Performance of an On-Site Wastewater Treatment System Using Reactive Filter Media and a Sequencing Batch Constructed Wetland
Abstract
1. Introduction
2. Materials and Methods
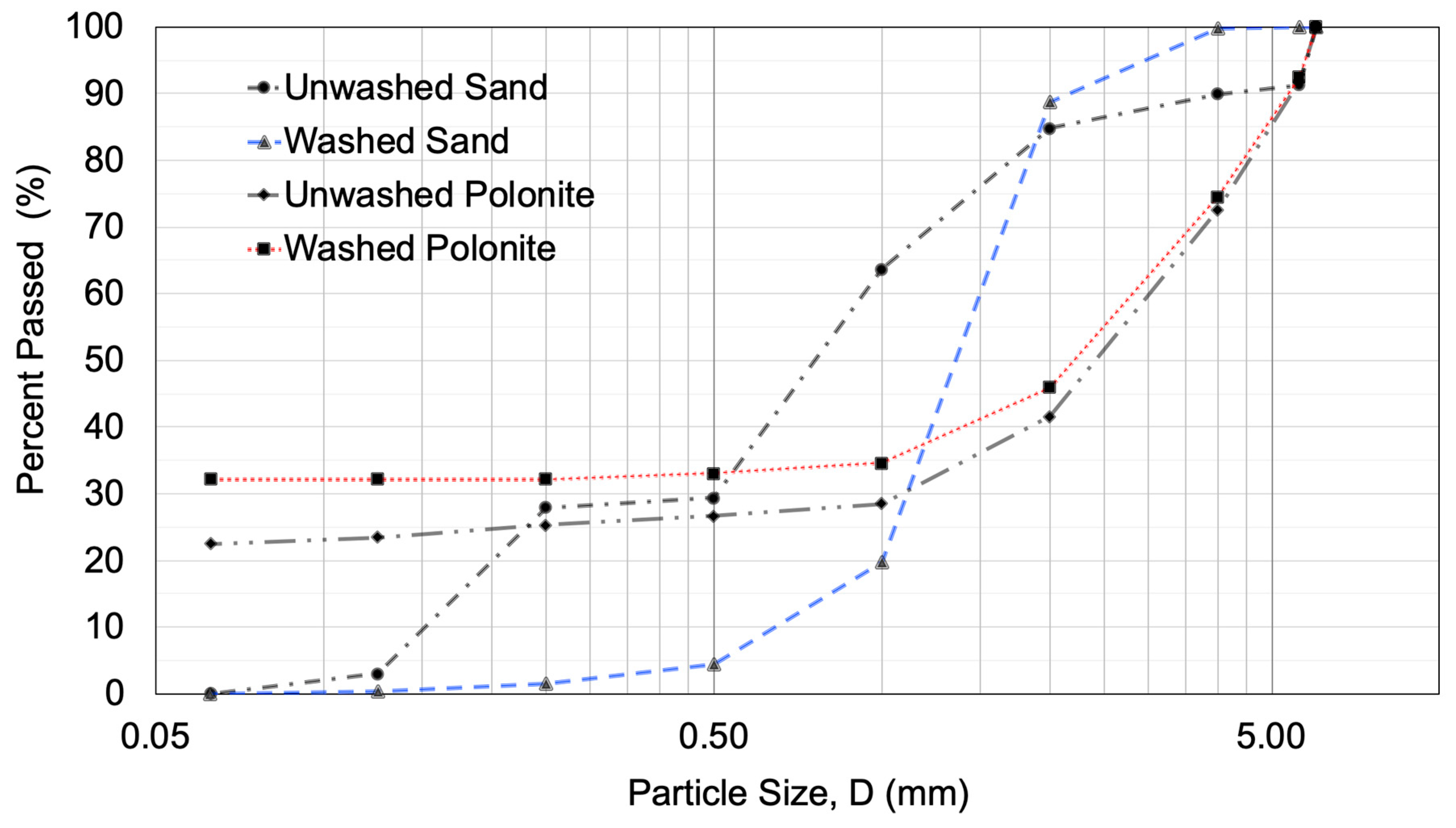
2.1. Study Site and Description of Wastewater Treatment System
2.2. Sampling and Water Quality Analyses
2.3. Analysis of P and N Removal Efficiency
2.4. Statistical Analysis
3. Results
3.1. Overall P, N, BOD, and Bacteria Removal Capacity of the On-site System
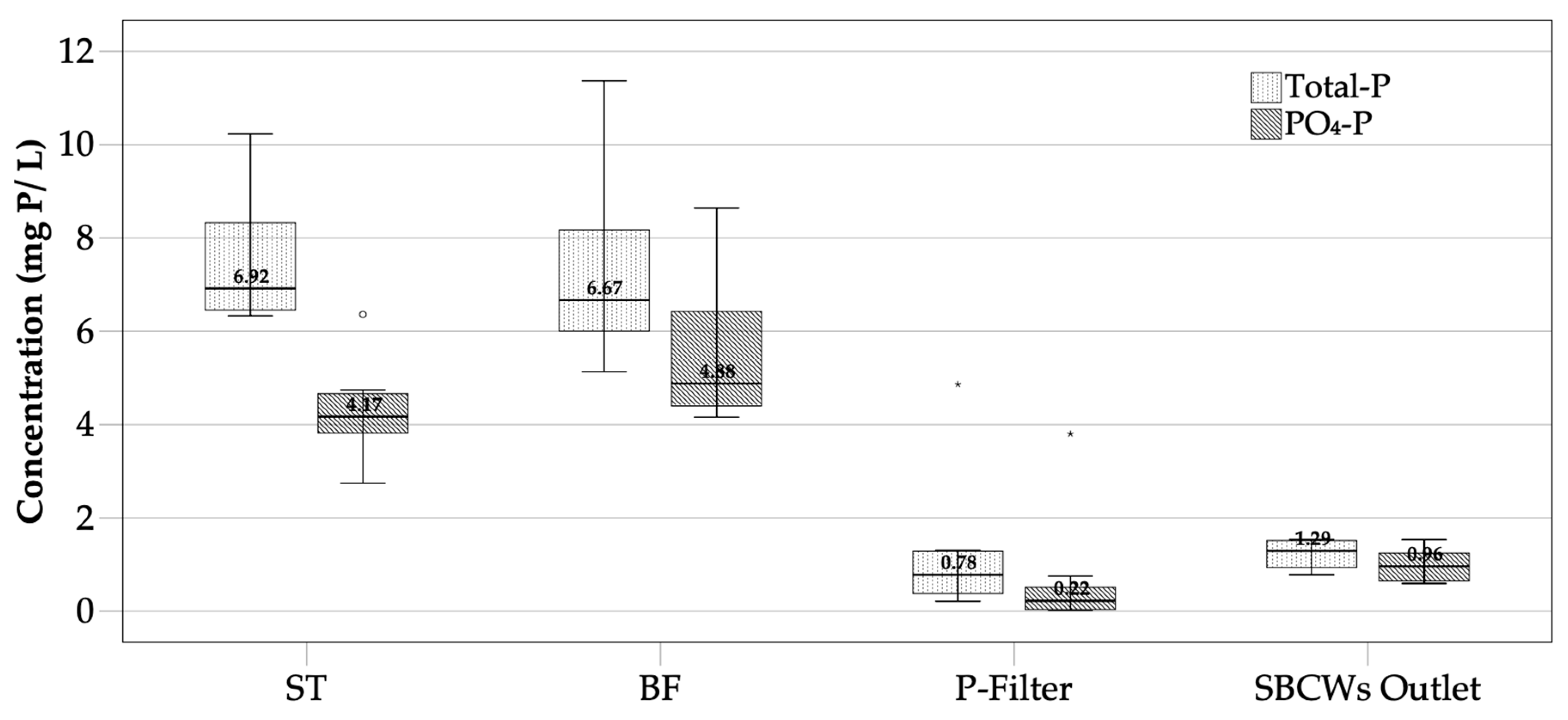
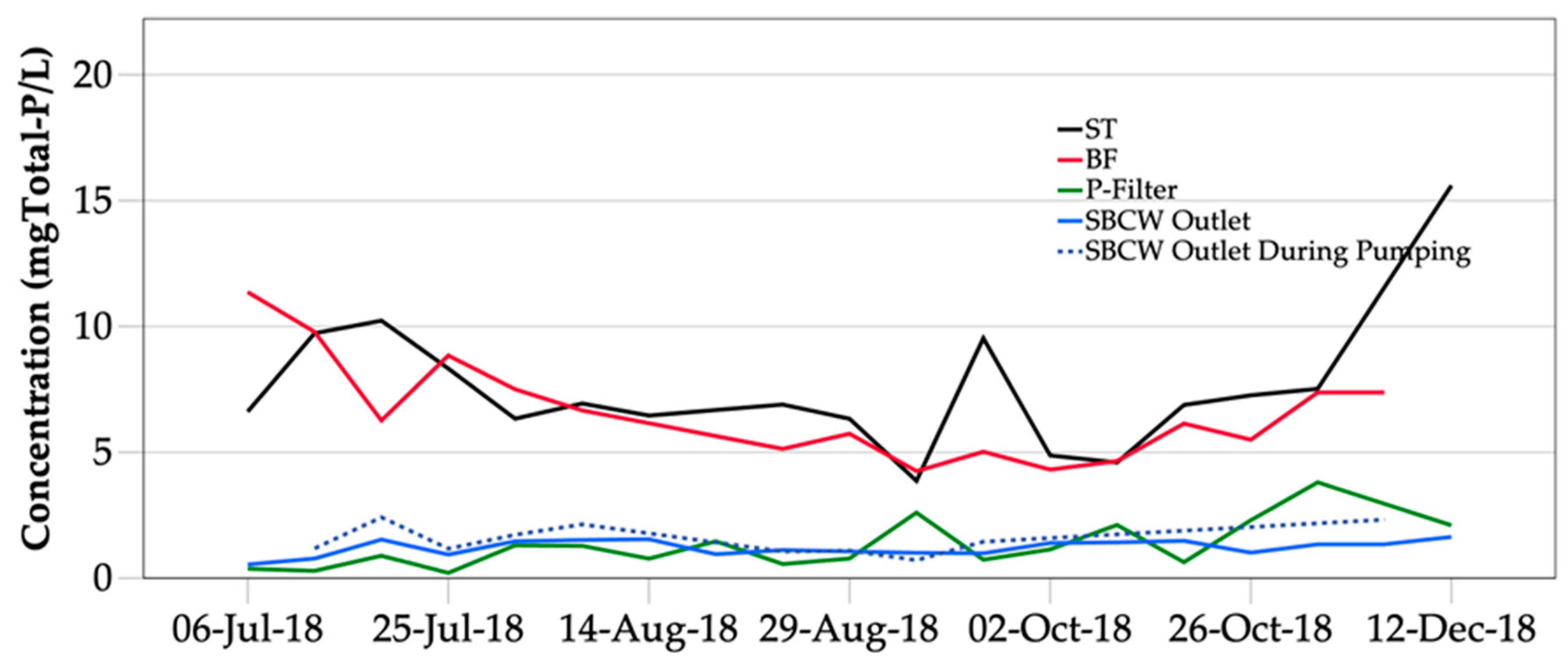
3.1.1. Phosphorus Removal
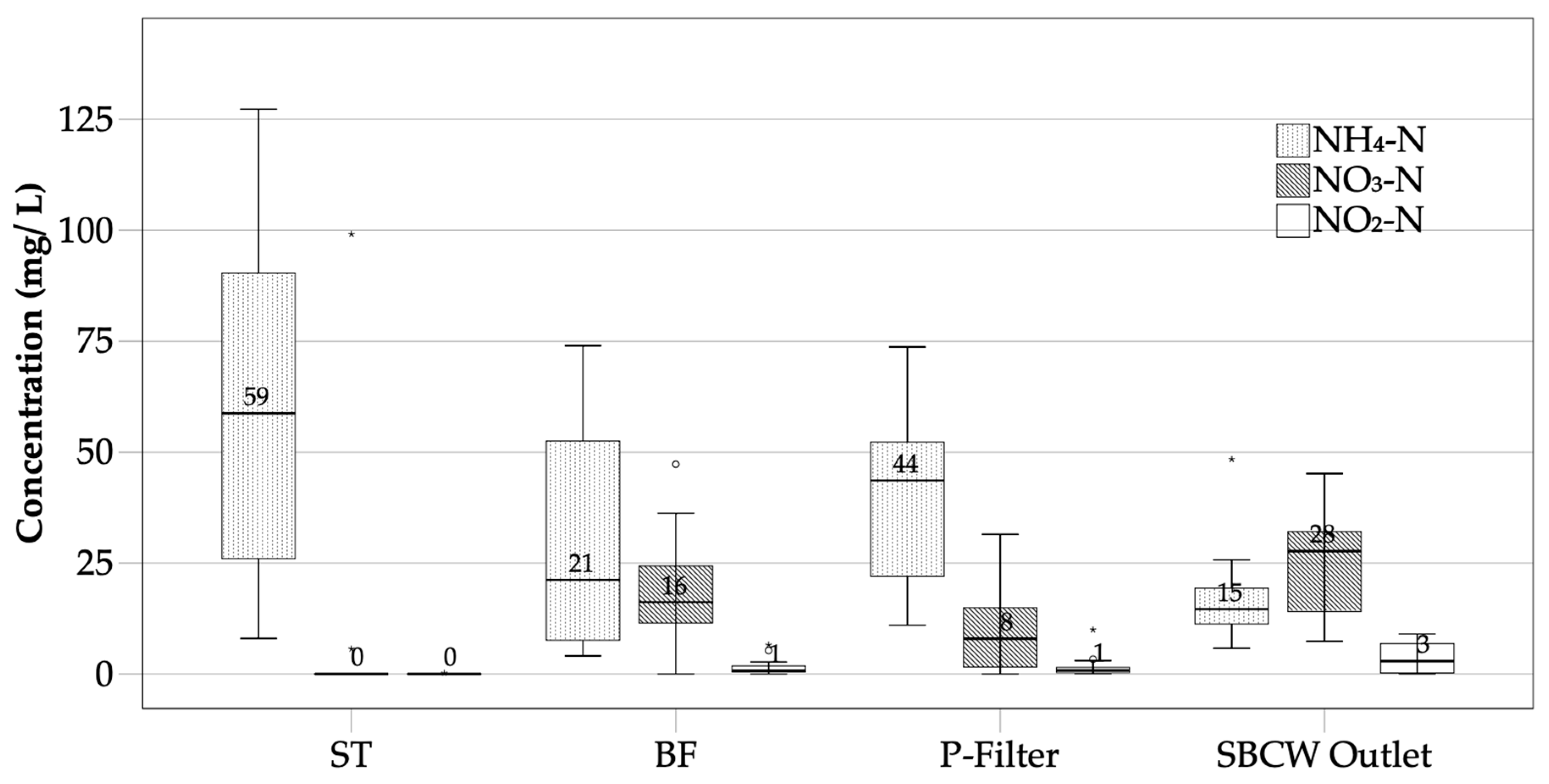
3.1.2. Nitrogen Removal
3.1.3. BOD and Bacteria Removal
3.2. Retained P in the On-Site System
3.3. Influence of Physical-Chemical Conditions
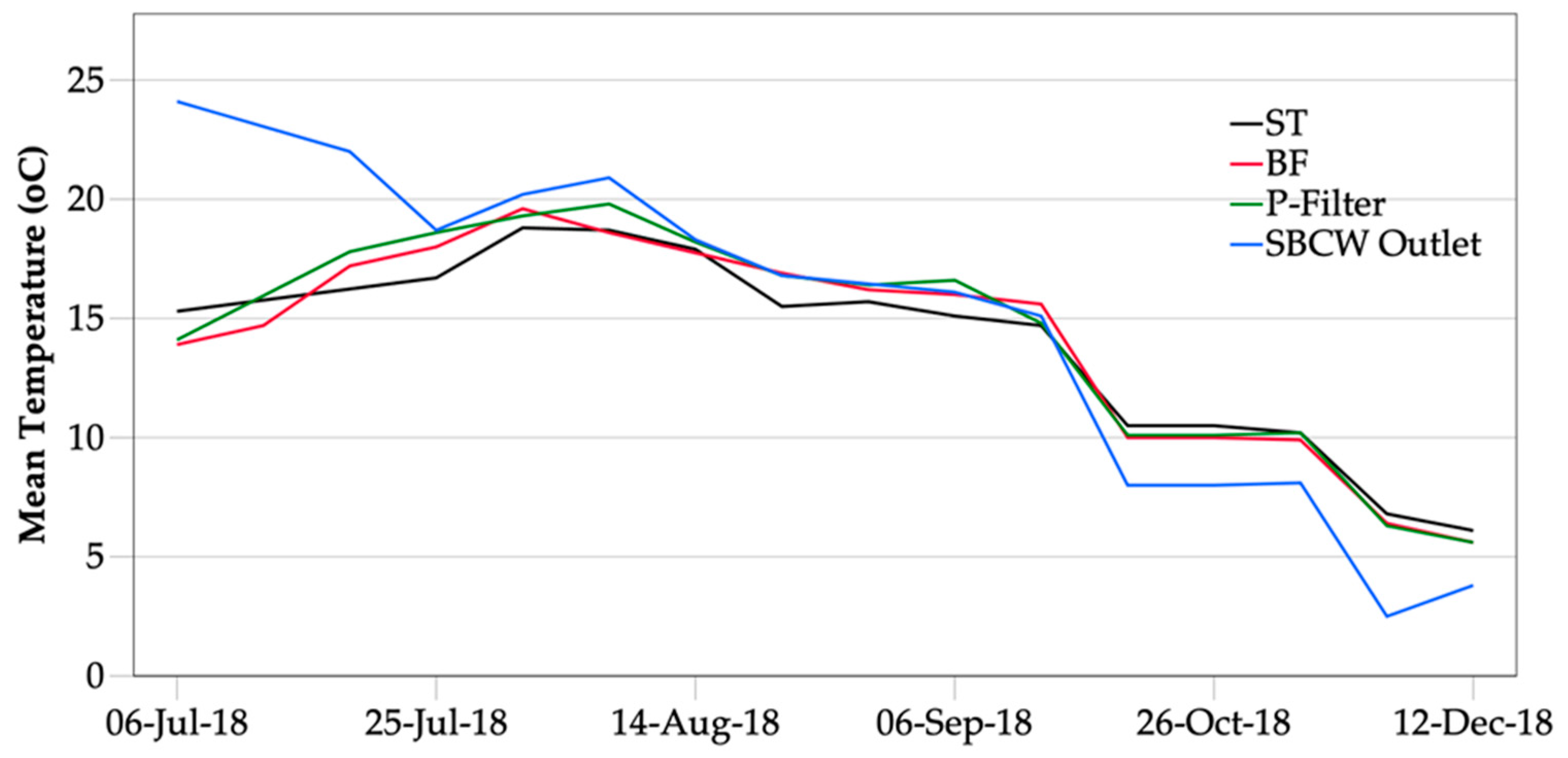
3.3.1. Water Temperature
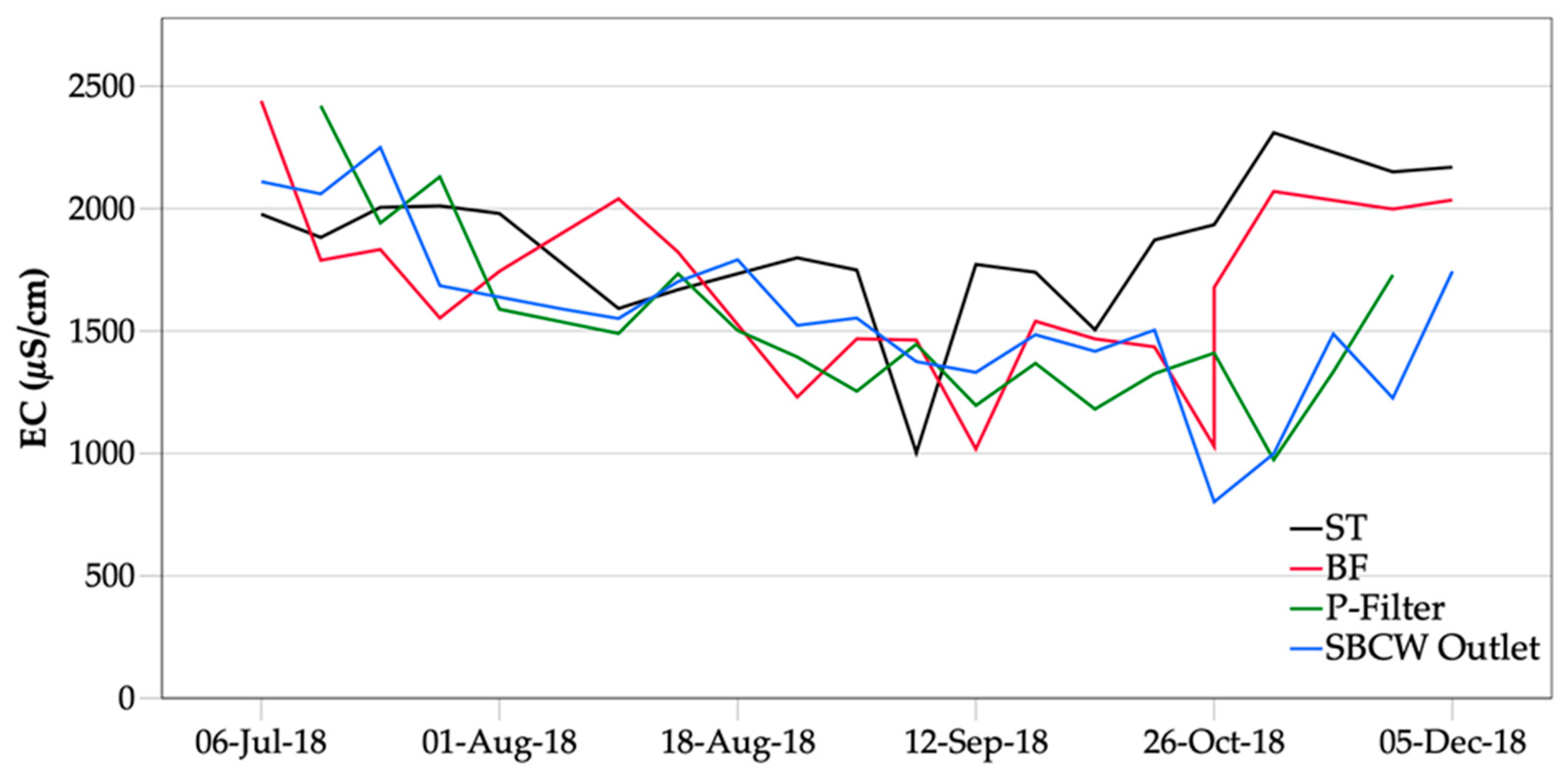
3.3.2. Electrical Conductivity
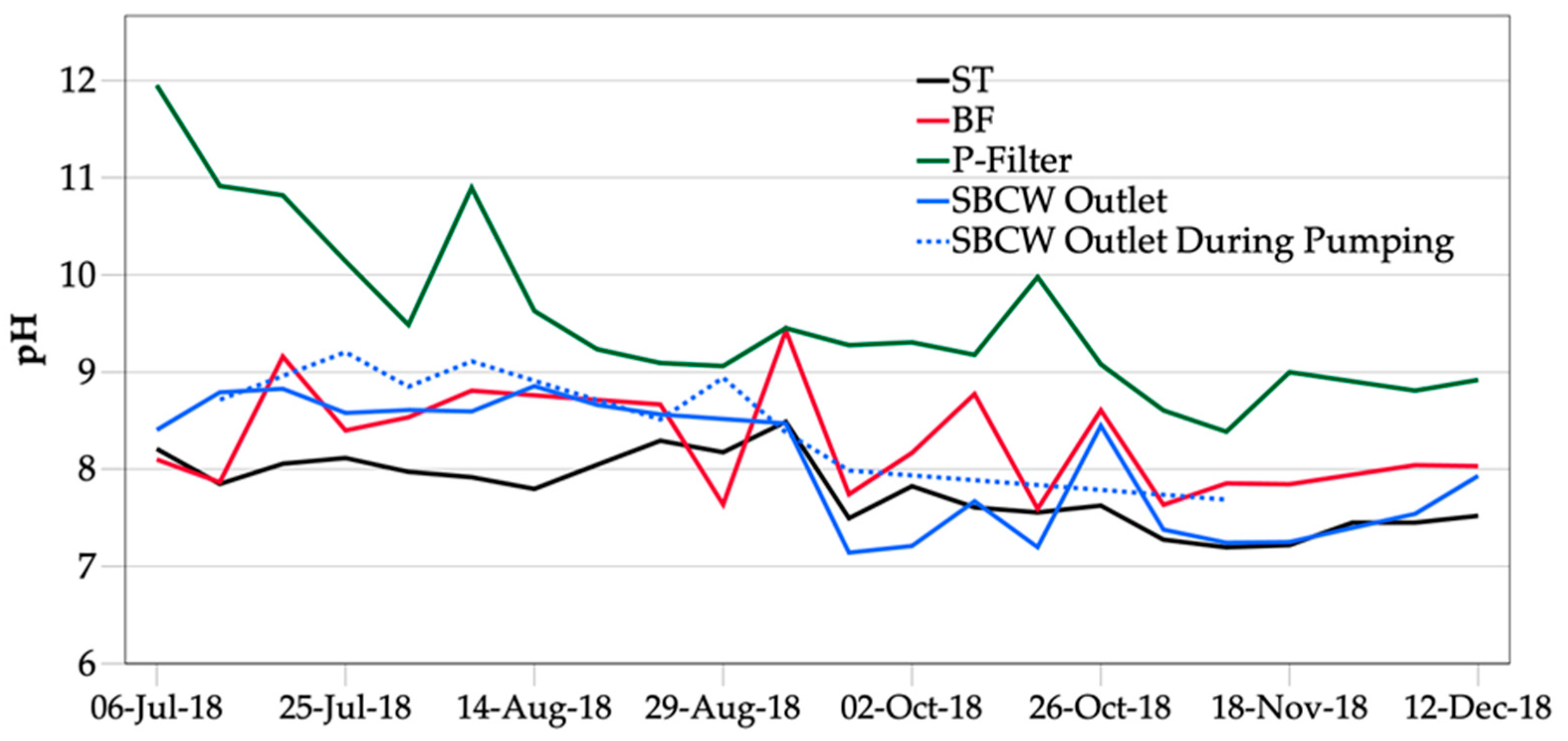
3.3.3. pH
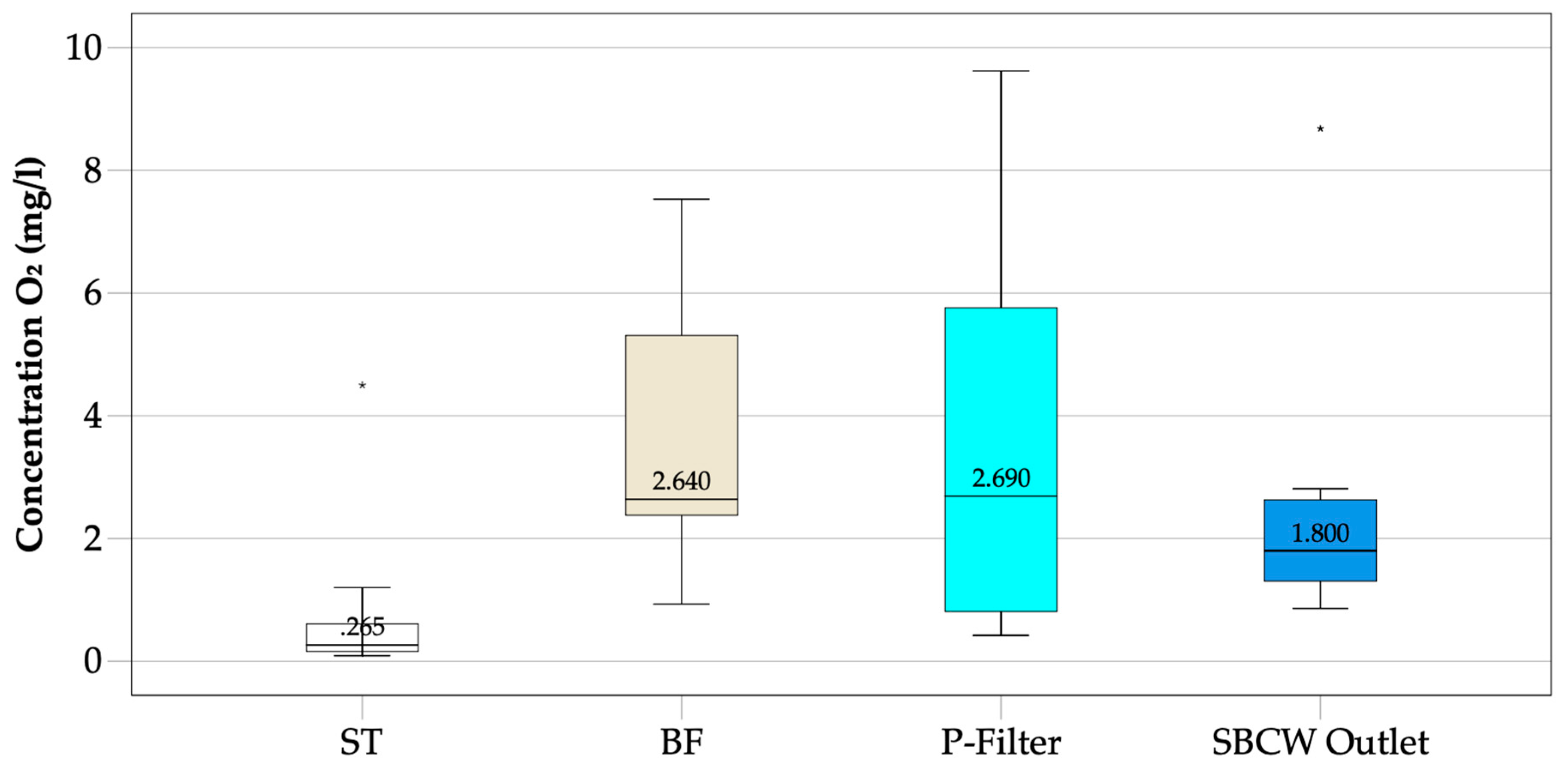
3.3.4. Dissolved Oxygen
4. Discussion
4.1. Nitrogen and Phosphorus—Transformation, Removal, Retention
4.2. Influence of Physical-Chemical Parameters on Nutrient Removal
4.3. The SBCW as a pH Reducer for the Alkaline Effluent and a P Post-Precipitation Step
4.4. Operation and Design Considerations of the SBCW
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eveborn, D.; Gustafsson, J.P.; Elmefors, E.; Yu, L.; Eriksson, A.K.; Ljung, E.; Renman, G. Phosphorus in soil treatment systems: Accumulation and mobility. Water Res. 2014, 64, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Blum, K.M.; Gago-Ferrero, P.; Wiberg, K.; Ahrens, L.; Andersson, P.L. Impact of on-site wastewater infiltration systems on organic contaminants in groundwater and recipient waters. Sci. Total Environ. 2019, 651, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, P.D.; Krogstad, T.; Paruch, A.M.; Mæhlum, T.; Adam, K.; Arias, C.A.; Heistad, A.; Jonsson, L.; Hellström, D.; Brix, H.; et al. Filter bed systems treating domestic wastewater in the Nordic countries—Performance and reuse of filter media. Ecol. Eng. 2010, 36, 1651–1659. [Google Scholar] [CrossRef]
- Mažeikienė, A. Improving small-scale wastewater treatment plant performance by using a filtering tertiary treatment unit. J. Environ. Manag. 2019, 232, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyk, A.; Bus, A.; Baryła, A. Influence of operation time, hydraulic load and drying on phosphate retention capacity of mineral filters treating natural swimming pool water. Ecol. Eng. 2019, 130, 176–183. [Google Scholar] [CrossRef]
- Cucarella, V.; Renman, G.; Zaleski, T.; Mazurek, R. Recycling of calcium-silicate material after wastewater filtration to agriculture-Soil condition impact. Ecol. Chem. Eng. S 2012, 19, 373–382. [Google Scholar] [CrossRef]
- Vohla, C.; Kõiv, M.; Bavor, H.J.; Chazarenc, F.; Mander, Ü. Filter materials for phosphorus removal from wastewater in treatment wetlands—A review. Ecol. Eng. 2011, 37, 70–89. [Google Scholar] [CrossRef]
- Renman, A.; Hylander, L.D.; Renman, G. Transformation and removal of nitrogen in reactive bed filter materials designed for on-site wastewater treatment. Ecol. Eng. 2008, 34, 207–214. [Google Scholar] [CrossRef]
- Renman, A.; Renman, G. Long-term phosphate removal by the calcium-silicate material Polonite in wastewater filtration systems. Chemosphere 2010, 79, 659–664. [Google Scholar] [CrossRef]
- Healy, M.G.; Rodgers, M.; Mulqueen, J. Treatment of dairy wastewater using constructed wetlands and intermittent sand filters. Bioresour. Technol. 2007, 98, 2268–2281. [Google Scholar] [CrossRef]
- House, C.H.; Bergmann, B.A.; Stomp, A.M.; Frederick, D.J. Combining constructed wetlands and aquatic and soil filters for reclamation and reuse of water. Ecol. Eng. 1999, 12, 27–38. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, Y.; Doherty, L.; Hu, Y.; Hao, X. A review of incorporation of constructed wetland with other treatment processes. Chem. Eng. J. 2015, 279, 220–230. [Google Scholar] [CrossRef]
- Wu, S.; Kuschk, P.; Brix, H.; Vymazal, J.; Dong, R. Development of constructed wetlands in performance intensifications for wastewater treatment: A nitrogen and organic matter targeted review. Water Res. 2014, 57, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Nivala, J.; Headley, T.; Wallace, S.; Bernhard, K.; Brix, H.; van Afferden, M.; Müller, R.A. Comparative analysis of constructed wetlands: The design and construction of the ecotechnology research facility in Langenreichenbach, Germany. Ecol. Eng. 2013, 61, 527–543. [Google Scholar] [CrossRef]
- Renman, G.; Kietlińska, A. A compact constructed wetland for treatment of landfill leachate. Inter. Ver. Theor. Angew. Limnol. Verhandl. 2000, 27, 629–632. [Google Scholar] [CrossRef]
- Kietlińska, A.; Renman, G.; Jannes, S.; Tham, G. Nitrogen removal from landfill leachate using a compact constructed wetland and the effect of chemical pretreatment. J. Environ. Sci. Health 2005, 40, 1493–1506. [Google Scholar] [CrossRef]
- Ilyas, H.; Masih, I. Intensification of constructed wetlands for land area reduction: A review. Environ. Sci. Pollut. Res. 2017, 24, 12081–12091. [Google Scholar] [CrossRef]
- Paranychianakis, N.V.; Tsiknia, M.; Kalogerakis, N. Pathways regulating the removal of nitrogen in planted and unplanted subsurface flow constructed wetlands. Water Res. 2016, 102, 321–329. [Google Scholar] [CrossRef]
- He, H.; Chen, Y.; Li, X.; Cheng, Y.; Yang, C.; Zeng, G. Influence of salinity on microorganisms in activated sludge processes: A review. Int. Biodeterior. Biodegrad. 2017, 119, 520–527. [Google Scholar] [CrossRef]
- Nilsson, C.; Lakshmanan, R.; Renman, G.; Rajarao, G.K. Efficacy of reactive mineral-based sorbents for phosphate, bacteria, nitrogen and TOC removal-Column experiment in recirculation batch mode. Water Res. 2013, 47, 5165–5175. [Google Scholar] [CrossRef]
- Brogowski, Z.; Renman, G. Characterization of opoka as a basis for its use in wastewater treatment. Pol. J. Environ. Stud. 2004, 13, 15–20. [Google Scholar]
- Vidal, B.; Hedström, A.; Herrmann, I. Phosphorus reduction in filters for on-site wastewater treatment. J. Water Process Eng. 2018, 22, 210–217. [Google Scholar] [CrossRef]
- Adera, S.; Drizo, A.; Twohig, E.; Jagannathan, K.; Benoit, G. Improving performance of treatment wetlands: Evaluation of supplemental aeration, varying flow direction, and phosphorus removing filters. Water Air Soil Pollut. 2018, 229, 100. [Google Scholar] [CrossRef]
- Eveborn, D.; Kong, D.; Gustafsson, J.P. Wastewater treatment by soil infiltration: Long-term phosphorus removal. J. Contamin. Hydrol. 2012, 140, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef]
- Maltais-Landry, G.; Maranger, R.; Brisson, J.; Chazarenc, F. Nitrogen transformations and retention in planted and artificially aerated constructed wetlands. Water Res. 2009, 43, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Hamisi, R.; Renman, G.; Renman, A.; Wörman, A. Modelling Phosphorus Sorption Kinetics and the Longevity of Reactive Filter Materials Used for On-site Wastewater Treatment. Water 2019, 11, 811. [Google Scholar] [CrossRef]
- Herrmann, I.; Nordqvist, K.; Hedström, A.; Viklander, M. Effect of temperature on the performance of laboratory-scale phosphorus-removing filter beds in on-site wastewater treatment. Chemosphere 2014, 117, 360–366. [Google Scholar] [CrossRef]
- Renman, A. On-Site Wastewater Treatment: Polonite and Other Filter Materials for Removal of Metals, Nitrogen and Phosphorus. Ph.D. Dissertation, KTH Royal Institute of Technology, Stockholm, Sweden, 2008. Available online: http://www.diva-portal.org/smash/get/diva2:14097/FULLTEXT01.pdf (accessed on 10 May 2019).
- Wang, M.; Zhang, D.Q.; Dong, J.W.; Tan, S.K. Constructed wetlands for wastewater treatment in cold climate—A review. J. Environ. Sci. 2017, 57, 293–311. [Google Scholar] [CrossRef]
- Uygur, A.; Kargı, F. Salt inhibition on biological nutrient removal from saline wastewater in a sequencing batch reactor. Enzyme Microb. Technol. 2004, 34, 313–318. [Google Scholar] [CrossRef]
- Eregno, F.E.; Heistad, A. On-site treated wastewater disposal systems—The role of stratified filter medias for reducing the risk of pollution. Environ. Int. 2019, 124, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.; Akkanen, J. Novel, Activated Carbon-Based Material for in-Situ Remediation of Contaminated Sediments. Environ. Sci. Technol. 2019, 53, 3217–3224. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.I.; Rogerson, M.; Burke, I.T.; Stewart, D.I.; Mayes, W.M. Hydraulic and biotic impacts on neutralisation of high-pH waters. Sci. Total Environ. 2017, 601, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Martikainen, K.; Matikka, V.; Veijalainen, A.M.; Pitkänen, T.; Heinonen-Tanski, H.; Miettinen, I.T. Sand filters for removal of microbes and nutrients from wastewater during a one-year pilot study in a cold temperate climate. J. Environ. Manag. 2014, 133, 206–213. [Google Scholar] [CrossRef]
- Wolthers, M.; Nehrke, G.; Gustafsson, J.P.; Van Cappellen, P. Calcite growth kinetics: Modeling the effect of solution stoichiometry. Geochim. Cosmochim. Acta 2012, 77, 121–134. [Google Scholar] [CrossRef]
- Drizo, A.; Forget, C.; Chapuis, R.P.; Comeau, Y. Phosphorus removal by electric arc furnace steel slag and serpentinite. Water Res. 2006, 40, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Niska, K.G.; (Skandinavisk Ecotech AB). Personal communication, 12 September 2018.
- Sun, G.; Zhao, Y.; Allen, S. Enhanced removal of organic matter and ammoniacal-nitrogen in a column experiment of tidal flow constructed wetland system. J. Biotechnol. 2005, 115, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.C.; D’Eugenio, J.; McBride, G.B.; Sukias, J.P.; Thompson, K. Effect of water level fluctuation on nitrogen removal from constructed wetland mesocosms. Ecol. Eng. 1999, 12, 67–92. [Google Scholar] [CrossRef]
- Pelissari, C.; Ávila, C.; Trein, C.M.; García, J.; de Armas, R.D.; Sezerino, P.H. Nitrogen transforming bacteria within a full-scale partially saturated vertical subsurface flow constructed wetland treating urban wastewater. Sci. Total Environ. 2017, 574, 390–399. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Septic Tank | P-filter | SBCW | ||||||
|---|---|---|---|---|---|---|---|---|---|
| July | Sept. | Dec. | July | Sept. | Dec. | July | Sept. | Dec. | |
| BOD7 (mg L−1) | 91.2 | 95.1 | 101.5 | 2.1 | 2.2 | 2.9 | <1.0 | <1.0 | 2.4 |
| E. coli (cfu/100 mL) | 79000 | 82000 | 96000 | 91 | 113 | 500 | 2 | 2 | 73 |
| Intestinal enterococci (cfu/100 mL) | 39000 | 36000 | 37000 | 210 | 241 | 2050 | 5 | 5 | 135 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamisi, R.; Renman, A.; Renman, G. Performance of an On-Site Wastewater Treatment System Using Reactive Filter Media and a Sequencing Batch Constructed Wetland. Sustainability 2019, 11, 3172. https://doi.org/10.3390/su11113172
Hamisi R, Renman A, Renman G. Performance of an On-Site Wastewater Treatment System Using Reactive Filter Media and a Sequencing Batch Constructed Wetland. Sustainability. 2019; 11(11):3172. https://doi.org/10.3390/su11113172
Chicago/Turabian StyleHamisi, Rajabu, Agnieszka Renman, and Gunno Renman. 2019. "Performance of an On-Site Wastewater Treatment System Using Reactive Filter Media and a Sequencing Batch Constructed Wetland" Sustainability 11, no. 11: 3172. https://doi.org/10.3390/su11113172
APA StyleHamisi, R., Renman, A., & Renman, G. (2019). Performance of an On-Site Wastewater Treatment System Using Reactive Filter Media and a Sequencing Batch Constructed Wetland. Sustainability, 11(11), 3172. https://doi.org/10.3390/su11113172






