Solubility, Diffusion and Crop Uptake of Phosphorus in Three Different Struvites
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Characterization
2.2. Struvite Sources and Characterisation
2.3. Agronomic Performance of Struvites in Comparison to Triple Superphosphate
2.4. Influence of pH and Organic Acids on Struvite Dissolution
2.5. Dissolution and Diffusion of Struvite and TSP Derived P in Soil
2.6. Data and Statistical Analysis
3. Results
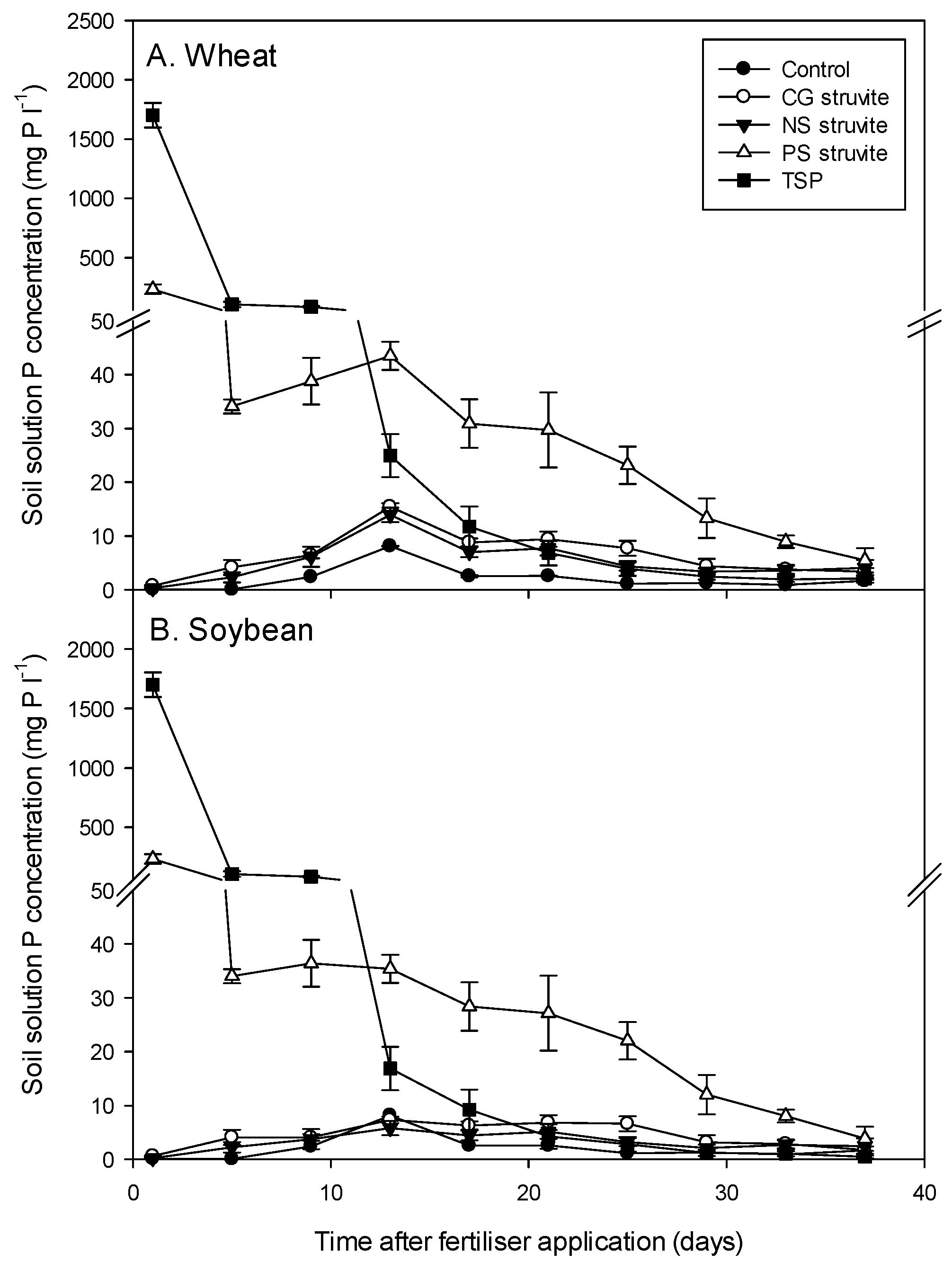
3.1. Solubilisation and Agronomic Performance of Struvites
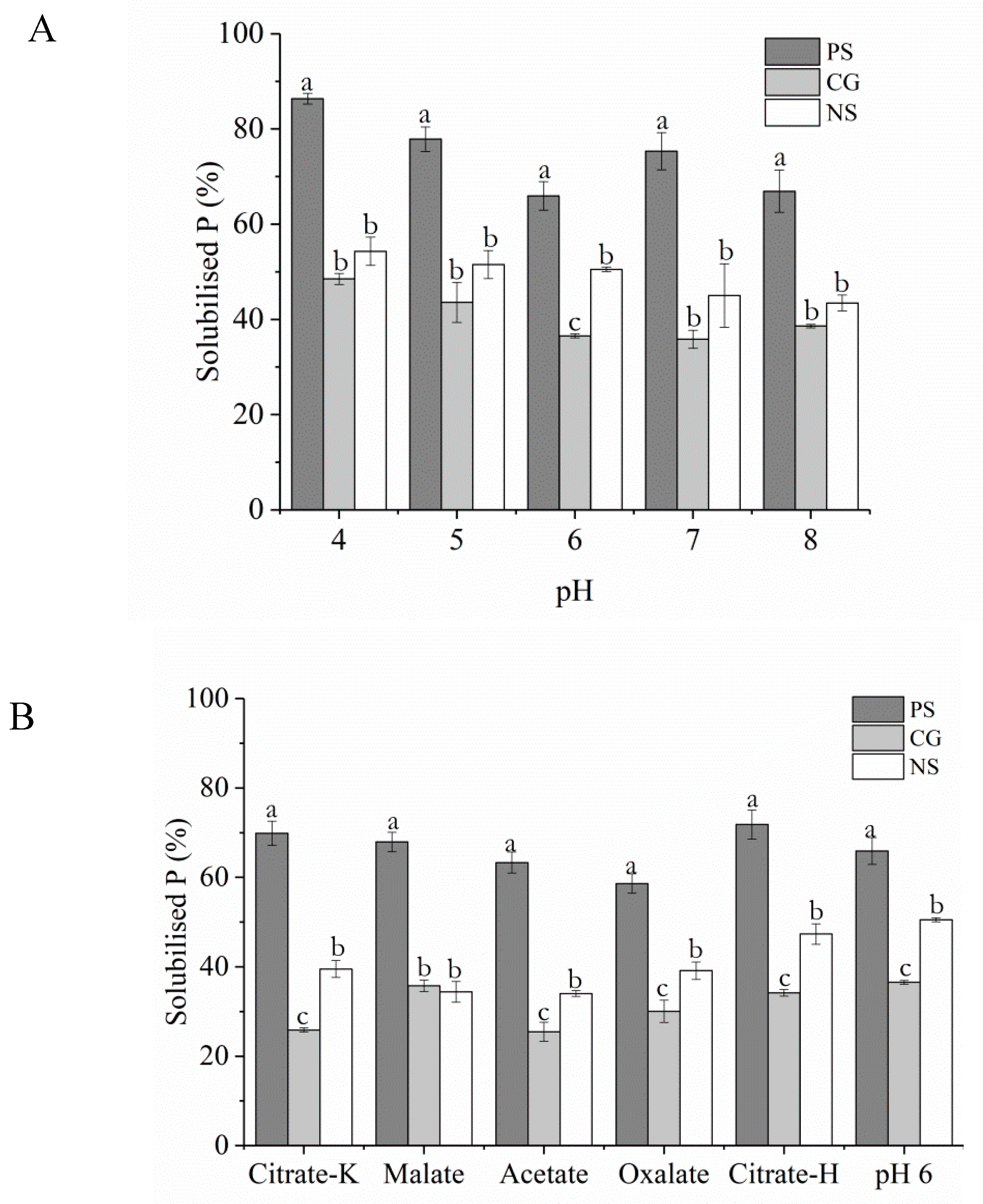
3.2. Influence of pH and Organic Acids on Struvite Dissolution
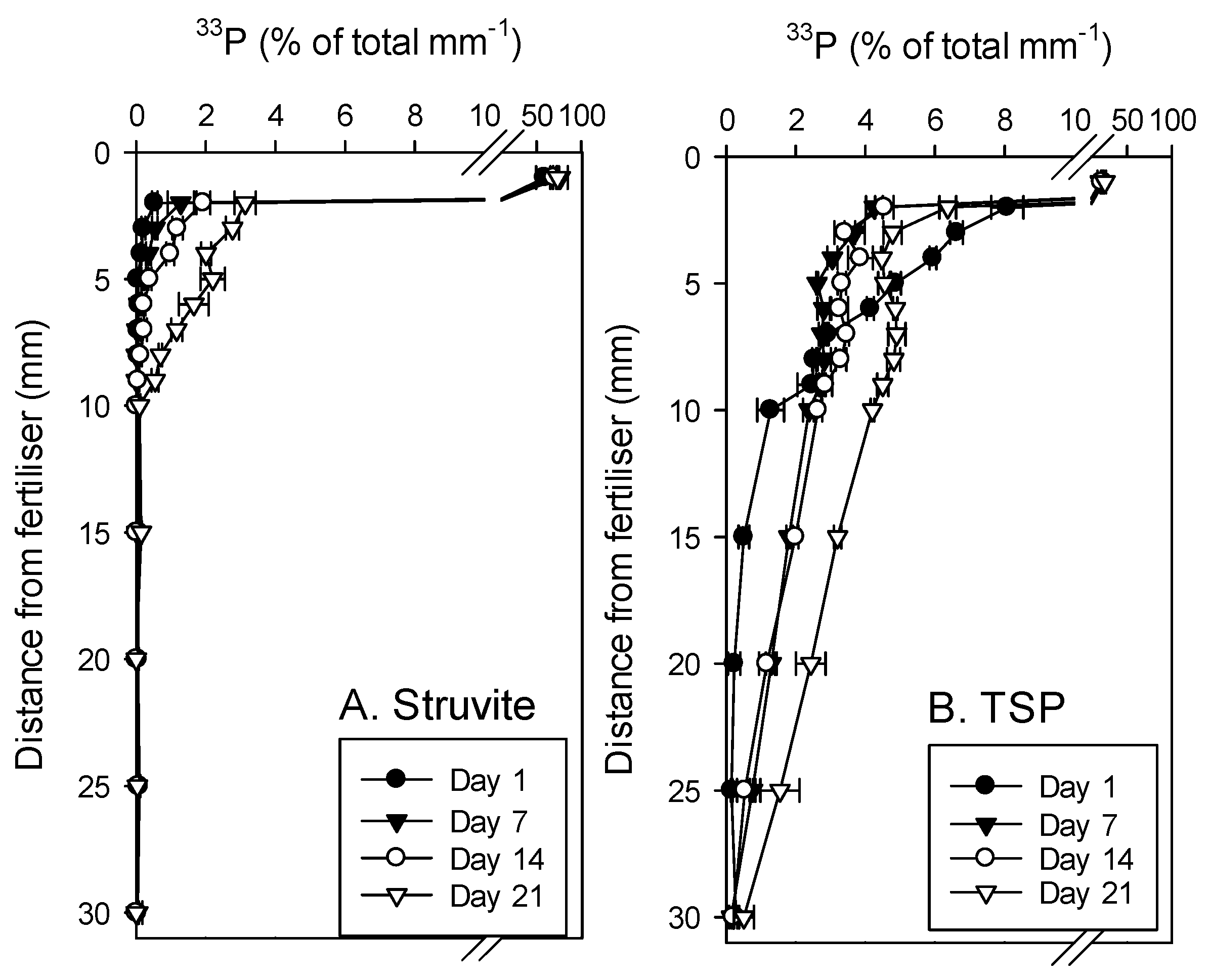
3.3. Phosphorus Diffusion from 33P-Labelled Struvite and TSP in Soil
4. Discussion
4.1. Solubility of the Struvites in Relation to TSP
4.2. Agronomic Performance of Struvites Relative to TSP
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; Kihara, J.; Koala, S.; Mukalama, J.; Waswa, B.; Bationo, A. Long-term effects of TSP and Minjingu phosphate rock applications on yield response of maize and soybean in a humid tropical maize–legume cropping system. Nutr. Cycl. Agroecosyst. 2016, 104, 79–91. [Google Scholar] [CrossRef]
- Khademi, Z.; Jones, D.L.; Malakouti, M.J.; Asadi, F. Organic acids differ in enhancing phosphorus uptake by Triticum aestivum L.—Effects of rhizosphere concentration and counterion. Plant Soil 2010, 334, 151–159. [Google Scholar] [CrossRef]
- Santner, J.; Mannel, M.; Burrell, L.D.; Hoefer, C.; Kreuzeder, A.; Wenzel, W.W. Phosphorus uptake by Zea mays L. is quantitatively predicted by infinite sink extraction of soil P. Plant Soil 2015, 386, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Hinsinger, P.; Brauman, A.; Devau, N.; Gérard, F.; Jourdan, C.; Laclau, J.P.; Le Cadre, E.; Jaillard, B.; Plassard, C. Acquisition of phosphorus and other poorly mobile nutrients by roots. Where do plant nutrition models fail? Plant Soil 2011, 348, 29–61. [Google Scholar] [CrossRef]
- Cordell, D.; Neset, T.S.S. Phosphorus vulnerability: A qualitative framework for assessing the vulnerability of national and regional food systems to the multidimensional stressors of phosphorus scarcity. Glob. Environ. Chang. Human Policy Dimens. 2014, 24, 108–122. [Google Scholar] [CrossRef]
- Cordell, D.; Metson, G.S.; Iwaniec, D.M.; Bui, T.T.; Childers, D.L.; Dao, N.; Dang, H.T.T.; Davidson, J.; Jacobs, B.; Kumwenda, S.; et al. Transforming cities: Securing food and clean waterways through a transdisciplinary phosphorus approach. In Transdisciplinary Research and Practice for Sustainable Outcomes; Routledge: Harlow, UK, 2016. [Google Scholar]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. Hum. Policy Dimens. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Cabeza, R.; Steingrobe, B.; Römer, W.; Claassen, N. Effectiveness of recycled P products as P fertilizers, as evaluated in pot experiments. Nutr. Cycl. Agroecosyst. 2011, 91, 173. [Google Scholar] [CrossRef]
- Achat, D.L.; Sperandio, M.; Daumer, M.L.; Santellani, A.C.; Prud’Homme, L.; Akhtar, M.; Morel, C. Plant-availability of phosphorus recycled from pig manures and dairy effluents as assessed by isotopic labeling techniques. Geoderma 2014, 232–234, 24–33. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus recovery from wastewater by struvite crystallization: A review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Kaikake, K.; Sekito, T.; Dote, Y. Phosphate recovery from phosphorus-rich solution obtained from chicken manure incineration ash. Waste Manag. 2009, 29, 1084–1088. [Google Scholar] [CrossRef]
- Doyle, J.D.; Parsons, S. Struvite, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- Yilmazel, Y.D.; Demirer, G.N. Removal and recovery of nutrients as struvite from anaerobic digestion residues of poultry manure. Environ. Technol. 2011, 32, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, C.K.; Vyas, P.M.; Joshi, M.J. Growth and Characterization of Struvite-K Crystals. Cryst. Res. Technol. 2011, 46, 187–194. [Google Scholar] [CrossRef]
- Suryawanshi, V.B.; Chaudhari, R.T. Synthesis and Characterization of Struvite-K Crystals by Agar Gel. J. Cryst. Process Technol. 2014, 4, 212–224. [Google Scholar] [CrossRef]
- Bhuiyan, M.I.H.; Mavinic, D.S.; Beckie, R.D. A solubility and thermodynamic study of struvite. Environ. Technol. 2007, 28, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Degryse, F.; Baird, R.; da Silva, R.C.; McLaughlin, M.J. Dissolution rate and agronomic effectiveness of struvite fertilizers—Effect of soil pH, granulation and base excess. Plant Soil 2016, 410, 139–152. [Google Scholar] [CrossRef]
- Antonini, S.; Arias, M.A.; Eichert, T.; Clemens, J. Greenhouse evaluation and environmental impact assessment of different urine-derived struvite fertilizers as phosphorus sources for plants. Chemosphere 2012, 89, 1202–1210. [Google Scholar] [CrossRef]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J.A. Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef]
- Gahoonia, T.S.; Nielsen, N.E. The effects of root-induced ph changes on the depletion of inorganic and organic phosphorus in the rhizosphere. Plant Soil 1992, 143, 185–191. [Google Scholar] [CrossRef]
- Nye, P.H. Changes of pH across the rhizosphere induced by roots. Plant Soil 1981, 61, 7–26. [Google Scholar] [CrossRef]
- Song, Y.W.; Wang, H.R.; Cao, Y.X.; Li, F.; Cui, C.H.; Zhou, L.X. Inhibition of low molecular organic acids on the activity of acidithiobacillus species and its effect on the removal of heavy metals from contaminated soil. Huanjing Kexue/Environ. Sci. 2016, 37, 1960–1967. [Google Scholar]
- Ryan, P.R.; James, R.A.; Weligama, C.; Delhaize, E.; Rattey, A.; Lewis, D.C.; Bovill, W.D.; McDonald, G.; Rathjen, T.M.; Wang, E.; et al. Can citrate efflux from roots improve phosphorus uptake by plants? Testing the hypothesis with near-isogenic lines of wheat. Physiol. Plant. 2014, 151, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, M.; Jalali, M. Effect of low-molecular-weight organic acids on kinetics release and fractionation of phosphorus in some calcareous soils of western Iran. Environ. Monit. Assess. 2013, 85, 5471–5482. [Google Scholar] [CrossRef] [PubMed]
- Weast, R. CRC Handbook of Chemistry and Physics; CRC Press: Cleveland, OH, USA, 1970. [Google Scholar]
- UK-ALA. Lime Calculator; Agricultural Lime Association: London, UK, 2015. [Google Scholar]
- Van Raij, B.; Cantarella, H.; Quaggio, J.A.; Furlani, A.M.C. Recomendações de Adubação e Calagem Para o Estado de São Paulo; Instituto Agronômico (IAC. Boletim Técnico, 100): Campinas, Brazil, 1997; Volume 2, 285p. [Google Scholar]
- EMBRAPA. Manual de Métodos de Análise Químicas de Solos, Plantas e Fertilizantes; Embrapa Informação Tecnológica: Rio de Janeiro, Brazil, 1997; Volume 2, 212p. [Google Scholar]
- Rowell, D.L. Soil Science: Methods and Applications; Routledge: Harlow, UK, 2015. [Google Scholar]
- Oburger, E.; Jones, D.L.; Wenzel, W.W. Phosphorus saturation and pH differentially regulate the efficiency of organic acid anion-mediated P solubilization mechanisms in soil. Plant Soil 2011, 341, 363–382. [Google Scholar] [CrossRef]
- Ministério da Agricultura, Pecuária e Abastecimento. Manual de Métodos Analíticos Oficiais Para Fertilizantes Minerais, Orgânicos, Organominerais e Corretivos. Coordenação-Geral de Apoio Laboratorial; Murilo Carlos Muniz Veras (Org.) MAPA—Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brasil, 2014; 220p. [Google Scholar]
- AHDB. Nutrient Management Guide (RB209); Agriculture and Horticulture Development Board: Kenilworth, UK, 2017. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação do Estado Nutricional das Plantas; POTAFOS: Piracicaba, Brazil, 1997. [Google Scholar]
- Jones, D.L. Organic acids in the rhizosphere—A critical review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Ryan, P.; Delhaize, E.; Jones, D.L. Function and mechanism of organic anion exudation from plant roots. Ann. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 527–560. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Mulvaney, R.L. Nitrogen—Inorganic forms. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954.
- Levy, E.T.; Schlesinger, W.H. A comparison of fractionation methods for forms of phosphorus in soils. Biogeochemistry 1999, 47, 25–38. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciênc. Agrotecnol. (UFLA) 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Massey, M.S.; Davis, J.G.; Ippolito, J.A.; Sheffield, R.E. Effectiveness of recovered magnesium phosphates as fertilizers in neutral and slightly alkaline soils. Agron. J. 2009, 101, 323–329. [Google Scholar] [CrossRef]
- Achat, D.L.; Daumer, M.L.; Sperandio, M.; Santellani, A.C.; Morel, C. Solubility and mobility of phosphorus recycled from dairy effluents and pig manures in incubated soils with different characteristics. Nutr. Cycl. Agroecosyst. 2014, 99, 1–15. [Google Scholar] [CrossRef]
- Yu, R.T.; Ren, H.Q.; Wang, Y.R.; Ding, L.L.; Geng, J.J.; Xu, K.; Zhang, Y. A kinetic study of struvite precipitation recycling technology with NaOH/Mg(OH)2 addition. Bioresour. Technol. 2013, 143, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, G.; Fuess, H.; Joswig, W. Neutron diffraction study of Mg(NH4)(PO4)·6H2O (struvite) and survey of water molecules donating short hydrogen bonds. Acta Crystallogr. B 1986, 42, 253–258. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Mavinic, D.S.; Bhuiyan, M.I.H.; Koch, F.A. Exploring the determination of struvite solubility product from analytical results. Environ. Technol. 2006, 27, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Schuiling, R.D.; Andrade, A. Recovery of struvite from calf manure. Environ. Technol. 1999, 20, 765–768. [Google Scholar] [CrossRef]
- Phan, L.; Weichgrebe, D.; Urban, I.; Rosenwinkel, K.; Gunther, L.; Dockhorn, T.; Dichtl, N.; Muller, J.; Bayerle, N. Empirical evaluation of nutrient recovery using seaborne technology at the wastewater treatment plant Gifhorn. In International Conference on Nutrient Recovery from Wastewater Streams; Ashley, K., Mavinic, D., Koch, F., Eds.; WA Publishing: London, UK, 2009. [Google Scholar]
- Nascimento, C.A.C.; Pagliari, P.H.; Faria, L.A.; Vitti, G.C. Phosphorus mobility and behaviour in soils treated with calcium, ammonium and magnesium phosphates. Soil Sci. Soc. Am. J. 2018, 82, 622–631. [Google Scholar] [CrossRef]
- Vogel, T.; Kruse, J.; Siebers, N.; Nelles, M.; Eichler-Lobermann, B. Recycled products from municipal wastewater: Composition and effects on phosphorus mobility in a sandy soil. J. Environ. Q. 2017, 46, 443–451. [Google Scholar] [CrossRef]
- Bonvin, C.; Etter, B.; Udert, K.M.; Frossard, E.; Nanzer, S.; Tamburini, F.; Oberson, A. Plant uptake of phosphorus and nitrogen recycled from synthetic source-separated urine. Ambio 2015, 44, 217–227. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Sylvester-Bradley, R.; Jones, D.L.; Healey, J.R.; Talboys, P.J. Feed the crop not the soil: Rethinking phosphorus management in the food chain. Environ. Sci. Technol. 2014, 48, 6523–6530. [Google Scholar] [CrossRef]
| Soil Parameter | Value |
|---|---|
| pHCaCl2 | 5.3 |
| OM (g kg−1) | 34 |
| P (mg kg−1) | 24 |
| S (mg kg−1) | 7 |
| Ca (mmolc kg−1) | 36 |
| Mg (mmolc kg−1) | 3 |
| K (mmolc kg−1) | 2.2 |
| Al (mmolc kg−1) | <1 |
| CEC (mmolc kg−1) | 79.2 |
| V (%) | 52 |
| B (mg kg−1) | 0.25 |
| Cu (mg kg−1) | 1.2 |
| Fe (mg kg−1) | 110 |
| Mn (mg kg−1) | 21.8 |
| Zn (mg kg−1) | 1.2 |
| Clay (g kg−1) | 178 |
| Silt (g kg−1) | 235 |
| Sand (g kg−1) | 587 |
| Struvite | pH CaCl2 0.01 M | Salt Index | Total | Citric Acid | NAC + H2O | H2O | ||||
| P2O5(%) | ||||||||||
| PS | 7.2 | 60.6 | 17.5 | 17.4 | 17.5 | 3.8 | ||||
| NS | 7.8 | 10.2 | 29.1 | 28.4 | 28.9 | 2.0 | ||||
| CG | 7.6 | 10.4 | 29.3 | 29.1 | 28.8 | 2.5 | ||||
| Struvite | Mg | Ca | K | S | N | |||||
| % | ||||||||||
| PS | 7.1 | 2.0 | 17.3 | 6.5 | 1.8 | |||||
| NS | 11.3 | 0.35 | <0.1 | <0.1 | 5.9 | |||||
| CG | 11.6 | <0.1 | <0.1 | <0.1 | 5.8 | |||||
| Wheat | ||||||
| Treatment | Shoot DM (g plant−1) | Root DM (g plant−1) | Shoot P (mg plant−1) | Root P (mg plant−1) | P uptake (mg plant−1) | PUE% |
| TSP | 1.67 a | 1.07 a | 3.01 a | 2.06 a | 5.07 a | |
| PS | 1.30 bc | 0.91 ab | 2.40 a | 2.00 a | 4.40 ab | 74 ns |
| CG | 1.32 b | 0.83 ab | 2.24 a | 1.63 a | 3.86 b | 53 |
| NS | 1.24 bc | 0.83 ab | 2.20 a | 2.06 a | 4.26 ab | 69 |
| Control | 0.97 c | 0.62 b | 1.83 b | 0.63 b | 2.46 c | |
| * CV | 9.3 | 14.4 | 9.4 | 17.0 | 10.2 | 24.5 |
| Soybean | ||||||
| Treatment | Shoot DM (g plant−1) | Root DM (g plant−1) | Shoot P (mg plant−1) | Root P (mg plant−1) | P uptake (mg plant−1) | PUE% |
| TSP | 2.42 a | 0.74ns | 3.85 a | 1.86ns | 5.72 a | |
| PS | 2.11 ab | 0.71 | 3.18 ab | 1.80 | 4.98 a | 84 ns |
| CG | 2.03 ab | 0.61 | 3.11 ab | 1.41 | 4.52 ab | 77 |
| NS | 2.23 a | 0.61 | 3.33 ab | 1.34 | 4.67 ab | 74 |
| Control | 1.54 b | 0.48 | 2.20 b | 1.17 | 3.37 b | |
| * CV | 11.9 | 21.8 | 14.6 | 19.8 | 10.5 | 6.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rech, I.; Withers, P.J.A.; Jones, D.L.; Pavinato, P.S. Solubility, Diffusion and Crop Uptake of Phosphorus in Three Different Struvites. Sustainability 2019, 11, 134. https://doi.org/10.3390/su11010134
Rech I, Withers PJA, Jones DL, Pavinato PS. Solubility, Diffusion and Crop Uptake of Phosphorus in Three Different Struvites. Sustainability. 2019; 11(1):134. https://doi.org/10.3390/su11010134
Chicago/Turabian StyleRech, Ioná, Paul J. A. Withers, Davey L. Jones, and Paulo S. Pavinato. 2019. "Solubility, Diffusion and Crop Uptake of Phosphorus in Three Different Struvites" Sustainability 11, no. 1: 134. https://doi.org/10.3390/su11010134
APA StyleRech, I., Withers, P. J. A., Jones, D. L., & Pavinato, P. S. (2019). Solubility, Diffusion and Crop Uptake of Phosphorus in Three Different Struvites. Sustainability, 11(1), 134. https://doi.org/10.3390/su11010134





