Response of Corals Acropora pharaonis and Porites lutea to Changes in pH and Temperature in the Gulf
Abstract
1. Introduction
2. Materials and Methods
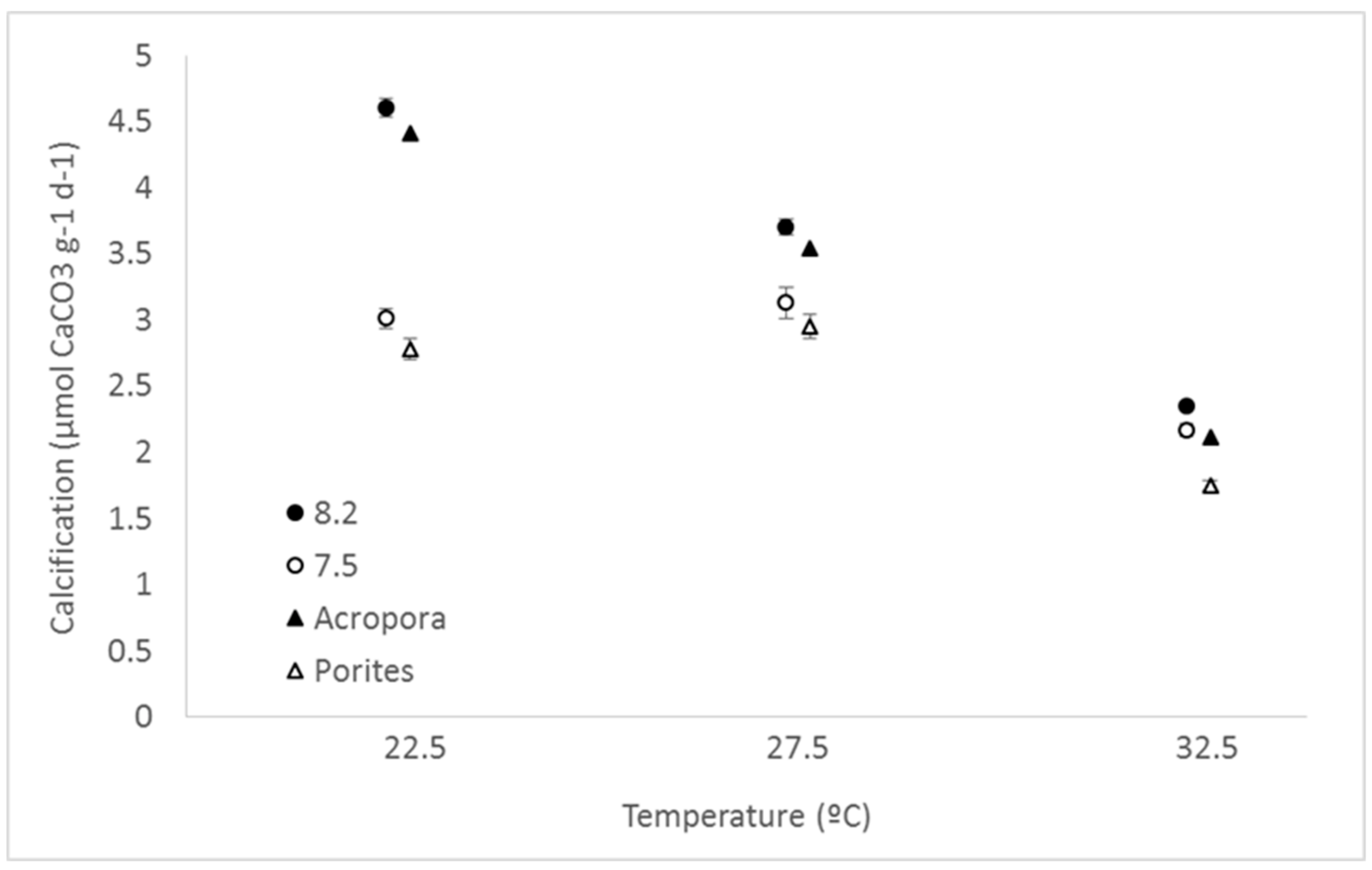
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feary, D.A.; Burt, J.A.; Cavalcante, G.H.; Bauman, A.G. Extreme Physical Factors and the Structure of Gulf Fish and Reef Communities. In Coral Reefs of the Gulf; Riegl, B.M., Purkis, S.J., Eds.; Springer: New York, NY, USA, 2012; Volume 3, pp. 163–170. [Google Scholar]
- Bahr, K.; Jokiel, P.; Ku’ulei, S. Seasonal and annual calcification rates of the Hawaiian reef coral, Montipora capitata, under present and future climate change scenarios. ICES J. Mar. Sci. 2017, 74, 1083–1091. [Google Scholar]
- Schiedek, D.; Sundelin, B.; Readman, J.W.; Macdonald, R.W. Interactions between climate change and contaminants. Mar. Poll. Bull. 2007, 54, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Al-Ghadban, A.N.; Al Khabbaz, A. Localized Hyper Saline Waters in Arabian Gulf from Desalination activity—An example from South Kuwait. Environ. Monit. Assess. 2010, 181, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Gevao, B.; Al-Ghadban, A.N.; Nithyanandan, M.; Al-Shamroukh, D. Acidification in Arabian Gulf—Insights from pH and temperature measurements. J. Environ. Monit. 2012, 14, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- Anlauf, H.; D’Croz, L.; O’Dea, A.A. corrosive concoction: the combined effects of ocean warming and acidification on the early growth of a stony coral are multiplicative. J. Exp. Mar. Biol. Ecol 2011, 397, 13–20. [Google Scholar] [CrossRef]
- Uddin, S.; Al Shamroukh, D.; Al-Khabaz, A.; Al-Yagoub, A. Assessment, and Monitoring of Water Quality for the Sabah Al Ahmed Sea City Project, Phase II; Technical Report KISR10980; Kuwait Institute for Scientific Research: Safat, Kuwait, 2014 2014; 30p. [Google Scholar]
- Andersson, A.J.; Mackenzie, F.T.; Gattuso, J.-P. Effects of ocean acidification on benthic processes, organisms, and ecosystems. In Ocean acidification, Gattuso J.-P.; Hanson, L., Ed.; Oxford University Press: New York, NY, USA, 2011; pp. 122–153. [Google Scholar]
- Eyre, B.; Andersson, A.; Cyronak, T. Benthic coral reef calcium carbonate dissolution in an acidifying ocean. Nat. Clim. Chang. 2014, 4, 969–976. [Google Scholar] [CrossRef]
- Allemand, D.; Tambutte, E.; Zaccola, D.; Tambutte, S. Coral calcification, cells to reefs. In Coral reefs: An ecosystem in transition; Dubinsky, Z., Stambler, N., Eds.; Springer: New York, NY, USA, 2011; pp. 119–150. [Google Scholar]
- Anderson, L.; Dyrssen, D. Alkalinity and total carbonate in the Arabian Sea. Carbonate depletion in the Red Sea and Persian Gulf. Mar. Chem. 1994, 47, 195–202. [Google Scholar] [CrossRef]
- Riegl, B. Corals in a non-reef setting in the southern Persian Gulf (Dubai, UAE): fauna and community structure in response to recurring mass mortality. Coral Reefs 1999, 18, 63–73. [Google Scholar] [CrossRef]
- Purkis, S.J.; Riegl, B. Spatial and temporal dynamics of Arabian Gulf coral assemblages quantified from remote-sensing and in situ monitoring data (Jebel Ali, Dubai, U.A.E.). Mar Ecol Prog Ser 2005, 287, 99–113. [Google Scholar] [CrossRef]
- Tumbutte, E.; Allemand, D.; Bourge, I.; Gattuso, J.-P. An improved 45Ca protocol for investigating physiological mechanisms in coral calcification. Mar. Biol. 1995, 122, 453–459. [Google Scholar] [CrossRef]
- Lewis, E.; Wallace, D.; Allison, L.J. Program Developed for CO2 System Calculations; ORNL/CDIAC-105; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy: Oak Ridge, TN, USA, 1998.
- Pierrot, D.; Lewis, E.; Wallace, D. MS Excel Program Developed for CO2 System Calculations; ORNL/CDIAC-105a; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy: Oak Ridge, TN, USA, 2006.
- Castillo, K.; Ries, J.; Bruno, J.; Westfield, I. The reef-building coral Siderastrea siderea exhibits parabolic responses to ocean acidification and warming. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141856. [Google Scholar] [CrossRef] [PubMed]
- Hoeke, R.; Jokiel, P.; Buddemeier, R.; Brainard, R. Projected changes to growth and mortality of Hawaiian corals over the next 100 years. PLoS ONE 2011, 6, e18038. [Google Scholar] [CrossRef] [PubMed]
- Jokiel, P. Predicting the impact of ocean acidification on coral reefs: evaluating the assumptions involved. ICES J. Mar. Sci. 2016, 73, 550–557. [Google Scholar] [CrossRef]
- Coles, S.L. Coral species diversity and environmental factors in the Arabian Gulf and the Gulf of Oman: a comparison to the Indo-Pacific region. Atoll. Res. Bull. 2003, 507, 1–19. [Google Scholar] [CrossRef]
- Sheppard, C.; Al-Husiani, M.; Al-Jamali, F.; Al-Yamani, F.; Baldwin, R.; Bishop, J.; Benzoni, F.; Dutrieux, E.; Dulvy, N.K.; Durvasula, S.R.V.; et al. Environmental Concerns for the Future of Gulf Coral Reefs. In Coral Reefs of the Gulf: Adaptation to Climatic Extremes; Riegl, B.M., Purkis, S.J., Eds.; Springer Science and Business Media B.V.: New York, NY, USA, 2012. [Google Scholar]
- Bauman, A.G.; Baird, A.H.; Cavalcante, G.H. Coral reproduction in the world’s warmest reefs: southern Persian Gulf (Dubai, United Arab Emirates). Coral Reefs 2011, 30, 405–413. [Google Scholar] [CrossRef]
- Schoepf, V.; Stat, M.; Falter, J.L.; McCulloch, M.T. Limits to the thermal tolerance of corals adapted to a highly fluctuating, naturally extreme temperature environment. Scientific Reports 2015, 5, 1–14. [Google Scholar] [CrossRef]
- Purkis, S.; Renegar, D.; Riegl, B. The most temperature-adapted corals have an Achilles’ Heel. Mar. Poll. Bull. 2011, 62, 246–250. [Google Scholar] [CrossRef]
- Riegl, B.; Purkis, S.J. Coral Reefs of the Gulf Adaptation to Climatic Extremes; Springer: New York NY, USA, 2012. [Google Scholar]
- Burt, J.; Bartholomew, A.; Usseglio, P. Recovery of corals a decade after a bleaching event in Dubai, United Arab Emirates. Mar. Biol. 2008, 154, 27–36. [Google Scholar] [CrossRef]
- Wilkinson, C.; Hodgson, G. Coral reefs and the 1997–1998 mass bleaching and mortality. Nat. Resour. 1999, 35, 16–25. [Google Scholar]
- Wilkinson, C.R.; Linden, O.; Cesar, H.; Hodgson, G.; Rubens, J.; Strong, A.E. Ecological and socio-economic impacts of 1998 coral mortality in the Indian Ocean: an ENSO (El Nino-Southern Oscillation) impact and a warning of future change? Ambio 1999, 28, 188–196. [Google Scholar]
- Edmunds, P.J.; Cumbo, V.; Fan, T.Y. Effect of temperature on the respiration of brooded larvae from tropical reef corals. J. Exp. Biol. 2011, 138, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Baria, M.; Kurihara, H.; Harii, S. Tolerance to elevated temperature and ocean acidification of the larvae of the solitary corals Fungia fungites (Linnaues, 1758) and Lithophyllon repanda (Dana, 1846). Zool Sci. 2015, 32, 447–454. [Google Scholar]
| Nominal pH | Temperature [°C] | pHnbs | pCO2 [atm] | Ωcalc | Ωar |
|---|---|---|---|---|---|
| 8.2 | 22.5 | 8.18 ± 0.003 | 451 ± 6 | 6.48 ± 0.05 | 4.24 ± 0.04 |
| 27.5 | 437 ± 5 | 7.21 ± 0.04 | 4.80 ± 0.03 | ||
| 32.5 | 447 ± 6 | 7.81 ± 0.06 | 5.29 ± 0.04 | ||
| 7.5 | 22.5 | 7.50 ± 0.002 | 2453 ± 16 | 1.54 ± 0.01 | 1.01 ± 0.01 |
| 27.5 | 2525 ± 28 | 1.75 ± 0.02 | 1.17 ± 0.01 | ||
| 32.5 | 2688 ± 18 | 2.00 ± 0.01 | 1.36 ± 0.01 | ||
| ANOVA 3 | |||||
| Model (F11,83, p) | 4015.9 <0.0001 | 2422.5 <0.0001 | 2751.1 <0.0001 | 2763.4 <0.0001 | |
| pH (F1, p) | 44157.0 <0.0001 | 26529.3 <0.0001 | 29578.1 <0.0001 | 29454.0 <0.0001 | |
| Temperature (F2, p) | 1.39 0.26 | 28.38 <0.0001 | 272.2 <0.0001 | 372.2 <0.0001 | |
| pH x temperature (F2, p) | 0.63 0.54 | 28.95 <0.0001 | 65.52 <0.0001 | 95.0 <0.0001 | |
| Replicate (F6, p) | 0.38 0.89 | 0.47 0.83 | 1.47 0.20 | 1.45 0.21 | |
| F | P | |
|---|---|---|
| Acropora pharaonis | ||
| Model | F11,35 = 74.3 | <0.0001 |
| pH | F1 = 190.0 | <0.0001 |
| Temperature | F2 = 258.4 | <0.0001 |
| pH × Temperature | F2 = 51.0 | <0.0001 |
| Replicate | F6 = 1.41 | 0.25 |
| Porites lutea | ||
| Model | F11,35 = 114.71 | <0.0001 |
| pH | F1 = 298.4 | <0.0001 |
| Temperature | F2 = 415.6 | <0.0001 |
| pH × Temperature | F2 = 61.83 | <0.0001 |
| Replicate | F6 = 1.44 | 0.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behbehani, M.; Uddin, S.; Dupont, S.; Sajid, S.; Al-Musalam, L.; Al-Ghadban, A. Response of Corals Acropora pharaonis and Porites lutea to Changes in pH and Temperature in the Gulf. Sustainability 2019, 11, 3156. https://doi.org/10.3390/su11113156
Behbehani M, Uddin S, Dupont S, Sajid S, Al-Musalam L, Al-Ghadban A. Response of Corals Acropora pharaonis and Porites lutea to Changes in pH and Temperature in the Gulf. Sustainability. 2019; 11(11):3156. https://doi.org/10.3390/su11113156
Chicago/Turabian StyleBehbehani, Montaha, Saif Uddin, Sam Dupont, Sufiya Sajid, Lamya Al-Musalam, and Abdulnabi Al-Ghadban. 2019. "Response of Corals Acropora pharaonis and Porites lutea to Changes in pH and Temperature in the Gulf" Sustainability 11, no. 11: 3156. https://doi.org/10.3390/su11113156
APA StyleBehbehani, M., Uddin, S., Dupont, S., Sajid, S., Al-Musalam, L., & Al-Ghadban, A. (2019). Response of Corals Acropora pharaonis and Porites lutea to Changes in pH and Temperature in the Gulf. Sustainability, 11(11), 3156. https://doi.org/10.3390/su11113156







