Effects of Poultry-Litter Biochar on Soil Properties and Growth of Water Spinach (Ipomoea aquatica Forsk.)
Abstract
1. Introduction
2. Materials and Methods
- (I)
- NP: no plant growth and no amendment
- (II)
- CK: plant growth with no amendment
- (III)
- CF: urea and monobasic potassium phosphate applied as N: P2O5: K2O = 120: 60: 120 kg/ha
- (IV)
- RPL: raw poultry litter applied as P2O5 = 60 kg/ha
- (V)
- 350-1X, 350-0.5X, and 350-2X: 350 °C PLB applied as P2O5 = 60, 30, and 120 kg/ha, respectively
- (VI)
- 600-1X, 60-0.5X, and 600-2X: 600 °C PLB applied as P2O5 = 60, 30, and 120 kg/ha, respectively
- (VII)
- 350-0.5% and 350-1%: 350 °C PLB of 10 and 20 ton/ha, respectively, applied in the beginning of the experiments
- (VIII)
- 600-0.5% and 600-1%: 600 °C PLB of 10 and 20 ton/ha, respectively, applied in the beginning of the experiments
- (IX)
- CC-1X: poultry-litter compost applied as P2O5 = 60 kg/ha
3. Results
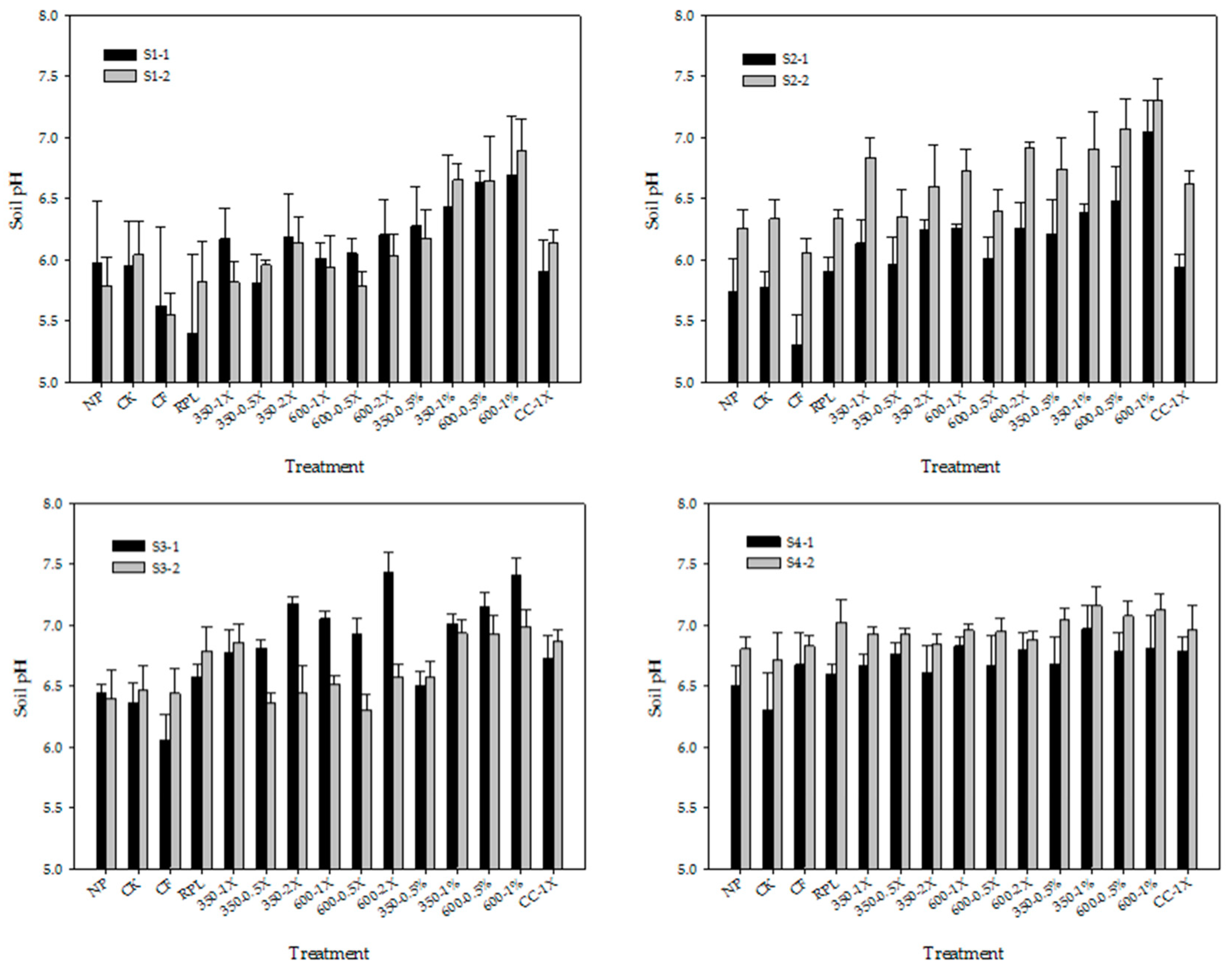
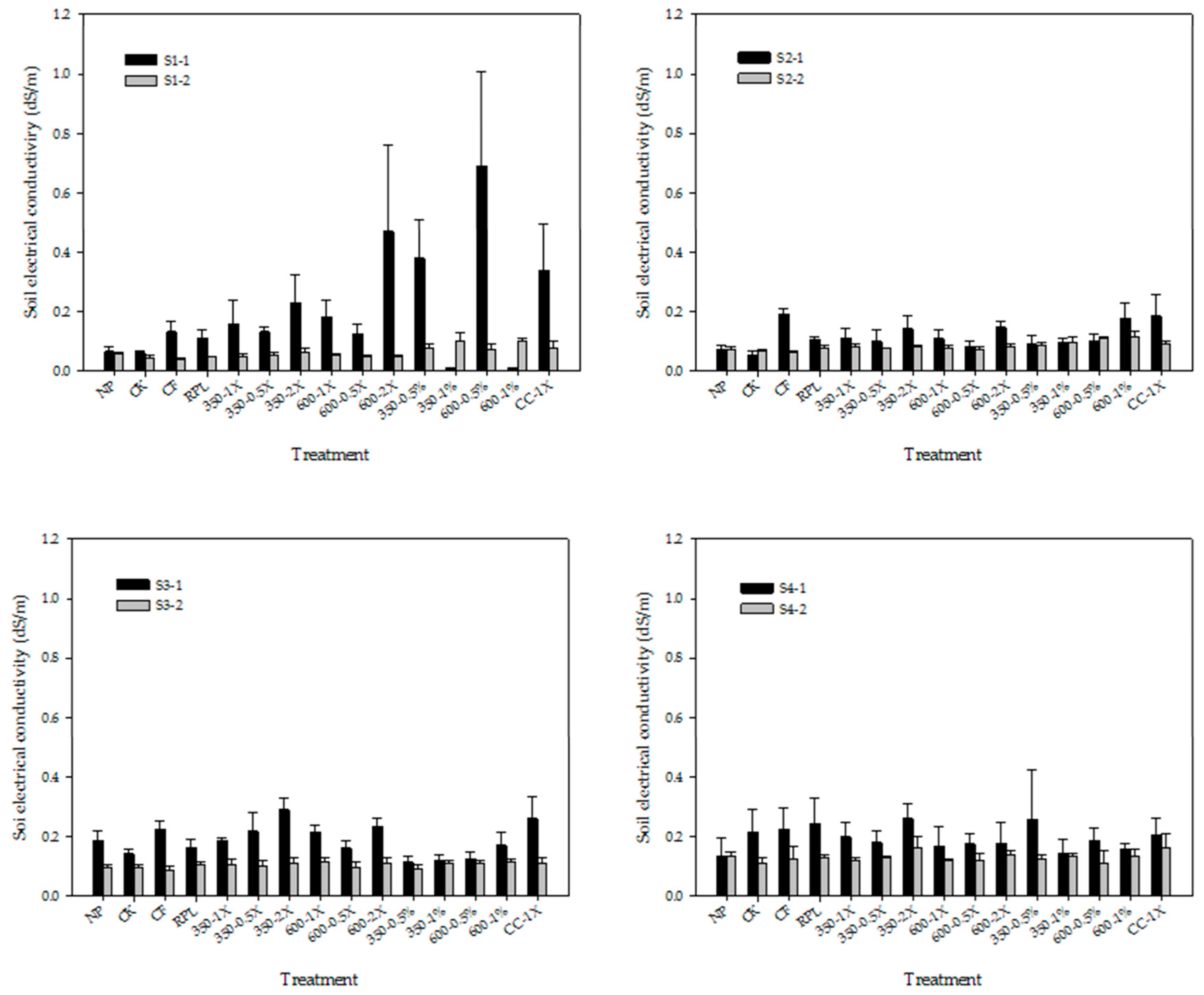
3.1. Soil pH and ECw
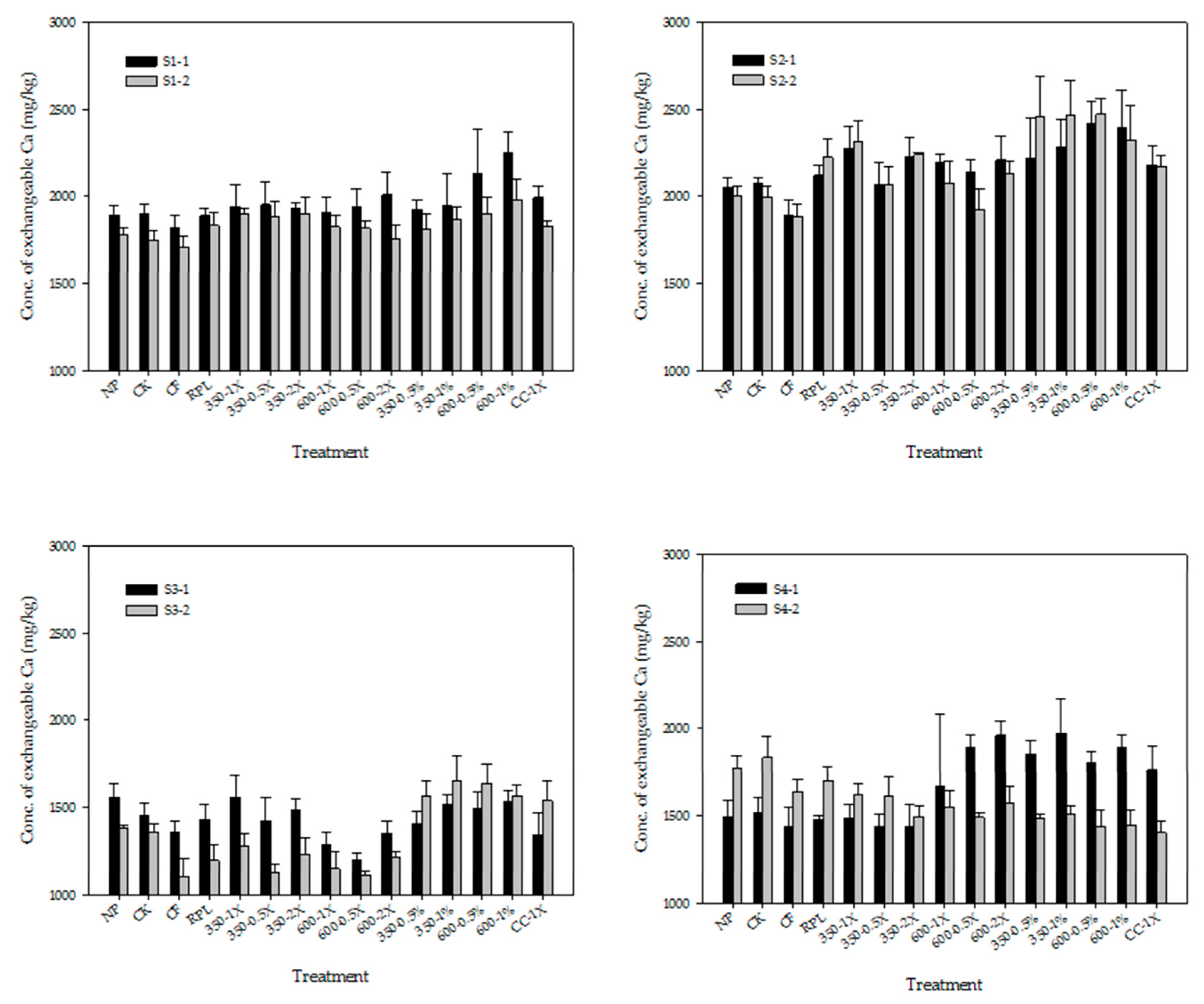
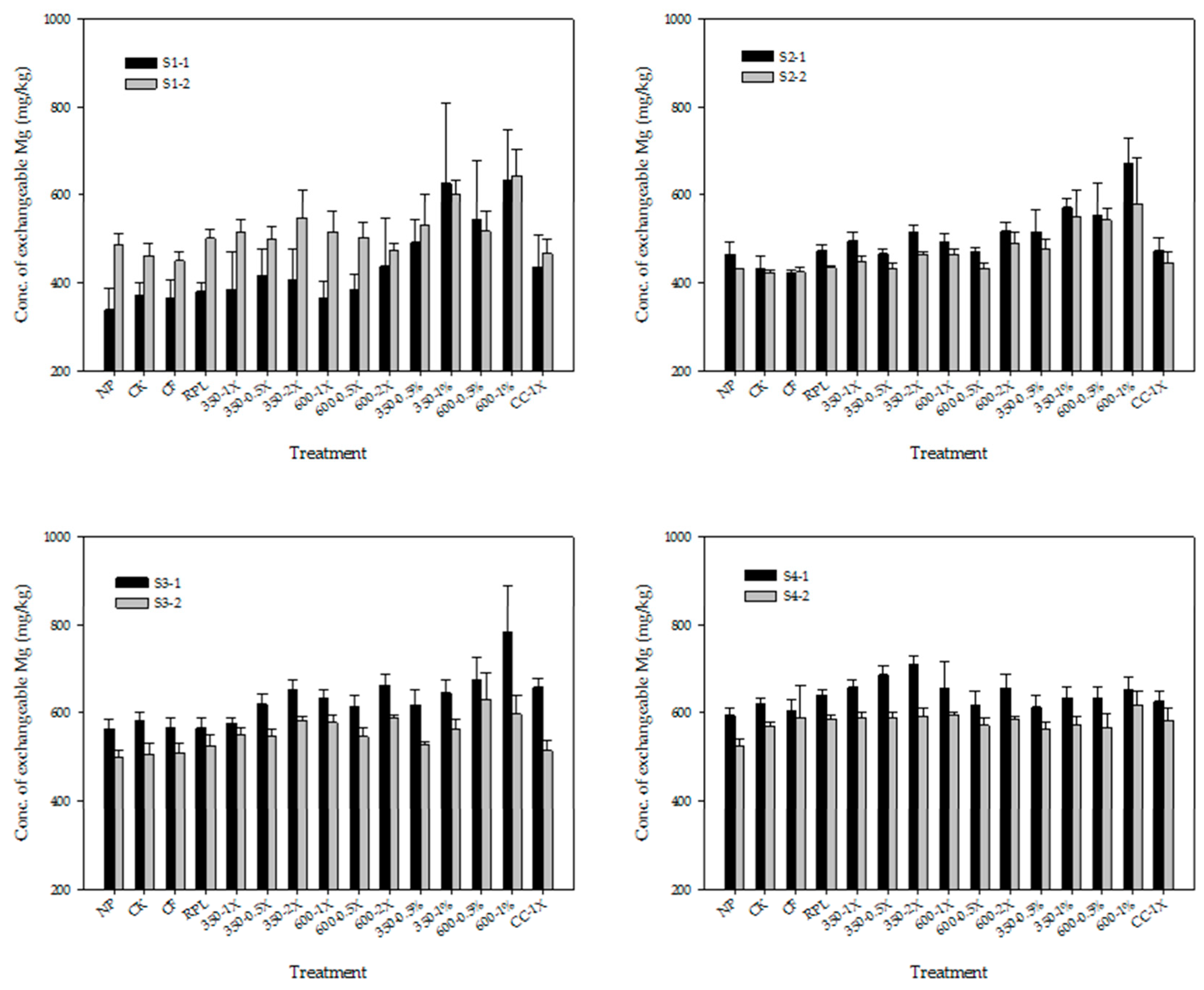
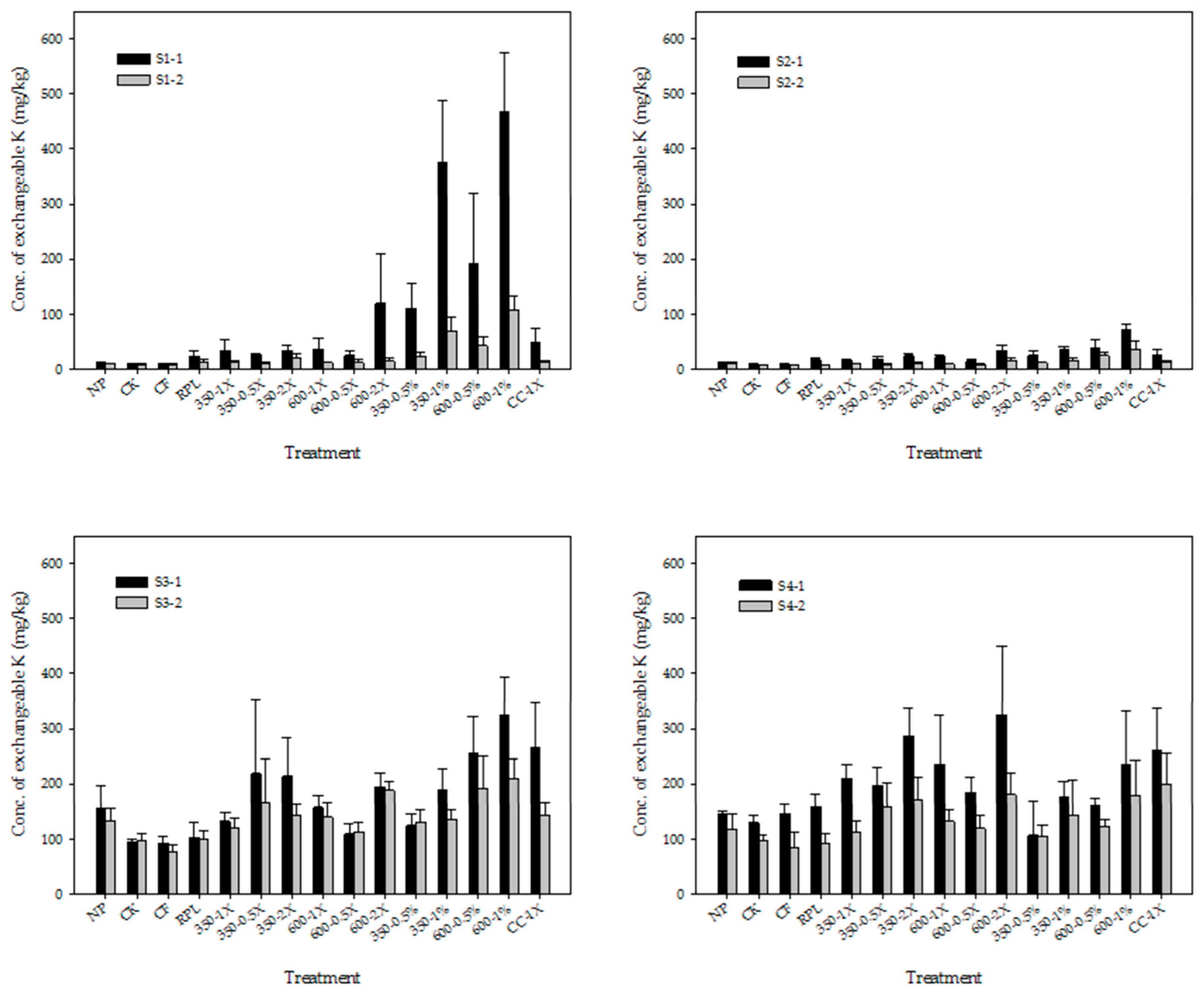
3.2. Exchangeable Cations
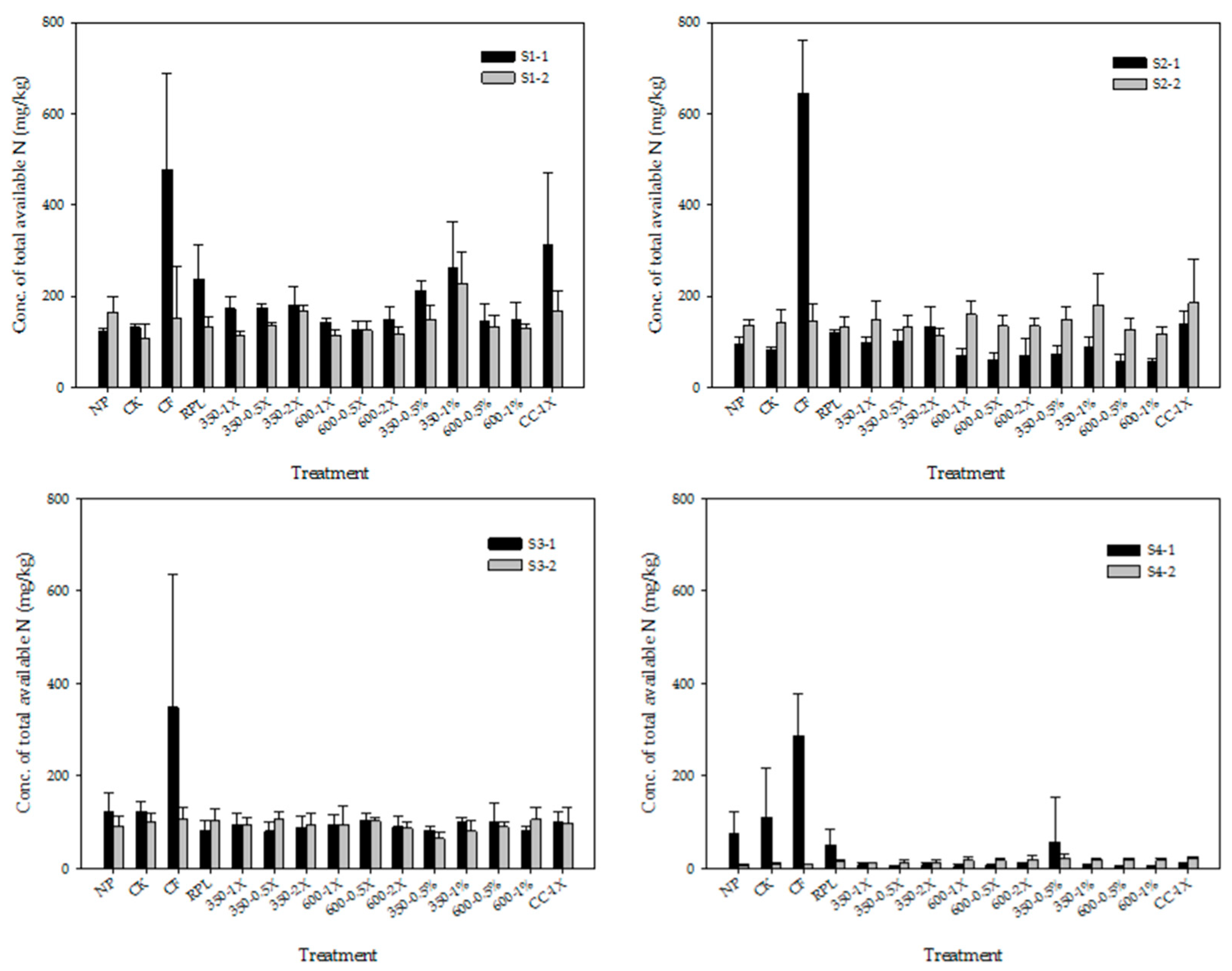
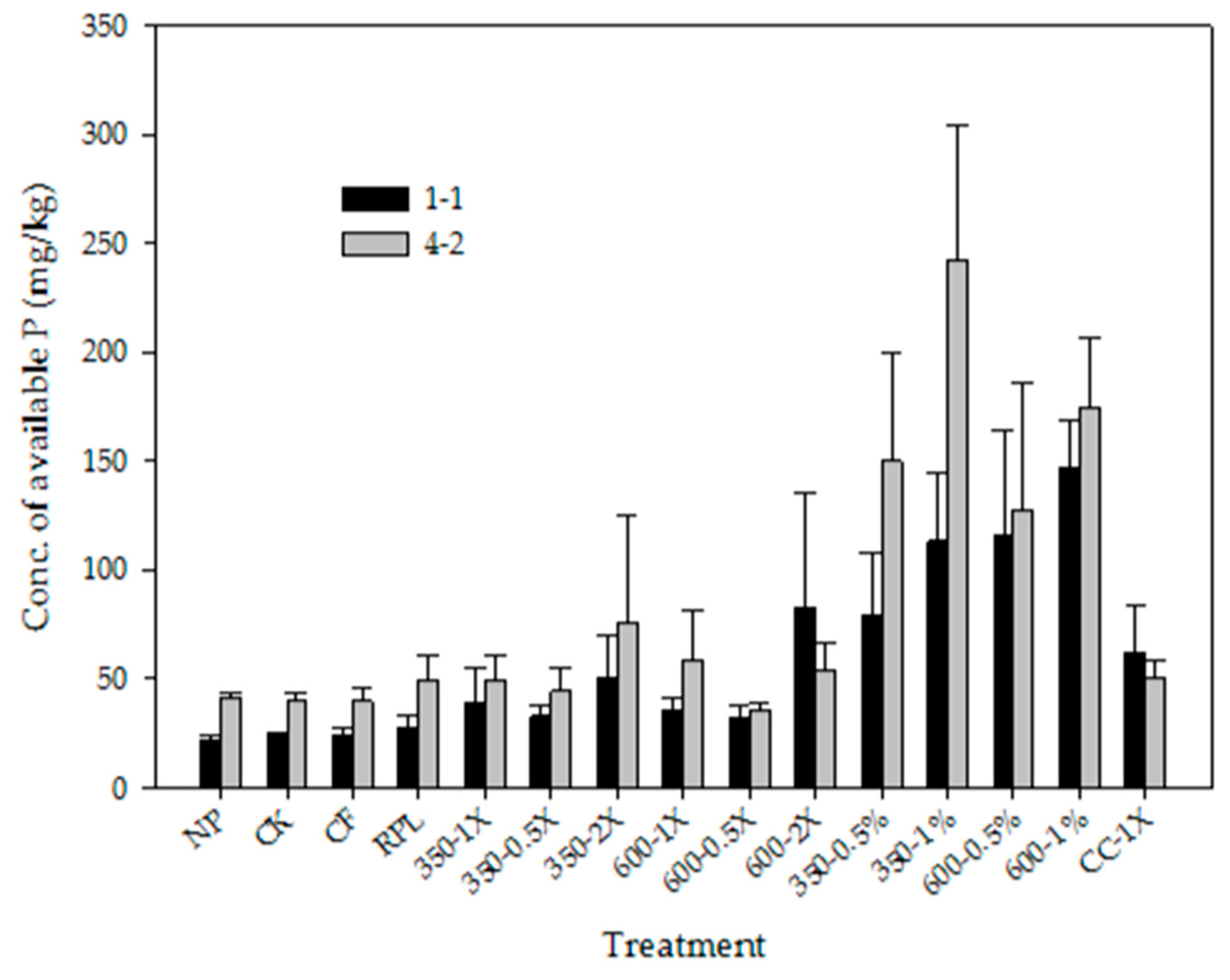
3.3. Available N and Available P
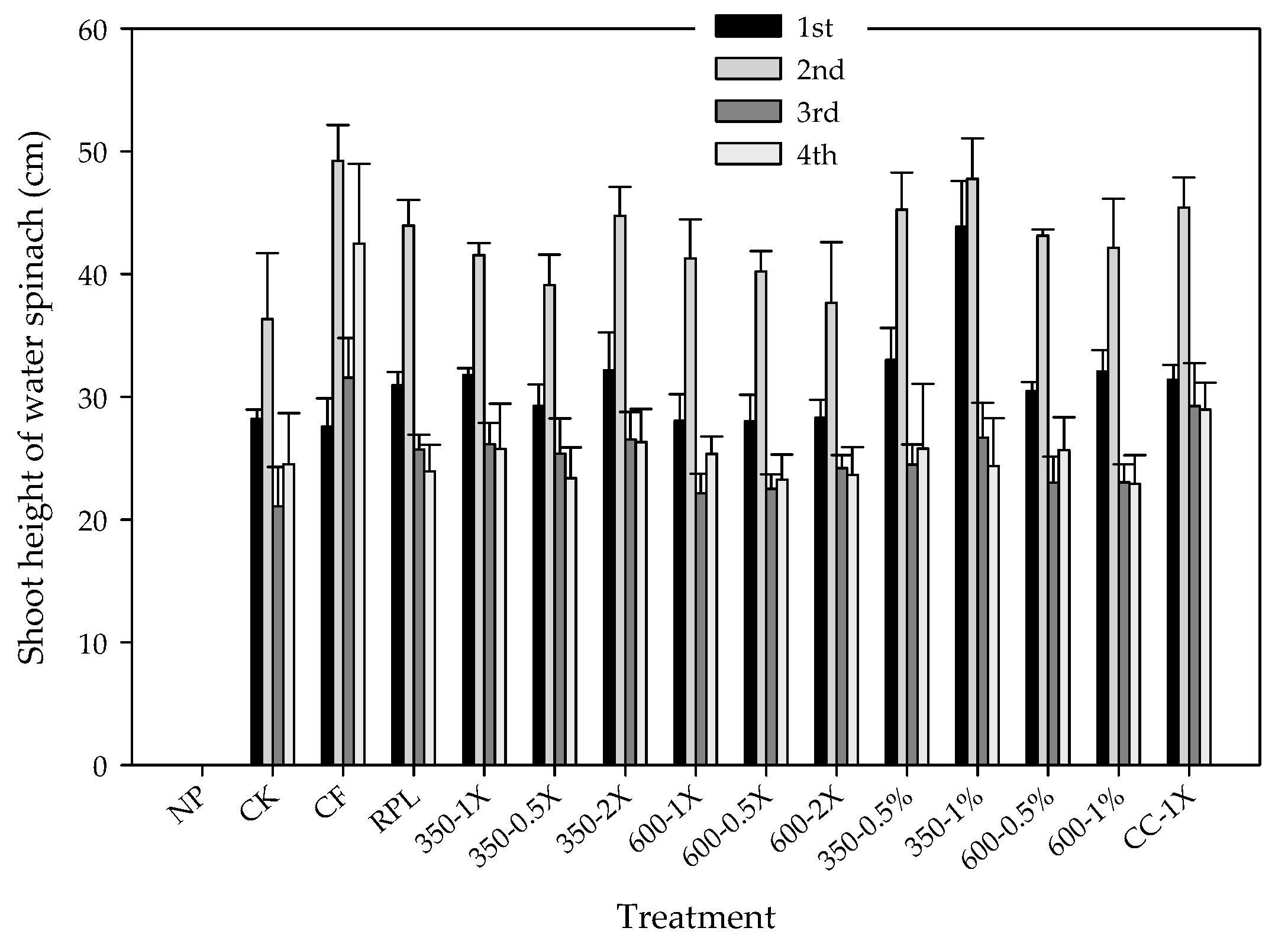
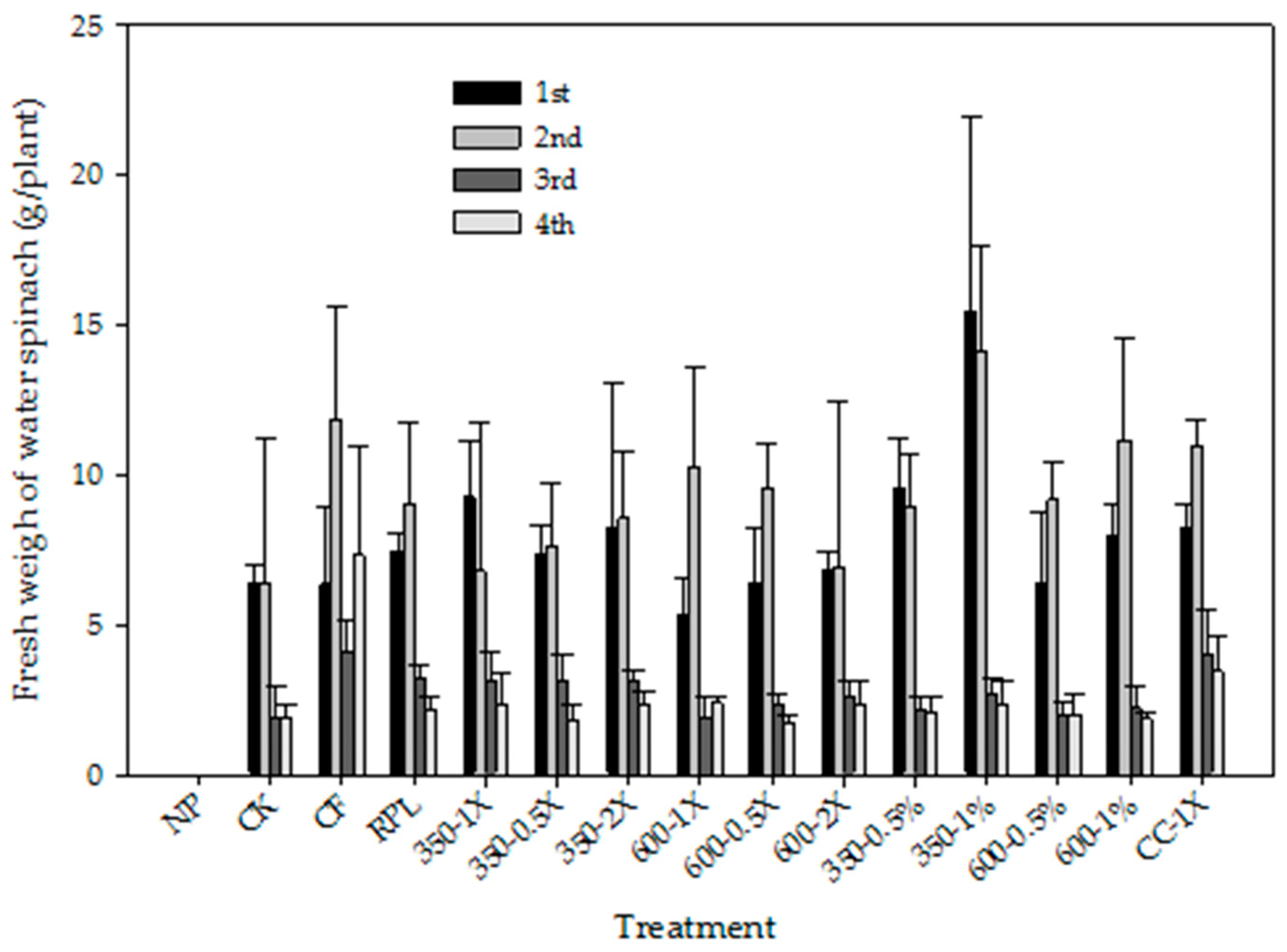
3.4. Plant Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McMichael, A.J.; Powles, J.W.; Butler, C.D.; Uauy, R. Energy and health 5—Food, livestock production, energy, climate change, and health. Lancet 2007, 370, 1253–1263. [Google Scholar] [CrossRef]
- Hsu, H.H.; Chen, C.T. Observed and projected climate change in Taiwan. Meteorol. Atmos. Phys. 2002, 79, 87–104. [Google Scholar] [CrossRef]
- Chen, H.; Petley, D.N. The impact of landslides and debris flows triggered by Typhoon Mindulle in Taiwan. Q. J. Eng. Geol. Hydrogeol. 2005, 38, 301–304. [Google Scholar] [CrossRef]
- Tewolde, H.; Adeli, A.; Sistani, K.R.; Rowe, D.E. Mineral nutrition of cotton fertilized with poultry litter or ammonium nitrate. Agron. J. 2011, 103, 1704–1711. [Google Scholar] [CrossRef]
- Jn-Baptiste, M.; Sistani, K.R.; Tewolde, H. Poultry manure application time impact on corn grain production in a crider silt Loam. Soil Sci. 2012, 177, 47–55. [Google Scholar] [CrossRef]
- Petersen, S.O.; Sommer, S.G.; Beline, F.; Burton, C.; Dach, J.; Dourmad, J.Y.; Leip, A.; Misselbrook, T.; Nicholson, F.; Poulsen, H.D.; et al. Recycling of livestock manure in a whole-farm perspective. Livest. Sci. 2007, 112, 180–191. [Google Scholar] [CrossRef]
- Blasi, C.D.; Buonanno, F.; Branca, C. Reactivities of some biomass chars in air. Carbon 1999, 37, 1227–1238. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Noack, A.G. Black carbon in soils and sediments: Analysis, distribution, implications and current challenges. Glob. Biogeochem. Cycle 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Radovic, L.R.; Moreno-Castilla, C.; Rivera-Ulrilla, J. Carbon materials as adsorbents in aqueous solutions. Chem. Phys. Carb. 2001, 27, 227–405. [Google Scholar]
- Mandal, K.G.; Misra, A.K.; Hati, K.M.; Bandyopadhyay, K.K.; Ghosh, P.K.; Mohanty, M. Rice residue-management options and effects on soil properties and crop productivity. J. Food Agric. Environ. 2004, 2, 224–231. [Google Scholar]
- Haefele, S.M.; Knoblauch, C.; Gummert, M.; Konboon, Y.; Koyama, S. Black carbon (biochar) in rice-based systems: Characterization and opportunities. In Amazonian Dark Earths: Wim Sombroek’s Vision; Woods, W.I., Teixeira, W.G., Lehmann, J., Steiner, C., WinklerPrins, A., Rebellato, L., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 445–463. ISBN 978-1-4020-9030-1. [Google Scholar]
- Beesley, L.; Moreno-Jimenez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Verheijen, F.; Jeffery, S.; Bastos, A.; van der Velde, M.; Diafas, I. A critical scientific review of effects on soil properties processes and functions. In Biochar Application to Soils; European Communities: Luxembourg, 2010; pp. 1018–5593. [Google Scholar]
- Anyanwu, I.N.; Alo, M.N.; Onyekwere, A.M.; Crosse, J.D.; Nworie, O.; Chamba, E.B. Influence of biochar aged in acidic soil on ecosystem engineers and two tropical agricultural plants. Ecotoxicol. Environ. Saf. 2018, 153, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Lal, R. Biochar impacts on soil physical properties and greenhouse gas emissions. Agron. J. 2013, 3, 313–339. [Google Scholar] [CrossRef]
- Amarasinghe, H.; Gunathilake, S.; Karunarathna, A. Ascertaining of optimum pyrolysis conditions in producing refuse tea biochar as a soil amendment. Proc. Food Sci. 2016, 6, 97–102. [Google Scholar] [CrossRef]
- Grossman, J.M.; O’Neill, B.E.; Tsai, S.M.; Liang, B.Q.; Neves, E.; Lehmann, J.; Thies, J.E. Amazonian anthrosols support similar microbial communities that differ distinctly from Those Extant in Adjacent, Unmodified Soils of the Same Mineralogy. Microb. Ecol. 2010, 60, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Allah, E.F.A.; Berg, G. Biochar treatment resulted in a combined effect on soybean growth promotion and a shift in plant growth promoting rhizobacteria. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Steiner, C.; Glaser, B.; Teixeira, W.G.; Lehmann, J.; Blum, W.E.H.; Zech, W. Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J. Plant Nutr. Soil Sci. 2008, 171, 893–899. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil—Concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Speir, A.; Morris, L.; Ogden, L.; Harris, K.; Lee, D.; Das, K. Potential for pyrolysis char to affect soil moisture and nutrient status of a loamy sand soil. In Proceedings of the 2007 Georgia Water Resources Conference, Athens, GA, USA, 27–29 March 2007. [Google Scholar]
- Zee, T.; Nelson, N.; Newdigger, G. Biochar and nitrogen effects on winter wheat growth. Kans. Agric. Exp. Stn. Res. Rep. 2017, 3, 6. [Google Scholar] [CrossRef]
- Xu, H.J.; Wang, X.H.; Li, H.; Yao, H.Y.; Su, J.Q.; Zhu, Y.G. Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environ. Sci. Technol. 2014, 48, 9391–9399. [Google Scholar] [CrossRef] [PubMed]
- Trupiano, D.; Cocozza, C.; Baronti, S.; Amendola, C.; Vaccari, F.P.; Lustrato, G.; Di Lonardo, S.; Fantasma, F.; Tognetti, R.; Scippa, G.S. The effects of biochar and its combination with compost on lettuce (Lactuca sativa L.) growth, soil properties, and soil microbial activity and abundance. Int. J. Agron. 2017, 2017, 3158207. [Google Scholar] [CrossRef]
- Vaccari, F.; Maienza, A.; Miglietta, F.; Baronti, S.; Di Lonardo, S.; Giagnoni, L.; Lagomarsino, A.; Pozzi, A.; Pusceddu, E.; Ranieri, R. Biochar stimulates plant growth but not fruit yield of processing tomato in a fertile soil. Agric. Ecosyst. Environ. 2015, 207, 163–170. [Google Scholar] [CrossRef]
- Carter, S.; Shackley, S.; Sohi, S.; Suy, T.B.; Haefele, S. The impact of biochar application on soil properties and plant growth of pot grown lettuce (Lactuca sativa) and cabbage (Brassica chinensis). Agronomy 2013, 3, 404–418. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Buss, W.; Mašek, O.; Graham, M.; Wüst, D. Inherent organic compounds in biochar—Their content, composition and potential toxic effects. J. Environ. Manag. 2015, 156, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Domene, X.; Hanley, K.; Enders, A.; Lehmann, J. Short-term mesofauna responses to soil additions of corn stover biochar and the role of microbial biomass. Appl. Soil Ecol. 2015, 89, 10–17. [Google Scholar] [CrossRef]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Belyaeva, O.N.; Haynes, R.J. Comparison of the effects of conventional organic amendments and biochar on the chemical, physical and microbial properties of coal fly ash as a plant growth medium. Environ. Earth Sci. 2012, 66, 1987–1997. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Zhu, Q.H.; Peng, X.H.; Huang, T.Q.; Xie, Z.B.; Holden, N.M. Effect of biochar addition on maize growth and nitrogen use efficiency in acidic red soils. Pedosphere 2014, 24, 699–708. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K. The amelioration effects of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use Manag. 2011, 27, 110–115. [Google Scholar] [CrossRef]
- Weikard, H.P.; Seyhan, D. Distribution of phosphorus resources between rich and poor countries: The effect of recycling. Ecol. Econ. 2009, 68, 1749–1755. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series No. 5, SSSA and ASA; SSSA Inc.; ASA Inc.: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis: Part 3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series No. 5, SSSA and ASA; SSSA Inc.; ASA Inc.: Madison, WI, USA, 1996; pp. 1201–1230. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Method; Klute, A., Ed.; ASA Inc.; SSSA Inc.: Madison, WI, USA, 1986; pp. 383–412. [Google Scholar]
- EPA/Taiwan. Method Code No: NIEA S321.64B; Environmental Protection Administration of Taiwan ROC: Taipei, Taiwan, 2015.
- Jones, J.B.; Case, V.W. Sampling, handling and analyzing plant tissue samples. In Soil Testing and Plant Analysis; Westerman, R.L., Ed.; SSSA Inc.: Madison, WI, USA, 1990; pp. 389–427. [Google Scholar]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis: Part 3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series No. 5, SSSA and ASA; SSSA Inc.; ASA Inc.: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis: Part 3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series No. 5, SSSA and ASA; SSSA Inc.; ASA Inc.: Madison, WI, USA, 1996; pp. 417–435. [Google Scholar]
- Mulvaney, R.L. Nitrogen—Inorganic forms. In Methods of Soil Analysis: Part 3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series No. 5, SSSA and ASA; SSSA Inc.; ASA Inc.: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Kuo, S. Phosphorus. In Methods of Soil Analysis: Part 3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series No. 5, SSSA and ASA; SSSA Inc.; ASA Inc.: Madison, WI, USA, 1996; pp. 869–919. [Google Scholar]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soil; Pearson Education: Upper Saddle River, NJ, USA, 2017; pp. 438–481. ISBN 978-0-13-325448-8. [Google Scholar]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar-root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Rillig, M.C.; Thies, J.E. Characteristics of biochar: Biological properties. In Biochar for Environmental Management; Joseph, S., Lehmann, J., Eds.; Routledge: London, UK, 2012; pp. 117–138. [Google Scholar]
- Huang, J.H.; Hsu, S.H.; Wang, S.L. Effects of rice straw ash amendment on Cu bioavailability in flooded rice paddy soils. J. Hazard. Mater. 2011, 186, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Wang, S.L.; Huang, J.H.; Huang, S.T.; Wang, M.K. Effects of rice straw ash amendment on Cd solubility and distribution in a contaminated paddy soil under submergence. Paddy Water Environ. 2015, 13, 135–143. [Google Scholar] [CrossRef]
- Liao, I.H.; Huang, J.H.; Wang, S.L.; Cheng, M.P.; Liu, J.C. Adsorptions of Cd(II) and Pb(II) in aqueous solution by rice-straw char. Desalin. Water Treat. 2015, 57, 21619–21626. [Google Scholar] [CrossRef]
- Lee, S.-E.; Ahn, H.-J.; Youn, S.-K.; Kim, S.-M.; Jung, K. Application effect of food waste compost abundant in NaCl on the growth and cationic balance of rice plant in paddy soil. Korean J. Soil Sci. Fertil. 2000, 33, 100–108. [Google Scholar]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. (Eds.) Soil Fertility and Fertilizers; Pearson Inc.: Upper Saddle River, NJ, USA, 2014; pp. 185–221. [Google Scholar]
- Major, J.; Steiner, C.; Downie, A.; Lehmann, J.; Joseph, S. Biochar effects on nutrient leaching. In Biochar for Environmental Management: Science and Technology; Joseph, S., Lehmann, J., Eds.; Earthscan: London, UK, 2009; p. 271. [Google Scholar]
- Mikan, C.J.; Abrams, M.D. Altered forest composition and soil properties of historic charcoal hearths in Southeastern Pennsylvania. Can. J. For. Res. 1995, 25, 687–696. [Google Scholar] [CrossRef]
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar production by sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 72–78. [Google Scholar] [CrossRef]
- Antal, M.J.; Grønli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Guo, M.X.; Shen, Y.F. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Am. Chem. Soc. 2011, 94, 138–145. [Google Scholar]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- He, Y.D.; Zhai, Y.B.; Li, C.T.; Yang, F.; Chen, L.; Fan, X.P.; Peng, W.F.; Fu, Z.M. The fate of Cu, Zn, Pb and Cd during the pyrolysis of sewage sludge at different temperatures. Environ. Technol. 2010, 31, 567–574. [Google Scholar] [CrossRef] [PubMed]
| Pyrolysis Temperature | Unit | ||
|---|---|---|---|
| 350 °C | 600 °C | ||
| pH 1 | 8.13 | 7.78 | - |
| EC 1 | 10.07 | 16.18 | dS/m |
| Carbon (C) | 23.43 | 21.07 | % |
| Hydrogen (H) | 3.14 | 1.13 | % |
| Oxygen (O) | 27.98 | 20.91 | % |
| Nitrogen (N) | 2.60 | 1.79 | % |
| Phosphorus (P) | 1.97 | 3.00 | % |
| Potassium (K) | 2.29 | 3.44 | % |
| Calcium (Ca) | 7.06 | 8.16 | % |
| Magnesium (Mg) | 1.40 | 1.91 | % |
| Sulfur (S) | 1.32 | 1.57 | % |
| Iron (Fe) | 802 | 2137 | mg/kg |
| Manganese (Mn) | 543 | 772 | mg/kg |
| Copper (Cu) | 91 | 126 | mg/kg |
| Zinc (Zn) | 803 | 1061 | mg/kg |
| Nickel (Ni) | ND 2 | ND | mg/kg |
| Arsenic (As) | ND | ND | mg/kg |
| Cadmium (Cd) | ND | ND | mg/kg |
| Chromium (Cr) | ND | ND | mg/kg |
| Lead (Pb) | ND | ND | mg/kg |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.-H.; Wang, S.-L.; Tongsiri, P.; Cheng, M.-P.; Lai, H.-Y. Effects of Poultry-Litter Biochar on Soil Properties and Growth of Water Spinach (Ipomoea aquatica Forsk.). Sustainability 2018, 10, 2536. https://doi.org/10.3390/su10072536
Yu C-H, Wang S-L, Tongsiri P, Cheng M-P, Lai H-Y. Effects of Poultry-Litter Biochar on Soil Properties and Growth of Water Spinach (Ipomoea aquatica Forsk.). Sustainability. 2018; 10(7):2536. https://doi.org/10.3390/su10072536
Chicago/Turabian StyleYu, Chun-Hui, Shan-Li Wang, Prapasiri Tongsiri, Mei-Ping Cheng, and Hung-Yu Lai. 2018. "Effects of Poultry-Litter Biochar on Soil Properties and Growth of Water Spinach (Ipomoea aquatica Forsk.)" Sustainability 10, no. 7: 2536. https://doi.org/10.3390/su10072536
APA StyleYu, C.-H., Wang, S.-L., Tongsiri, P., Cheng, M.-P., & Lai, H.-Y. (2018). Effects of Poultry-Litter Biochar on Soil Properties and Growth of Water Spinach (Ipomoea aquatica Forsk.). Sustainability, 10(7), 2536. https://doi.org/10.3390/su10072536







