1. Introduction
Volcanic activity has a significant impact on the world’s ecosystem. Its eruption is catastrophic, spewing lava and ashes, posing a serious risk to humans and their livelihoods. Ashes ejected from the volcano can cause much nuisance to farmers, burying agricultural lands, and destroying crops. The ashes can also present harmful impacts on human health and animals, contaminating infrastructures, and disrupting aviation and land transport. The land area threatened by volcanic eruptions in Indonesia is around 16,670 km2 and affects around 5,000,000 people.
However, the aftermath of volcanic eruptions leads to the world’s most productive soils, volcanic soils. Soils derived from volcanic ash or tephra have the highest capacity to store carbon due to their poorly crystalline minerals that have large surface areas enabling complexation and physical protection. These soils also retain the most persistent soil organic carbon pools. It was estimated that, while volcanic ash soils (Andosols) cover only 0.84% of the earth’s surface, they contain about 5% of global soil carbon [
1]. Soil organic carbon (SOC) stock for Andosols is estimated to be around 254 t C ha
−1 in the upper 100 cm [
2]. Soils derived from volcanic ash are also known to have a high human carrying capacity, as evidenced by the dense population in areas near volcanoes around the world [
3]. Dutch soil scientist E.C.J. Mohr in 1938 [
4] compared population densities of different districts near Mount Merapi, Central Java, Indonesia, and found higher population densities in areas with soils derived from volcanic ash. While SOC sequestration in volcanic ash soils (Andosols) have been widely discussed and reviewed, e.g., Reference [
5], the carbon sequestration potential of tephra is less discussed. This soil material is particularly important in areas with volcanoes such as Indonesia, Philippines, Japan, New Zealand, Hawaii and Pacific Islands, the Caribbean islands, Iceland, and South America.
This paper aims to examine volcanic ash (tephra) from a soil security viewpoint, its main capability. Tephra rejuvenates soil and provides nutrients reserve, and has a large potential to sequester carbon over a relatively short period. This capacity ensures the security of our soils in active volcanic regions of the world. Its carbon sequestration potential can fulfill and even exceed global soil carbon initiatives, such as the 4 per mille soil carbon [
6]. However, volcanic ash’s condition for agriculture is poor as it is still unweather. This paper reviews the role of volcanic ash in sequestering SOC. It provides two case studies from recent volcanic eruptions from Indonesia. It further discusses how a soil security framework may be applied in areas suffering from constant volcanic eruptions.
2. Volcanic Materials and Their Chemical Composition
Materials ejected from volcanic eruptions are classified according to their form. Tephra describes all pyroclasts that leaves a volcanic vent by air, regardless of their type, size, and shape. It is also called volcanic ash, but sensu stricto tephra refers to ashes that are less than 2 mm in diameter. Pyroclasts have a broader meaning than tephra, which includes consolidated and unconsolidated materials [
7].
The type and abundance of primary minerals in tephras depends on the volcano, but usually made up of volcanic glass (silica), quartz, plagioclase, pyroxenes, hornblende, biotite, olivine, etc. [
8], examined tephra from the eruptions from Ruapehu volcano on 11 and 14 October 1995 in New Zealand. Tephra from the two eruptions contained 3.0 and 0.7% by mass of sulphur (S), significantly raised soil sulphate levels in the affected area. Also, its fine grain caused it to be oxidised rapidly, lowering the soil pH. Whereas, the composition of tephra from Mt. Talang in West Sumatra, Indonesia was dominated by labradorite (35%) and rock fragments (21%) [
9,
10,
11]. The heavy mineral fraction consisted of hypersthene (11%), augite (3%), opaques (3%), and hornblende (traces). While the primary mineralogical composition of tephra from Merapi volcano (Central Java, Indonesia) consisted of more non-crystalline (53–60%) compared to crystalline components [
12].
3. Volcanic Soils and Carbon Storage Capacity
When first deposited from volcanic eruptions, pristine tephra contained no organic carbon, but may contain some inorganic carbon. The inorganic carbon originated from volatile elements emitted during a volcanic blast in the form of carbon dioxide, carbon monoxide, methane, and carbonyl sulphide. In the absence of microorganisms during and after a volcanic eruption, this inorganic carbon flux immediately reacted with water molecule or calcium (Ca) to form carbonic acid or calcium carbonate [
13]. However, as the volcanic ash weathered, it produced non-crystalline and poorly crystalline minerals and oxides [
14]. The large specific surface area (∼700 mg
−1) and great reactivity of these poorly crystalline minerals are mostly responsible in the complexation and stabilization of organic matter. Accumulation of organic carbon in the volcanic ash depends on the interaction among a series of biotic and abiotic factors.
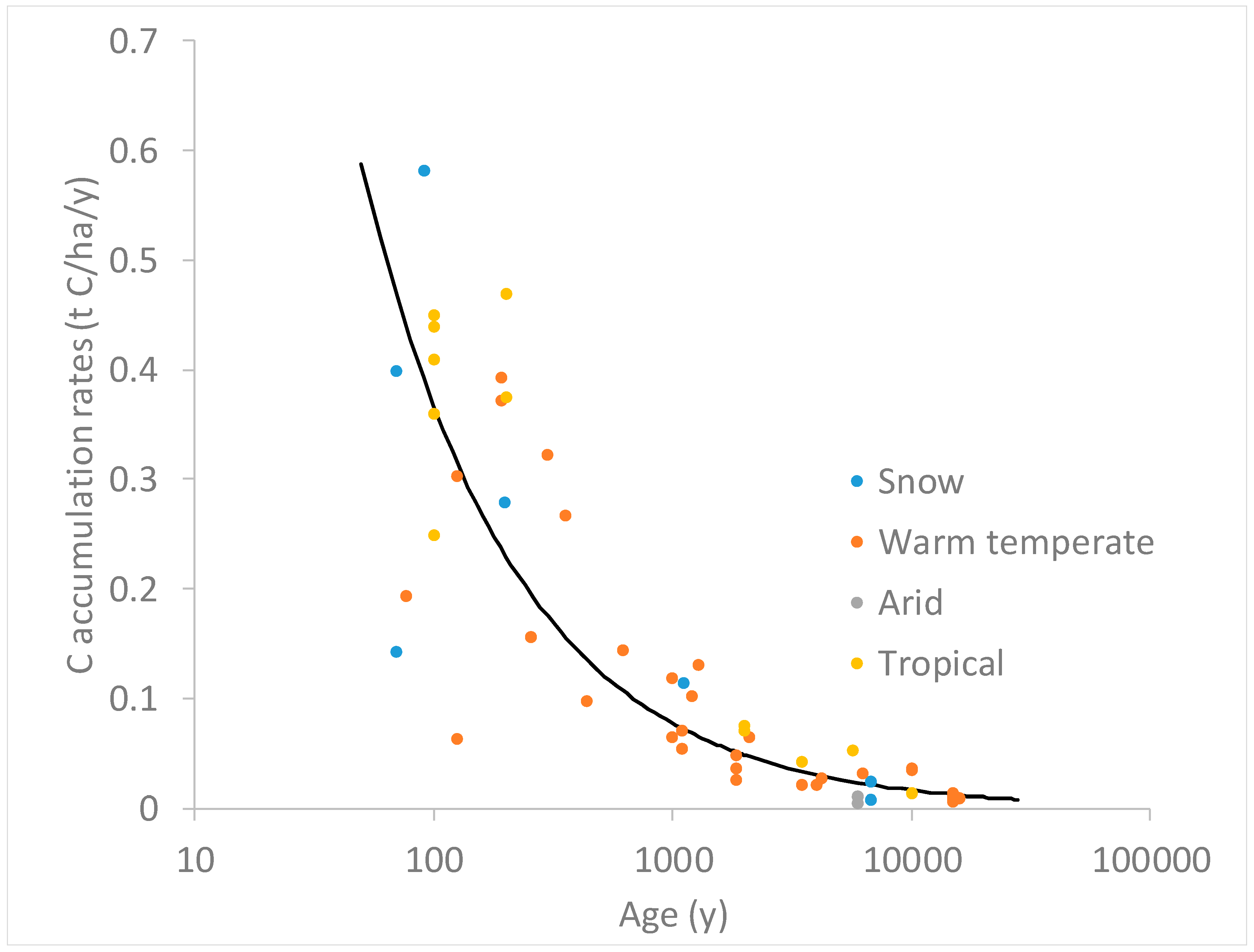
Zehetner [
15] examined if organic C accumulation in volcanic soils can offset CO
2 emissions from volcanic activities. He compiled data from studies conducted on Holocene volcanic deposits in different parts of the world to assess the SOC accumulation potential of volcanic soils (
Figure 1). He found that SOC accumulation rates decreased with increasing soil age, with the largest rate 0.5 t C/ha/year in the first 50 years and decreases to less than 0.1 t C/ha/year after 1000 years. While soil carbon content can be quite high in the surface horizons (up to 10%), the accumulation of organic matter was most rapid in the first 100 years. He further suggested that there is an upper limit of volcanic soils C sequestration at approximately 20 Tg/year, which is relatively insignificant in the global terrestrial C cycle. Nevertheless, we still believe that there is a potential to use volcanic ash as a terrestrial carbon sequester.
A study from a chronosequence in the Etna region in Italy [
16], where the soils were developed from lava, showed that a 125-year period of pedogenesis resulted in a SOC accumulation rate of 0.3 t C/ha/year. The rate of SOC accumulation in the 91-year-old tephra deposit of Mt. Bandai (Japan) is between 0.10 and 0.58 t C/ha/year, with a rapid accumulation rate during the early stage of soil development [
17]. However, another study on volcanic soils from Sierra del Chichinautzin Volcanic Field in Mexico indicated that, after 10,000 years, the C accumulation rates are only in the order of 0.02 to 0.07 t C/ha/year [
18].
Here we report studies that evaluated SOC accumulation since volcanic eruptions.
3.1. Krakatau, Indonesia
Mount Krakatau erupted on 27 August 1883 and formed a remnant Rakata Island, which was covered by ashes. The tropical island with high rainfall (2500–3000 mm/year) led to a rapid recolonization of vegetation and now a well-developed forest. Analysis of soils of Rakata, 110 years after the eruption showed that the SOC content ranges from 49 t C/ha at 720 m elevation to 140 t C ha at 480 m elevation [
19]. This is equivalent to rates of accumulation between 0.44 to 1.3 t C/ha/year.
Within the Krakatau complex, there are four islands: Sertung, Panjang, Rakata, and Anak Krakatau. Mt. Anak Krakatau meaning Child of Krakatau emerged from the caldera in the 1930s and is composed of lava and pyroclastic deposits from the late Mt. Krakatau. Since its emergence from the sea, Anak Krakatau has progressively grown and considered as one of the fastest growing volcano in Indonesia. Anak Krakatau erupted on 22nd December 2018, ejecting tons of ashes and caused a collapse of South-West slope of the island. When the authors visited Mount Anak Krakatau in 2015 (
Figure 2), the organic C content of the soil range from 1.3 to 1.7%, or approximately 49 t C/ha in the top 25 cm, or an average accumulation rate of 0.34 t C/ha/year.
3.2. Mount Pinatubo, Philippines
Mt Pinatubo erupted on June 1991 ejected a large volume of ashes and lahars covering large agricultural areas in Central Luzon. The ash contains a high amount of volcanic ash and about 1.7 g/kg of P
2O
5. It appears that lahar mixed with soils promote regrowth of vegetation, mainly grasses, and leguminous plants. Seven years after the eruption, the lahar deposit has an OC content of 0.8% and nitrogen content of 0.066% [
20].
3.3. Mount St. Helens, USA
Twenty-five years after the eruption of Mt. St. Helens, the concentration of total soil C and N has increased to 4 and 0.4 g kg
−1, respectively [
21]. Two species of perennial lupine (
Lupinus Lepidus and
Lupinus latifolius) were the first plant colonized the volcanic ash deposits. These two legumes are an important factor in ecosystem development and plant succession following volcanic disturbance. They were able to colonize and persist on volcanic deposits because of their ability to fix atmospheric N
2 via symbiosis with
Rhizobium, and subsequently, accumulate organic matter. Total C increased at an average annual rate of about 46 mg kg
−1 (29 ± 7 kg ha
−1) under bare soil, 128 mg kg
−1 (70 ± 17 kg ha
−1) under live lupines and 161 mg kg
−1 (88 ± 17 kg ha
−1) under dead lupines. The carbon content after 25 years of eruption is still quite small (4000 mg kg
−1) with small SOC accumulation rates. They hypothesised that the organic matter inputs are in balance with outputs [
21].
3.4. Iceland
Iceland has a high concentration of active volcanoes, and its soil is mainly Andosols derived from tephra and aeolian sediments of volcanic glass [
22]. SOC sequestration rate in a sandy desert following a seven-year restoration process was evaluated [
21]. The desert’s parent material is volcanic glass with a low carbon concentration, and they found an annual carbon accumulation of 0.4–0.63 t C/ha. Meanwhile, sequestration rates of 0.23 t C/ha/year following tree plantation in a degraded heathland was reported [
23]. Another study reported a long-term carbon accumulation rates of 0.17–0.3 t C/ha/year in Southwest Iceland [
24].
Another study [
25] reported SOC accumulation in a 50-year-old volcanic island Surtsey. Seagull colony on the island provided nutrient enriched areas, and thus SOC concentration has been increasing from 0.08% (taking the 1986 value as baseline SOC concentration, the first year of permanent seabird colonization) to 0.9 ± 0.3% on deep tephra sand and 4.6 ± 0.4% on shallow tephra sand. The accumulation rate is between 0.1-0.4 t C/ha/year.
3.5. Kasotchi Island, Alaska
Kasatochi volcano erupted in August 2008 and buried a small island in pyroclastic deposits and fine ash. Subsequently, microbes, plants, and birds begun to re-colonize the initially sterile surface. Five years post-eruption, SOC content is still relatively small (<0.2% C). However, microbial activities in pyroclastic materials with organic matter (OM) inputs were one or two order magnitude larger than in materials without OM input [
26].
3.6. Hawaii
Soil development’s effect on SOC stock along chronosequences in Hawaii was evaluated [
27]. The study found that SOC content followed a similar trend as the soil development where volcanic parent materials weathered to non-crystalline minerals during the first 150,000 years, followed by a decline in the amount of non-crystalline minerals and an increase in stable crystalline mineral accumulation. Similarly, SOC accumulated to a maximum after 150,000 years and then decreased by 50% over the next four million years. They explained this through a change in SOC-mineral stability mechanisms during soil formation [
28]. As these increase over time, the minerals lose the capacity to stabilize carbon or the soil becomes saturated.
Reference [
29] evaluated a chronosequence of 10, 52, and 142-year-old lava flows on Mauna Loa, Hawaii. They found aboveground biomass accumulated rapidly in the first decade of primary succession. However, SOC accumulation lagged behind biomass, with negligible SOC at the 10-year site. SOC accumulation rates at 0.13 and 0.27 t C/ha/year were found at the 52- and 142-year sites. They concluded that weathering rates of lava, in part, regulate rates of nitrogen fixation in these young ecosystems.
4. The Weathering of Volcanic Ash
Most studies on the primary succession of volcanic islands focused primarily on flora and fauna community changes and hardly look into the development of soil carbon. In early stage of weathering, cations were leached from volcanic ash when they were in contact with water and significantly increased the concentration of plant nutrients in soils. As such, re-vegetation or plant recovery prevailed immediately. Volcanic ash weathered rapidly to form short-range order alumino-silicate mineral (allophane, imogolite). These non-crystalline clay minerals have a high capacity to protect SOC in volcanic soils.
The establishment of plants and biomass led to the accumulation of SOC. Simultaneously, as pools of carbon developed, the volcanic ashes underwent weathering or decomposition processes. Nitrogen supply to pioneer plants was hypothesised as a key to the re-vegetation of volcanic ash affected areas [
20]. It is continuous feedback that the increase in SOC will enhance release of nutrients and increase in cation exchange capacity and water holding capacity. These have positive effects on plant growth. Once the soil has increased its fertility, secondary colonizers will establish, which in turn further enhance SOC accumulation.
The decomposition of volcanic ash is likely to be proportional to the quantity of inputs N particular from not highly lignified plant biomass of lupines [
30]. Net migration of plant nutrients from volcanic ash leads to progressive stages of plant succession from assembly to interaction [
31]. The quantity and quality of substrates released from volcanic ash during decomposition influence the rate and pattern of plant succession through physical and biological amelioration of bare sites and subsequent interaction among colonists [
31,
32]. They also affect the succession below ground as the numbers, diversity, and activity of microbial are increased and interaction between lupines and fungi.
Soil carbon storage on volcanic soils across a high-elevation climate gradient on Mauna Kea, Hawaii showed that, after 20 ky, pedogenic processes have altered the nature and composition of the volcanic ash such that it is capable of retaining SOC [
33]. The sites have a cold climate and low rainfall (~250–500 mm). Under such dry condition, the rate of carbon supply to the soil was driven by coupling of rainfall above ground plant production.
Tephra deposited on top of soil also provides a unique weathering pathway. It was hypothesised that buried organic-rich soil pumps protons upward to the tephra layer, which acts as an alkaline trap for CO
2 [
34]. Pioneer plant (blue-green algae or cyanobacteria) used the CO
2 to initiate environment recovery. Black and brown patches were observed on tephra layer as the growth of cyanobacteria progress. Then, the algae mat provides suitable habitats to support mosses and higher plants life [
35].
5. SOC Stabilisation Mechanisms
The ability of Andosols to store a large amount of C is mainly due to the dominance of short-range-ordered clay minerals (allophane, imogolite, and ferrihydrite) and metal-humus complexes (Al/Fe–humus complexes) in their colloidal fraction. However, there are also non-allophanic Andosols, where Al-humus complexes are dominant. The mineralogical properties of these non-crystalline materials present a high reactive surface area and are regarded as the major agent of C stabilisation.
A study on volcanic soils from Chile and found that SOM has the largest correlation with Al, rather than with clay content and climatic conditions [
5]. They concluded that Al is the principal factor for immobilization of SOC in acid volcanic soils.
Soil organic matter (SOM) density fractionation study [
36] on a soil horizon derived from tephra from the last eruptive phases of the Piton des Neiges in the Reunion Island, showed that the largest proportion (82.6%) of organic matter was associated with minerals in organo-mineral complexes. Imogolite-type materials bound 6-fold more OM than anorthoclase, and 3.5-fold more OM than iron oxides. Buried horizons, which were dominated by crystalline minerals (feldspars, gibbsite), have the least capacity to store organic matter and the fastest carbon turnover. In contrast, buried horizons dominated by poorly crystalline clay minerals (proto-imogolite and proto-imogolite allophane) store large amounts of organic matter with low carbon turnover.
Allophane spherules tend to form nanoaggregates up to about 100 nm in diameter [
37,
38]. The nano pores both within and between nanoaggregates provide physical protection to SOC, where they cannot be accessed by microbes [
39]. The organo-mineral complexes of volcanic soils can also protect soil carbon from disturbances caused by forest management, thus preventing potential carbon loss [
40].
Mechanisms for chemical and physical stabilization of SOC in volcanic ash soils can be summarised as Reference [
41]:
direct stabilization of SOC in organo-metallic (Al-humus) complexes,
indirect chemical protection of SOC (notably aliphatic compounds) through low soil pH and toxic levels of Al, and
physical protection of SOC by the very large micro- or nanopores.
6. Case Study—Indonesia
The following case study from two areas in Indonesia showed how tephra from a fresh eruption could accumulate a high amount of carbon in a relatively short period [
35].
6.1. Pot Experiment, Mount Talang
Mount Talang in West Sumatra, Indonesia erupted in April 2005 (
Figure 3), and ashes from the eruption were collected [
40]. The texture was a sandy loam, with coarse (2.0–0.05 mm), medium (0.05–0.002 mm) and fine (<0.002 mm) particles of 13, 68 and 19%, respectively. Based on the silica (SiO2) content of 57%, the tephra of Mt. Talang is considered as a basalto-andesitic ash. Cation exchange capacity (CEC) was low with a value of 5.50 cmol
c kg
−1.
A pot experiment was carried out to study C accumulation on the tephra. The experiment was conducted in wired-house. There were 2 main treatments: (1) A single tephra layer or without soil, (2) a tephra layer was placed above existing soil surface (15 cm of A horizon and 15 cm of B horizon). The thicknesses of the tephra layer were 0, 2.5, and 5.0 cm simulating tephra deposition. The experiment is a randomized design with tree replicates on each treatment. Five mm of filtered water was added daily to each pot throughout four years of experiment. The water added was approximately equal to 14 years of precipitation in the Mt. Talang area.
The first noticeable change on the tephra layers was transformation of tephra color. The moist color of the surface tephra layer changed from light gray to very pale brown after 24 months and becoming pale brown after 46 months (
Figure 4). This color transformation can be attributed to oxidation and liberation of Fe from tephra grains as well as accumulation of OC [
40].
After two months, blue-green algae (cyanobacteria) started to colonize the bare tephra layer formed an algae mat. After 16 months, the surface was transformed into a green film of lichen. Vascular plants (grasses and shrubs) started to establish after two years (
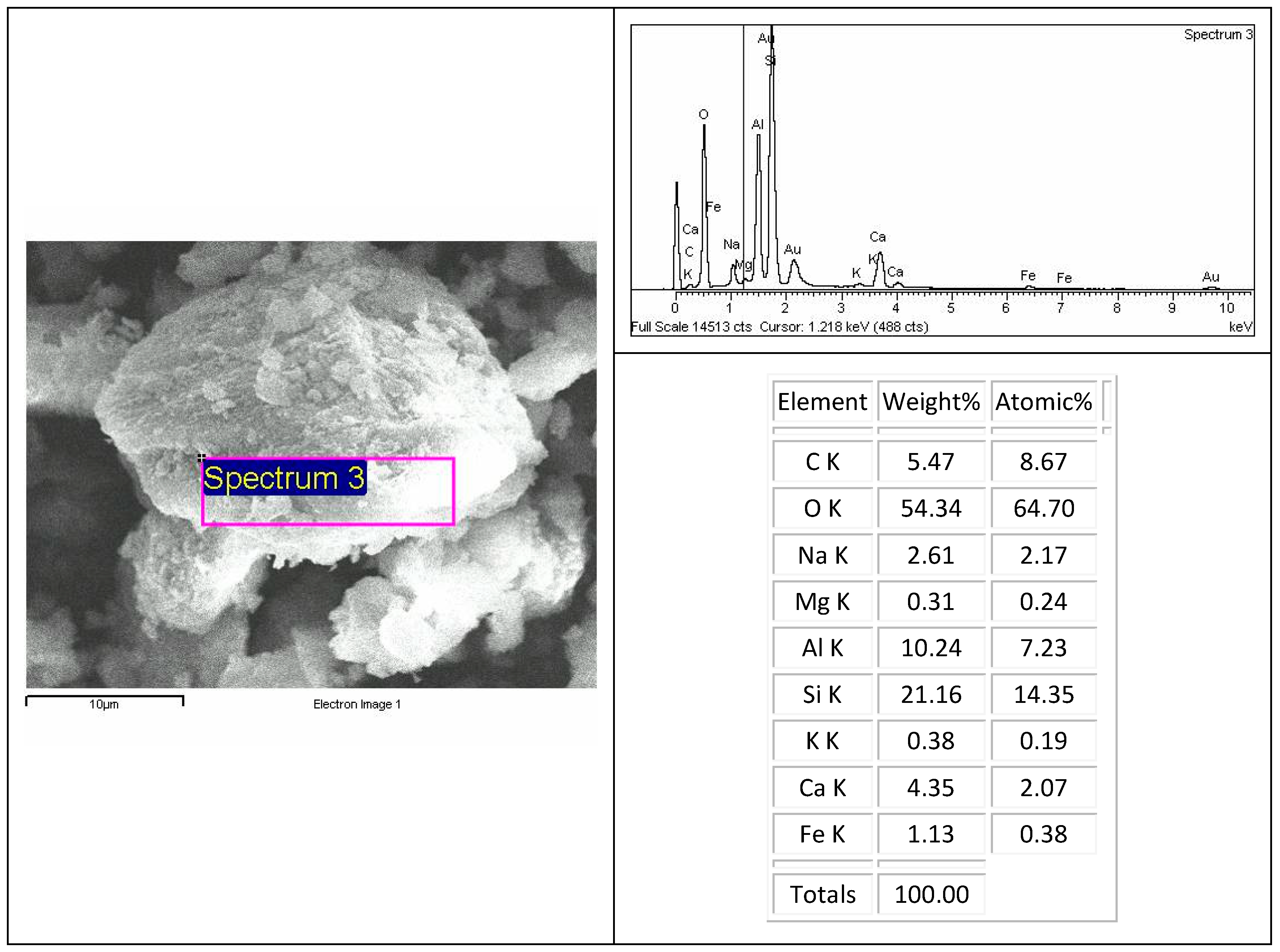
Figure 4). Inorganic carbon was detected by a SEM-EDX (
Figure 5).
The initial total C content of the tephra is 0.19%, and after 46 months, the C content of the 2.5 cm tephra layer on top of the soil increased to 1.75%, and the C content of the 5 cm tephra layer on top of the soil increased to 0.9%. Meanwhile, tephra by itself accumulates less carbon with a concentration of 0.6%. Sequestration rates of 0.2 to 0.5% C per year or 0.5–0.7 t C/ha/year were observed for volcanic ashes, which were added on top of soils (
Figure 6).
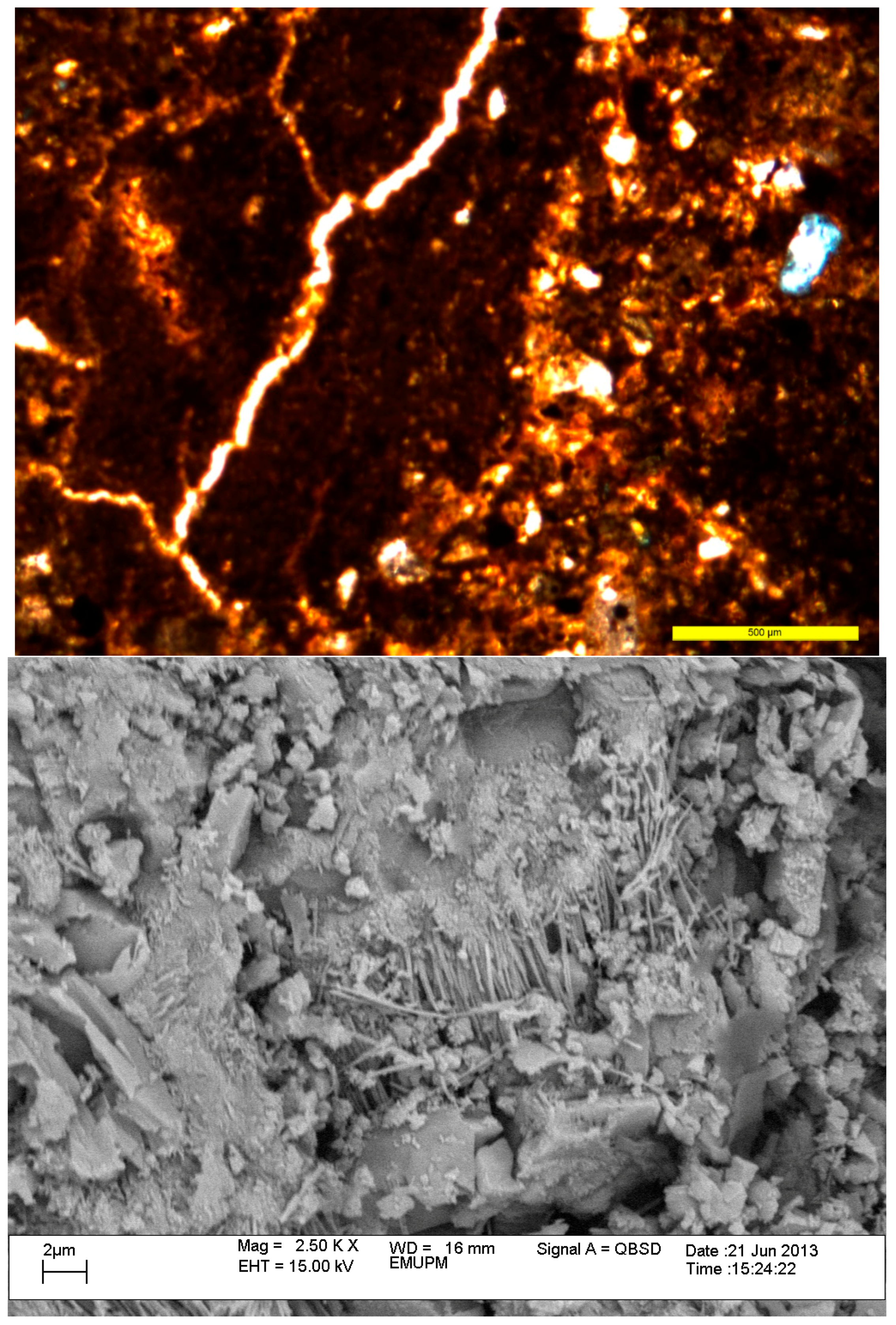
After 46 months root hyphae were observed with a SEM. The hyphae were attached to volcanic ash grains (
Figure 7 and
Figure 8) and may help in weathering of the materials and helped sequester more C in soil. The ashes contain light minerals include labradorite (35%) and rock fragments (21%), whereas the heavy mineral fractions consist of hypersthene (11%), augite (3%), opaques (3%) and hornblende (traces) (
Figure 9).
This study indicated that volcanic ash could sequester carbon rapidly and in large quantities. However, plants and microbial activities are required to enhance the C sequestration potential for pristine volcanic ashes.
Cyanobacteria have the ability to utilize both CO2 and bicarbonate (HCO3) as an inorganic carbon source to produce biomass. The growth of blue-green algae on the soil surface can significantly increase SOC.
The study [
40] found that soil OC accumulated approximately linearly with time (
Figure 5). The OC content of tephra layer increased significantly after 8 months and was nine times higher after 46 months as more vegetation emerged. The rate of OC accumulation on tephra on existing soil layer was 5–15 times faster than just only tephra. Rate on 2.5 cm tephra layer (with soil) was larger than 5.0 cm, highlighting the importance of life on existing soil. Sources of OC accumulated in the tephra layer during the first year was cyanobacteria, the second year was from cyanobacteria and lichens, and the third to fourth year was from shrubs or grasses.
6.2. A Natural Experiment, Mt Sinabung
Mt Sinabung in North Sumatra was dormant for more than 400 years and came active again in 2010 (
Figure 10). Eruptions were recorded in 2010, 2013, 2014, and 2016. On 28th January 2014, Sinabung erupted spewing pyroclastic flow and ash. Volcanic ash carried by rain-covered areas of the Sigarang Garang village, which is located Northeast of the foot of the volcano (
Figure 11). After deemed safe, the local people returned to the village and cleaned up the substantial amount of ash and bagged them.
We visited the village in January 2017 and found ashes that were exposed to rainfall were already colonised by lichens, and some has established grasses. Ashes that were stored and not exposed to rainfall were in a dry condition without any plants (
Figure 12).
Table 1 and
Table 2 show some chemical analysis of these samples.
The ash has an inorganic C between 0.1–0.2%. After three years of lichens colonisation, the C content increased to 4.1% (
Table 1). Additionally, in materials covered with grass, the C content increased to 1.6%. This limited data demonstrated the high capacity to accumulate Cover a short period.
6.3. Volcanic Ash, the Role of Soil Security
Here we would like to link volcanic ash in the framework of soil security [
41] in the case study of Sinabung based on the framework outlined in Bouma [
42].
Farmers in the area near Sinabung suffered because of repeated volcanic eruptions since 2010 and ashes constantly being deposited on their farming land. While the volcanic materials are valuable in terms of ecosystem services and boosting soil’s capability, the ashes caused farmers lost their income. An estimated 53,000 hectares of farmland was destroyed by volcanic ash in 2014. Local farmers adaptation includes reducing cultivation area, regularly hosing off the ashes from plants, and planting quick maturing vegetables [
43]. Some farmers were resettled to new area by the local government in Siosar area, Karo regency, with each family getting a 500 m
2 plot for farming. In the new area, social, cultural, and economic life have flourished.
To ensure soil security for farmers in the future, the volcanic ash possess high capability for supplying nutrients and absorbing carbon, however, it’s weathering took time (2–4 years). The current condition is an economical threat for farmers and if eruption continued it will be dangerous to live in the area. The role of connectivity is to inform farmers on the danger of volcanic threats and educating them about the risks. Farmers’ perception of risk of volcanic hazards is a complex interaction between cultural beliefs and socio-economic constraints [
44]. They also need to be informed on the valuable ashes that will keep their soil fertile for centuries. Farmers need to be trained to have a diverse range of cash crops as buffer. Or temporary shift to other types of occupation [
45]. Policy from the local government need to make sure farmers are well-equipped to handle the disaster.
An example from Kinali village in Siau Island, part of the archipelagic district of Sitaro, North Sulawesi Province, Indonesia demonstrated that people have adapted to volcanic eruptions from Mount Karangetang [
46]. This volcano is classified as both category 2 (high risk of lava, lahars, dense volcanic ash, and the possibility of pyroclastic flows) and category 3 (frequently affected by pyroclastic flows, lava, lahars, dense volcanic ash) by the Indonesian Government. However, the villagers realized that the volcano eruption bring fertility to the soil and in turn produce high-quality nutmeg that generates good income. The keys to such adaptation is social cohesion, strength knowledge and skills to face hazards, availability of good infrastructure, availability of food and water to cope with shortages, enough saving, and appropriate political support [
46].
7. Ecosystem Recovery after Volcanic Eruption
Tephra, which initially does not contain organic carbon and life form, has a high capacity to capture CO2 from the atmosphere through plants. The fine-size tephra, when exposed to the natural atmospheric environment, can store moisture, which enabled blue-green algae to colonise the bare surface layer forming an algae mat. This succession is followed by the development of layers of lichen, and then colonisation of vascular plants.
Ecosystem recovery after volcanic ash deposition depends on the thickness of the deposit. Re-vegetation was faster on shallow ash layer (2.5 cm) compared to thicker tephra layer, with a pedogenic time between 6 to 12 months [
36]. The thin volcanic ash layer has positive nutrient responses to vegetation. The area around Mount St. Helens in Washington State USA with 10–20 cm thick tephra after the eruption in 1980 started to recover after ten years. Meanwhile, in Krakatau islands in Indonesia with >100 cm thick of ash layer, plant recovery and new soil development only commenced after 100 years [
47]. While both areas are quite different in terms of climate and parent materials, it shows the importance of the thickness of the ashes.
Survival of vegetation underneath of tephra layer plays an important role on the reestablishment of ecosystem. Response capability of the buried plants is related to rhizome, shoots, and seeds, which survived after the eruption. Annual plants recovered faster than perennial plants [
48]. Initially, plants suffered nutrient deficiency such as N and P to growth [
20] and the presence of nitrogen-fixing pioneer plants can accelerate plant recovery or applying nitrogen fertilizer [
49].. Organic matter in these volcanic soils tends to be stabile as they formed stabile organomineral complexes and physically protected.
8. Conclusions
Tephra deposited to the soil surface renew the soil and sustain its productivity. Also, tephra has a large capacity to sequester atmospheric carbon. As the ash weathers to secondary minerals more organic carbon can be preserved and protected in the soil. The protective mechanisms of SOC in these soils include organo-metallic complexes, chemical protection through low soil pH and toxic levels of Al, and physical protection by the micro- or nanopores.
Most studies on plant succession of volcanic ash regions focused on flora and fauna community changes and rarely look into the development of soil carbon. Accumulation of SOC in these newly-deposited tephra plays an important role in the development of plants. The increase in SOC in tephra will enhance weathering and release nutrients that have positive effects on plant growth. Research on C dynamics and sequestration potential on newly deposited tephra is still rare. Beside its large C sequestration potential, it may provide clues on the initial pedogenesis.
Volcanic ash plays an important role in the global carbon cycle and ensures soil security in volcanic regions of the world. However, farmers living in the volcano area also need to be considered. Volcanic ash will severely affect agricultural area and farmers’ livelihood. As the value of tephra as new soil fertile materials is yet to be quantified, it is underappreciated and becomes a nuisance for local farmers. Connectivity and codification needs to ensure farming in the area to take into account risk and build appropriate adaptation strategies and resilience.
Author Contributions
Conceptualization, B.M. and D.F.; methodology, D.F., F.I.G., G., M.N.; investigation, D.F., F.I.G., G., M.N.; resources, D.F., F.I.G., G., M.N.; writing—original draft preparation, B.M., F.I.G., D.F.; writing—review and editing, B.M., F.I.G., D.F.; visualization, F.I.G., M.N.
Funding
D.F., G., and M.N. were funded by the Ministry of research, Technology and Higher Education of Indonesia with grant number 08/UN.16.17/PP.PBK.K/LPPM/2018. The support from Sydney Institure of Agriculture—The University of Sydney enabled some analyses were carried out there.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eswaran, H.; Van Den Berg, E.; Reich, P. Organic carbon in soils of the world. Soil Sci. Soc. Am. J. 1993, 57, 192–194. [Google Scholar] [CrossRef]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Small, C.; Naumann, T. The global distribution of human population and recent volcanism. Global Environ. Chang. Part B Environ. Hazards 2001, 3, 93–109. [Google Scholar] [CrossRef]
- Mohr, E.C.J. The relation between soil and population density in the Netherlands Indies. Comptes Rendus Du Congres Int. Du Geogr. 1938, 103, 478–493. [Google Scholar]
- Matus, F.; Rumpel, C.; Neculman, R.; Panichini, M.; Mora, M. Soil carbon storage and stabilisation in andic soils: A review. Catena 2014, 120, 102–110. [Google Scholar] [CrossRef]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.-S.; Cheng, K.; Das, B.S. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Arnalds, O. The Influence of Volcanic Tephra (Ash) on Ecosystems. Adv. Agron. 2013, 121, 331–380. [Google Scholar]
- Cronin, S.; Hedley, M.; Neall, V.; Smith, R. Agronomic impact of tephra fallout from the 1995 and 1996 Ruapehu Volcano eruptions, New Zealand. Environ. Geol. 1998, 34, 21–30. [Google Scholar] [CrossRef]
- Fiantis, D.; Nelson, M.; Shamshuddin, J.; Goh, T.B.; Van Ranst, E. Leaching experiments in recent tephra deposits from Talang volcano (West Sumatra), Indonesia. Geoderma 2010, 156, 161–172. [Google Scholar] [CrossRef]
- Fiantis, D.; Nelson, M.; Shamshuddin, J.; Goh, T.B.; Van Ranst, E. Determination of the Geochemical Weathering Indices and Trace Elements Content of New Volcanic Ash Deposits from Mt. Talang (West Sumatra) Indonesia. Eurasian Soil Sci. 2010, 43, 1477–1485. [Google Scholar] [CrossRef]
- Fiantis, D.; Nelson, M.; Shamshuddin, J.; Goh, T.B.; Van Ranst, E. Changes in the Chemical and Mineralogical Properties of Mt. Talang Volcanic Ash in West Sumatra during the Initial Weathering Phase. Commun. Soil Sci. Plant Anal. 2011, 42, 569–585. [Google Scholar] [CrossRef]
- Fiantis, D.; Nelson, M.; Van Ranst, E.; Shamshuddin, J.; Qafoku, N.P. Chemical Weathering of New Pyroclastic Deposits from Mt. Merapi (Java), Indonesia. J. Mountain Sci. 2009, 6, 240–254. [Google Scholar] [CrossRef]
- Dahlgren, R.A.; Saigusa, M.; Ugolini, F.C. The nature, properties and management of volcanic soils. Adv. Agron. 2004, 82, 113–182. [Google Scholar]
- Shoji, S.; Nanzyo, M.; Dahlgren, R.A. Volcanic Ash Soils—Genesis, Properties and Utilization; Elsevier: Amsterdam, The Netherland, 1993; p. 288. [Google Scholar]
- Zehetner, F. Does organic carbon sequestration in volcanic soils offset volcanic CO2 emissions? Quat. Sci. Rev. 2010, 29, 1313–1316. [Google Scholar] [CrossRef]
- Egli, M.; Nater, M.; Mirabella, A.; Raimondi, S.; Plötze, M.; Alioth, L. Clay minerals, oxyhydroxide formation, element leaching and humus development in volcanic soils. Geoderma 2008, 143, 101–114. [Google Scholar] [CrossRef]
- Morisada, K.; Imaya, A.; Ono, K. Temporal changes in organic carbon of soils developed on volcanic andesitic deposits in Japan. Forest Ecol. Manag. 2002, 171, 113–120. [Google Scholar] [CrossRef]
- Peña-Ramírez, V.M.; Vázquez-Selem, L.; Siebe, C. Soil organic carbon stocks and forest productivity in volcanic ash soils of different age (1835–30,500 years BP) in Mexico. Geoderma 2009, 149, 224–234. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Bruijnzeel, L.; Bush, M.B.; Klein, E.M.; Mace, K.A.; Raikes, J.A.; Whittaker, R. The biogeochemistry of phosphorus after the first century of soil development on Rakata Island, Krakatau, Indonesia. Biogeochemistry 1998, 40, 37–55. [Google Scholar] [CrossRef]
- Shoji, S.; Takahashi, T. Environmental and agricultural significance of volcanic ash soils. Glob. Environ. Res. 2002, 6, 113–135. [Google Scholar]
- Halvorson, J.J.; Smith, J.L. Carbon and nitrogen accumulation and microbial activity in Mount St. Helens pyroclastic substrates after 25 years. Plant Soil 2009, 315, 211–228. [Google Scholar] [CrossRef]
- Arnalds, O. Volcanic soils of Iceland. Catena 2004, 56, 3–20. [Google Scholar] [CrossRef]
- Ritter, E. Carbon, nitrogen and phosphorus in volcanic soils following afforestation with native birch (Betula pubescens) and introduced larch (Larix sibirica) in Iceland. Plant Soil 2007, 295, 239–251. [Google Scholar] [CrossRef]
- Gísladóttir, G.; Erlendsson, E.; Lal, R.; Bigham, J. Erosional Effects on Terrestrial Resources over the last Millennium in Reykjanes, Southwest Iceland✶. Quat. Res. 2010, 73, 20–32. [Google Scholar] [CrossRef]
- Leblans, N.I.W.; Sigurdsson, B.D.; Roefs, P.; Thuys, R.; Magnusson, B.; Janssens, I.A. Effects of seabird nitrogen input on biomass and carbon accumulation after 50 years of primary succession on a young volcanic island, Surtsey. Biogeosciences 2014, 11, 6237–6250. [Google Scholar] [CrossRef]
- Zeglin, L.H.; Wang, B.; Waythomas, C.; Rainey, F.; Talbot, S.L. Organic matter quantity and source affects microbial community structure and function following volcanic eruption on K asatochi I sland, A laska. Environ. Microbiol. 2016, 18, 146–158. [Google Scholar] [CrossRef]
- Torn, M.S.; Trumbore, S.E.; Chadwick, O.A.; Vitousek, P.M.; Hendricks, D.M. Mineral control of soil organic carbon storage and turnover. Nature 1997, 389, 170–173. [Google Scholar] [CrossRef]
- Masiello, C.; Chadwick, O.; Southon, J.; Torn, M.; Harden, J. Weathering controls on mechanisms of carbon storage in grassland soils. Global Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Crews, T.E.; Kitayama, K.; Fownes, J.H.; Riley, R.H.; Herbert, D.A.; Muellerdombois, D.; Vitousek, P.M. Changes in Soil-Phosphorus Fractions and Ecosystem Dynamics across a Long Chronosequence in Hawaii. Ecology 1995, 76, 1407–1424. [Google Scholar] [CrossRef]
- Gill, R.A.; Boie, J.A.; Bishop, J.G.; Larsen, L.; Apple, J.L.; Evans, R.D. Linking community and ecosystem development on Mount St. Helens. Oecologia 2006, 148, 312–324. [Google Scholar] [CrossRef] [PubMed]
- del Moral, R. Limits to convergence of vegetation during early primary succession. J. Veg. Sci. 2007, 18, 479–488. [Google Scholar] [CrossRef]
- Del Moral, R.; Rozzell, L.R. Long-term effects of Lupinus lepidus on vegetation dynamics at Mount St. Helens. Plant Ecol. 2005, 181, 203–215. [Google Scholar] [CrossRef]
- Kramer, M.G.; Chadwick, O.A. Controls on carbon storage and weathering in volcanic soils across a high-elevation climate gradient on Mauna Kea, Hawaii. Ecology 2016, 97, 2384–2395. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, F.C.; Dahlgren, R.A. Soil development in volcanic ash. Global Environ. Res. 2002, 6, 69–82. [Google Scholar]
- Basile-Doelsch, I.; Amundson, R.; Stone, W.; Masiello, C.; Bottero, J.Y.; Colin, F.; Masin, F.; Borschneck, D.; Meunier, J.D. Mineralogical control of organic carbon dynamics in a volcanic ash soil on La Réunion. Eur. J. Soil Sci. 2005, 56, 689–703. [Google Scholar] [CrossRef]
- Calabi-Floody, M.; Bendall, J.S.; Jara, A.A.; Welland, M.E.; Theng, B.K.; Rumpel, C.; de la Luz Mora, M. Nanoclays from an Andisol: Extraction, properties and carbon stabilization. Geoderma 2011, 161, 159–167. [Google Scholar] [CrossRef]
- Basile-Doelsch, I.; Amundson, R.; Stone, W.E.E.; Borschneck, D.; Bottero, J.Y.; Moustier, S.; Masin, F.; Colin, F. Mineral control of carbon pools in a volcanic soil horizon. Geoderma 2007, 137, 477–489. [Google Scholar] [CrossRef]
- Panichini, M.; Neculman, R.; Godoy, R.; Arancibia-Miranda, N.; Matus, F. Understanding carbon storage in volcanic soils under selectively logged temperate rainforests. Geoderma 2017, 302, 76–88. [Google Scholar] [CrossRef]
- Tonneijck, F.; Jansen, B.; Nierop, K.; Verstraten, J.; Sevink, J.; De Lange, L. Towards understanding of carbon stocks and stabilization in volcanic ash soils in natural Andean ecosystems of northern Ecuador. Eur. J. Soil Sci. 2010, 61, 392–405. [Google Scholar] [CrossRef]
- Fiantis, D.; Nelson, M.; Shamshuddin, J.; Goh, T.B.; Ranst, E.V. Initial carbon storage in new tephra layers of Mt. Talang in Sumatra as affected by pioneer plants. Commun. Soil Sci. Plant Anal. 2016, 47, 1792–1812. [Google Scholar]
- McBratney, A.; Field, D.J.; Koch, A. The dimensions of soil security. Geoderma 2014, 213, 203–213. [Google Scholar] [CrossRef]
- Bouma, J. Soil Security in Sustainable Development. Soil Syst. 2019, 3, 5. [Google Scholar] [CrossRef]
- Tampubolon, J.; Nainggolan, H.; Ginting, A.; Aritonang, J. Mount Sinabung Eruption: Impact on Local Economy and Smallholder Farming in Karo Regency, North Sumatra. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Singapore, 14–16 December 2017; p. 012039. [Google Scholar]
- Lavigne, F.; De Coster, B.; Juvin, N.; Flohic, F.; Gaillard, J.-C.; Texier, P.; Morin, J.; Sartohadi, J. People’s behaviour in the face of volcanic hazards: Perspectives from Javanese communities, Indonesia. J. Volcanol. Geotherm. Res. 2008, 172, 273–287. [Google Scholar] [CrossRef]
- Bachri, S.; Stötter, J.; Monreal, M.; Sartohadi, J. The calamity of eruptions, or an eruption of benefits? Mt. Bromo human–volcano system a case study of an open-risk perception. Nat. Hazards Earth Syst. Sci. 2015, 15, 277–290. [Google Scholar] [CrossRef]
- Rampengan, M.M.F.; Boedhihartono, A.K.; Margules, C.; Sayer, J.; Law, L.; Gaillard, J.C.; Tien, O.T.N.; Linh, T.T.M. Agroforestry on an Active Volcanic Small Island in I ndonesia: Prospering with Adversity. Geogr. Res. 2016, 54, 19–34. [Google Scholar] [CrossRef]
- Arnalds, O. The influence of volcanic tephra (ash) on ecosystems. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2013; Volume 121, pp. 331–380. [Google Scholar]
- Swedberg, K.C. Effects of Mount St. Helens ash on Plant Populations of Shallow Stony Soils; Keller, S.A.C., Ed.; Eastern Washington University Press: Washington, DC, USA, 1986; pp. 256–260. [Google Scholar]
- Del Moral, R.; Wood, D.M. Subalpine Vegetation Recovery Five Years After the Mount St. Helens Eurptions; Eastern Washington University Press: Washington, DC, USA, 1986; pp. 215–221. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).