Inhibition of Photosynthetic Activity in Wastewater-Borne Microalgal–Bacterial Consortia under Various Light Conditions
Abstract
1. Introduction
2. Materials and Methods
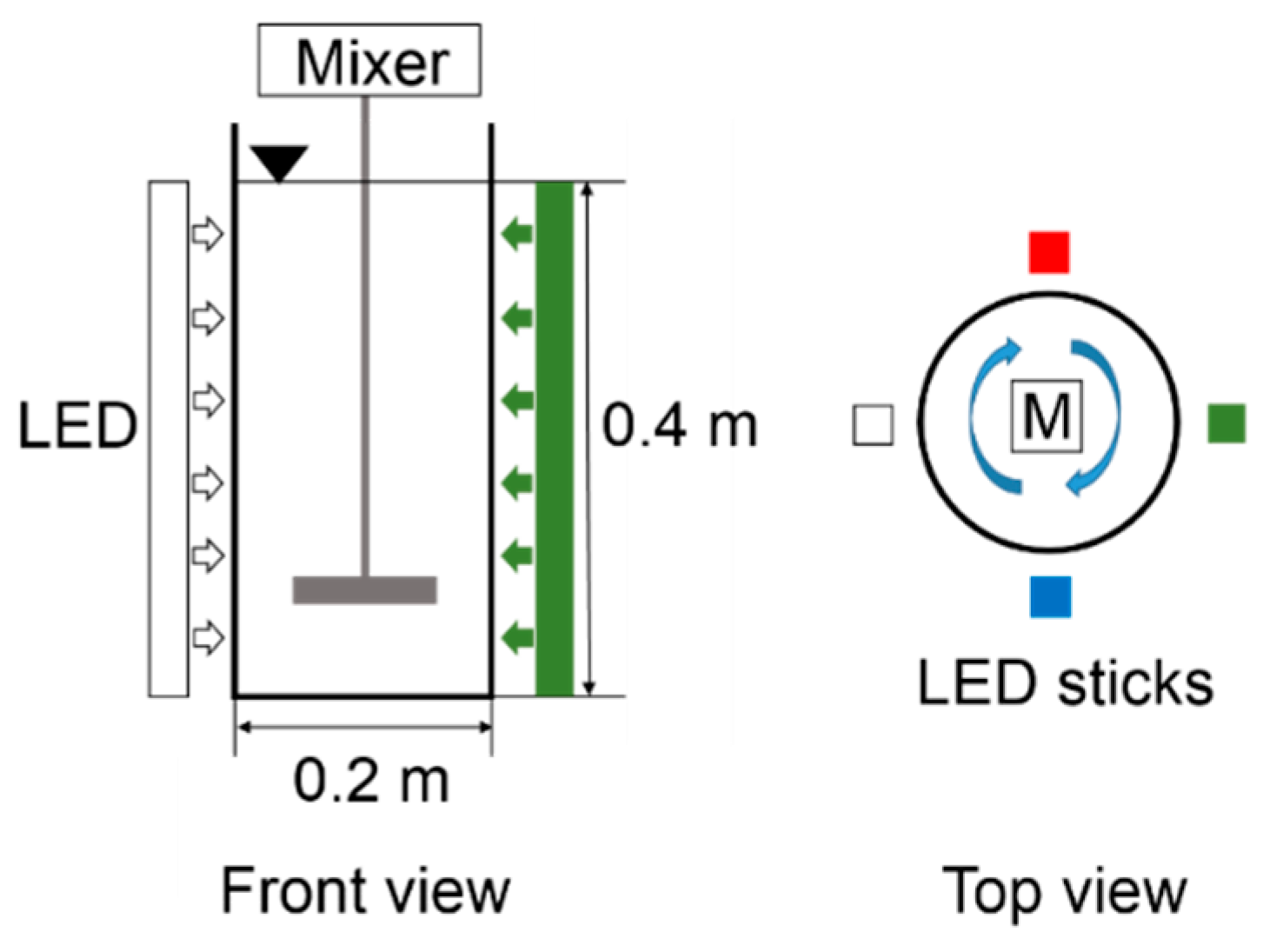
2.1. Cultivation of Microalgal–Bacterial Consortia
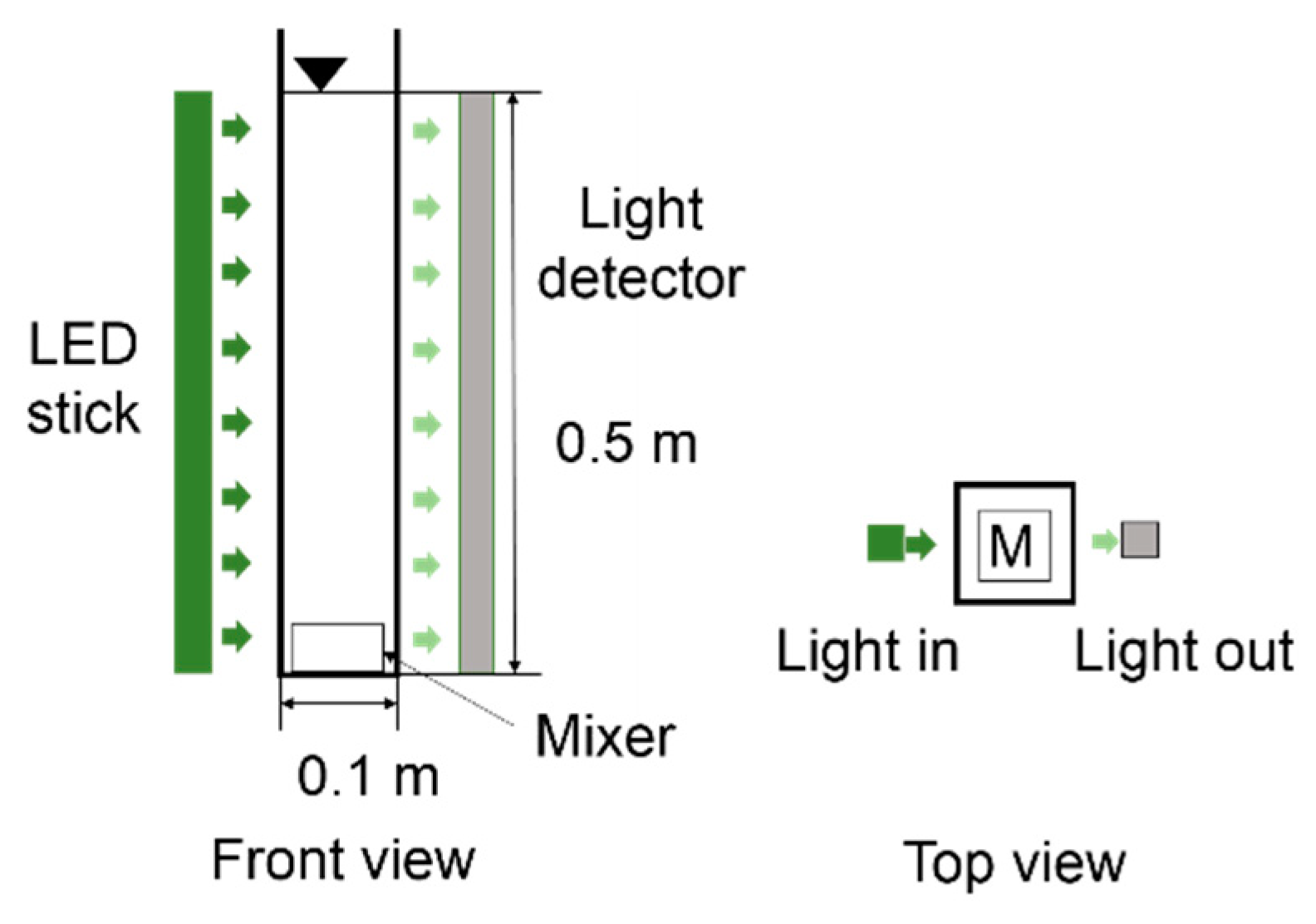
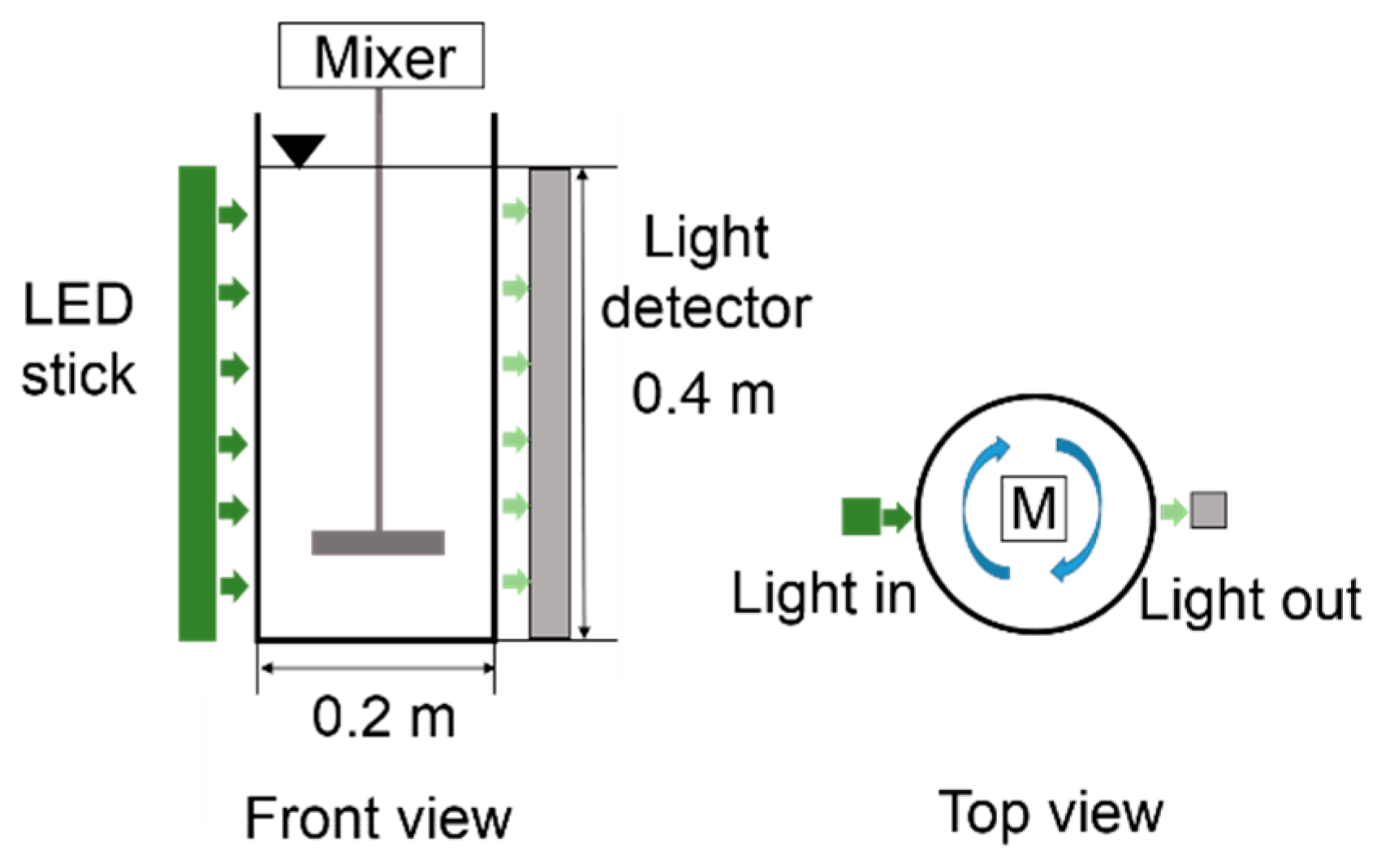
2.2. Transmittance of Light
2.3. Inhibition of Photosynthetic Activity
2.4. Statistical Analysis
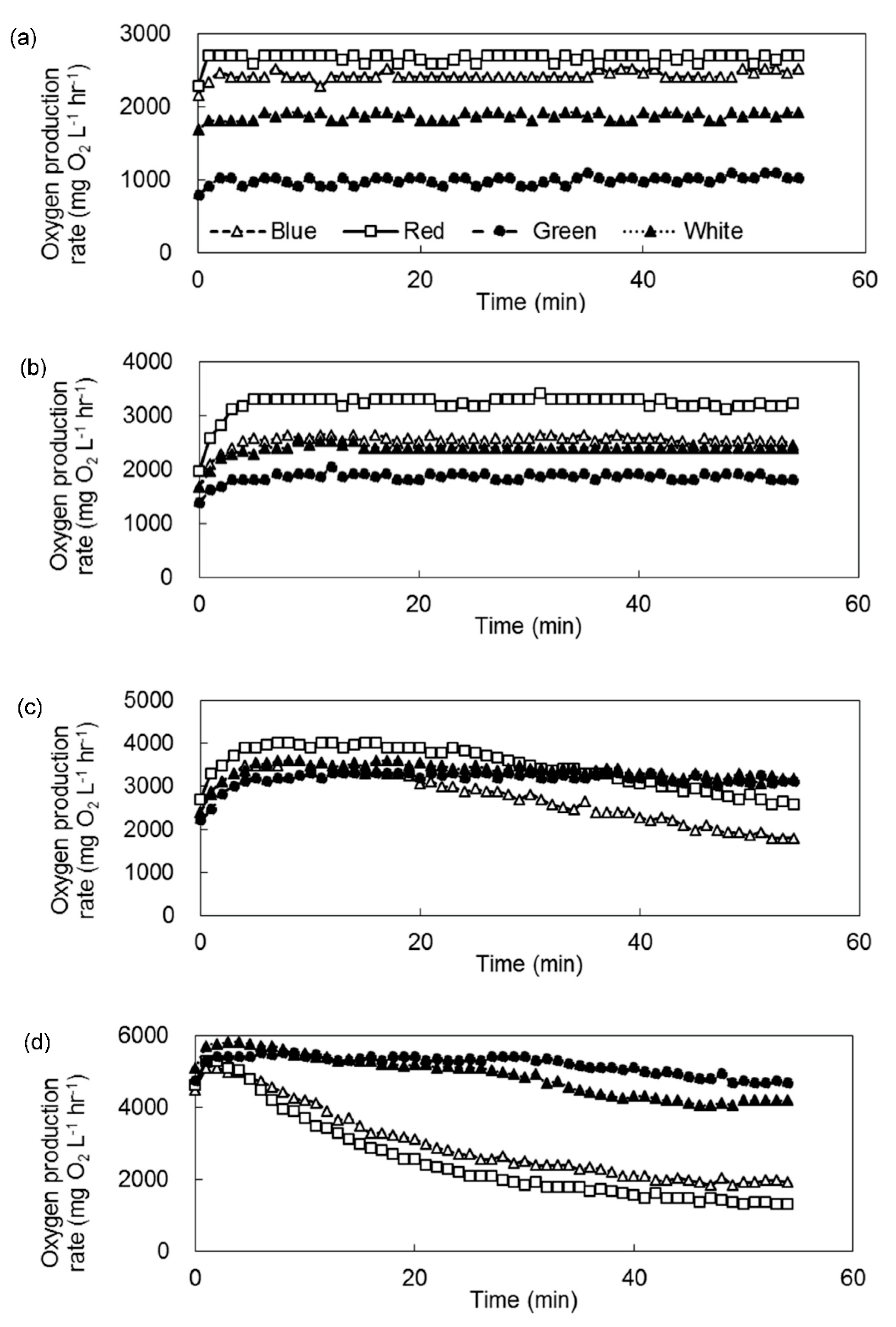
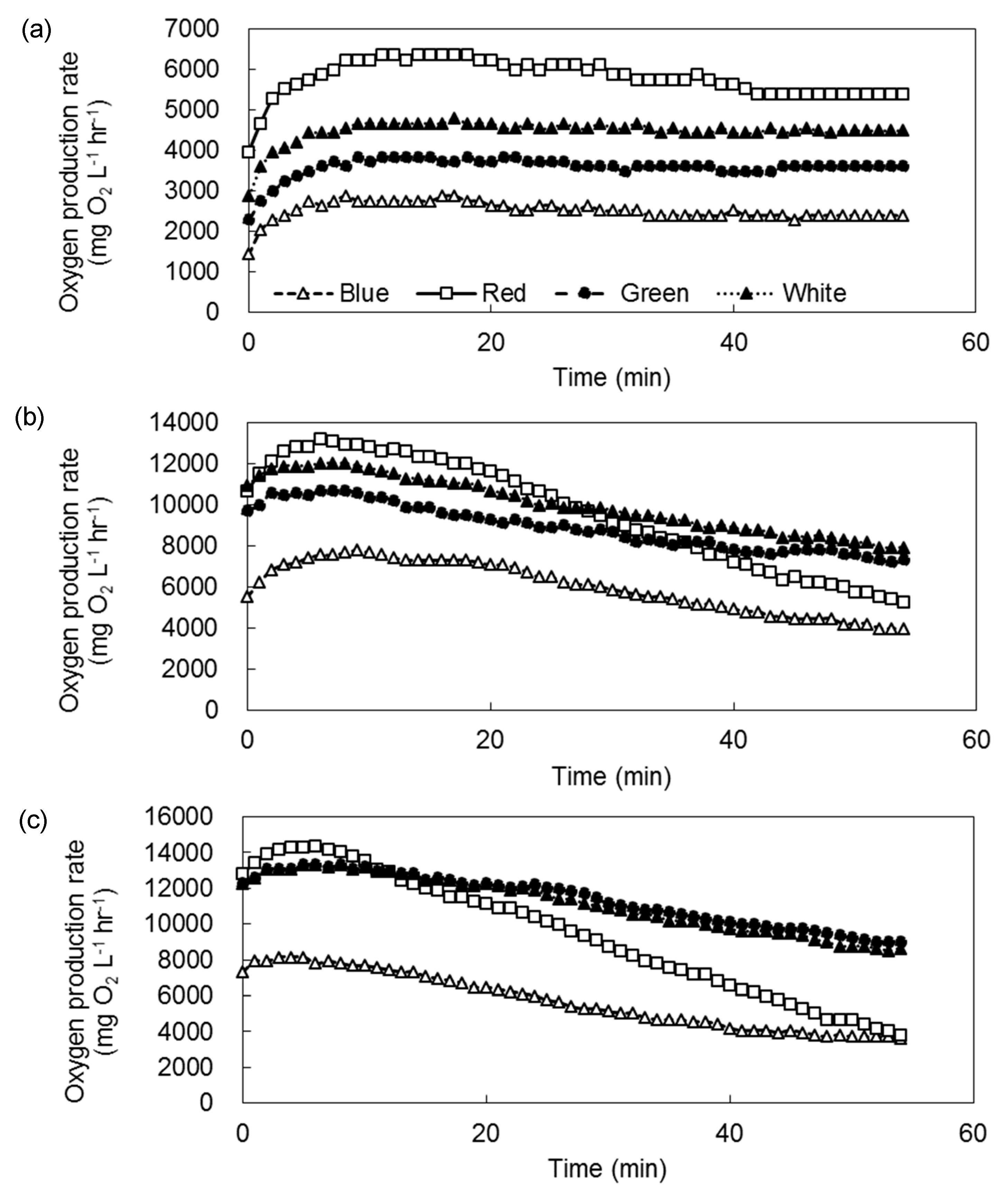
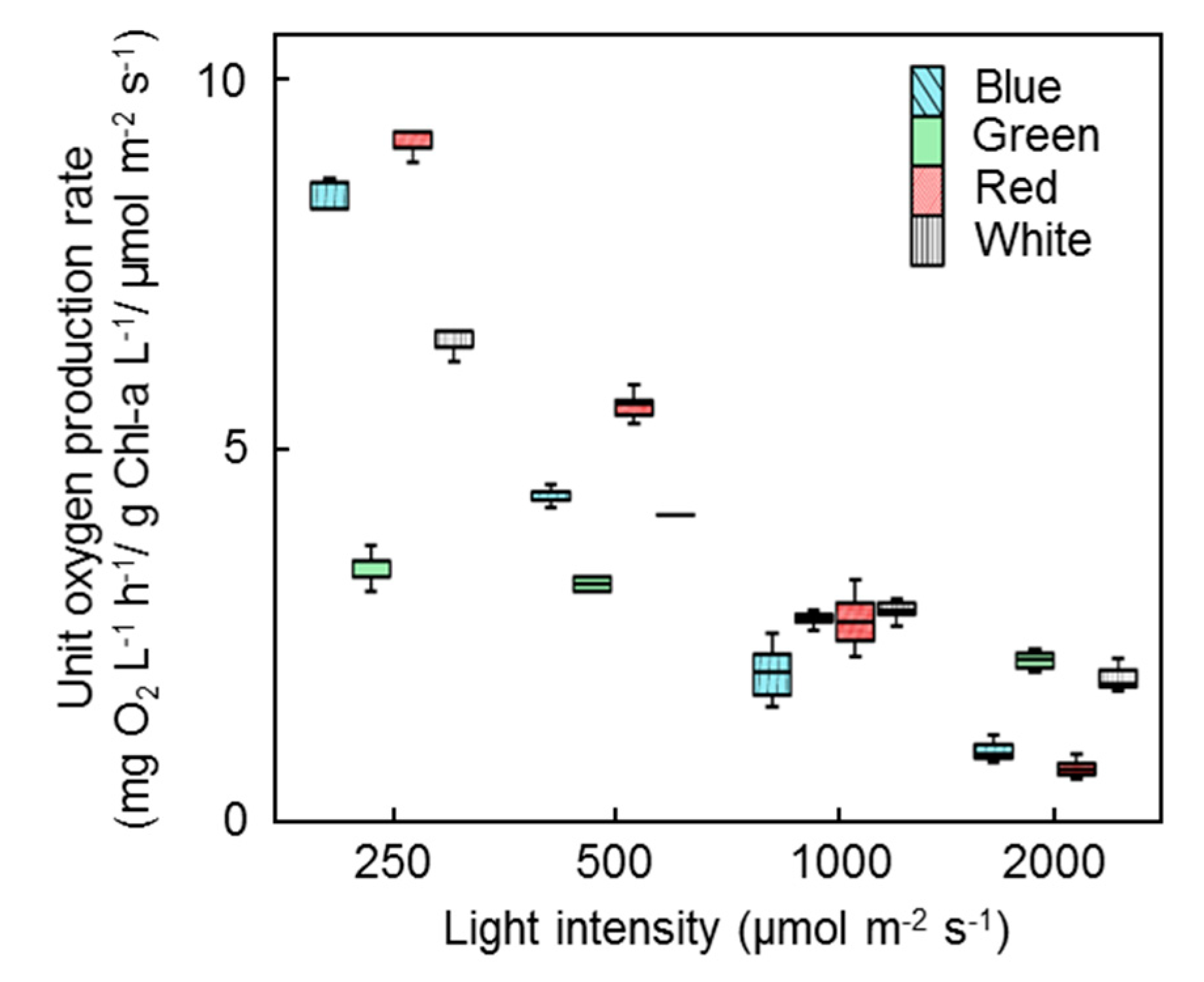
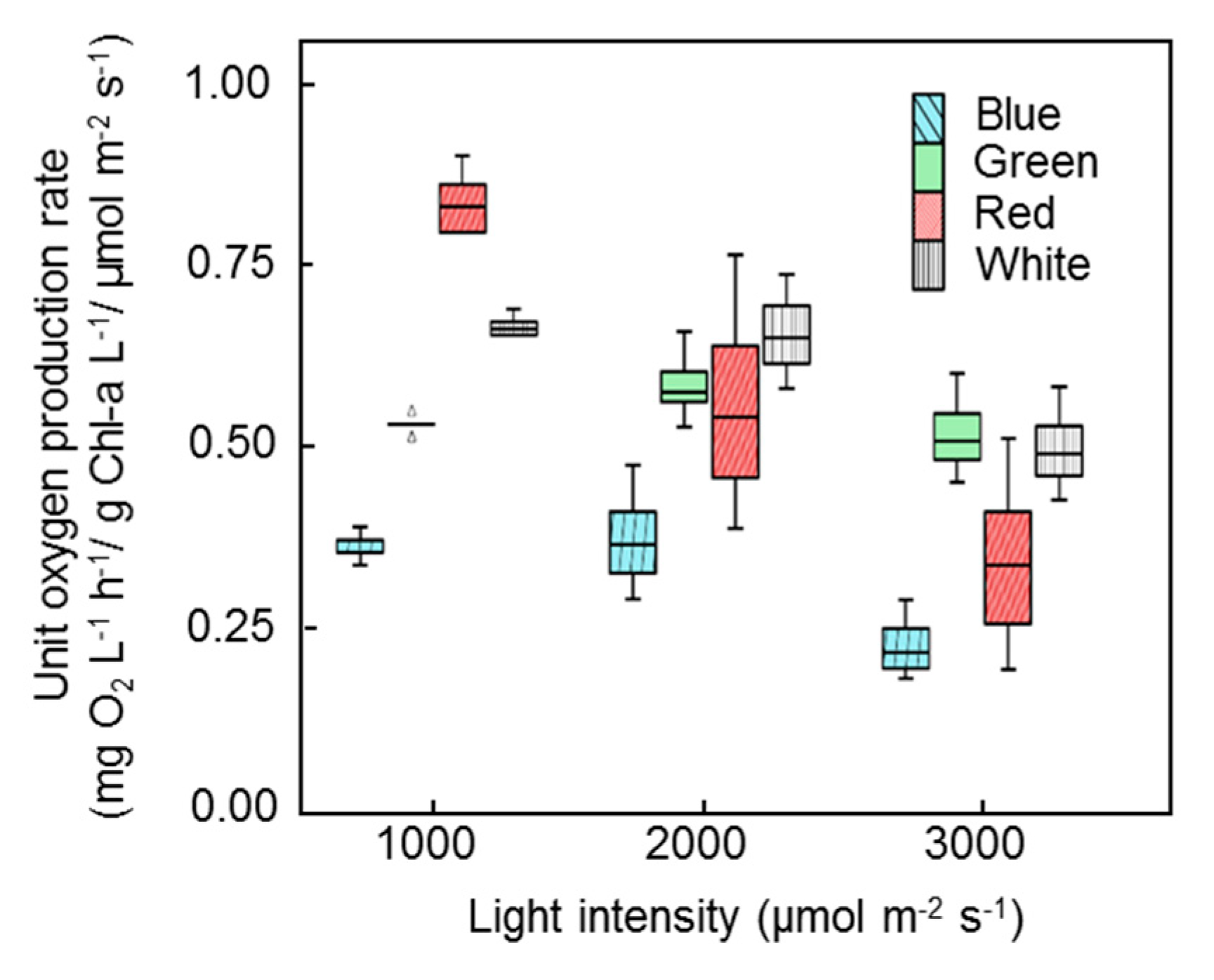
3. Results
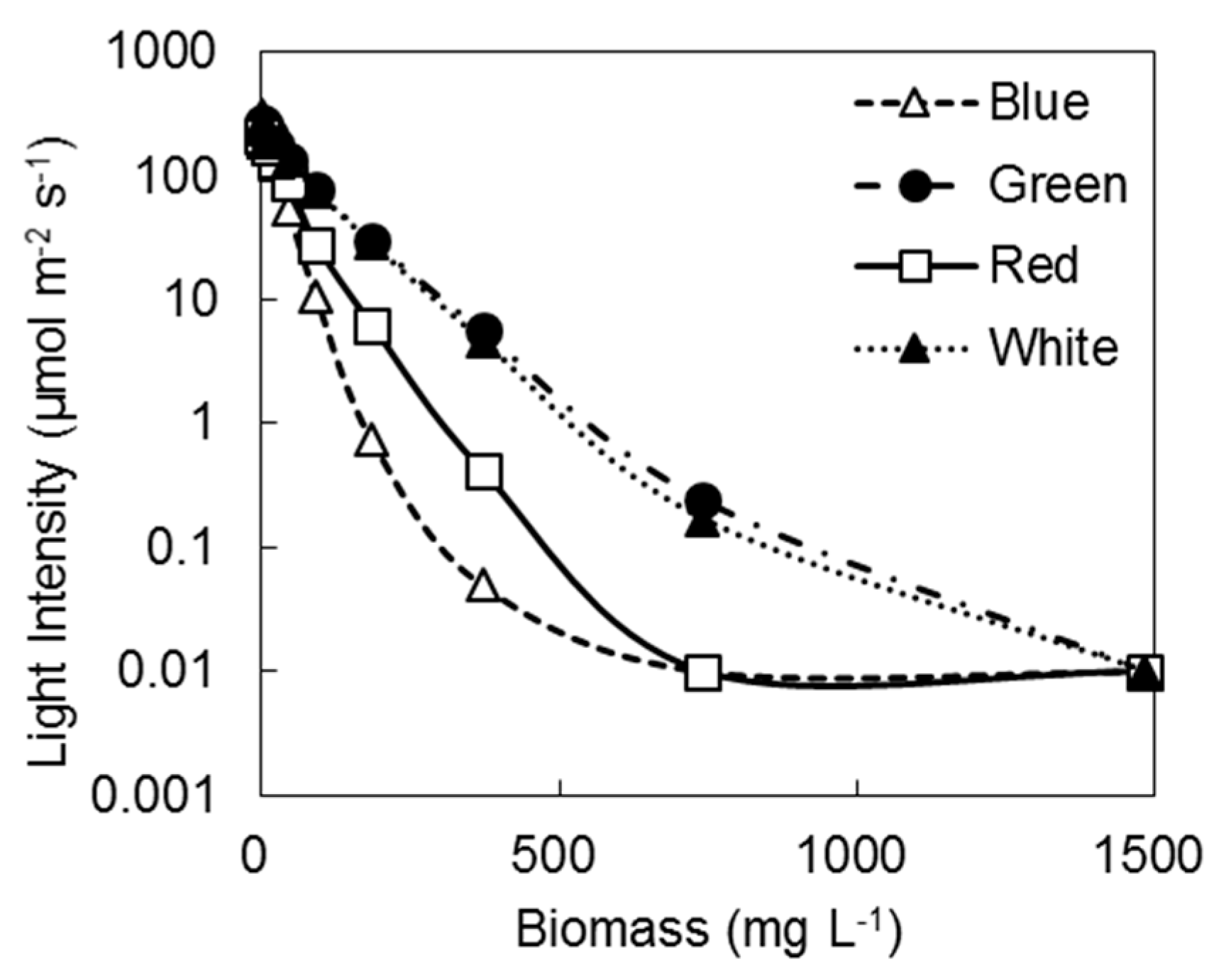
3.1. Transmittance of Light
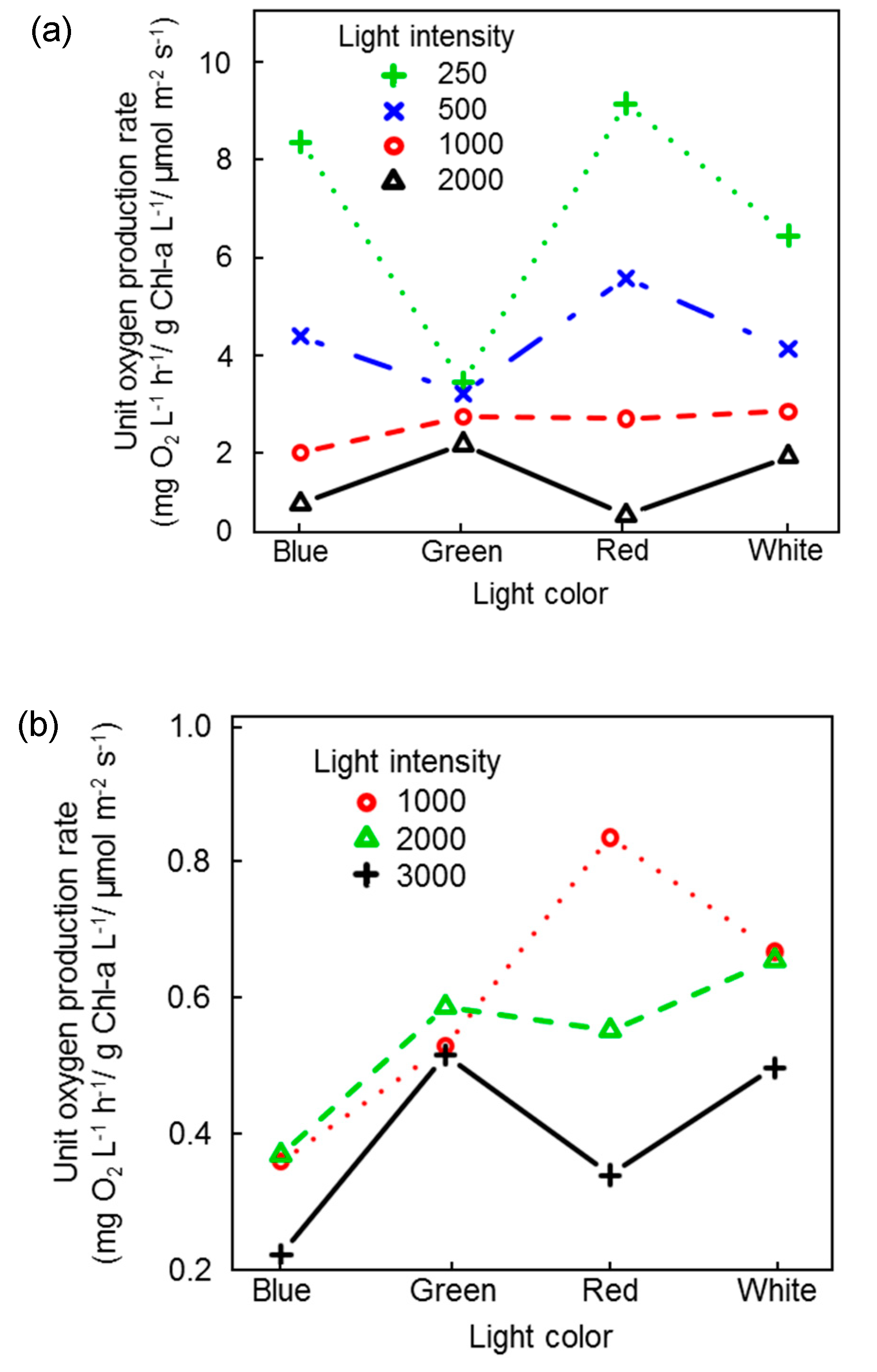
3.2. Inhibition of Photosynthetic Activities
3.3. Statistical Analysis of the Effect of Light Conditions on Photosynthetic Activities
3.3.1. Low Microalgal–Bacterial Consortia Concentration
3.3.2. High Microalgal–Bacterial Consortia Concentration
3.3.3. Interaction of Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Biomass (mg/L) | Transmittance Rate (%) | |||
|---|---|---|---|---|
| Blue | Green | Red | White | |
| 1482.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 741.0 | 0.0 | 0.1 | 0.0 | 0.1 |
| 370.5 | 0.0 | 2.1 | 0.2 | 1.8 |
| 185.3 | 0.2 | 11.4 | 2.9 | 10.8 |
| 92.6 | 3.5 | 29.2 | 12.9 | 28.5 |
| 46.3 | 17.6 | 50.8 | 40.9 | 50.8 |
| 23.2 | 40.0 | 67.9 | 62.9 | 68.8 |
| 11.6 | 58.8 | 81.5 | 81.0 | 83.4 |
| 5.8 | 73.5 | 89.1 | 89.6 | 91.0 |
| 2.9 | 85.4 | 95.2 | 90.0 | 98.1 |
| 0.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Source | Sum of Squares | DF | Mean Square | F Value | Pr > F |
|---|---|---|---|---|---|
| Model | 2747.421 | 15 | 183.1614 | 7039.51 | <0.0001 |
| Error | 12.07284 | 464 | 0.026019 | ||
| Total | 2759.494 | 479 | |||
| R2 = 0.9956 |
| Source | Type I SS | DF | Mean Square | F Value | Pr > F |
|---|---|---|---|---|---|
| Intensity | 2014.585 | 3 | 671.5282 | 25809.1 | <0.0001 |
| Color | 168.1905 | 3 | 56.06351 | 2154.71 | <0.0001 |
| Intensity * Color | 564.6458 | 9 | 62.73843 | 2411.25 | <0.0001 |
| Source | Sum of Squares | DF | Mean Square | F Value | Pr > F |
|---|---|---|---|---|---|
| Model | 9.392768 | 11 | 0.853888 | 284.2 | <0.0001 |
| Error | 1.045591 | 348 | 0.003005 | ||
| Total | 10.43836 | 359 | |||
| R2 = 0.8998 |
| Source | Type I SS | DF | Mean Square | F Value | Pr > F |
|---|---|---|---|---|---|
| Intensity | 2.654785 | 2 | 1.327393 | 441.79 | <0.0001 |
| Color | 4.662361 | 3 | 1.55412 | 517.25 | <0.0001 |
| Intensity * Color | 2.075622 | 6 | 0.345937 | 115.14 | <0.0001 |
References
- Dismukes, G.C.; Carrieri, D.; Bennette, N.; Ananyev, G.M.; Posewitz, M.C. Aquatic phototrophs: Efficient alternatives to land-based crops for biofuels. Curr. Opin. Biotechnol. 2008, 19, 235–240. [Google Scholar] [CrossRef]
- Christenson, L.B.; Sims, R.C. Rotating algal biofilm reactor and spool harvester for wastewater treatment with biofuels by-products. Biotechnol. Bioeng. 2012, 109, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-H.; Church, J.; Lee, S.-J.; Park, J.; Lee, W.H. Use of microalgae for advanced wastewater treatment and sustainable bioenergy generation. Environ. Eng. Sci. 2016, 33, 882–897. [Google Scholar] [CrossRef]
- Liu, X.; Saydah, B.; Eranki, P.; Colosi, L.M.; Mitchell, B.G.; Rhodes, J.; Clarens, A.F. Pilot-scale data provide enhanced estimates of the life cycle energy and emissions profile of algae biofuels produced via hydrothermal liquefaction. Bioresour. Technol. 2013, 148, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Lananan, F.; Hamid, S.H.A.; Din, W.N.S.; Ali, N.; Khatoon, H.; Jusoh, A.; Endut, A. Symbiotic bioremediation of aquaculture wastewater in reducing ammonia and phosphorus utilizing Effective Microorganism (EM-1) and microalgae (Chlorella sp.). Int. Biodeterior. Biodegrad. 2014, 95, 127–134. [Google Scholar] [CrossRef]
- Beuckels, A.; Smolders, E.; Muylaert, K. Nitrogen availability influences phosphorus removal in microalgae-based wastewater treatment. Water Res. 2015, 77, 98–106. [Google Scholar] [CrossRef]
- Rosso, D.; Larson, L.E.; Stenstrom, M.K. Aeration of large-scale municipal wastewater treatment plants: State of the art. Water Sci. Technol. 2008, 57, 973–978. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Norvill, Z.N.; Shilton, A.; Guieysse, B. Emerging contaminant degradation and removal in algal wastewater treatment ponds: Identifying the research gaps. J. Hazard. Mater. 2016, 313, 291–309. [Google Scholar] [CrossRef]
- Matamoros, V.; Gutiérrez, R.; Ferrer, I.; García, J.; Bayona, J.M. Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: A pilot-scale study. J. Hazard. Mater. 2015, 288, 34–42. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aleman-Nava, G.S.; Chandra, R.; Garcia-Perez, J.S.; Contreras-Angulo, J.R.; Markou, G.; Muylaert, K.; Rittmann, B.E.; Parra-Saldivar, R. Nutrients utilization and contaminants removal. A review of two approaches of algae and cyanobacteria in wastewater. Algal Res. 2017, 24, 438–449. [Google Scholar] [CrossRef]
- Oswald, W.J. My sixty years in applied algology. J. Appl. Phycol. 2003, 15, 99–106. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Shafiee, S.; Topal, E. A long-term view of worldwide fossil fuel prices. Appl. Energy 2010, 87, 988–1000. [Google Scholar] [CrossRef]
- Kumar, K.; Mishra, S.K.; Shrivastav, A.; Park, M.S.; Yang, J.-W. Recent trends in the mass cultivation of algae in raceway ponds. Renew. Sustain. Energy Rev. 2015, 51, 875–885. [Google Scholar] [CrossRef]
- Craggs, R.; Park, J.; Sutherland, D.; Heubeck, S. Economic construction and operation of hectare-scale wastewater treatment enhanced pond systems. J. Appl. Phycol. 2015, 27, 1913–1922. [Google Scholar] [CrossRef]
- Handler, R.M.; Canter, C.E.; Kalnes, T.N.; Lupton, F.S.; Kholiqov, O.; Shonnard, D.R.; Blowers, P. Evaluation of environmental impacts from microalgae cultivation in open-air raceway ponds: Analysis of the prior literature and investigation of wide variance in predicted impacts. Algal Res. 2012, 1, 83–92. [Google Scholar] [CrossRef]
- Dahmani, S.; Zerrouki, D.; Ramanna, L.; Rawat, I.; Bux, F. Cultivation of Chlorella pyrenoidosa in outdoor open raceway pond using domestic wastewater as medium in arid desert region. Bioresour. Technol. 2016, 219, 749–752. [Google Scholar] [CrossRef]
- Arias, D.M.; Uggetti, E.; García-Galán, M.J.; García, J. Cultivation and selection of cyanobacteria in a closed photobioreactor used for secondary effluent and digestate treatment. Sci. Total Environ. 2017, 587, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Kumar, P.; Malik, A.; Vijay, V.K. Bioconversion of algae to methane and subsequent utilization of digestate for algae cultivation: A closed loop bioenergy generation process. Bioresour. Technol. 2014, 158, 174–180. [Google Scholar] [CrossRef]
- Tredici, M.R.; Bassi, N.; Prussi, M.; Biondi, N.; Rodolfi, L.; Zittelli, G.C.; Sampietro, G. Energy balance of algal biomass production in a 1-ha “Green Wall Panel” plant: How to produce algal biomass in a closed reactor achieving a high Net Energy Ratio. Appl. Energy 2015, 154, 1103–1111. [Google Scholar] [CrossRef]
- Li, T.; Liu, L.-N.; Jiang, C.-D.; Liu, Y.-J.; Shi, L. Effects of mutual shading on the regulation of photosynthesis in field-grown sorghum. J. Photochem. Photobiol. B Biol. 2014, 137, 31–38. [Google Scholar] [CrossRef]
- Yan, C.; Luo, X.; Zheng, Z. Effects of various LED light qualities and light intensity supply strategies on purification of slurry from anaerobic digestion process by Chlorella vulgaris. Int. Biodeterior. Biodegrad. 2013, 79, 81–87. [Google Scholar] [CrossRef]
- Yan, C.; Muñoz, R.; Zhu, L.; Wang, Y. The effects of various LED (light emitting diode) lighting strategies on simultaneous biogas upgrading and biogas slurry nutrient reduction by using of microalgae Chlorella sp. Energy 2016, 106, 554–561. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Barreira, L.A.; Pereira, H.G.C.; Perales, J.A.; Varela, J.C.S. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef]
- Wagner, I.; Steinweg, C.; Posten, C. Mono-and dichromatic LED illumination leads to enhanced growth and energy conversion for high-efficiency cultivation of microalgae for application in space. Biotechnol. J. 2016, 11, 1060–1071. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Zhang, H.; Yan, C.; Zhang, Y. Effects of various LED light wavelengths and intensities on microalgae-based simultaneous biogas upgrading and digestate nutrient reduction process. Bioresour. Technol. 2013, 136, 461–468. [Google Scholar] [CrossRef]
- Blair, M.F.; Kokabian, B.; Gude, V.G. Light and growth medium effect on Chlorella vulgaris biomass production. J. Environ. Chem. Eng. 2014, 2, 665–674. [Google Scholar] [CrossRef]
- Kang, D.; Kim, K.; Jang, Y.; Moon, H.; Ju, D.; Jahng, D. Nutrient removal and community structure of wastewater-borne algal-bacterial consortia grown in raw wastewater with various wavelengths of light. Int. Biodeterior. Biodegrad. 2018, 126, 10–20. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, J.; Cha, M. Energy efficient growth control of microalgae using photobiological methods. Renew. Energy 2013, 54, 161–165. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hahida, S.; Yoshihara, T. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ. Exp. Bot. 2012, 75, 128–133. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant. Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.; Kim, K.T.; Heo, T.-Y.; Kwon, G.; Lim, C.; Park, J. Inhibition of Photosynthetic Activity in Wastewater-Borne Microalgal–Bacterial Consortia under Various Light Conditions. Sustainability 2019, 11, 2951. https://doi.org/10.3390/su11102951
Kang D, Kim KT, Heo T-Y, Kwon G, Lim C, Park J. Inhibition of Photosynthetic Activity in Wastewater-Borne Microalgal–Bacterial Consortia under Various Light Conditions. Sustainability. 2019; 11(10):2951. https://doi.org/10.3390/su11102951
Chicago/Turabian StyleKang, Donghan, Keug Tae Kim, Tae-Young Heo, Gyutae Kwon, Chaeseung Lim, and Jungsu Park. 2019. "Inhibition of Photosynthetic Activity in Wastewater-Borne Microalgal–Bacterial Consortia under Various Light Conditions" Sustainability 11, no. 10: 2951. https://doi.org/10.3390/su11102951
APA StyleKang, D., Kim, K. T., Heo, T.-Y., Kwon, G., Lim, C., & Park, J. (2019). Inhibition of Photosynthetic Activity in Wastewater-Borne Microalgal–Bacterial Consortia under Various Light Conditions. Sustainability, 11(10), 2951. https://doi.org/10.3390/su11102951





