Abstract
In order to utilize seaweeds as a natural therapeutic agent in aquatic cultures, it is important to evaluate their antimicrobial activities. We analyzed that of the typical seaweeds of the Zhejiang Coast in order to improve their potential utilization. Six species of seaweeds were collected from the Zhejiang coast—Ulva pertusa, Ulva prolifera, Gloiopeltis furcata, Gracilariopsis lemaneiformis, Sargassum fusiforme and Ishige okamurae—and their ethanolic extracts were tested for antibacterial effects as well as antiviral activity against the white spot syndrome virus (WSSV). The seaweed extracts inhibited bacterial growth in vitro, and increased the immune responsiveness and survival of the crab Scylla paramamosain infected with WSSV in a concentration-dependent manner. U. prolifera, G. lemaneiformis, and S. fusiforme showed the most potent antibacterial activities and most significant enhancement of the innate immunity in healthy crabs. In conclusion, our study showed that the seaweed extracts have therapeutic effects and are a potential natural medicine for aquatic animals.
1. Introduction
Seaweeds are important primary producers in the ocean, and thus play an important role in the marine ecosystem. According to the 2016 FAO report, about 28.5 million tons of seaweeds were harvested in 2014 globally, mainly as a source of food [1]. From 1977 to 1987, most seaweeds were discovered to have medicinal value [2]. In the 1980s and 1990s, many bioactive substances were also discovered from marine bacteria, invertebrates, and algae [3]. Several active substances isolated from seaweeds, such as polysaccharides, fatty acids, proteins, phenolics, terpenes, etc., show significant antitumor, antibacterial, antiviral, and anti-oxidative activities [4].
Polysaccharides isolated from the seaweeds have been shown to decrease the growth of tumor cells [5,6], while terpenes from the red alga Portieria hornemannii exhibited cytotoxicity against human cancer cells [7]. In addition, seaweed polysaccharides can prevent viral attachment to target cells and inhibit viral replication, and this antiviral activity depends on the degree of sulfation of the polysaccharides [8,9,10]. Apart from the polysaccharides, antiviral fatty acids, alkaloids, and terpenes have also been isolated from algae [11,12]. Seaweeds contain a large number of antibacterial compounds such as fatty acids [13,14,15], phenols [16,17], and steroids [18,19,20]. Several antibacterial fatty acids are known in the red alga Gloiopeltis furcata [13,14]. Since seaweeds reside in complex marine habitats with continuous exposure to oxidative stress, they produce antioxidant secondary metabolites [4] such as polyphenols, polysaccharides, unsaturated fatty acids, chlorophyll, and carotenoids [21,22,23].
In recent years, seaweed extracts have been widely used for the prevention and treatment of bacterial and viral diseases in aquatic animals [24]. Seaweed extracts have shown strong antibacterial activity against aquatic pathogenic bacteria [25,26]. The white spot syndrome virus (WSSV) was first isolated in Taiwan in 1992, and it causes extensive deaths in crustaceans such as shrimps and crabs, and other aquatic species [27,28]. Recent studies have reported anti-WSSV virus activity in several seaweed species [29,30]. Gracilaria tenuistipitata extracts significantly increased the immunological activity of Litopenaeus vannamei against WSSV and reduced shrimp mortality [30]. Methanolic extract of the red macroalga Hypnea spinella showed significant antiviral activity in WSSV-infected freshwater crab Paratelphusa hydrodomous [31]. Therefore, seaweeds have been widely used in recent years in integrated multi-trophic aquaculture (IMTA), and have significantly reduced eutrophication and increased productivity [32,33,34].
The Zhejiang coastline along the East China Sea is more than 6600 km long, with 3061 islands encompassing a total area of 500 m2. The coastline has an abundant seaweed repository, totaling almost 262 species [35]. Although the different seaweed species have diverse active substances [11,36,37,38], there is limited knowledge regarding their biological effects. Therefore, detailed studies are needed to distinguish antimicrobial and other activities of the different seaweeds from the Zhejiang coast. We have evaluated the antimicrobial activities of the typical seaweeds found along the Zhejiang coast, in order to increase the efficiency of their therapeutic utilization for aquatic animals.
2. Materials and Methods
2.1. Collection of the Seaweeds
Seaweeds were collected from four sampling points along the Zhejiang coast (N30°42′, S122°46′; N29°32′, S121°45′; N29°25′, S122°11′; N28°53′, S122°15′) (Figure 1). All of the seaweeds were collected manually in the intertidal zone, and preserved on ice until further processing. After identifying the samples using previous literature, six species of seaweeds were selected for the study: Ulva pertus, Ulva prolifera (green algae), Gloiopeltis furcata, Gracilariopsis lemaneiformis (red algae), Ishige okamurae, and Sargassum fusiforme (brown algae) (Figure 2). The selected seaweeds had large biomass and insufficient utilization.
Figure 1.
Map showing sampling sites.

Figure 2.
Six species of seaweeds.
2.2. Preparation of Extracts
The seaweeds were rinsed with sterile seawater to remove any adherents and necrotic parts, and then dried in the shade at room temperature. The dried samples were then powdered in a grinder, and sieved through a 40-mesh screen. Each powdered sample (20 g) was suspended in 300 mL 85% ethanol, and after 72 h, the mixture was filtered. The process was repeated once more, and the two filtrates were combined. The filtrates were concentrated under reduced pressure at 40 °C with a rotary evaporator to near dryness. The dried extracts were freeze-dried and stored at −40 °C [39].
2.3. Antibacterial Activity Assay
2.3.1. Bacterial Strains
Gram-negative Escherichia coli, Aeromonas hydrophila, Pseudomonas aeruginosa, and Vibrio alginolyticus, and Gram-positive Staphylococcus aureus, were selected for testing the antibacterial effects of the seaweed extracts. The bacterial strains were acquired from the key laboratory of Marine Biotechnology in Ningbo University, and maintained in liquid medium consisting of 1% (w/v) sodium chloride, 0.5% (w/v) yeast extract, and 1% (w/v) Bacto-peptone, pH 7.2–7.4.
2.3.2. Antibacterial Activity by Disc Diffusion Assay
Antibacterial effects of the ethanolic extracts of seaweed were assayed using a modified version of the disc diffusion method [39]. Bacterial-agar medium was first prepared by mixing 20 mL of bacterial suspension with 150 mL of sterile nutrient agar media, which had the same composition as the liquid culture medium described above, along with 1.5% (w/v) agar. A basal agar layer was prepared in each petri dish with 5 mL of the agar medium, and then 5 mL of the bacteria medium was overlaid onto the pre-poured plates. Filter-paper discs (6-mm diameter) impregnated with 10 mg/mL of the 85% ethanol extracts solution were placed on the surface of the agar plates. Chloramphenicol solution (5 mg/mL) was used as positive control, and 85% ethanol was used as negative control. The petri dishes were incubated at 37 °C for 24 h, and the diameter (mm) of the zone of bacterial-growth inhibition was measured as an index of antibacterial activity. Three independent disc diffusion tests were performed.
2.4. Crabs Stock and Pathogen Challenge
Scylla paramamosain, a cultured crustacean of high economic value, was used as the in vivo experimental model. Crabs weighing approximately 300 g were reared in a 50-L tank equipped with an aerated pump at room temperature, and were randomized into 20 groups (n = 16 per group). The crabs were infected with the white spot syndrome virus (WSSV), as described previously [40,41,42]. Both healthy and WSSV-challenged crabs were injected with 1 mg/kg, 5 mg/kg, and 10 mg/kg seaweed extracts dissolved in phosphate buffer solution (PBS) (test groups). The negative control or PBS group was injected with only PBS. The positive control or WSSV group was injected with WSSV and PBS. The crabs were observed twice daily after infection; their clinical symptoms were observed, and mortality was recorded.
2.5. Immunological Analysis of the Crab Hemolymph
The hemolymph was withdrawn from the healthy and WSSV-challenged crabs 24 h after seaweed extract or PBS injection, and the immunological parameters were measured as described below.
2.5.1. Total Hemocyte Count (THC) Assay
As previously described [40,41], 100 μL of hemolymph was withdrawn into a 1 mL of sterile syringe containing 0.9 mL of anticoagulant solution (trisodium citrate 30 mM, sodium chloride 0.34 M, ethylenediaminetetraacetic acid 10 mM, pH 7.55), placed on a hemocytometer and THC was performed.
2.5.2. Prophenoloxidase (proPO) Assay
Prophenoloxidase activity was measured spectrometrically, as previously described [40,41]. The hemolymph was briefly centrifuged at 800× g at 4 °C for 20 min to separate hemocytes and serum. The latter was incubated with an equal volume of L-dihydroxyphenylalanine (L-DOPA) for 30 min, and then, optical density was measured at 490 nm.
2.5.3. Superoxide Dismutase (SOD) Assay
SOD activity was measured using nitro blue tetrazolium (NBT) chloride in the presence of riboflavin, as described previously [40,41]. Briefly, 100 mL of hemolymph was homogenized with 0.5 mL of phosphate buffer (50 mM, pH 7.8), and the homogenate was centrifuged at 5724× g for 5 min at 4 °C. The supernatant was heated at 65 °C for 5 min, centrifuged again, and the supernatants were stored at −20 °C for later use. A reaction solution (50 mM, pH 7.8) was prepared with 0.1 mM of ethylenediamine tetra-acetic acid (EDTA), 13 mM of methionine, 0.75 mM of NBT, and 20 mM of riboflavin in phosphate buffer. The supernatants were incubated with the reaction solution under 4000Lx fluorescent light for 2 min to activate the photoreduction of riboflavin, and the optical density at 560 nm was measured.
2.6. Statistical Analysis
Analysis of variance (ANOVA) was used to estimate the total hemocyte count, proPO activity, and SOD activity of the crabs injected with different seaweed extracts. Two-way ANOVA was used to identify gaps in means between factor level combinations. All of the experiments were repeated three times, and statistical analysis was conducted using SPSS version 24 (IBM, Armonk, NY, USA) [43].
3. Results
3.1. Extraction of the Seaweeds
The quantities of different seaweed extracts were 2.58 ± 0.30 g (U. pertusa), 2.92 ± 0.22 g (U. prolifera), 2.86 ± 0.14 g (G. furcata), 1.98 ± 0.17 g (G. lemaneiformis), 2.16 ± 0.18 g (I. okamurae), and 3.11 ± 0.25 g (S. fusiforme). The highest quantity was obtained from U. prolifera, and the lowest from G. lemaneiformis.
3.2. Antibacterial Activities of Seaweed Extracts
The antibacterial activities of the six seaweed species against five bacterial species are shown in Table 1. In addition to the differences in antibacterial effects across the seaweeds, each species showed a varying degree of inhibition against different bacteria. All of the seaweeds showed antibacterial activity against S. aureus and A. hydrophila. G. lemaneiformis displayed the greatest zone of inhibition (12.50 ± 0.87 mm) against S. aureus, while U. prolifera displayed the greatest zone of inhibition (12.25 ± 1.77 mm) against A. hydrophila. Only S. fusiforme showed antibacterial activity against P. aeruginosa, and against four out of the five species of bacteria.

Table 1.
The antibacterial activities of the seaweed extracts.
3.3. Effect of the Seaweed Extract on the Crabs Infected by WSSV
The cumulative mortality of the crabs is shown in Figure 3. The PBS group showed a cumulative mortality of 6%, while that of the WSSV group was 100% over 204 h of infection. At the end of 204 h, the final mortality rates of the experimental group injected with 1 mg/kg, 5 mg/kg, and 10 mg/kg S. fusiforme extract were 88%, 81%, and 75%, respectively. Crabs treated with I. okamurae (10 mg/kg) also survived, and the mortality rate was 94%. No surviving crabs were seen in the remaining experimental groups. Therefore, S. fusiforme extract significantly improved the survival of the WSSV-challenged crabs compared with the other seaweed species (p < 0.05).
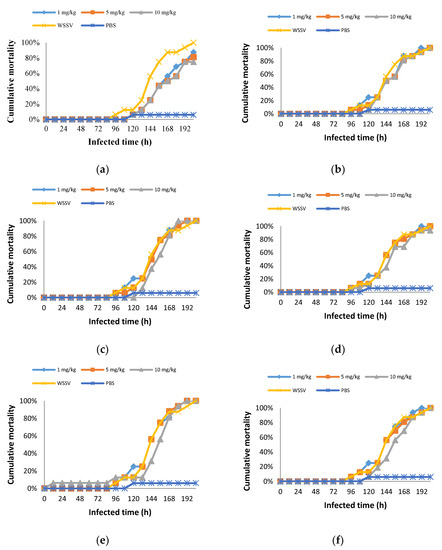
Figure 3.
Cumulative mortality of crabs injected with S. fusiforme (a), U. pertusa (b), G. lemaneiformis (c), I. okamurae (d), G. furcata (e), U. prolifera (f).
3.4. Immunological Parameters
3.4.1. Total Hemocyte Count (THC)
THC was measured 24 h after the healthy (Figure 4a) or infected (Figure 4b) crabs were injected with seaweed extracts. The THC of the healthy crabs injected with S. fusiforme (5 mg/kg and 10 mg/kg) and U. prolifera (10 mg/kg) extracts were significantly high (two-way ANOVA, F (12, 42) = 2.204, p < 0.01), with 10 mg/kg S. fusiforme resulting in the highest THC values. Among the WSSV-challenged crabs, all of the test groups showed higher THC values compared with the positive control group, and THC of the crabs injected with S. fusiforme (1 mg/kg, 5 mg/kg, and 10 mg/kg), G. lemaneiformis (10 mg/kg) and U. pertusa (10 mg/kg) extract were significantly higher compared with those treated with other seaweed species (two-way ANOVA, F (12, 42) = 5.587, p < 0.01).
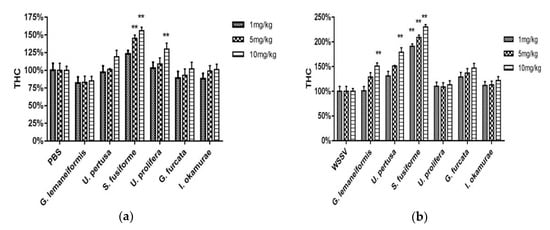
Figure 4.
Total hemocyte count (THC) of healthy crabs treated with seaweed extracts (a); THC of white spot syndrome virus (WSSV)-challenged crabs treated with seaweed extracts (b). Data are expressed as means ± standard deviation of three separate individuals. The asterisks indicate a significant difference (p < 0.01) between the treatment and control groups.
3.4.2. Prophenoloxidase (proPO) Activity
The proPO activity was measured in healthy (Figure 5a) and WSSV-infected crabs (Figure 5b) 24 h after the injection of seaweed extract. Among the healthy crabs, the proPO activity of those injected with seaweed extracts was higher than the PBS group, and directly proportional to the concentration of the extracts. The proPO values of crabs injected with S. fusiforme extracts were significantly higher compared with that induced by the other species (two-way ANOVA, F (6, 42) = 47.26, p < 0.01), and the highest activity was seen with 10 mg/kg S. fusiforme extract. Among the WSSV-challenged crabs, all of the test groups except for those injected with 1 mg/kg of U. pertusa, 1 mg/kg of I. okamurae, and 1 mg/kg and 5 mg/kg of U. prolifera exhibited higher proPO activity than the positive control group. The proPO values of crabs injected with S. fusiforme (5 mg/kg and 10 mg/kg) extract were significantly higher compared with the other species (two-way ANOVA, F (6, 42) = 11.78, p < 0.01).
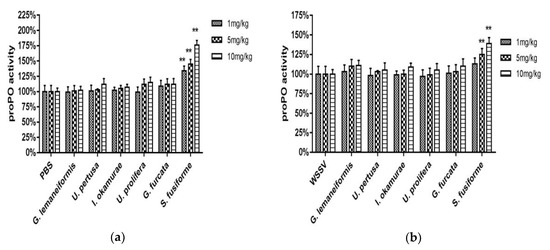
Figure 5.
Prophenoloxidase (proPO) of healthy crabs treated with seaweed extracts (a); proPO of WSSV-challenged crabs treated with seaweed extracts (b). Data are expressed as means ± standard deviation of three separate individuals. The asterisks indicate a significant difference (p < 0.01) between the treatment and control groups.
3.4.3. SOD Activity
SOD activity was measured in the healthy (Figure 6a) and WSSV-challenged (Figure 6b) crabs 24 h after being injected with seaweed extracts. The SOD activity of healthy crabs injected with extracts of S. fusiforme (1 mg/kg, 5 mg/kg, and 10 mg/kg), G. lemaneiformis (10 mg/kg), G. furcata (10 mg/kg), and U. pertusa (10 mg/kg) were significantly high (two-way ANOVA, F (12, 42) = 4.404, p < 0.01), and that of the 10 mg/kg S. fusiforme-injected group was the highest. In the WSSV-challenged crabs, all of the seaweed extract-injected groups showed higher SOD values than the positive control group, and the highest SOD values were seen in crabs injected with S. fusiforme (1 mg/kg, 5 mg/kg, and 10 mg/kg), G. furcata (10 mg/kg), and U. pertusa (10 mg/kg) extracts (two-way ANOVA, F (12, 42) = 2.909, p < 0.01).
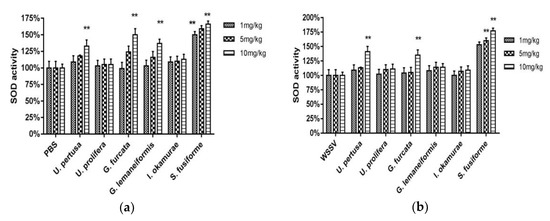
Figure 6.
Superoxide dismutase (SOD) of healthy crabs treated with seaweed extracts (a); SOD of WSSV-challenged crabs treated with seaweed extracts (b). Data are expressed as means ± standard deviation of three separate individuals. The asterisks indicate a significant difference (p < 0.01) between the treatment and control groups.
4. Discussion and Conclusions
Bacterial growth inhibition assay showed the inhibitory effects of all of the seaweed extracts against S. aureus, which is consistent with previous reports demonstrating the sensitivity of gram-positive strains to seaweed extracts [44,45,46]. The extract of G. lemaneiformis was effective against S. aureus and V. alginolyticus. A previous report showed antibacterial activity of the hot water extract of G. lemaneiformis against S. aureus and E. coli, and in contrast to our results, stronger effects against the latter [45]. The extract of S. fusiforme showed a relatively broad-spectrum antibacterial effect against four of the five bacterial strains tested, and it was the only seaweed that inhibited P. aeruginosa. Among the three common aquatic pathogenic bacteria—A. hydrophila, P. aeruginosa and V. alginolyticus—A. hydrophila was the most sensitive, and P. aeruginosa was the least sensitive to the seaweed extracts. The different potency of these extracts may be due to differences in their composition. According to previous reports, diverse biomolecules of seaweeds such as fatty acids, polysaccharides, and polyphenols have antibacterial activities, which in turn depend on the extraction and sampling methods, as well as the sampling season [47,48,49,50]. Salvador et al. (2007) studied the antibacterial activity of 82 species of seaweeds in different seasons, and found that the extracts of brown and red algae had stronger effects in autumn, while green algae had stronger effects during summer [51].
Several studies have also shown the presence of antiviral components in seaweeds [52,53]. In this study, the extracts from S. fusiforme significantly reduced the mortality of WSSV-infected crabs, and the final mortality rate of crabs injected with 10 mg/kg of S. fusiforme extract was 75%. S. fusiforme extracts in our study were not purified, but their antiviral effect was comparable with that of pure polysaccharides of S. fusiforme that were shown to kill viruses directly and prevent cellular adsorption [54].
In addition, the seaweed extracts improved the immune response of the crabs in our study. Invertebrates such as crabs lack immunoglobulin in body fluids, and mainly rely on non-specific innate immune mechanisms to resist pathogenic invasions. U. prolifera, G. lemaneiformis, and S. fusiforme significantly enhanced the innate immunity of healthy crabs. This is consistent with a previous study showing that the bioactive components of seaweed extracts, such as polysaccharides, fatty acids, alkaloids, and steroids, not only inhibited pathogens, but also improved the immunity of animals and therefore improved disease resistance [55]. Evidence from previous studies indicate that Gracilaria tenuistipitata and Gracilaria fisheri increased THC, proPO, SOD, and lysozyme activities and other immunological parameters of shrimps, thereby improving disease resistance [29,56,57].
The uncontrolled use of antibiotics in aquacultures in recent years has led to the emergence of resistant fish pathogens, compelling us to search for new antimicrobial compounds from natural sources such as seaweeds [20]. This study confirmed the potential therapeutic use of seaweed extracts on bacteria and viruses. Among them, S. fusiforme showed highly potent antimicrobial activity, and enhanced the immune response and reduced the mortality rate of WSSV-infected crabs. These seaweed extracts are therefore promising alternatives for the prevention and control of aquatic animal diseases.
Author Contributions
Conceptualization, Y.L. and N.X.; Methodology, Y.L.; Software, Y.L. and S.S.; Validation, Y.L., S.S., X.P., Y.Y. and F.Z.; Formal Analysis, Y.L.; Investigation, Y.L.; Resources, Y.L.; Data Curation, Y.L. and S.S.; Writing-Original Draft Preparation, Y.L.; Writing-Review & Editing, Y.L. and N.X.; Supervision, S.Z. and N.X.; Project Administration, S.Z. and N.X.; Funding Acquisition, S.Z. and N.X.
Funding
This research was funded by China Agriculture Research System CARS-50 and the key program of NSF of Zhejiang LZ17D06001.
Acknowledgments
The authors would like to thank China Agriculture Research System and the key program of NSF of Zhejiang for funding this research. The views expressed herein are those of the authors and do not necessarily reflect the views of any funding agencies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. The State of World Fisheries and Aquaculture in 2016: Contributing to the Full Realization of Food and Nutrition Security; FAO: Rome, Italy, 2016; p. 50. [Google Scholar]
- Ireland, C.M.; Copp, B.R.; Foster, M.P.; McDonald, L.A.; Radisky, D.C.; Swersey, J.C. Biomedical Potential of Marine Natural Products. In Pharmaceutical and Bioactive Natural Products; Springer: Boston, MA, USA, 1993; pp. 1–43. [Google Scholar]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 1999: Compounds with antibacterial, anticoagulant, antifungal, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities affecting the cardiovascular, endocrine, immune and nervous systems, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 132, 315–339. [Google Scholar] [PubMed]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.F.; Siqueira, J.M., Jr.; Vendruscolo, L.F.; de Jesus Neiva, T.; Gagliardi, A.R.; Maraschin, M.; Ribeiro-do-Valle, R.M. Antiangiogenic and antitumoral properties of a polysaccharide isolated from the seaweed Sargassum stenophyllum. Cancer Chemother. Pharmacol. 2005, 56, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R.W.; Cardellina, J.H.; Kato, Y.; Brinen, L.S.; Clardy, J.; Snader, K.M.; Boyd, M.R. A pentahalogenated monoterpene from the red alga Portieria hornemannii produces a novel cytotoxicity profile against a diverse panel of human tumor cell lines. J. Med. Chem. 1992, 35, 3007–3011. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Pujol, C.A.; Damonte, E.B.; Sinha, S.; Ray, B. Sulfated xylomannans from the red seaweed Sebdenia polydactyla: Structural features, chemical modification and antiviral activity. Antivir. Chem. Chemother. 2009, 19, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Plouguerné, E.; de Souza, L.M.; Sassaki, G.L.; Cavalcanti, J.F.; Villela Romanos, M.T.; da Gama, B.A.; Pereira, R.C.; Barreto-Bergter, E. Antiviral Sulfoquinovosyldiacylglycerols (SQDGs) from the Brazilian brown seaweed Sargassum vulgare. Mar. Drugs 2013, 11, 4628–4640. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.R.; Robaina, M.C.S.; Mendes, G.S.; Silva, T.S.L.; Gestinari, L.M.S.; Pamplona, O.S.; Yoneshigue-Valentin, Y.; Kaiser, C.R.; Romanos, M.T.V. Antiviral activity of extracts from Brazilian seaweeds against herpes simplex virus. Rev. Bras. Farmacogn. 2012, 22. [Google Scholar] [CrossRef]
- Pinto, A.M.V.; Leite, J.P.G.; Ferreira, W.J.; Cavalcanti, D.N.; Villaça, R.C.; Giongo, V.; Teixeira, V.L.; Paixão, I.C.N.D. Marine natural seaweed products as potential antiviral drugs against Bovine viral diarrhea virus. Rev. Bras. Farmacogn. 2012, 22. [Google Scholar] [CrossRef]
- Rosell, K.G.; Srivastava, L.M. Fatty acids as antimicrobial substances in brown algae. Hydrobiologia 1987, 151, 471–475. [Google Scholar] [CrossRef]
- Kurihara, H.; Goto, Y.; Aida, M.; Hosokawa, M.; Takahashi, K. Antibacterial activity against cariogenic bacteria and inhibition of insoluble glucan production by free fatty acids obtained from dried Gloiopeltis furcata. Fish. Sci. 1999, 65, 129–132. [Google Scholar] [CrossRef]
- Xu, N.; Fan, X.; Yan, X.; Li, X.; Niu, R.; Tseng, C.K. Antibacterial bromophenols from the marine red alga Rhodomela confervoides. Phytochemistry 2003, 62, 1221–1224. [Google Scholar] [CrossRef]
- Sandsdalen, E.; Haug, T.; Stensvåg, K.; Styrvold, O.B. The antibacterial effect of a polyhydroxylated fucophlorethol from the marine brown alga, Fucus vesiculosus. World J. Microbiol. Biotechnol. 2003, 19, 777–782. [Google Scholar] [CrossRef]
- Elshouny, W.; Gaafar, R.; Ismail, G.; Elzanaty, M. Seasonal variation of the antibacterial activity of some seaweeds against multi drug resistant pathogenic bacterial strains. Egypt. J. Exp. Biol. (Bot.) 2017, 13, 341–351. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Vagias, C.; Rahman, M.M.; Gibbons, S.; Roussis, V. Brominated diterpenes with antibacterial activity from the red alga Sphaerococcus coronopifolius. J. Nat. Prod. 2008, 71, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Vairappan, C.S.; Suzuki, M.; Ishii, T.; Okino, T.; Abe, T.; Masuda, M. Antibacterial activity of halogenated sesquiterpenes from Malaysian Laurencia spp. Phytochemistry 2008, 69, 2490–2494. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Lipton, A.P.; Paulraj, R.; Chakraborty, R.D. Guaiane sesquiterpenes from seaweed Ulva fasciata Delile and their antibacterial properties. Eur. J. Med. Chem. 2010, 45, 2237–2244. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Wang, R.; Paul, V.J.; Luesch, H. Seaweed extracts and unsaturated fatty acid constituents from the green alga Ulva lactuca as activators of the cytoprotective Nrf2-ARE pathway. Free Radic. Biol. Med. 2013, 57, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Fino, N.; Rodrigues, A.I.; Mendes, S.; Pedrosa, R. Cytoprotective effect of seaweeds with high antioxidant activity from the Peniche coast (Portugal). Food Chem. 2017, 218, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Cortés, Y.; Hormazábal, E.; Leal, H.; Urzúa, A.; Mutis, A.; Parra, L.; Quiroz, A. Novel antimicrobial activity of a dichloromethane extract obtained from red seaweed Ceramium rubrum (Hudson) (Rhodophyta: Florideophyceae) against Yersinia ruckeri and Saprolegnia parasitica, agents that cause diseases in salmonids. Electron. J. Biotechnol. 2014, 17, 126–131. [Google Scholar] [CrossRef]
- Bansemir, A.; Blume, M.; Schröder, S.; Lindequist, U. Screening of cultivated seaweeds for antibacterial activity against fish pathogenic bacteria. Aquaculture 2006, 252, 79–84. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Vijayakumar, S.; Mukherjee, A.; Chandrasekaran, N.; Thomas, J. Antioxidant and antibacterial activity of Chaetomorpha antennina against shrimp pathogen Vibrio parahaemolyticus. Aquaculture 2014, 433, 467–475. [Google Scholar] [CrossRef]
- Lo, C.F.; Ho, C.H.; Peng, S.E.; Chen, C.H.; Hsu, H.C.; Chiu, Y.L.; Chang, C.F.; Liu, K.F.; Su, M.S.; Wang, C.H.; et al. White spot syndrome baculovirus (WSBV) detected in cultured and captured shrimp, crabs and other arthropods. Dis. Aquat. Org. 1996, 27, 215–225. [Google Scholar] [CrossRef]
- Hossain, M.S.; Chakraborty, A.; Joseph, B.; Otta, S.K.; Karunasagar, I.; Karunasagar, I. Detection of new hosts for white spot syndrome virus of shrimp using nested polymerase chain reaction. Aquaculture 2001, 198, 1–11. [Google Scholar] [CrossRef]
- Lin, Y.; Yeh, S.; Li, C.; Chen, L.; Cheng, A.; Chen, J. An immersion of Gracilaria tenuistipitata extract improves the immunity and survival of white shrimp Litopenaeus vannamei challenged with white spot syndrome virus. Fish Shellfish Immunol. 2011, 31, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Sirirustananun, N.; Chen, J.; Lin, Y.; Yeh, S.; Liou, C.; Chen, L.; Sim, S.S.; Chiew, S.L. Dietary administration of a Gracilaria tenuistipitata extract enhances the immune response and resistance against Vibrio alginolyticus and white spot syndrome virus in the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2011, 31, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, S.; Manasi, K.; Vinodhini, S.; Vidhya, G.; Hemalatha, K.; Sudhakaran, R. Confirmation of Anti-WSSV activity from Red Algae Hypnae spinella in freshwater crab Paratelphusa hydrodomous. Int. J. ChemTech Res. 2014, 8, 4022–4026. [Google Scholar]
- Abreu, M.H.; Pereira, R.; Yarish, C.; Sousa-Pinto, A.H.B.A. IMTA with Gracilaria vermiculophylla: Productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 2011, 312, 77–87. [Google Scholar] [CrossRef]
- Shpigel, M.; Shauli, L.; Odintsov, V.; Ben-Ezra, D.; Neori, A.; Guttman, L. The sea urchin, Paracentrotus lividus, in an Integrated Multi-Trophic Aquaculture (IMTA) system with fish (Sparus aurata) and seaweed (Ulva lactuca): Nitrogen partitioning and proportional configurations. Aquaculture 2018, 260–269. [Google Scholar] [CrossRef]
- Kang, Y.H.; Shin, J.A.; Kim, M.S.; Chung, I.K. A preliminary study of the bioremediation potential of Codium fragile applied to seaweed integrated multi-trophic aquaculture (IMTA) during the summer. J. Appl. Phycol. 2008, 20, 183–190. [Google Scholar] [CrossRef]
- Sun, J.; Yu, H.; Chen, W. Records of benthic seaweeds in Zhejiang. J. Zhejiang Ocean Univ. (Nat. Sci.) 2006, 3, 312–321. [Google Scholar]
- Zhang, W.; Duan, X.; Huang, H.; Zhang, Y.; Wang, B. Evaluation of 28 marine algae from the Qingdao coast for antioxidative capacity and determination of antioxidant efficiency and total phenolic content of fractions and subfractions derived from Symphyocladia latiuscula (Rhodomelaceae). J. Appl. Phycol. 2007, 19, 97–108. [Google Scholar] [CrossRef]
- Alghazeer, R.; Whida, F.; Abduelrhman, E.; Gammoudi, F.; Azwai, S. Screening of antibacterial activity in marine green, red and brown macroalgae from the western coast of Libya. Nat. Sci. 2013, 5, 7–14. [Google Scholar] [CrossRef]
- Zaid, S.A.A.L.; Abdel-Wahab, K.S.E.D.; Nermine, A. Screening for Antiviral activities of aqueous extracts of some egyptian seaweeds. Egypt. J. Hosp. Med. 2016, 430–435. [Google Scholar] [CrossRef]
- Bouhlal, R.; Riadi, H.; Lopez, J.M.; Bourgougnon, N. The antibacterial potential of the Seaweeds (Rhodophyceae) of the Strait of Gibraltar and the Mediterranean Coast of Morocco. Afr. J. Biotechnol. 2010, 9, 6365–6372. [Google Scholar]
- Sun, B.; Quan, H.; Zhu, F. Dietary chitosan nanoparticles protect crayfish Procambarus clarkii against white spot syndrome virus (WSSV) infection. Fish Shellfish Immunol. 2016, 54, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wang, Z.; Wang, Z.; Ma, X.; Zhu, F. A proteomic study of hemocyte proteins from mud crab (Scylla paramamosain) infected with white spot syndrome virus (WSSV) or Vibrio alginolyticus. Front. Immunol. 2017, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, B.; Zhu, F. Epigallocatechin-3-gallate inhibit replication of white spot syndrome virus in Scylla paramamosain. Fish Shellfish Immunol. 2017, 67, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Levesque, R. SPSS Programming and Data Management: A Guide for SPSS and SAS Users; SPSS: Chicago, IL, USA, 2006. [Google Scholar]
- González Del Val, A.; Platas, G.; Basilio, A.; Cabello, A.; Gorrochategui, J.; Suay, I.; Vicente, F.; Portillo, E.; Jiménez Del Río, M.; Reina, G.G.; et al. Screening of antimicrobial activities in red, green and brown macroalgae from Gran Canaria (Canary Islands, Spain). Int. Microbiol. 2001, 4, 35. [Google Scholar] [PubMed]
- Etahiri, S.; Bultel-Poncé, V.; Elkouri, A.E.; Assobhei, O.; Zaoui, D.; Guyot, M. Antibacterial activities of marine algae from the atlantic coast of morocco. Mar. Life 2003, 13, 3–9. [Google Scholar]
- Morales, J.L.; Cantillo-Ciau, Z.O.; Sánchez-Molina, I.; Mena-Rejón, G.J. Screening of antibacterial and antifungal activities of six marine macroalgae from coasts of Yucatán Peninsula. Pharm. Biol. 2008, 44, 632–635. [Google Scholar] [CrossRef]
- Stirk, W.A.; Reinecke, D.L.; van Staden, J. Seasonal variation in antifungal, antibacterial and acetylcholinesterase activity in seven South African seaweeds. J. Appl. Phycol. 2007, 19, 271–276. [Google Scholar] [CrossRef]
- Lee, J.; Eom, S.; Lee, E.; Jung, Y.; Kim, H.; Jo, M.; Son, K.; Lee, H.; Kim, J.H.; Lee, M.; et al. In vitro antibacterial and synergistic effect of phlorotannins isolated from edible brown seaweed Eisenia bicyclis against acne-related bacteria. Algae 2014, 29, 47–55. [Google Scholar] [CrossRef]
- Ogasawara, K.; Yamada, K.; Hatsugai, N.; Imada, C.; Nishimura, M. Hexose oxidase-mediated hydrogen peroxide as a mechanism for the antibacterial activity in the red seaweed Ptilophora subcostata. PLoS ONE 2016, 11, e0149084. [Google Scholar] [CrossRef] [PubMed]
- Ward, F.M.E.; Deyab, M.A. Evaluation of antibacterial activity of the brown Seaweed Turbinaria ornata (Turner) J. Agardh from Egypt. J. Coast. Life Med. 2016, 4, 603–607. [Google Scholar] [CrossRef]
- Salvador, N.; Gómez Garreta, A.; Lavelli, L.; Ribera, M.A. Antimicrobial activity of Iberian macroalgae. Sci. Mar. 2007, 71, 101–114. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakano, T.; Hashimoto, M.; Kanekiyo, K.; Hayashi, T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008, 8, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lim, J.; Sohn, E.; Choi, Y.; Han, E. Growth-inhibitory effect of a fucoidan from brown seaweed Undaria pinnatifida on Plasmodium parasites. Parasitol. Res. 2009, 104, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Cen, Y.; Wang, L.; Ma, X.; Xu, S.; Zhang, M.; Wang, Y. Antivirus effects of polysaccharides from Sargassum fusiforme in vitro. Chin. J. Pathophysiol. 2004, 20, 765–768. [Google Scholar]
- Arts, J.A.J.; Taverne-Thiele, A.J.; Savelkoul, H.F.J.; Rombout, J.H.W.M. Haemocyte reactions in WSSV immersion infected Penaeus monodon. Fish Shellfish Immunol. 2007, 23, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Chen, J. The immunostimulatory effect of hot-water extract of Gracilaria tenuistipitata on the white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus. Fish Shellfish Immunol. 2005, 19, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Wongprasert, K.; Rudtanatip, T.; Praiboon, J. Immunostimulatory activity of sulfated galactans isolated from the red seaweed Gracilaria fisheri and development of resistance against white spot syndrome virus (WSSV) in shrimp. Fish Shellfish Immunol. 2014, 36, 52–60. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).