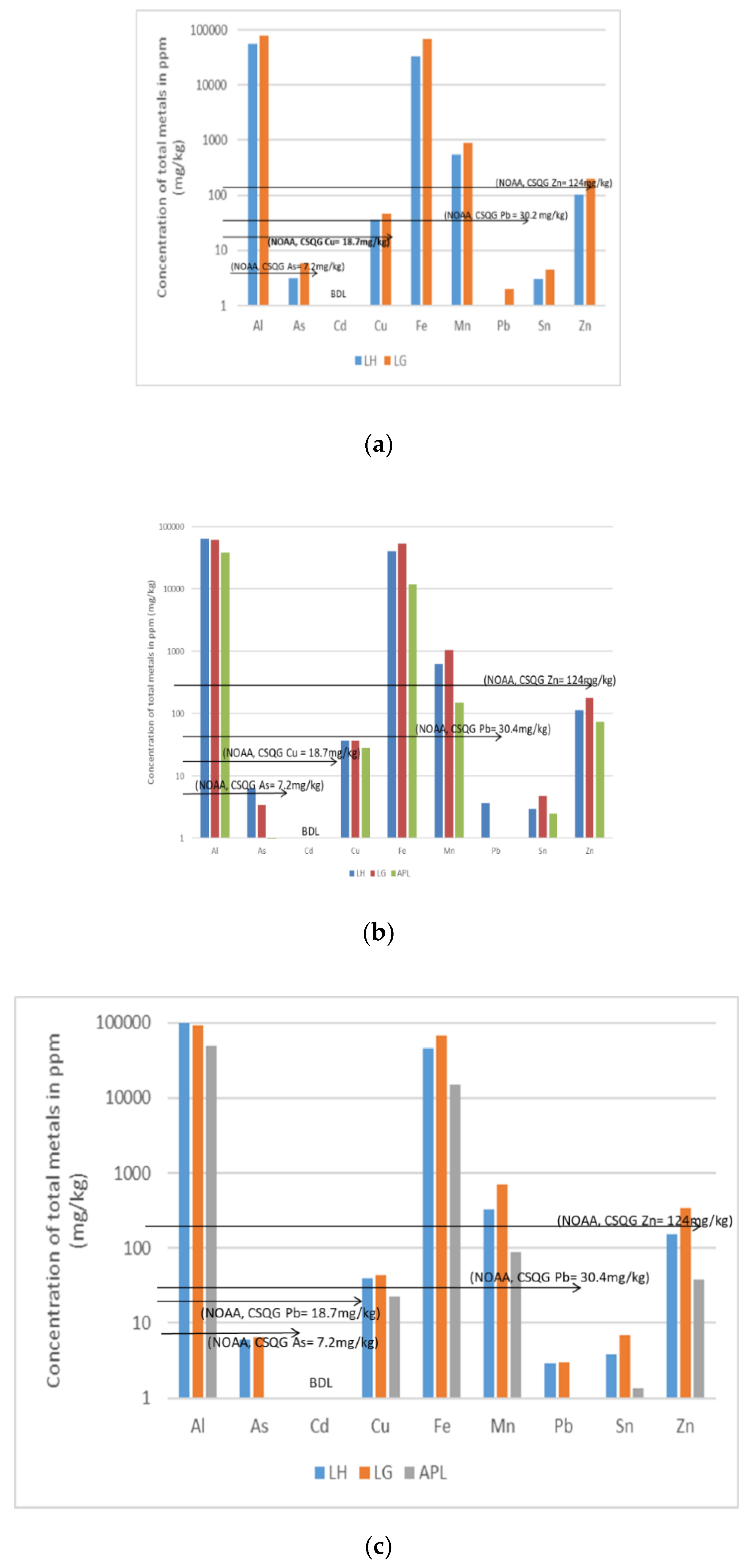
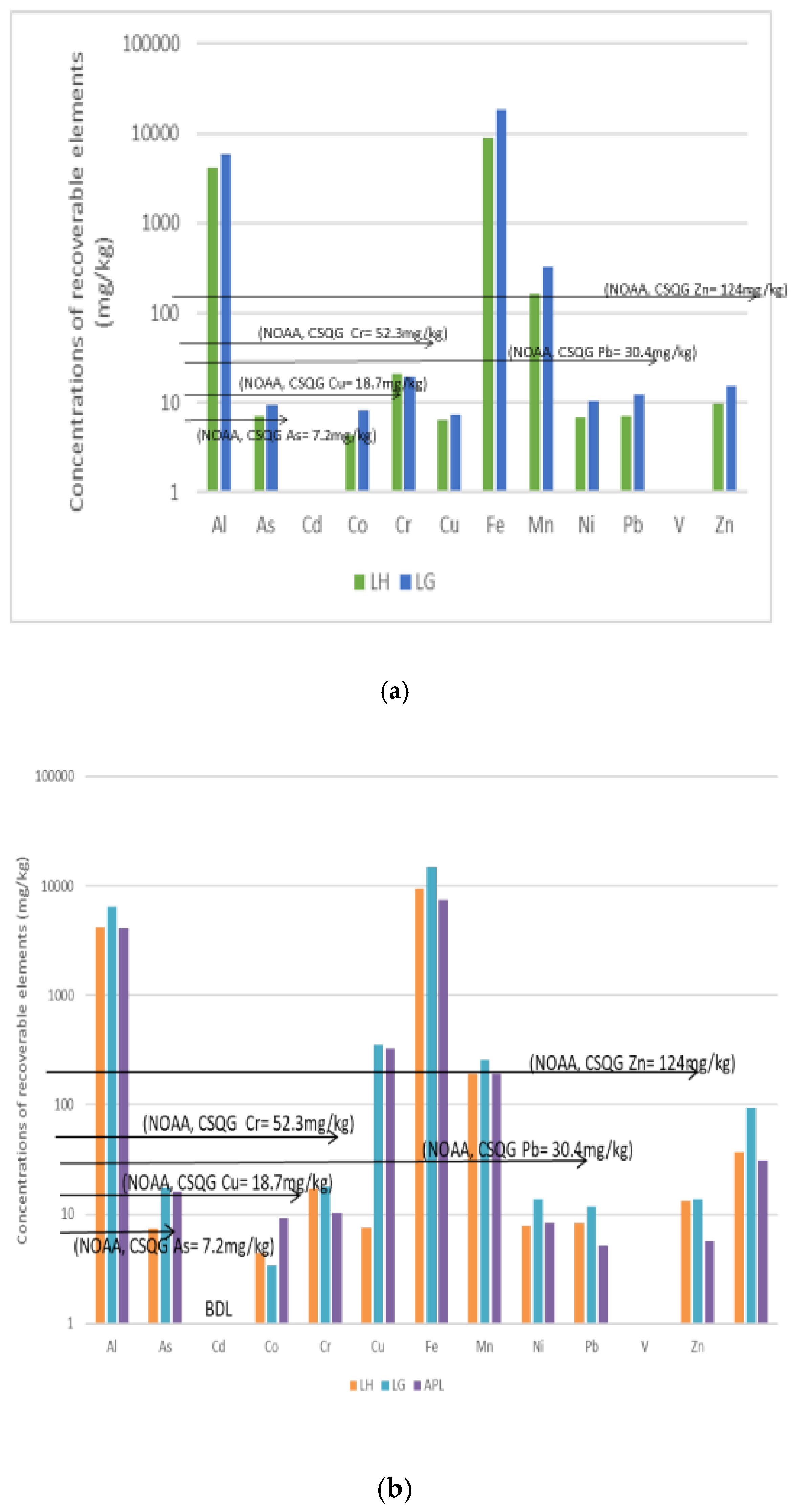
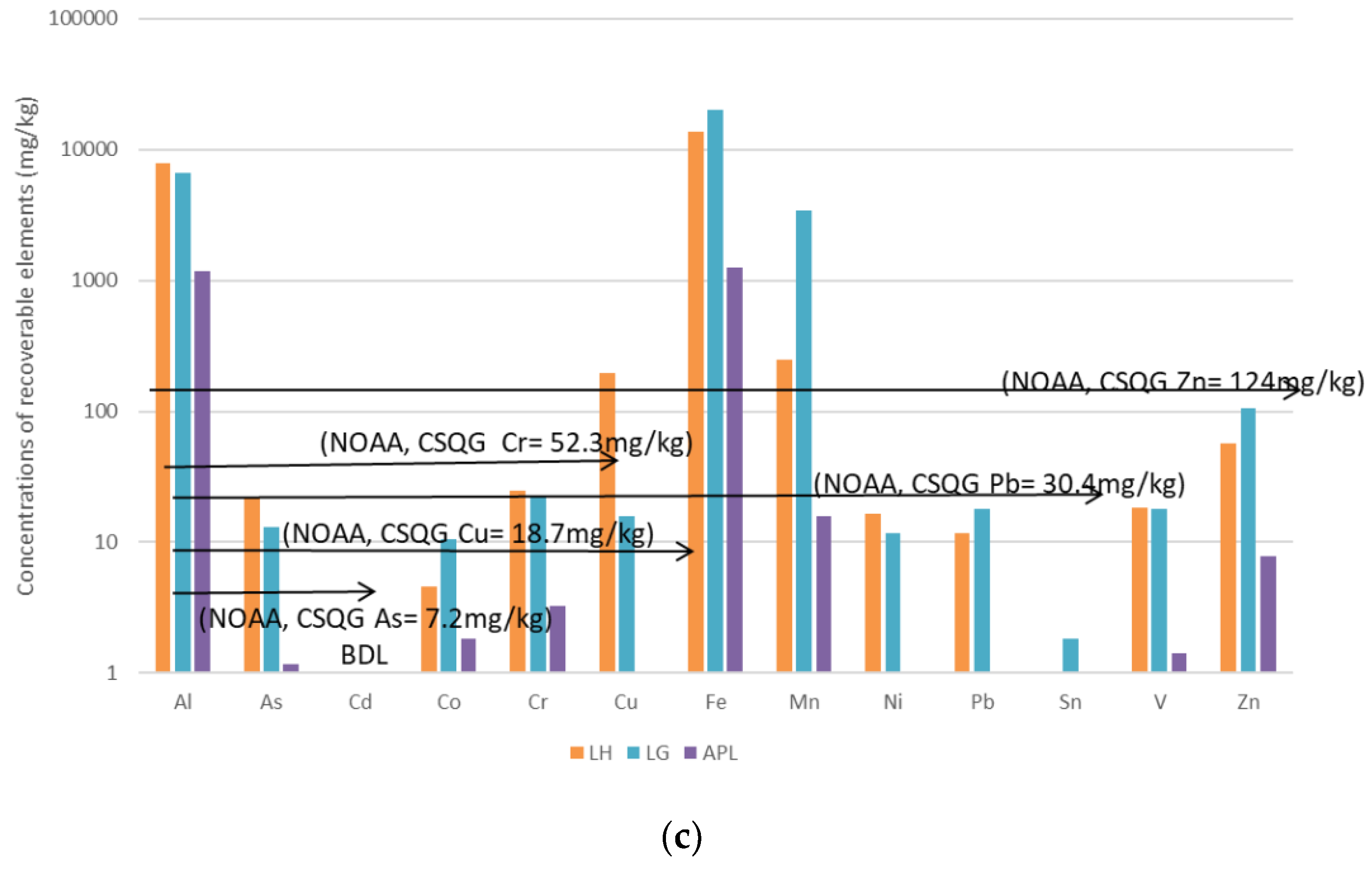
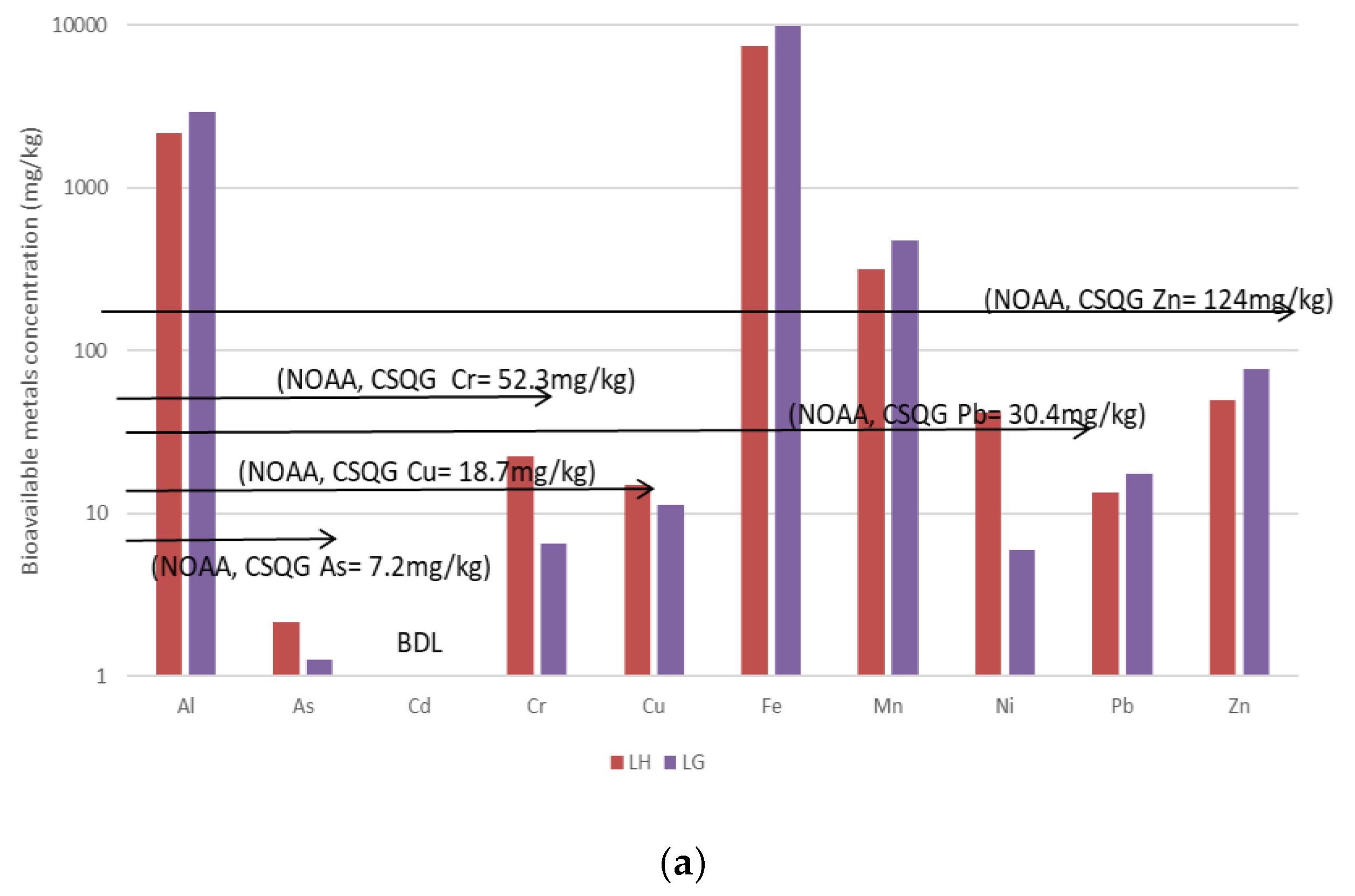
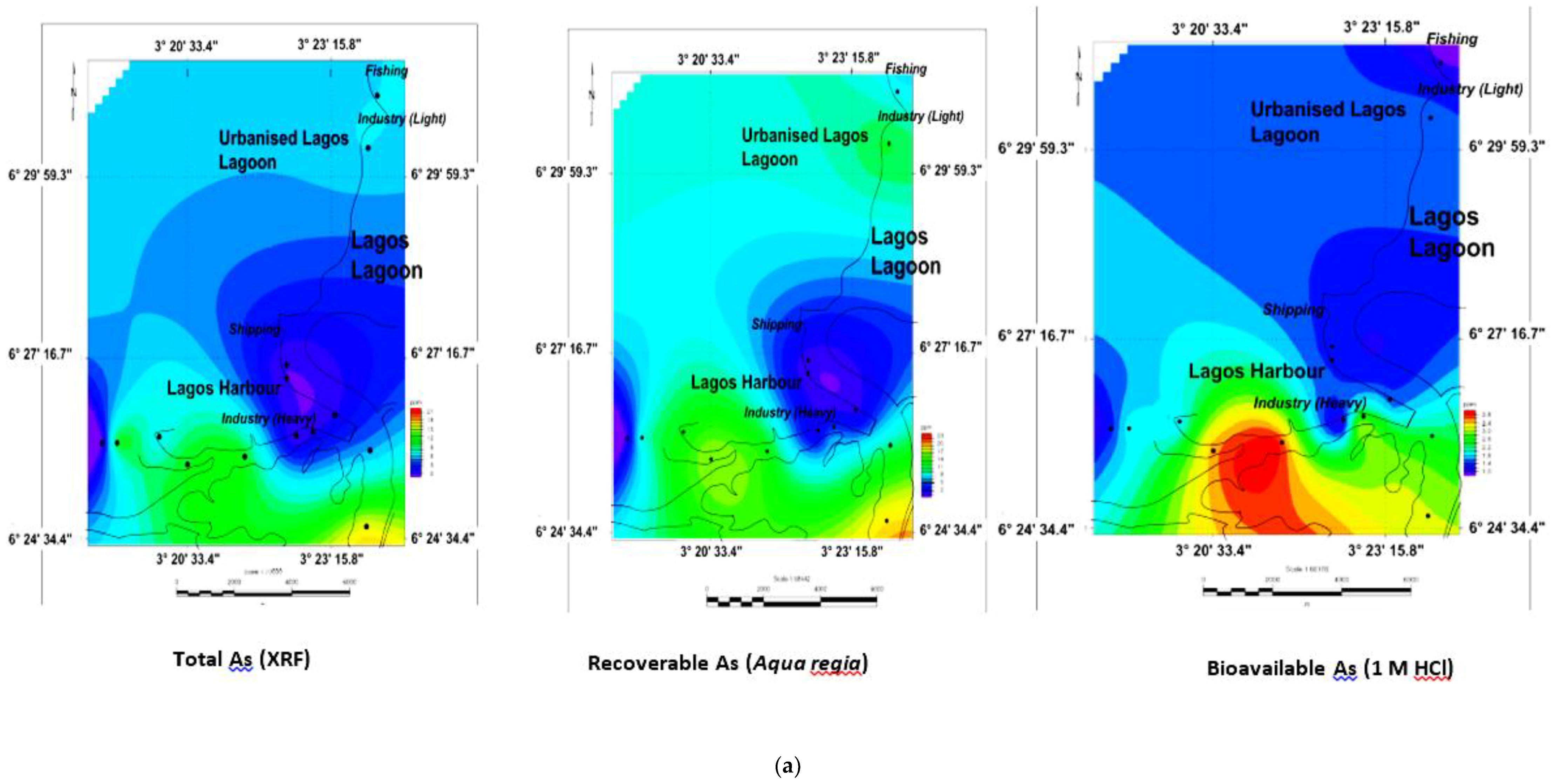
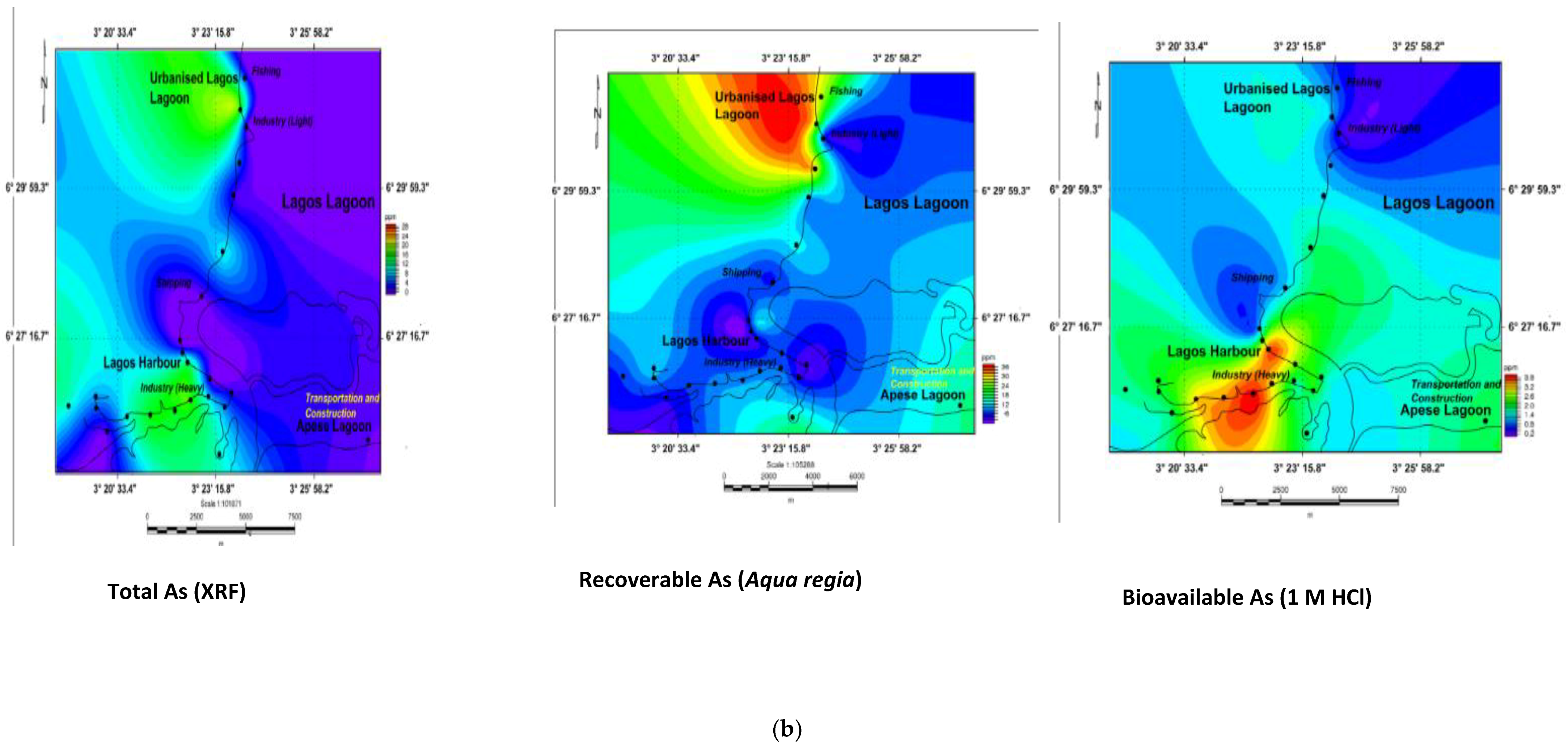
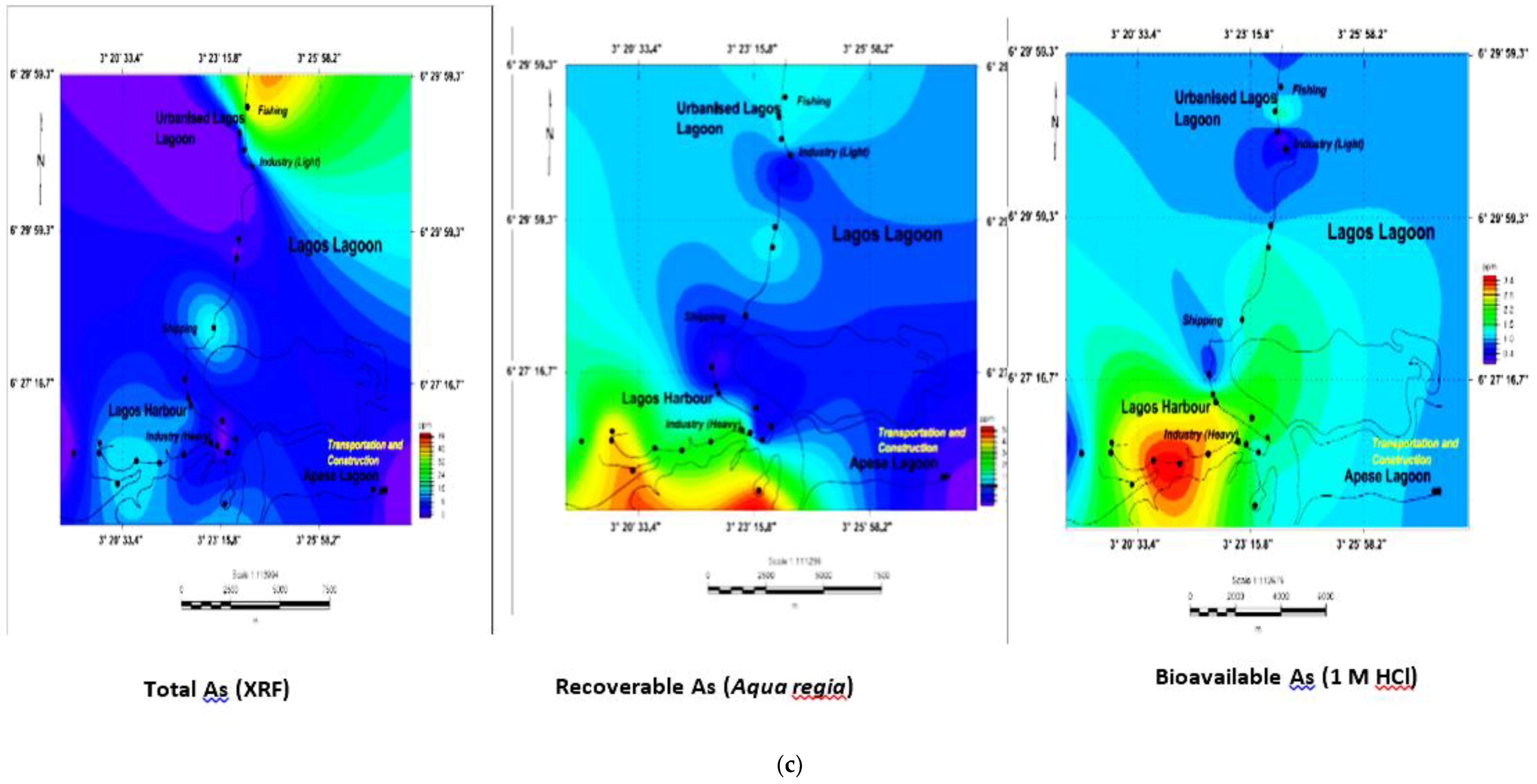
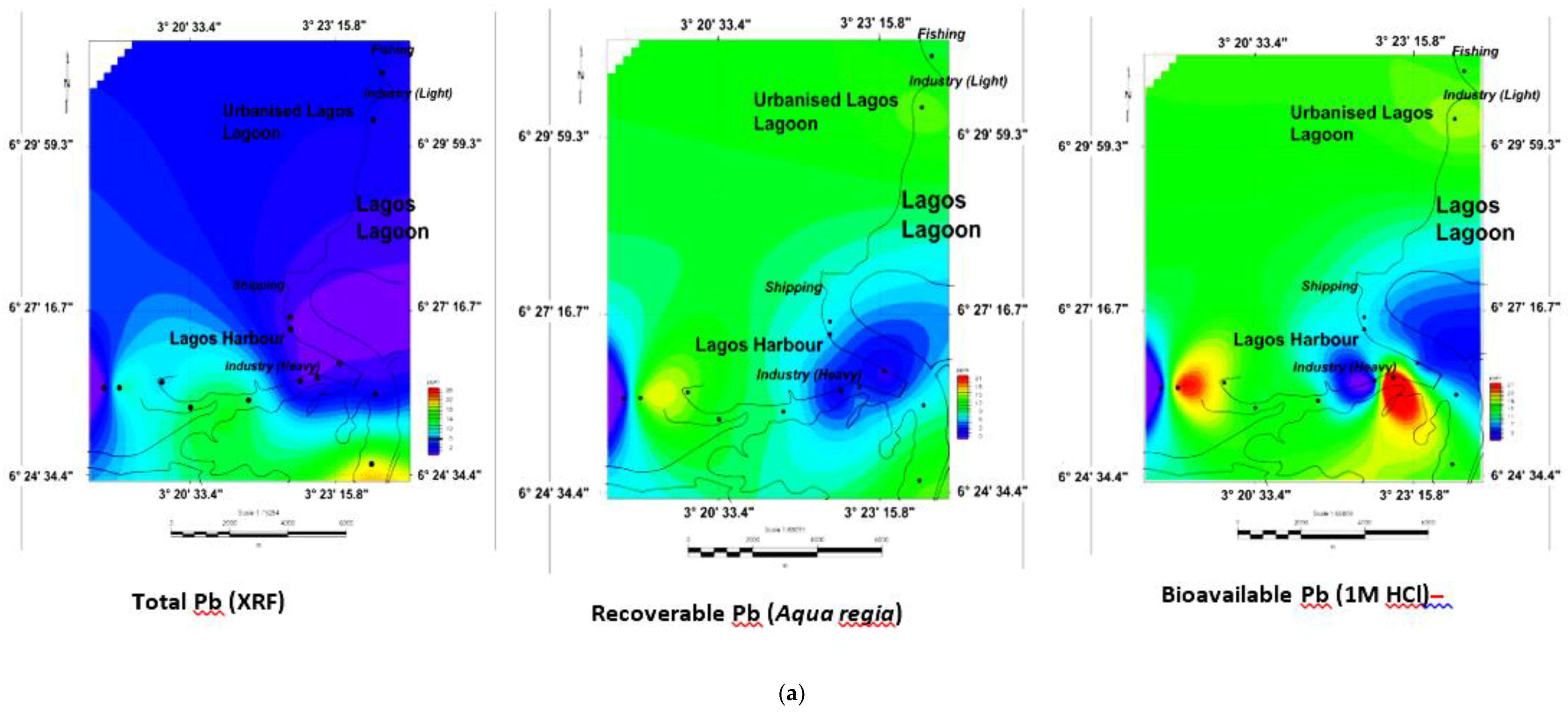
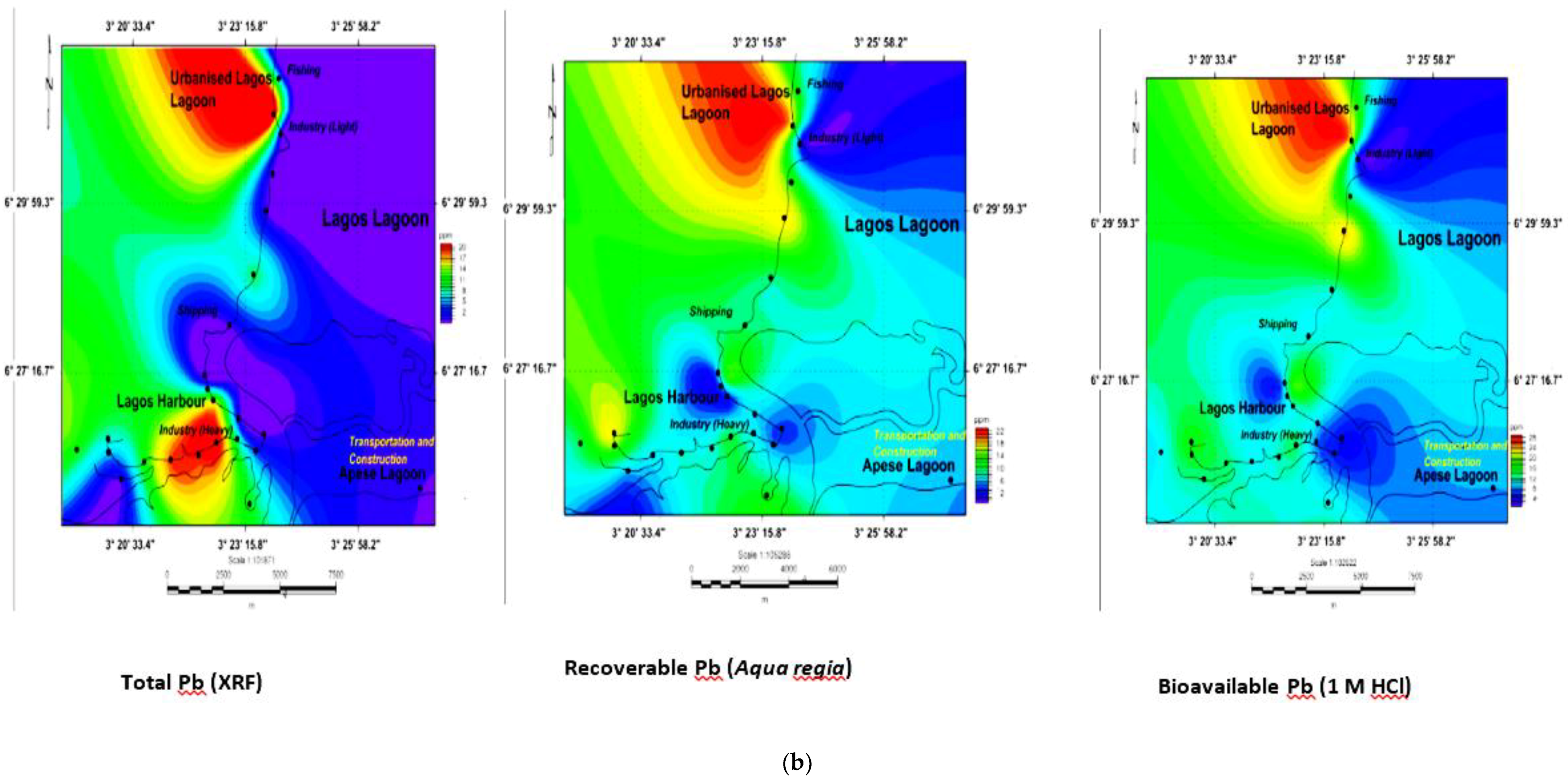
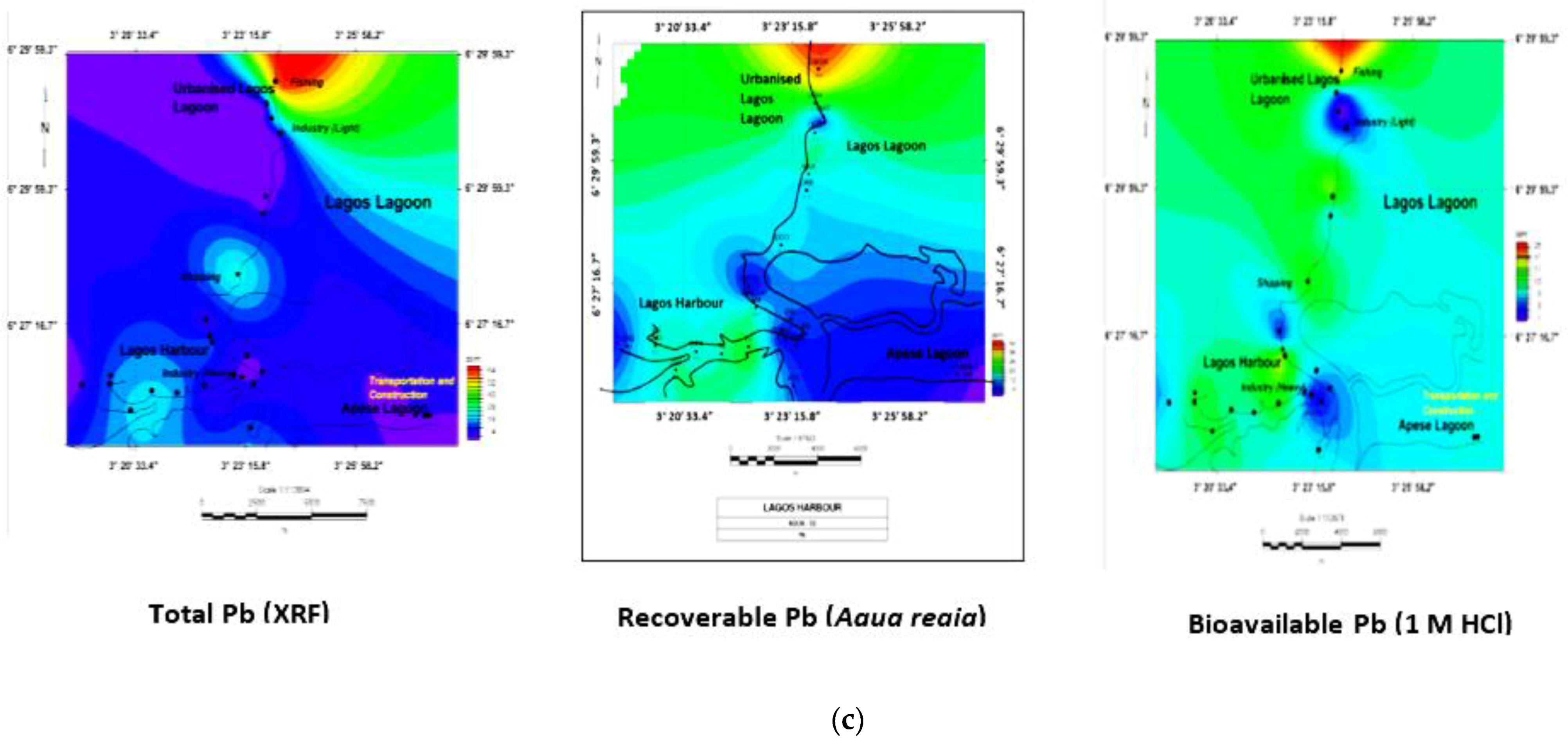
| Location | Methods and Elements | Sources | Comments | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lagos Harbour | Total (XRF) | Recoverable (Aqua Regia) | Bioavailable (I M HCl) | ||||||||
| DS1 | DS2 | WS | DS1 | DS2 | WS | DS1 | DS2 | WS | |||
| Cu, Sn, | Cu, Sn, Zn | Cu, Sn | As, Cu, Sn, Ni | Sn | Ni | Ni | Ni |
| Cu, Sn, Zn, Ni and As exceeded the NOAA/CSQGs TEL | ||
| Lagos Lagoon | Total (XRF) | Recoverable (Aqua-Regia) | Bioavailable (I M HCl) | ||||||||
| DS1 | DS2 | WS | DS1 | DS2 | WS | DS1 | DS2 | WS | |||
| Cu, Sn, Zn | Cu, Sn, Zn | Cu, Sn, Zn | As, Sn | As, Sn | As, Cu | Cd | Ni |
| Cu, Sn, Zn, Ni and As exceeded the NOAA/CSQGs TEL | ||
| Apese Lagoon | Total (XRF) | Recoverable (Aqua-Regia) | Bioavailable (I M HCl) | ||||||||
| DS1 | DS2 | WS | DS1 | DS2 | WS | DS1 | DS2 | WS | |||
| No Sampling | Cu, Sn | Cu, Sn | No Sampling | As, Cu | No Sampling | No Significant value |
| Cu, Sn and As exceeded the NOAA/CSQGs TEL | |||
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamanga, A.; Amaeze, N.H.; Al-Anzi, B. Comparative Investigation of Total, Recoverable and Bioavailable Fractions of Sediment Metals and Metalloids in the Lagos Harbour and Lagoon System. Sustainability 2019, 11, 4339. https://doi.org/10.3390/su11164339
Bamanga A, Amaeze NH, Al-Anzi B. Comparative Investigation of Total, Recoverable and Bioavailable Fractions of Sediment Metals and Metalloids in the Lagos Harbour and Lagoon System. Sustainability. 2019; 11(16):4339. https://doi.org/10.3390/su11164339
Chicago/Turabian StyleBamanga, Awwal, Nnamdi Henry Amaeze, and Bader Al-Anzi. 2019. "Comparative Investigation of Total, Recoverable and Bioavailable Fractions of Sediment Metals and Metalloids in the Lagos Harbour and Lagoon System" Sustainability 11, no. 16: 4339. https://doi.org/10.3390/su11164339
APA StyleBamanga, A., Amaeze, N. H., & Al-Anzi, B. (2019). Comparative Investigation of Total, Recoverable and Bioavailable Fractions of Sediment Metals and Metalloids in the Lagos Harbour and Lagoon System. Sustainability, 11(16), 4339. https://doi.org/10.3390/su11164339





