Life Cycle Assessment of Seabass (Dicentrarchus labrax) Produced in Offshore Fish Farms: Variability and Multiple Regression Analysis
Abstract
1. Introduction
2. Materials and Methods
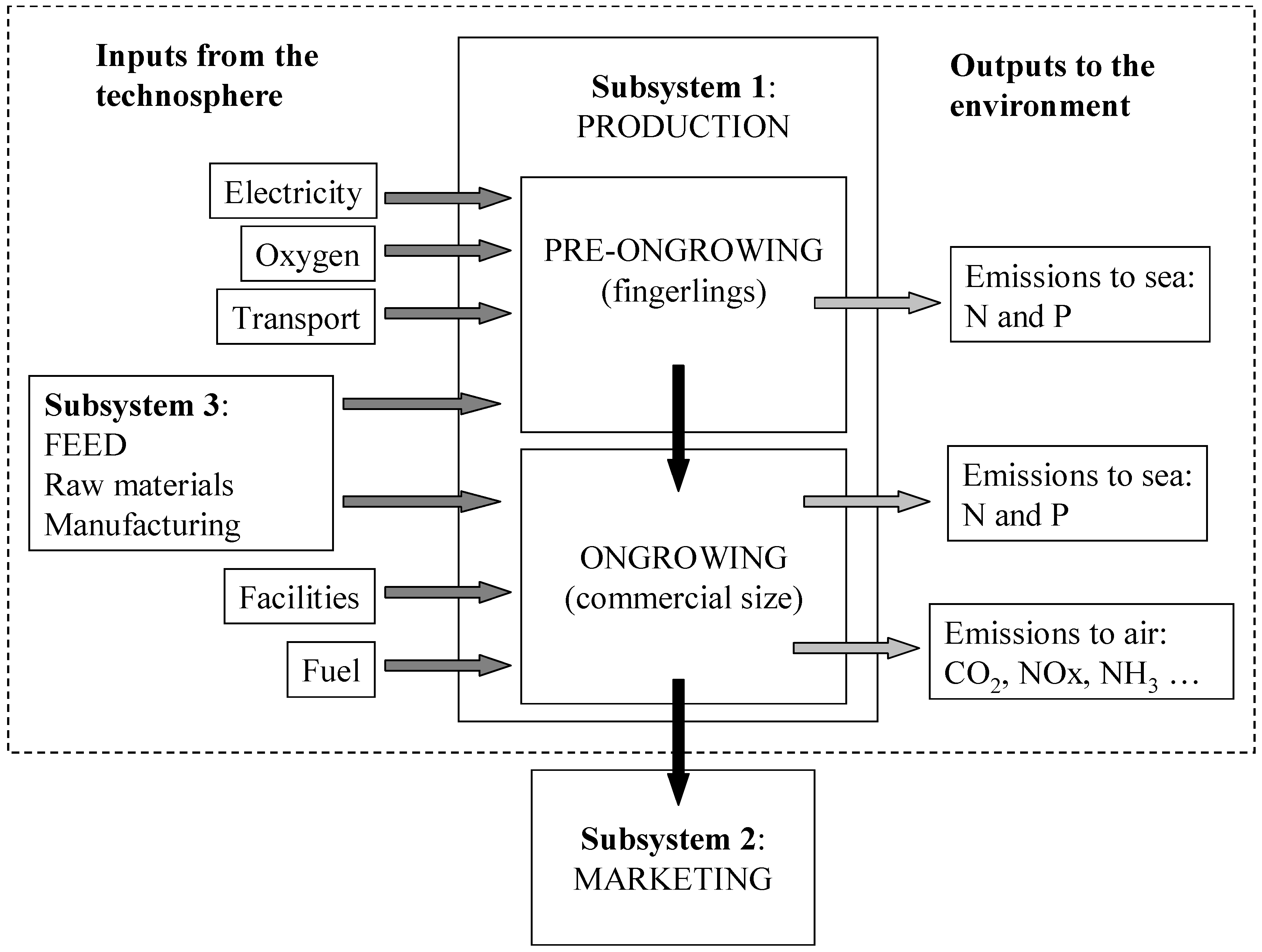
2.1. Description of the System
2.2. Goal and Scope
2.3. Data Collection and Life Cycle Inventory
2.3.1. Pre-Ongrowing
2.3.1.1. Fingerlings Feed
2.3.1.2. Electricity and Oxygen
- D: density of the liquid (kg·L−1), in this case seawater (1.027 kg·L−1).
- H: Manometric height (m). A value of 10 m was considered.
- R: Pump performance, which can vary between 0.65 and 0.75. A value of 0.7 was assumed.
- WF: Water flow (L·s−1).
2.3.2.3. Emissions of N and P
2.3.2.4. Transport
2.3.2. Facilities
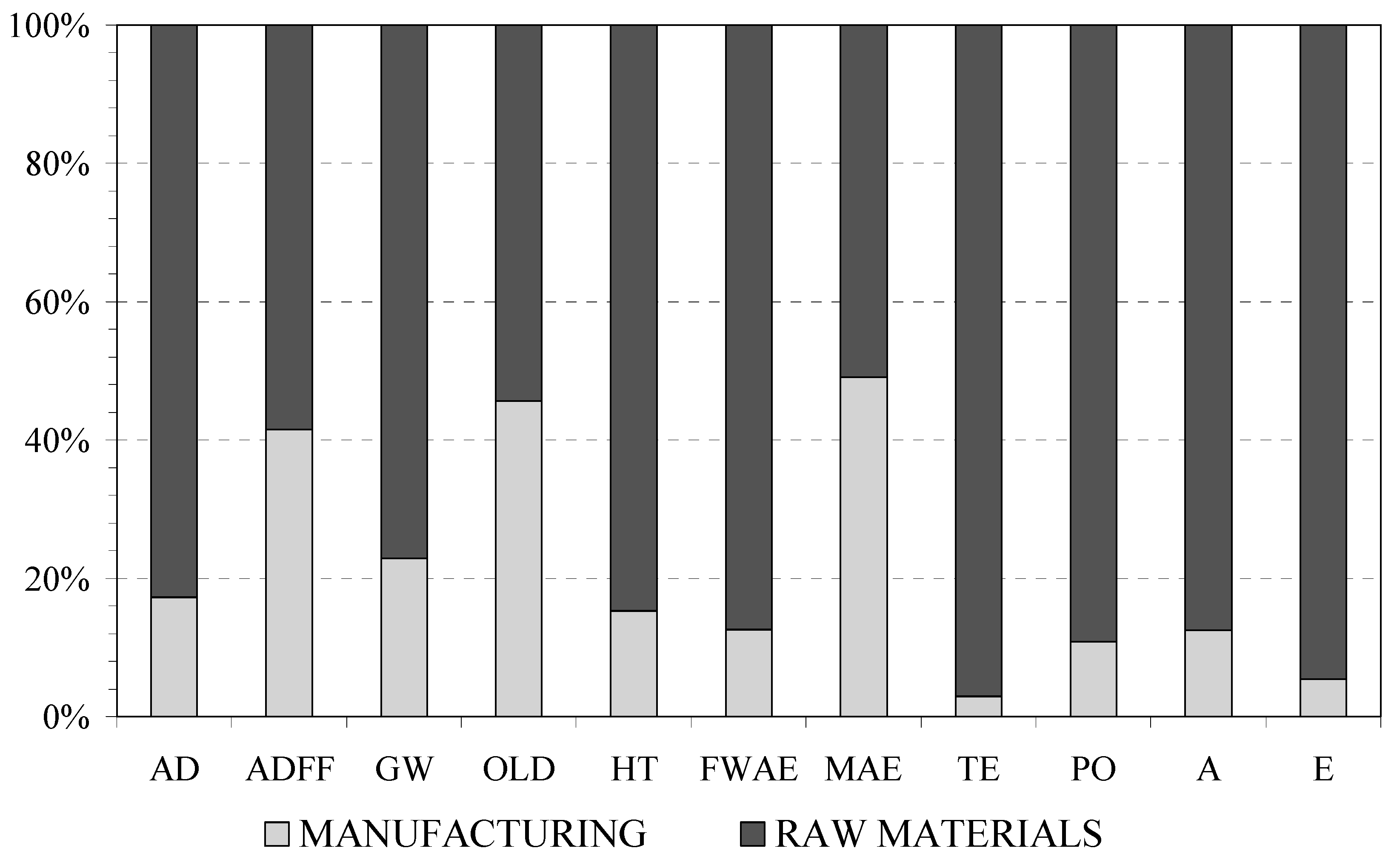
2.3.3. Feed
2.3.4. Growth
2.3.5. Fuel
2.4. Evaluation of the Impact of the Life Cycle
2.5. Interpretation of the Results
2.5.1. Uncertainty Analysis
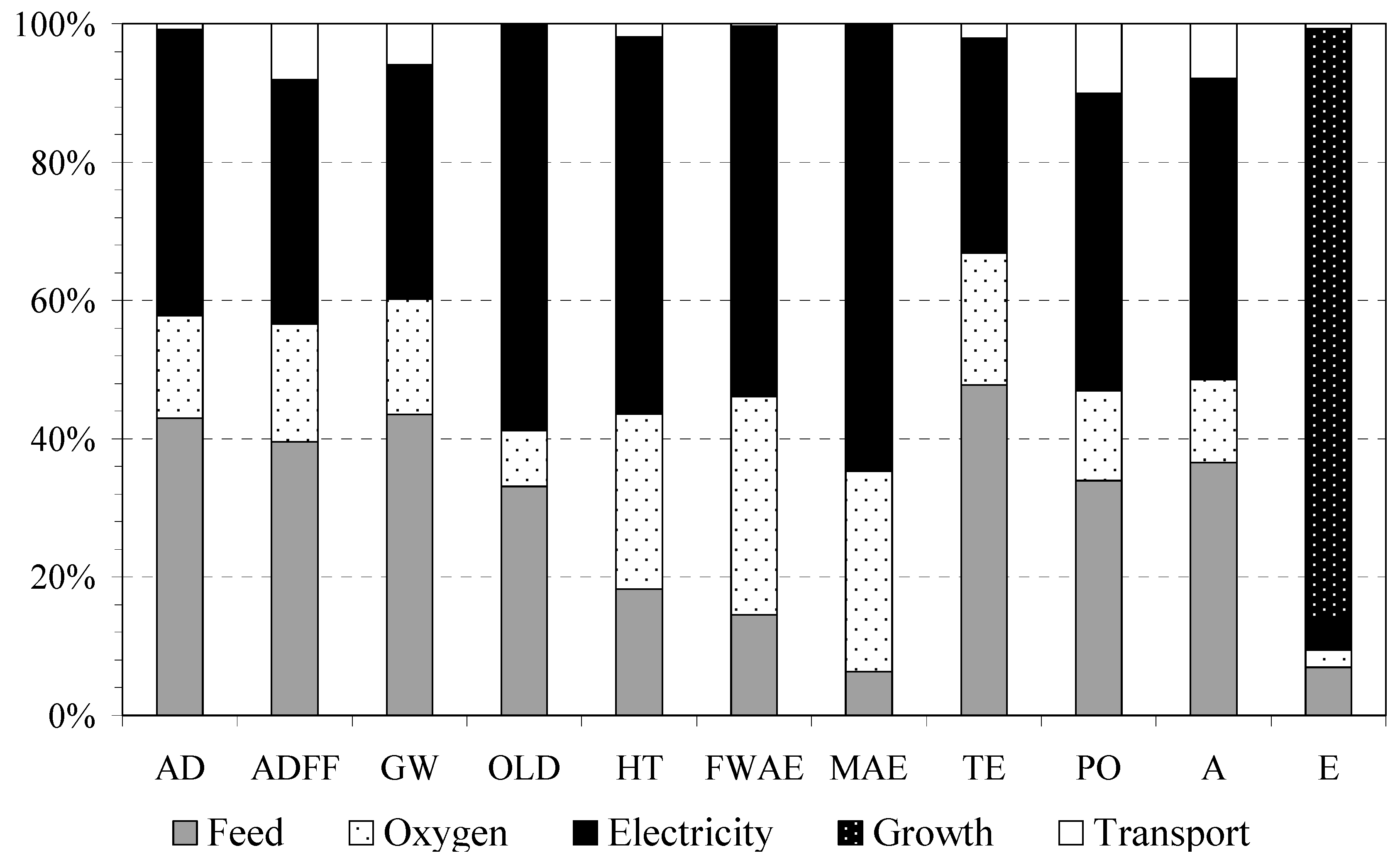
2.5.2. Contribution Analysis
2.5.3. Sensitivity Analysis and Multiple Regression
3. Results
3.1. Uncertainty Analysis
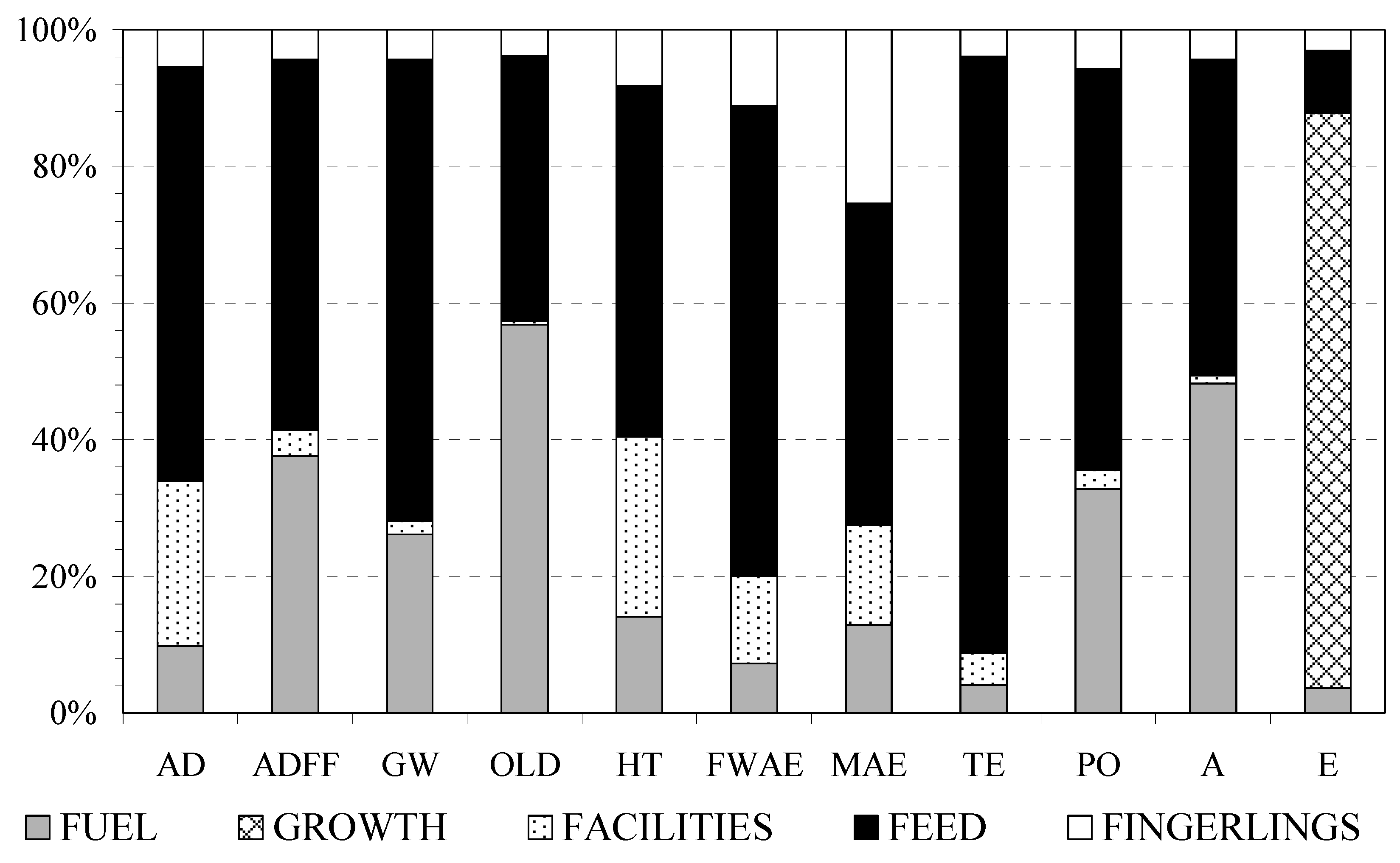
3.2. Contribution Analysis
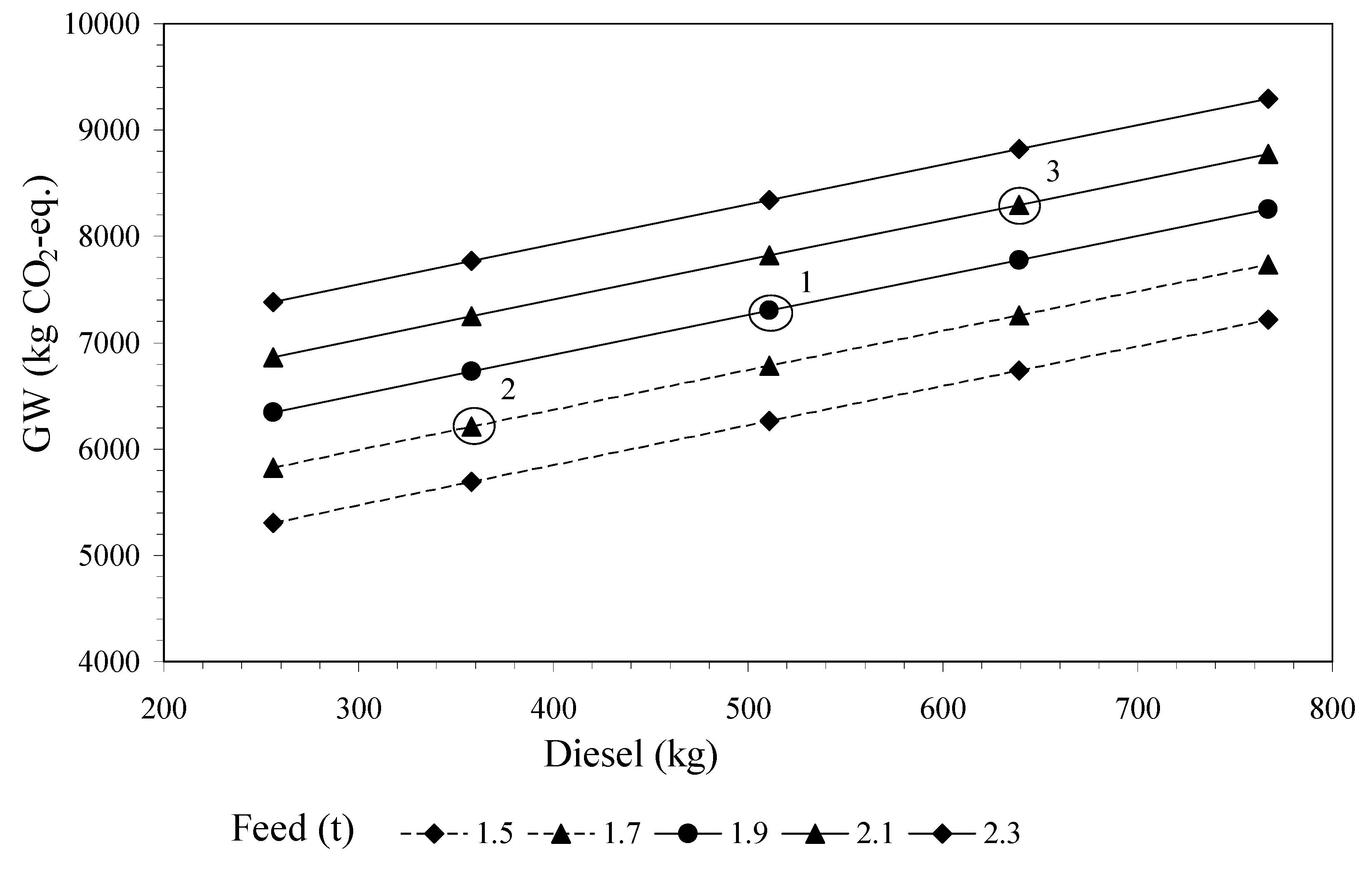
3.3. Sensitivity Analysis and Development of Equations
3.3.1. Selection of Independent Variables
3.3.2. Multiple Regression Analysis
4. Discussion
4.1. System Components
4.1.1. Fingerlings
4.1.2. Facilities
4.1.3. Feed
4.1.4. Growth
4.1.5. Fuel
4.2. Sensitivity Analysis
4.2.1. Variability of the Independent Variables: Feed and Diesel
4.2.2. The Explanatory Equations of the Variability of the Impacts
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ISO Environmental Management-Life Cycle Assessment: Principles and Framework; ISO 14040; ISO-International Organization for Standards: Geneva, Switzerland, 2006.
- Jerbi, M.A.; Aubin, J.; Achour, L.; Kacem, A. Life cycle assessment (LCA) of two rearing techniques of sea bass (Dicentrarchus labrax). Aquac. Eng. 2012, 46, 1–9. [Google Scholar] [CrossRef]
- Ayer, N.W.; Tyedmers, P.H. Assesing alternative aquaculture technologies: Life cycle assessment of salmonid culture systems in Canada. J. Clean. Prod. 2009, 17, 362–373. [Google Scholar] [CrossRef]
- Samuel-Fitwi, B.; Schroeder, J.P.; Schulz, C. System delimitation in life cycle assessment (LCA) of aquaculture: Striving for valid and comprehensive environmental assessment using rainbow trout farming as a case study. Int. J. Life Cycle Assess. 2013, 18, 577–589. [Google Scholar] [CrossRef]
- Aubin, J.; Papatryphon, E.; Van der Werf, H.M.G.; Chatzifotis, S. Assesment of the environmental impact of carnivorous finfish production systems using life cycle assessment. J. Clean. Prod. 2009, 17, 354–361. [Google Scholar] [CrossRef]
- Aubin, J.; Papatryphon, E.; Van der Werf, H.M.G.; Petit, J.; Morvan, Y.M. Characterisation of the environmental impact of a turbot (Scophthalmus maximus) re-circulating production system using Life Cycle Assessment. Aquaculture 2006, 261, 1259–1268. [Google Scholar] [CrossRef]
- Iribarren, D.; Moreira, M.T.; Feijoo, G. Life Cycle Assessment of Aquaculture Feed and Application to the Turbot Sector. Int. J. Environ. Res. 2012, 6, 837–848. [Google Scholar]
- García García, B.; Rosique Jiménez, C.; Aguado-Giménez, F.; García García, J. Life cycle assessment of gilthead seabream (Sparus aurata) production in offshore fish farms. Sustainability 2016, 8, 1228. [Google Scholar] [CrossRef]
- Pelletier, N.; Tyedmers, P.; Sonesson, U.; Scholz, A.; Ziegler, F.; Flysjo, A.; Kruse, S.; Cancino, B.; Silverman, H. Not all salmon are created equal: Life cycle assessment (LCA) of global salmon farming systems. Environ. Sci. Technol. 2009, 43, 8730–8736. [Google Scholar] [CrossRef]
- Abdou, K.; Aubin, J.; Romdhane, M.S.; Le Loc’h, F.; Lasram, F.B.R. Environmental assessment of seabass (Dicentrarchus labrax) and seabream (Sparus aurata) farming from a life cycle perspective: A case study of a Tunisian aquaculture farm. Aquaculture 2017, 471, 204–212. [Google Scholar] [CrossRef]
- Boissy, J.; Aubin, J.; Drissi, A.; Van der Werf, H.M.G.; Bell, G.J.; Kaushik, S.J. Environmental impacts of plant-based salmonid diets at feed and farm scales. Aquaculture 2011, 321, 61–70. [Google Scholar] [CrossRef]
- Basto-Silva, C.; Guerreiro, I.; Oliva-Teles, A.; Neto, B. Life cycle assessment of diets for gilthead seabream (Sparus aurata) with different protein/carbohydrate ratios and fishmeal or plant feedstuffs as main protein sources. Int. J. Life Cycle Assess. 2019. [Google Scholar] [CrossRef]
- Roque d’Orbcastel, E.; Blancheton, J.P.; Aubin, J. Towards environmentally sustainable aquaculture: Comparison between two trout farming systems using Life Cycle Assessment. Aquac. Eng. 2009, 40, 113–119. [Google Scholar] [CrossRef]
- Bohnes, F.A.; Hauschild, M.Z.; Shlundt, J.; Laurent, A. Life cycle assessments of aquaculture systems: A critical review of reported findings with recommendations for policy and system development. Rev. Aquac. 2018, 1–19. [Google Scholar] [CrossRef]
- Samuel-Fitwi, B.; Nagel, F.; Meyer, S.; Schroeder, J.P.; Schulz, C. Comparative life cycle assessment (LCA) of raising rainbow trout (Oncorhynchus mykiss) in different production systems. Aquac. Eng. 2013, 54, 85–92. [Google Scholar] [CrossRef]
- Cerezo Valverde, J.; García García, B. The effect of oxygen levels on oxygen consumption, survival and ventilatory frequency of sharpsnout sea bream (Diplodus puntazzo Gmelin, 1789) at different conditions of temperature and fish weiht. J. Appl. Ichthyol. 2004, 20, 488–492. [Google Scholar] [CrossRef]
- Muller-Feuga, A.; Petit, J.; Sabaut, J.J. The influence of temperature and wet weight on the oxygen demand of rainbow trout (Salmo gairdneri R.) in fresh water. Aquaculture 1978, 14, 355–363. [Google Scholar] [CrossRef]
- García García, B.; Cerezo Valverde, J.; Gómez, E.; Hernández, M.D.; Aguado-Giménez, F. Ammonia excretion of octopus (Octopus vulgaris) in relation to body weight and protein intake. Aquaculture 2011, 319, 162–167. [Google Scholar] [CrossRef]
- Aguado-Giménez, F.; García García, B. Growth and food intake models in Octopus vulgaris Cuvier (1797): Influence of body weight, temperature, sex and diet. Aquac. Int. 2003, 10, 361–377. [Google Scholar] [CrossRef]
- Björnsson, B.; Steinarsson, A.; Árnason, T. Growth model for Atlantic Cod (Gadus morhua): Effects of temperature and body weight on growth rate. Aquaculture 2007, 271, 216–226. [Google Scholar] [CrossRef]
- García García, B.; Cerezo Valverde, J.; Aguado-Giménez, F.; García García, J.; Hernádez, M.D. Effect of the interaction between body weight and temperature on growth and maximum daily food intake in sharpsnout sea bream (Diplodus puntazzo). Aquac. Int. 2011, 19, 131–141. [Google Scholar] [CrossRef]
- García García, B.; Cerezo Valverde, J.; Aguado-Giménez, F.; García García, J.; Hernádez, M.D. Growth and mortality of common octopus Octopus vulgaris reared at different stoking densities in Mediterranean cages. Aquac. Res. 2009, 40, 1202–1212. [Google Scholar] [CrossRef]
- Handeland, S.O.; Imsland, A.; Stefansson, S. The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 2008, 283, 36–42. [Google Scholar] [CrossRef]
- Imsland, A.K.; Sunde, L.M.; Folkvord, A.; Stefansson, S.O. The interaction between temperature and size on growth of juvenile turbot (Scophthalmus maximus Rafinesque). J. Fish Biol. 1996, 49, 926–940. [Google Scholar] [CrossRef]
- Ballester-Molto, M.; García García, B.; García García, J.; Cerezo Valverde, J.; Aguado-Giménez, F. Controlling feed losses by chewing in gilthead sea bream (Sparus aurata) ongrowing may improve the environmental sustainability of the aquacultural activity. Aquaculture 2016, 464, 111–116. [Google Scholar] [CrossRef]
- García García, J.; García García, B. Econometric model of viability/profitability of octopus (Octopus vulgaris) ongrowing in sea cages. Aquac. Int. 2011, 19, 1177–1191. [Google Scholar] [CrossRef]
- García García, J.; García García, B. Econometric model of viability/profitability of ongrowing sharp snout sea bream (Diplodus puntazzo) in sea cages. Aquac. Int. 2010, 18, 955–971. [Google Scholar] [CrossRef]
- García García, J.; García García, B. An econometric viability model for ongrowing sole (Solea senegalensis) in tanks using pumped well sea water. Span. J. Agric. Res. 2006, 4, 304–315. [Google Scholar] [CrossRef]
- APROMAR. La Acuicultura en España; Ministerio de Agricultura, Alimentación y Medio Ambiente, Gobierno de España: Madrid, Spain, 2017.
- ISO Environmental Management–Life Cycle Assessment: Requirements and Guidelines; ISO 14044; ISO-International Organization for Standards: Geneva, Switzerland, 2006.
- PRé Consultants Introduction to LCA with SimaPro; PRé: Amersfoort, The Netherland, 2016.
- Guinée, J.B.; Gorrée, M.; Heijungs, R.; Huppes, G.; Kleijn, R.; de Koning, A.; van Oers, L.; Wegener Sleeswijk, A.; Suh, S.; Udo de Haes, H.A.; et al. Handbook on Life Cycle Assessment. Operational Guide to the ISO Standards. I: LCA in Perspective. IIa: Guide. IIb: Operational Annex. III: Scientific Background; Kluwer Academic Publishers: Dordrecht, The Netherland, 2002; ISBN 1-4020-0228-9. [Google Scholar]
- Henriksson, P.J.G.; Guinée, J.B.; Kleijn, R.; de Snoo, G.R. Life cycle assessment of aquaculture systems-a review of methodologies. Int. J. Life Cycle Assess. 2012, 17, 304–313. [Google Scholar] [CrossRef]
- Samuel-Fitwi, B.; Meyer, S.; Reckmann, K.; Schroeder, J.P. Aspiring for environmentally conscious aquafeed: Comparative LCA of aquafeed manufacturing using different protein sources. J. Clean. Prod. 2013, 52, 225–233. [Google Scholar] [CrossRef]
- Grönroos, J.; Seppälä, J.; Silvenius, F.; Mäkinen, T. Life cycle assessment of Finnish cultivated rainbow trout. Boreal Environ. Res. 2006, 11, 401–414. [Google Scholar]
- Baez Paleo, J.D. Ingeniería de la Acuicultura marina: Instalaciones de Peces en el Mar; Publicaciones Científicas y Tecnológicas de la Fundación OESA: Madrid, Spain, 2009; ISBN 978-84-00-08865-1. [Google Scholar]
- Eroldogan, O.T.; Kumlu, M.; Aktas, M. Optimun feeding rates for European sea bass Dicentrarchus labrax L. reared in seawater and freshwater. Aquaculture 2004, 231, 501–525. [Google Scholar] [CrossRef]
- García García, B.; Bermúdez, L.; Gómez, O.; Rosique, M.J. Consumo de oxígeno en juveniles de lubina (Dicentrarchus labrax L.). In Acuicultura Intermareal; Yúfera, M., Ed.; Instituto de Ciencias del Mar de Andalucía: Cádiz, Spain, 1989; pp. 297–303. [Google Scholar]
- Murcia, P.D.P. Economía de escala en las explotaciones de engorde de dorada (Sparus aurata) en jaulas flotantes en el Mediterráneo. An. Vet. Murcia 2005, 21, 69–76. [Google Scholar]
- García García, J.; Rouco Yañez, A.; García García, B. Directrices generales de diseño de explotaciones de engorde de especies acuícolas en jaulas en mar. Arch. Zootec. 2002, 51, 469–472. [Google Scholar]
- Fréon, P.; Durand, H.; Avadí, A.; Huaranca, S. Life cycle assessment of three Peruvian fishmeal plants: Toward a cleaner production. J. Clean. Prod. 2017, 145, 50–63. [Google Scholar] [CrossRef]
- Mosnier, E.; Van der Werf, H.M.G.; Boissy, J.; Dourmad, J.Y. Evaluation of the environmental implications of the incorporation of feed-use amino acids in the manufacturing of pig and broiler feeds using Life Cycle Assessment. Animal 2011, 5, 1972–1983. [Google Scholar] [CrossRef]
- Ballester-Molto, M.; Follana-Berná, G.; Sanchez-Jerez, P.; Aguado-Giménez, F. Total nitrogen, carbon and phosphorus digestibility in gilthead seabream (Sparus aurata) and European seabass (Dicentrarchus labrax) fed with conventional and organic commercial feeds: Implications for particulate waste production. Aquac. Res. 2017, 48, 3450–3463. [Google Scholar] [CrossRef]
- García García, J. Análisis del Sector del Limonero y Evaluación Económica de su Cultivo; Consejería de Agricultura y Agua: Murcia, Spain, 2014. [Google Scholar]
- Aguado-Giménez, F.; García García, B.; Hernández, M.D.; Cerezo Valverde, J. Gross metabolic waste output estimates using a nutritional approach in Atlantic bluefin tuna (Thunnus thynnus) under intensive fattening conditions in western Mediterranean Sea. Aquac. Res. 2006, 37, 1254–1258. [Google Scholar] [CrossRef]
- Cho, C.Y.; Hynes, J.D.; Wood, K.R.; Yoshida, H.K. Quantification of fish culture wastes by biological (nutritional) and chemical (limnological) methods: The development of high nutrient dense (HND) diets. In Nutritional Strategies and Aquaculture Waste; Cowey, C.B., Cho, C.Y., Eds.; University of Guelph: Guelph, ON, Canada, 1991; pp. 37–50. [Google Scholar]
- Cho, C.Y.; Bureau, D. Development of bioenergetic models and the Fish-PrFEQ software to estimate production feeding ration and waste output in aquaculture. Aquat. Living Resour. 1998, 11, 199–210. [Google Scholar] [CrossRef]
- Leung, K.M.I.; Chu, J.C.W.; Wu, R.S.S. Nitrogen budgets for the aerolated grouper Epinephelus areolatus, cultured under laboratory conditions and in open-sea cages. Mar. Ecol. Prog. Ser. 1999, 186, 271–281. [Google Scholar] [CrossRef]
- Lupatsch, Y.; Kissil, G.W. Predicting aquaculture waste from gilthead seabream (Sparus aurata) culture using a nutritional approach. Aquat. Living Resour. 1998, 11, 265–268. [Google Scholar] [CrossRef]
- Mazón, M.J.; Piedecausa, M.A.; Hernádez, M.D.; García García, B. Evaluation of environmental nitrogen and phosphorus contributions as a result of intensive ongrowing of common octopus (Octopus vulgaris). Aquaculture 2007, 266, 226–235. [Google Scholar] [CrossRef]
- Piedecausa, M.A.; Aguado-Giménez, F.; Cerezo Valverde, J.; Hernández, M.D.; García García, B. Simulating the temporal pattern of waste production in farmed gilthead seabream (Sparus aurata), European seabass (Dicentrarchus labrax) and Atlantic bluefin tuna (Thunnus thynnus). Ecol. Model. 2010, 221, 634–640. [Google Scholar] [CrossRef]
- Klein, J.; Geilenkirchen, G.; Hulskotte, J.; Ligterink, N.; Fortuin, P.; Mlnár-in’t Veld, H. Methods for Calculating Transport Emission in the Netherland; Task Force on Transportation of the Dutch Pollutant Release and Transfer Register: The Hague, The Netherland, 2014. [Google Scholar]
- Cooper, J.; Diesburg, S.; Babej, A.; Noon, M.; Kahn, E.; Puettmann, M.; Colt, J. Life Cycle Assessment of products from Alaskan salmon processing wastes: Implication of coproduction, intermittent landings, and storage time. Fish. Res. 2014, 151, 26–38. [Google Scholar] [CrossRef]
- Pelletier, N.; Tyedmers, P. Feeding farmed salmon: Is organic better? Aquaculture 2007, 272, 399–416. [Google Scholar] [CrossRef]
- Hargrave, B.T.; Holmer, M.; Newcombre, C.P. Towards a classification of organic enrichment in marine sediments based on biogeochemical indicators. Mar. Pollut. Bull. 2008, 56, 810–824. [Google Scholar] [CrossRef]
- Hargrave, B.T.; Duplisea, D.E.; Pfeiffer, E.; Wildish, D.J. Seasonal changes in benthic fluxes of dissolved oxygen and ammonium associated with marine cultured Atlantic salmon. Mar. Ecol. Prog. Ser. 1993, 96, 249–257. [Google Scholar] [CrossRef]
- Karakassis, I.; Hatziyanni, E.; Tsapakis, M.; Plaiti, W. Benthic recovery following cessation of fish farming: A series of sucesses and catastrophes. Mar. Ecol. Prog. Ser. 1999, 184, 205–218. [Google Scholar] [CrossRef]
- Mazzola, A.; Mirto, S.; La Rosa, T.; Fabiano, M.; Danovaro, R. Fish farming effects on benthic community structure in coastal sediments: Analysis of meiofaunal resilience. ICES J. Mar. Sci. 2000, 57, 1454–1461. [Google Scholar] [CrossRef]
- Ballester-Moltó, M. Dinámica de la Producción de Residuos Particulados en Granjas de Peces Mediterráneas: Influencia de la Ictiofauna Salvaje. Ph.D. Thesis, Universidad de Alicante, Alicante, Spain, 2016. [Google Scholar]
- Aguado-Giménez, F.; García García, B. Assessment of some chemical parameters in marine sediments exposed to offshore cage fish farming influece. Aquaculture 2004, 242, 283–296. [Google Scholar] [CrossRef]
- Black, K.D. Sustainability of Aquaculture. In Environmental Impacts of Aquaculture; Black, K.D., Ed.; Sheffield Academic Press: Sheffield, UK, 2001; pp. 199–212. [Google Scholar]
- Cromey, C.J.; Nickell, T.D.; Black, K.D. DEPOMOD-modeling the deposition and biological effects of waste solids from marine cage farm. Aquaculture 2002, 214, 211–239. [Google Scholar] [CrossRef]
- Pitta, P.; Tsapakis, M.; Apostolaki, E.T.; Tsagaraki, T.; Holmer, M.; Karakassis, I. “Ghost nutrients” from fish farms are transferred up the food web by phytoplankton grazers. Mar. Ecol. Prog. Ser. 2009, 374, 1–6. [Google Scholar] [CrossRef]
- Pitta, P.; Karakassis, I.; Tsapakis, M.; Zivanovic, S. Natural vs. Mariculture induced variability in nutrients and plankton in the Eastern Mediterranean. Hydrobiologia 1999, 391, 181–184. [Google Scholar]
- Thigstad, T.F.; Krom, M.D.; Mantoura, R.F.C.; Flaten, G.A.G.; Groom, S.; Herut, B.; Kress, N.; Law, C.S.; Pasternak, A.; Pitta, P.; et al. Nature of phosphorous limitation in the ultraoligotrophic eastern Mediterranean. Science 2005, 309, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, V.; Aguado-Giménez, F.; Gairin, J.I.; Sánchez-Jerez, P. Exploring patterns of variation in amphipod assemblages at multiple spatial scales: Natural variability versus coastal aquaculture effect. Aquac. Environ. Interact. 2013, 3, 93–105. [Google Scholar] [CrossRef]
- Karakassis, I.; Tsapakis, M.; Hatziyanni, E.; Papadopoulou, K.N. Impact of cage farming of fish on the seabed in three Mediterranean coastal areas. ICES J. Mar. Sci. 2000, 57, 1462–1471. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Pérez, M.; Romero, J. Effects of fish farm loading on seagrass (Posidonia oceanica) distribution, growth and photosynthesis. Mar. Pollut. Bull. 2001, 42, 749–760. [Google Scholar] [CrossRef]
- Piedecausa, M.A.; Aguado-Giménez, F.; García García, B.; Ballester, G.; Telfer, T. Settling velocity and total ammonia nitrogen leaching from commercial feed and faecal pellets of gilthead seabream (Sparus aurata L. 1758) and seabass (Dicentrarchus labrax L. 1758). Aquac. Res. 2009, 40, 1703–1714. [Google Scholar] [CrossRef]
- Gowen, R.; Bradbury, N. The ecological impact of salmon farming in coastal waters: A review. Oceanogr. Mar. Biol. Annu. Rev. 1987, 25, 563–575. [Google Scholar]
- Gasca-Leyva, E.; León, C.; Hernádez, J.M.; Vergara, J.M. Bioeconomic analysis of production location of sea bream (Sparus aurata) cultivation. Aquaculture 2005, 213, 219–232. [Google Scholar] [CrossRef]
- Merinero, S.; Martínez, S.; Tomás, A.; Jover, M. Análisis económico de alternativas de producción de Dorada en jaulas marinas en el litoral Mediterráneo español. Rev. Aquat. 2005, 23, 1–19. [Google Scholar]
- Aguado-Giménez, F.; Piedecausa, M.A.; Carrasco, C.; Gutiérrez, J.M.; Aliaga, V.; García García, B. Do benthic biofilters contribute to sustainability and restoration of the benthic environment impacted by offshore cage finfish aquaculture? Mar. Pollut. Bull. 2011, 62, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
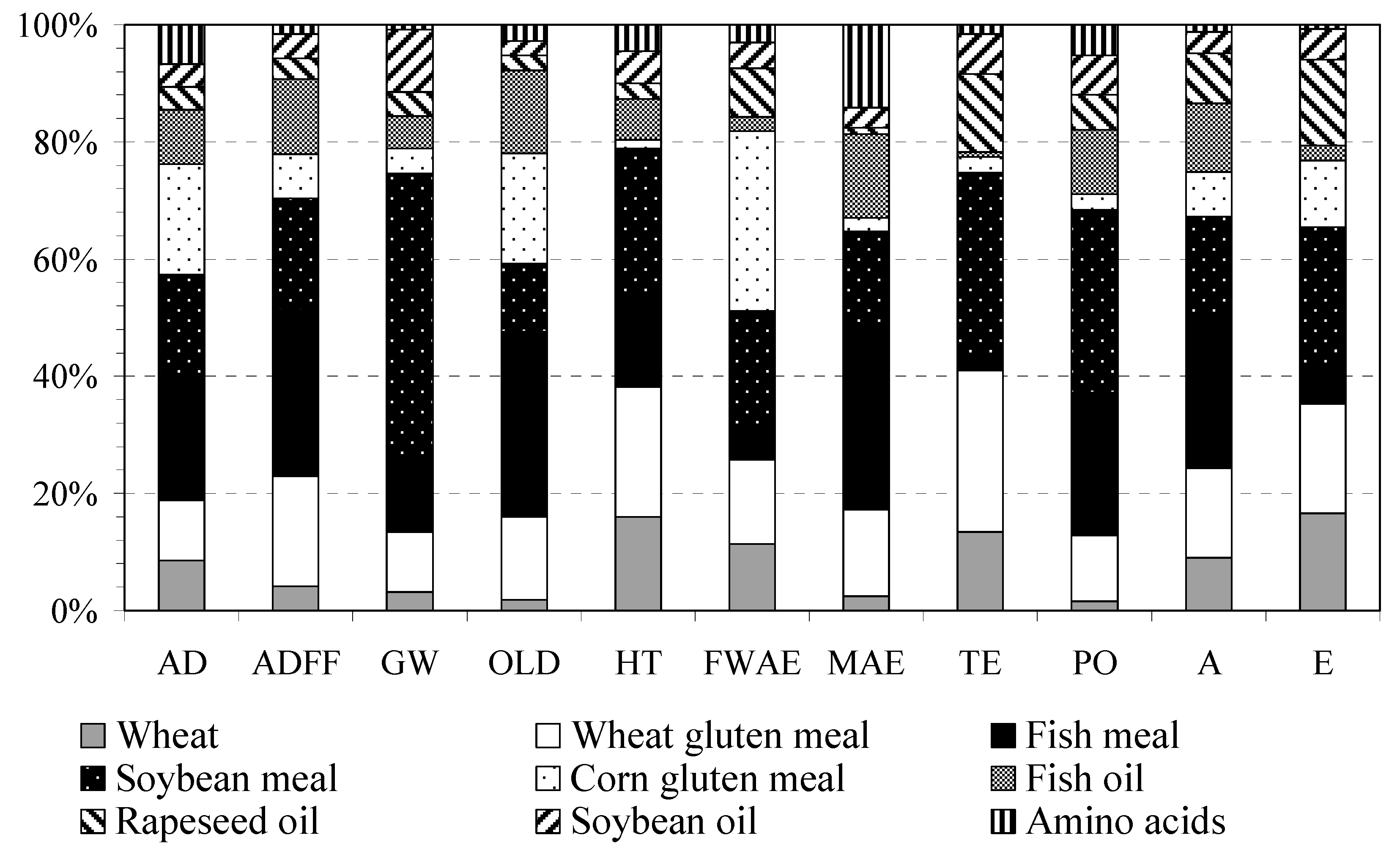


| Raw Materials (kg) (1 t Feed) | Pre-Ongrowing | Ongrowing | Origin |
|---|---|---|---|
| Wheat | 130 | 130 | Spain |
| Fish meal | 490 | 200 | Peru [41] |
| Soybean meal | 150 | 210 | 76% Brazil, 21% the USA |
| Wheat gluten meal | 50 | 150 | 50% Spain, 11% France, EU |
| Corn gluten meal | 50 | 110 | France |
| Fish oil | 40 | 90 | Peru [41] |
| Soybean oil | 40 | 40 | 76% Brazil, 21 the USA |
| Rapeseed oil | 40 | 50 | 90% France, EU |
| Vitamins and mineral | 10 | 10 | |
| Monocalcium phosphate | 5 | Ecoinvent | |
| Amino acids | 5 | [42] | |
| Manufacture of feed (1 t) | [7] | ||
| Chemical composition | |||
| Protein (%) | 50.1 | 42.1 | |
| Lipids (%) | 18.4 | 21.5 | |
| N (%) | 8.1 | 6.7 | |
| P (%) | 1.2 | 1.1 | |
| Nutritional indexes | |||
| Gross energy (MJ) | 214 | 222 | |
| Protein/Energy | 23.5 | 19.0 | |
| FCR | 1.5 | 1.9 | Confidential surveys |
| ADCN (%) | 94.52 | 95.35 | [43] |
| ADCP (%) | 41.40 | 51.70 | [43] |
| BW (g) | SOC (mg O2·kg−1·h−1) | SWF (L·kg−1·s−1) | OS (g O2·kg−1·h−1) | Q (kwh) |
|---|---|---|---|---|
| 0.5 | 1907 | 0.05792 | 1.17334 | 0.00834 |
| 1.0 | 1283 | 0.03898 | 0.78954 | 0.00561 |
| 5.0 | 514 | 0.01563 | 0.31655 | 0.00225 |
| 10.0 | 348 | 0.01057 | 0.21411 | 0.00152 |
| 15.0 | 277 | 0.00842 | 0.17047 | 0.00121 |
| Average | 866 | 0.02630 | 0.53280 | 0.00379 |
| Time (Days) | Electricity (kw·h) | Oxygen (g O2) | ||
| 1 kg of fingerlings | 90 | 8.18 | 1151 | |
| System Components | Values | Observations |
|---|---|---|
| Feed (kg) | 1.5 | Raw materials, manufacturing and transportation |
| Electricity (kw·h) | 8.18 | Pumping seawater to the culture tanks |
| Oxygen (g O2) | 1151 | Supply of oxygen to the culture tanks |
| N emission (g) | 97.29 | 91.71 dissolved, 5.58 particulate |
| P emission (g) | 13.07 | 4.42 dissolved, 8.64 particulate |
| Transport by truck (kg·km) | 1813.36 | 34% of 5333.42 kg·km |
| Oxygen in the truck (g O2) | 0.39 | 34% of 1.15 |
| Transportation by boat (kg·km) | 3777.18 | 66% of 5723 kg·km |
| Oxygen in the boat (g O2) | 3.59 | 66% of 5.44 |
| Input Pre-Ongrowing | Output Pre-Ongrowing | ||
|---|---|---|---|
| Feed (kg) | 56.25 | Fingerlings (kg) | 37.50 |
| Electricity (kw·h) | 306.75 | ||
| Oxygen (kg O2) | 43.16 | Emissions to sea water | |
| Transport by truck (kg·km) | 68,001 | N (kg) | 3.65 |
| Oxygen in the truck (g O2) | 14.63 | P (kg) | 0.49 |
| Transport by boat (kg·km) | 141,644 | ||
| Oxygen in the boat (kg O2) | 134.63 | ||
| Input Ongrowing | Output Ongrowing | ||
| Facilities | Commercial seabass (kg) | 1000 | |
| Polyethylene (kg) | 21.028 | ||
| Polystyrene (kg) | 0.294 | Emissions to sea water | |
| Nylon (kg) | 5.037 | N (kg) | 105.27 |
| Polypropylene (kg) | 5.675 | P (kg) | 17.21 |
| Polyurethane (kg) | 0.084 | ||
| Polyvinylchloride (kg) | 0.026 | Emissions to air | |
| Concrete block (kg) | 1.067 | CO2 (kg) | 1622.270 |
| Cast iron (kg) | 2.000 | NOx (kg) | 30.169 |
| Steel, low-alloyed (kg) | 1.623 | CO (kg) | 4.091 |
| Steel, chromium steel (kg) | 2.318 | NMVOC (kg) | 1.329 |
| VOL (kg) | 1.381 | ||
| Feed (kg) | 1900 | PM10 (kg) | 0.716 |
| CH4 (kg) | 0.056 | ||
| Fuel | SO2 (kg) | 2.812 | |
| Diesel (kg) | 511.34 | N2O (kg) | 0.041 |
| Lubricating oil (kg) | 1.44 | NH3 (kg) | 0.005 |
| PEI | Equivalency Unit | Mean | SD | CV (%) | 95% CI | SEM | |
|---|---|---|---|---|---|---|---|
| AD | kg Sb-eq | 0.00181 | 0.00024 | 13.16 | 0.00145 | 0.00239 | 0.000008 |
| ADFF | MJ | 72,591 | 6586 | 9.07 | 61,823 | 87,068 | 208 |
| GW | kg CO2-eq | 7293 | 155 | 2.13 | 7037 | 7649 | 4.90 |
| OLD | kg CFC-11-eq | 0.00062 | 0.00022 | 34.83 | 0.00036 | 0.00119 | 0.000007 |
| HT | kg 1,4-DB-eq | 837 | 74 | 8.87 | 724 | 1010 | 2.35 |
| FWAE | kg 1,4-DB-eq | 514 | 69 | 13.46 | 428 | 661 | 2.19 |
| MAE | kg 1,4-DB-eq | 1,003,519 | 169,740 | 16.91 | 739,240 | 1,409,041 | 5368 |
| TE | kg 1,4-DB-eq | 11.99 | 0.41 | 3.43 | 11.47 | 12.98 | 0.013 |
| PO | kg C2H4-eq | 1275 | 0.055 | 4.28 | 1195 | 1.409 | 0.0017 |
| A | kg SO2-eq | 44.15 | 1.41 | 3.19 | 42.06 | 47.58 | 0.045 |
| E | kg PO−4-eq | 115.23 | 0.39 | 0.34 | 114.68 | 116.14 | 0.012 |
| PEI | a | b | c | R2adj | ANOVA |
|---|---|---|---|---|---|
| SE | SE | SE | RSE | F-Statistic | |
| p-Level | p-Level | p-Level | p-Level | p-Level | |
| AD (kg Sb-eq) | 5.38E-04 | 5.82E-04 | 3.50E-07 | 1 | 4.90E+07 |
| 1.31E-07 | 6.30E-08 | 9.86E-11 | 6.43E-08 | 0.001 | |
| 0.001 | 0.001 | 0.001 | |||
| ADFF (MJ) | 5.92E+03 | 2.08E+04 | 5.34E+01 | 1 | 8.60E+06 |
| 2.00E+01 | 9.63E+00 | 1.51E-02 | 9.82E+00 | 0.001 | |
| 0.001 | 0.001 | 0.001 | |||
| GW (kg CO2-eq) | 4.53E+02 | 2.60E+03 | 3.74E+00 | 1 | |
| 1.40E+00 | 6.74E-01 | 1.06E-03 | 0.69E+00 | 1.37E+07 | |
| 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| OLD (kg CFC-11-eq) | 2.61E-05 | 1.27E-04 | 6.87E-07 | 1 | |
| 2.57E-07 | 1.24E-07 | 1.94E-10 | 1.26E-07 | 6.81E+06 | |
| 0.001 | 0.001 | 0.001 | 0.001 | ||
| HT (kg 1,4-DB-eq) | 2.87E+02 | 2.25E+02 | 2.30E-01 | 1 | |
| 8.62E-02 | 4.14E-02 | 6.49E-05 | 4.23E-02 | 2.11E+07 | |
| 0.001 | 0.001 | 0.001 | 0.001 | ||
| FWAE (kg 1,4-DB-eq) | 1.24E+02 | 1.87E+02 | 7.37E-02 | 1 | 1.05E+08 |
| 2.76E-02 | 1.33E-02 | 2.08E-05 | 1.35E-02 | 0.001 | |
| 0.001 | 0.001 | 0.001 | |||
| MAE (kg 1,4-DB-eq) | 4.05E+05 | 2.50E+05 | 2.56E+02 | 1 | 2.10E+07 |
| 9.60E+01 | 4.61E+01 | 7.22E-02 | 4.71E+01 | 0.001 | |
| 0.001 | 0.001 | 0.001 | |||
| TE (kg 1,4-DB-eq) | 1.03E+00 | 5.50E+00 | 9.56E-04 | 1 | |
| 3.58E-04 | 1.72E-04 | 2.70E-07 | 1.76E-04 | 5.16E+08 | |
| 0.001 | 0.001 | 0.001 | 0.001 | ||
| PO (kg C2H4-eq) | 1.09E-01 | 3.94E-01 | 8.17E-04 | 1 | |
| 3.07E-04 | 1.47E-04 | 2.31E-07 | 1.50E-04 | 9.86E+06 | |
| 0.001 | 0.001 | 0.001 | 0.001 | ||
| A (kg SO2-eq) | 2.41E+00 | 1.08E+01 | 4.18E-02 | 1 | |
| 1.56E-02 | 7.51E-03 | 1.18E-05 | 7.66E-03 | 7.31E+06 | |
| 0.001 | 0.001 | 0.001 | 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García García, B.; Rosique Jiménez, C.; Aguado-Giménez, F.; García García, J. Life Cycle Assessment of Seabass (Dicentrarchus labrax) Produced in Offshore Fish Farms: Variability and Multiple Regression Analysis. Sustainability 2019, 11, 3523. https://doi.org/10.3390/su11133523
García García B, Rosique Jiménez C, Aguado-Giménez F, García García J. Life Cycle Assessment of Seabass (Dicentrarchus labrax) Produced in Offshore Fish Farms: Variability and Multiple Regression Analysis. Sustainability. 2019; 11(13):3523. https://doi.org/10.3390/su11133523
Chicago/Turabian StyleGarcía García, Benjamín, Caridad Rosique Jiménez, Felipe Aguado-Giménez, and José García García. 2019. "Life Cycle Assessment of Seabass (Dicentrarchus labrax) Produced in Offshore Fish Farms: Variability and Multiple Regression Analysis" Sustainability 11, no. 13: 3523. https://doi.org/10.3390/su11133523
APA StyleGarcía García, B., Rosique Jiménez, C., Aguado-Giménez, F., & García García, J. (2019). Life Cycle Assessment of Seabass (Dicentrarchus labrax) Produced in Offshore Fish Farms: Variability and Multiple Regression Analysis. Sustainability, 11(13), 3523. https://doi.org/10.3390/su11133523





