Effects of Forest Restoration on Soil Carbon, Nitrogen, Phosphorus, and Their Stoichiometry in Hunan, Southern China
Abstract
1. Introduction
2. Materials and Methods
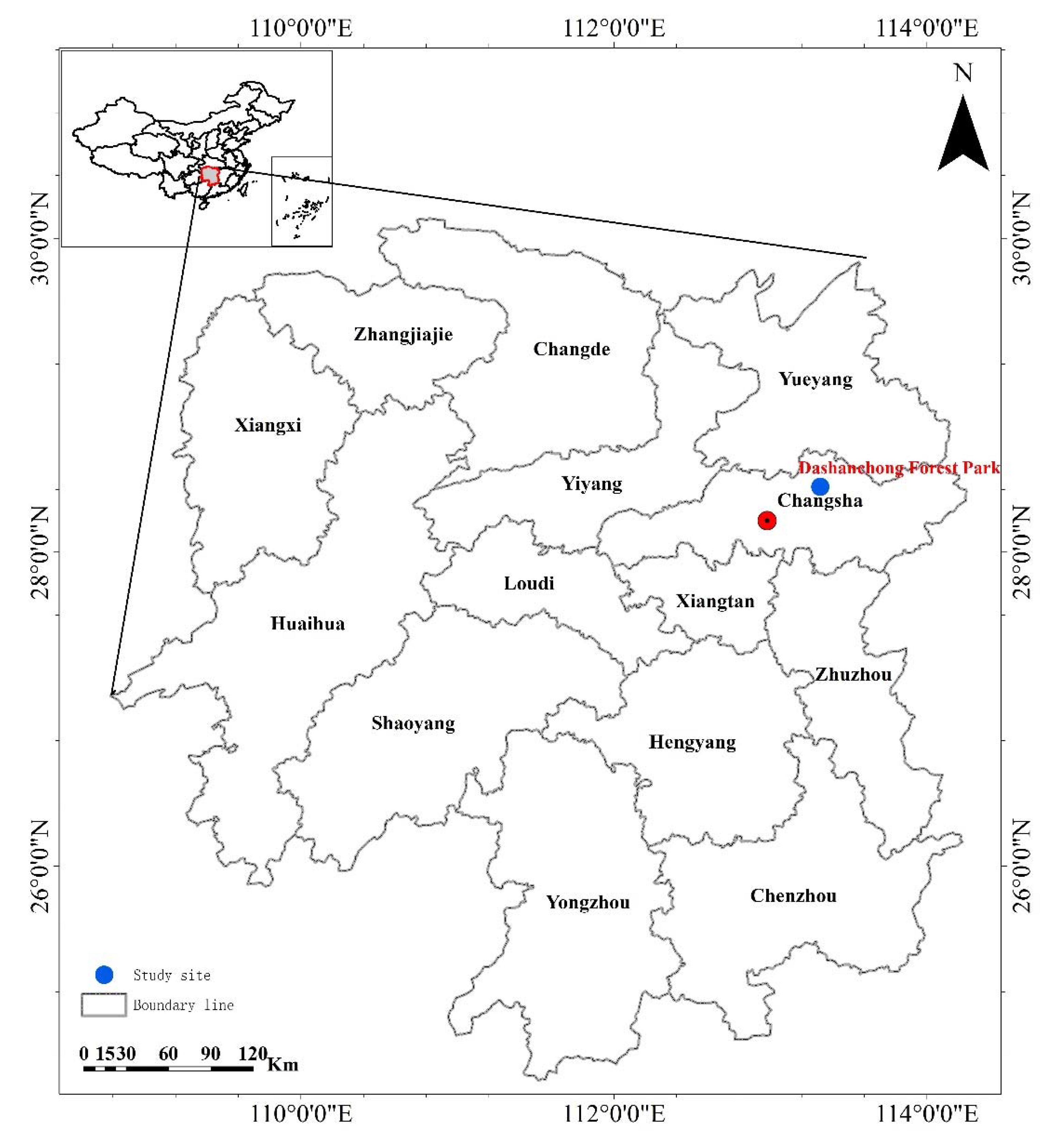
2.1. Description of Study Site
2.2. Field Soil Sampling and Laboratory Analysis
2.3. Data Analyses
3. Results
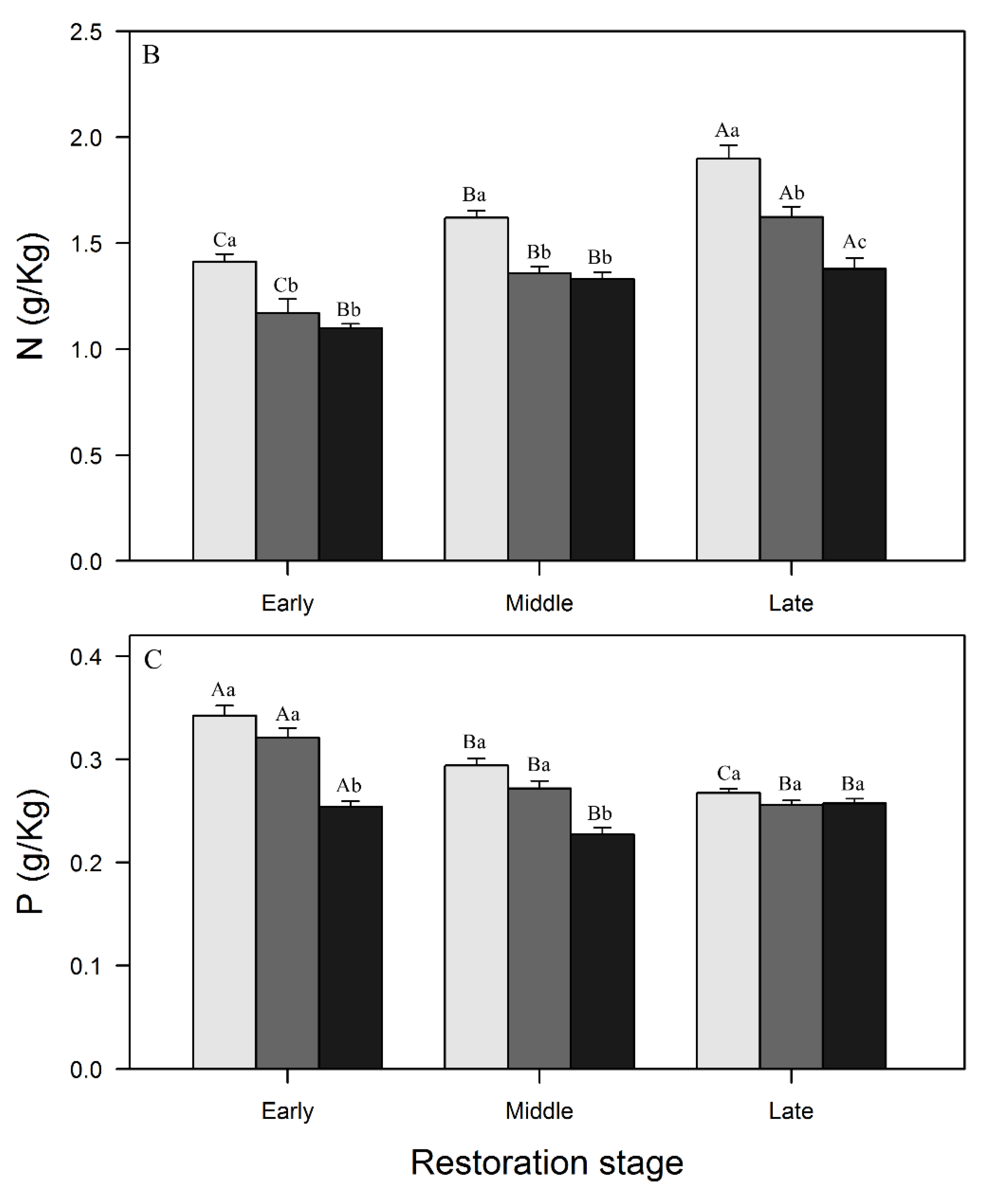
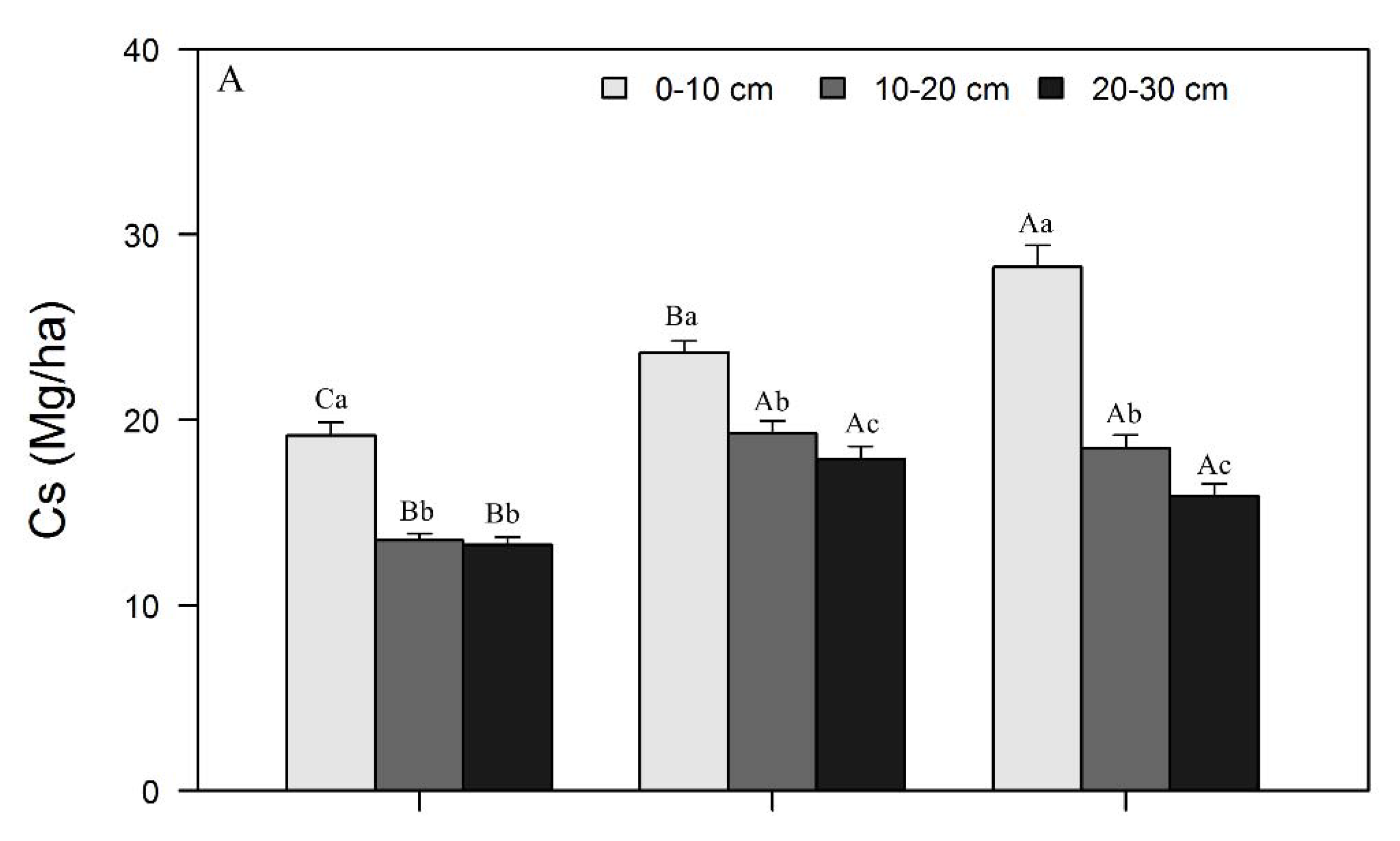
3.1. Variations in SOC, N, and P Concentrations and Stocks across Restoration Stages and Soil Depth
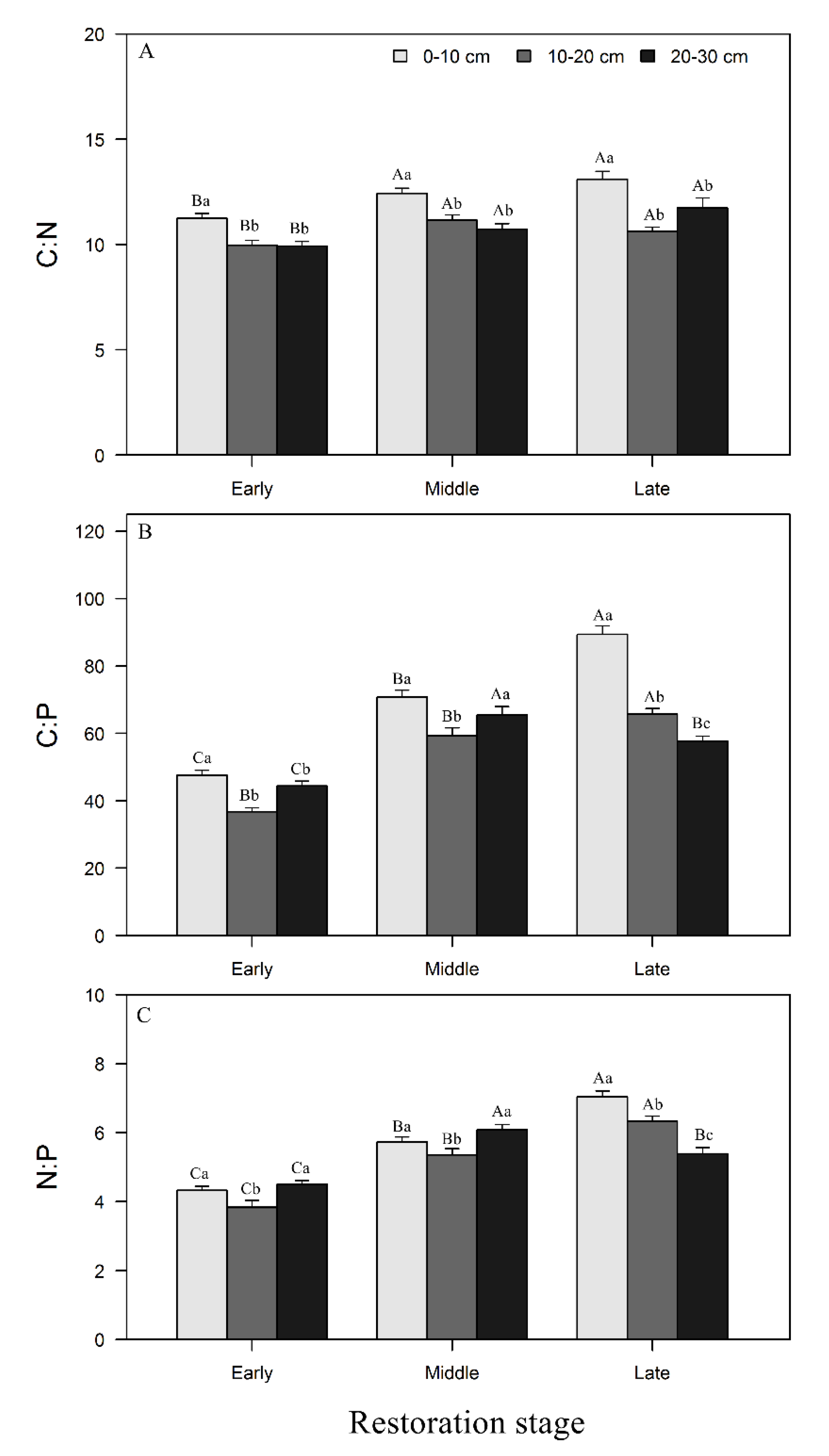
3.2. Changes in Soil Stoichiometry across Forest Restoration Stages and Soil Depth
4. Discussion
4.1. Changes in SOC, N, and P Concentrations and Stocks with Restoration and Soil Depth
4.2. Variation of Soil Stoichiometry with Forest Restoration and Soil Depth
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yang, Y.H.; Luo, Y.Q. Carbon: Nitrogen stoichiometry in forest ecosystems during stand development. Glob. Ecol. Biogeogr. 2011, 20, 354–361. [Google Scholar] [CrossRef]
- Hume, A.; Chen, H.Y.; Taylor, A.R.; Kayahara, G.J.; Man, R. Soil C:N:P dynamics during secondary succession following fire in the boreal forest of central Canada. For. Ecol. Manag. 2016, 369, 1–9. [Google Scholar] [CrossRef]
- Andrews, S.; Karlen, D.L.; Cambardella, C.A. The soil management assessment framework: A quantitative soil quality evaluation method. Soil Sci. Soc. Am. J. 2004, 68, 1945–1196. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Penuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Adhikari, K.; Jia, S.; Jin, X.; Liu, H. Spatial-temporal changes of soil organic carbon content in Wafangdian, China. Sustainability 2016, 8, 1154. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wang, X.; Niu, Y.; Lian, J. Improvements in soil carbon and nitrogen capacities after shrub planting to stabilize sand dunes in China’s horqin sandy land. Sustainability 2017, 9, 662. [Google Scholar] [CrossRef]
- Chang, R.Y.; Fu, B.J.; Liu, G.H.; Liu, S.G. Soil carbon sequestration potential for “Grain for Green” project in Loess Plateau, China. Environ. Manag. 2011, 48, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, K.B.; Chen, M.L.; Shangguan, Z.P.; Sweeney, S. Soil organic carbon storage capacity positively related to forest succession on the Loess Plateau, China. Catena 2013, 110, 1–7. [Google Scholar] [CrossRef]
- Wang, B.; Liu, G.B.; Xue, S.; Zhu, B.B. Changes in soil physic-chemical and microbiological properties during natural succession on abandoned farmland in the Loess Plateau. Environ. Earth Sci. 2011, 62, 91–925. [Google Scholar] [CrossRef]
- Chen, C.R.; Condron, L.M.; Davis, M.R.; Sherlock, R.R. Effects of afforestation on phosphorus dynamics and biological properties in a New Zealand grassland soil. Plant Soil 2000, 220, 151–163. [Google Scholar] [CrossRef]
- Wen, Z.M.; Jiao, F.; Liu, B.Y.; Bu, Y.J.; Jiao, J.Y. Natural vegetation succession and soil nutrient dynamics of abandoned farmlands in forest-steppe zone on Loess Plateau, China. J. Appl. Ecol. 2005, 16, 2025–2029. [Google Scholar]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tian, H.; Liu, J.; Wang, S.; Liu, M.; Pan, S.; Shi, X. Pools and distributions of soil phosphorus in China. Glob. Biogeochem. Cycles 2005, 19, GB1020. [Google Scholar] [CrossRef]
- Zeng, Y.L.; Fang, X.; Xiang, W.H.; Deng, X.W.; Peng, C.H. Stoichiometric and nutrient resorption characteristics of dominant tree species in subtropical Chinese forests. Ecol. Evol. 2017, 7, 11033–11043. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Farrington, H. Nutrient limitation and soil development: Experimental test of a biogeochemical theory. Biogeochemistry 1997, 37, 63–75. [Google Scholar] [CrossRef]
- Wardle, D.A.; Walker, L.R.; Bardgett, R.D. Ecosystem properties and forest decline in contrasting long–term chronosequences. Science 2004, 305, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, J.; Wang, Y.; Zhou, G.; Han, T.; Li, Y. Increasing phosphorus limitation along three successional forests in southern China. Plant Soil 2013, 364, 181–191. [Google Scholar] [CrossRef]
- Aponte, C.; Marañón, T.; García, L. Microbial, C, N and P in soils of Mediterranean oak forests: Influence of season, canopy cover and soil depth. Biogeochemistry 2010, 101, 77–92. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Liu, S.; Shen, J.; Huang, D.; Su, Y.; Syers, J.K. Is the C:N:P stoichiometry in soil and soil microbial biomass related to the landscape and land use in southern subtropical China? Glob. Biogeochem. Cycles 2012, 26. [Google Scholar] [CrossRef]
- Gao, Y.; He, N.P.; Yu, G.R.; Chen, W.L.; Wang, Q.F. Long-term effects of different land use types on C, N, and P stoichiometry and storage in subtropical ecosystems: A case study in China. Ecol. Eng. 2014, 67, 171–181. [Google Scholar] [CrossRef]
- Zhao, F.; Sun, J.; Ren, C.; Kang, D.; Deng, J.; Han, X.; Ren, G. Land use change influences soil C, N, and P stoichiometry under ‘Grain-to-Green Program’ in China. Sci. Rep. 2015, 5, 10195. [Google Scholar]
- Tian, H.; Chen, G.; Zhang, C.; Melillo, J.M.; Hall, C.A.S. Land use change influences soil C, N, and P stoichiometry under ‘Grain-to-Green Program’ in China. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Liu, W.; Su, J.; Li, S.; Zhang, Z.; Li, Z. Stoichiometry study of C, N and P in plant and soil at different successional stages of monsoon evergreen broad-leaved forest in Pu’er, Yunnan Province. Acta Ecol. Sin. 2010, 30, 6581–6590. (In Chinese) [Google Scholar]
- Jiao, F.; Wen, Z.M.; An, S.S.; Yuan, Z. Successional changes in soil stoichiometry after land abandonment in Loess Plateau, China. Ecol. Eng. 2013, 58, 249–254. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, M.; Huang, Z.; Lin, T.C.; Vadeboncoeur, M.A.; Searle, E.B.; Chen, H.Y. Temporal changes in soil C-N-P stoichiometry over the past 60 years across subtropical China. Glob. Chang. Biol. 2017, 13939, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Jia, X.X.; Wei, X.R.; Shao, M.A.; Li, X.Z. Distribution of soil carbon and nitrogen along a revegetational succession on the Loess Plateau of China. Catena 2012, 95, 160–168. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.B.; Shangguan, Z.P. Long-term natural succession improves nitrogen storage capacity of soil on the Loess Plateau, China. Soil Res. 2014, 52, 262–270. [Google Scholar] [CrossRef]
- Liu, S.J.; Zhang, W.; Wang, K.L.; Pan, F.J.; Yang, S.; Shu, S.Y. Factors controlling accumulation of soil organic carbon along vegetation succession in a typical karst region in Southwest China. Sci. Total. Environ. 2015, 521, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Yan, E.R.; Wang, X.H.; Guo, M.; Zhong, Q.; Zhou, W. C:N:P stoichiometry across evergreen broad-leaved forests, evergreen coniferous forests and deciduous broad-leaved forests in the Tiantong region, Zhejiang Province, eastern China. J. Plant Ecol. 2010, 34, 48–57. (In Chinese) [Google Scholar]
- Jiang, F.; Wu, X.; Xiang, W.H.; Fang, X.; Zeng, Y.L.; Ouyang, S.; Lei, P.F.; Deng, X.W.; Peng, C.H. Spatial variations in soil organic carbon, nitrogen and phosphorus concentrations related to stand characteristics in subtropical areas. Plant Soil 2017, 413, 289–301. [Google Scholar] [CrossRef]
- Ouyang, S.; Xiang, W.H.; Wang, X.P.; Zeng, Y.L.; Lei, P.F.; Deng, X.W.; Peng, C.H. Significant effects of biodiversity on forest biomass during the succession of subtropical forest in south China. For. Ecol. Manag. 2016, 372, 291–302. [Google Scholar] [CrossRef]
- Zhu, W.J.; Xiang, W.H.; Pan, Q.; Zeng, Y.L.; Ouyang, S.; Lei, P.F.; Deng, X.W.; Fang, X.; Peng, C.H. Spatial and seasonal variations of leaf area index (LAI) in subtropical secondary forests related to floristic composition and stand characters. Biogeosciences 2016, 13, 3819–3831. [Google Scholar] [CrossRef]
- IUSS Working Group. World Reference Base for Soil Resources 2006. In World Soil Resources Reports; No. 103; FAO: Rome, Italy, 2006; ISBN 92951055114. [Google Scholar]
- Jia, G.M.; Cao, J.; Wang, C.Y.; Wang, G. Microbial biomass and nutrients in soil at the different stages of secondary forest succession in Ziwulin, Northwest China. For. Ecol. Manag. 2005, 217, 117–125. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Ed.; Association of Canadian Search, Employment & Staffing Services: Madison, WI, USA, 1996. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1958. [Google Scholar]
- Institute of Soil Science, Chinese Academy of Sciences. Analytical Methods of Soil Physics and Chemistry; Shanghai Scientific and Technical Publishers: Shanghai, China, 1978. [Google Scholar]
- Li, C.; Zhao, L.; Sun, P.; Zhao, F.; Kang, D.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Deep Soil C, N, and P storage and stoichiometry in response to land use patterns in the Loess Hilly Region of China. PLoS ONE 2016, 11, e015907. [Google Scholar]
- Warton, D.I.; Duursma, R.A.; Falster, D.S.; Taskinen, S. SMART 3-an R package for estimation and inference about allometric lines. Methods Ecol. Evol. 2012, 3, 257–259. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Davis, M.; Nordmeyer, A.; Henley, D.; Watt, M. Ecosystem carbon accretion 10 years after afforestation of depleted subhumid grassland planted with three densities of Pinus nigra. Glob. Chang. Biol. 2007, 13, 1414–1422. [Google Scholar] [CrossRef]
- Hooper, D.U.; Vitousek, P.M. The effects of plant composition and diversity on ecosystem processes. Science 1997, 277, 1302–1305. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bonner, K.I.; Nicholson, K.S. Biodiversity and plant litter: Exper imental evidxence which does not support the view that enhanced species richness improves ecosystem function. Oikos 1997, 79, 247–258. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69. [Google Scholar] [CrossRef]
- Yang, L.Y.; Wu, S.T.; Zhang, L.B. Fine root biomass dynamics and carbon storage along a successional gradient in Changbai Mountains, China. Forestry 2010, 83, 379–387. [Google Scholar] [CrossRef]
- Guo, J.; Yu, L.H.; Fang, X.; Xiang, W.H.; Deng, X.W.; Lu, X. Litter production and turnover in four types of subtropical forests in China. Acta Ecol. Sin. 2015, 35, 4668–4677. (In Chinese) [Google Scholar]
- Liu, C.; Xiang, W.H.; Lei, P.F.; Deng, X.W.; Tian, D.L.; Fang, X.; Peng, C.H. Standing fine root mass and production in four Chinese subtropical forests along a succession and species diversity gradient. Plant Soil 2014, 376, 445–459. [Google Scholar] [CrossRef]
- Wu, J.G. The mineralization of soil organic carbon under different land uses in the Liupan mountain forest zone. J. Plant Ecol. 2004, 28, 530–538. (In Chinese) [Google Scholar]
- Justine, M.F.; Yang, W.; Wu, F.; Tan, B.; Naeem Khan, M.; Li, Z. Dissolved organic matter in soils varies across a chronosequence of Pinus massoniana plantations. Ecosphere 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Hooper, D.U.; Bignell, D.E.; Brown, V.K. Interactions between aboveground and belowground biodiversity in terrestrial ecosystems: Patterns, mechanisms and feedbacks. BioScience 2000, 50, 1049–1061. [Google Scholar] [CrossRef]
- Wei, X.; Shao, M.; Fu, X.; Horton, R.; Li, Y.; Zhang, X. Distribution of soil organic C, N and P in three adjacent land use patterns in the northern Loess Plateau, China. Biogeochemistry 2009, 96, 149–162. [Google Scholar] [CrossRef]
- Saikh, H.; Varadachari, C.; Ghosh, K. Changes in carbon, nitrogen and phosphorus levels due to deforestation and cultivation: A case study in Simlipal National Park, India. Plant Soil 1998, 198, 137–145. [Google Scholar] [CrossRef]
- Yimer, F.; Ledin, S.; Abdelkadir, A. Changes in soil organic carbon and total nitrogen contents in three adjacent land use types in the Bale Mountains, south-eastern highlands of Ethiopia. For. Ecol. Manag. 2007, 242, 337–342. [Google Scholar] [CrossRef]
- Paul, E.A. Soil Microbiology, Ecology, and Biochemistry, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Fan, H.; Wu, J.; Liu, W.; Yuan, Y.; Hu, L.; Cai, Q. Linkages of plant and soil C: N: P stoichiometry and their relationships to forest growth in subtropical plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]


| Forest Type | Altitude (m) | Aspect | Slope (o) | Stand Density (Tree ha−1) | Total BA (m−2 ha−1) | Dominant Species |
|---|---|---|---|---|---|---|
| Early (PM-LG) | 220–262 | SW | 15 | 2492 | 33.66 | Pinus massoniana Lithocarpus glaber Diospyros glaucifolia |
| Middle (CA) | 245–321 | W | 35 | 1696 | 18.79 | Choerospondiasaxillaris Loropetalum chinensis Symplocos setchuensis |
| Late (LG-CG) | 225–254 | NW | 22 | 1340 | 23.10 | Lithocarpus glaber Cyclobalanopsis glauca Sassafras tzumu |
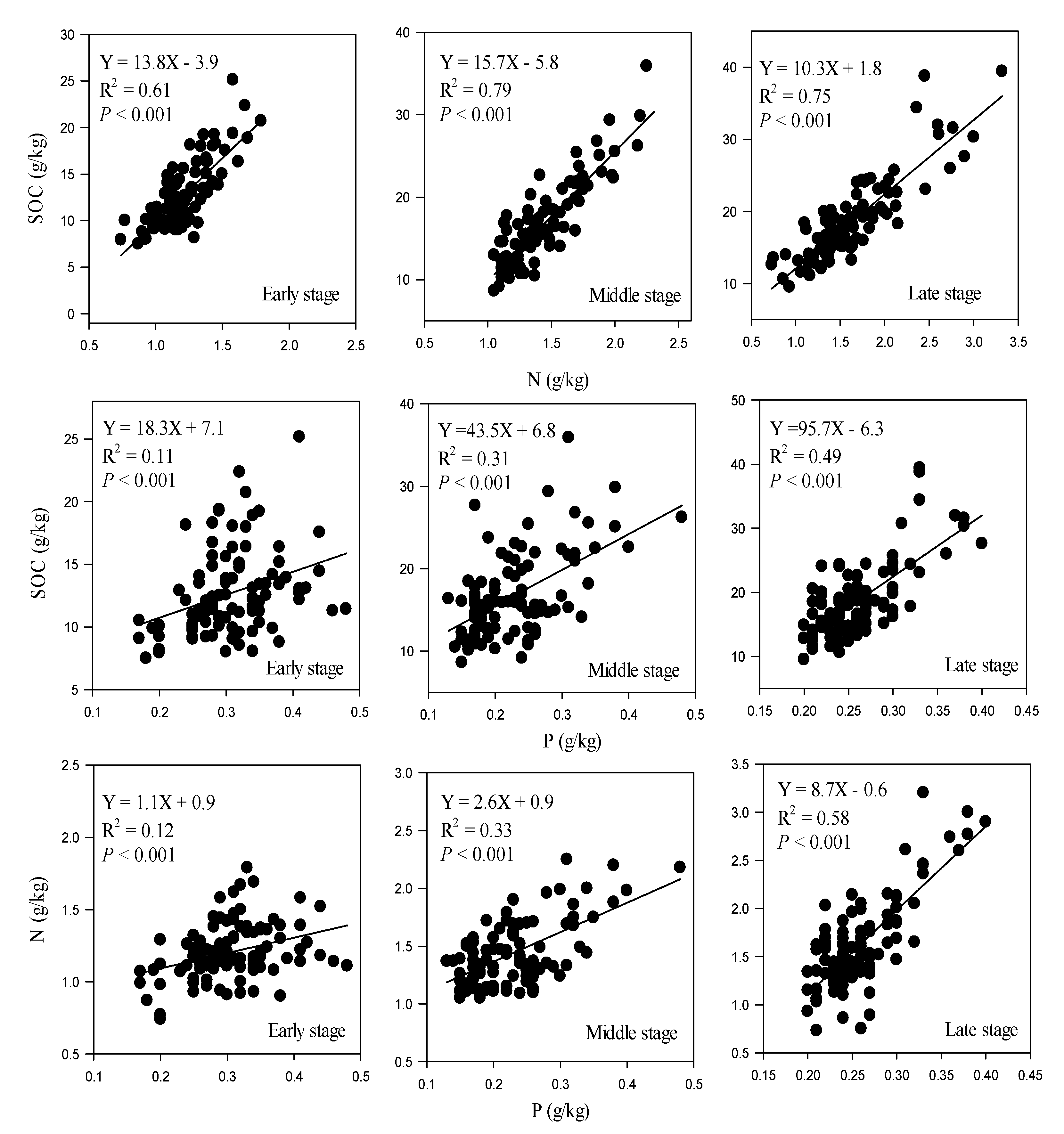
| Soil Stoichiometry | Restoration Stage | n | Slope [95% CI] | p | Intercept [95% CI] | p |
|---|---|---|---|---|---|---|
| C:N | Early | 100 | 13.8 b [11.5, 16.0] | <0.01 | −3.9 a,b [−6.6, −1.2] | 0.03 |
| Middle | 100 | 15.7 a [14.3, 17.7] | −5.8 b [−8.7, −3.8] | |||
| Late | 100 | 10.3 c [9.1, 11.5] | 1.8 a [−0.2, 3.8] | |||
| C:P | Early | 100 | 18.3 c [8.1, 28.4] | <0.01 | 7.1 a [3.9, 10.2] | <0.01 |
| Middle | 100 | 43.5 b [30.3, 56.6] | 6.8 b [3.7, 9.9] | |||
| Late | 100 | 95.7 a [76.4, 114.9] | −6.3 c [−11.3, −1.2] | |||
| N:P | Early | 100 | 1.1 c [0.48, 1.6] | <0.01 | 0.9 a [0.7, 1.1] | <0.01 |
| Middle | 100 | 2.6 b [2.0, 3.34] | 0.9 a [0.6, 1.0] | |||
| Late | 100 | 8.7 a [7.3, 10.2] | −0.6 b [−1.0, −0.2] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Xiang, W.; Gou, M.; Chen, L.; Lei, P.; Fang, X.; Deng, X.; Ouyang, S. Effects of Forest Restoration on Soil Carbon, Nitrogen, Phosphorus, and Their Stoichiometry in Hunan, Southern China. Sustainability 2018, 10, 1874. https://doi.org/10.3390/su10061874
Xu C, Xiang W, Gou M, Chen L, Lei P, Fang X, Deng X, Ouyang S. Effects of Forest Restoration on Soil Carbon, Nitrogen, Phosphorus, and Their Stoichiometry in Hunan, Southern China. Sustainability. 2018; 10(6):1874. https://doi.org/10.3390/su10061874
Chicago/Turabian StyleXu, Chuanhong, Wenhua Xiang, Mengmeng Gou, Liang Chen, Pifeng Lei, Xi Fang, Xiangwen Deng, and Shuai Ouyang. 2018. "Effects of Forest Restoration on Soil Carbon, Nitrogen, Phosphorus, and Their Stoichiometry in Hunan, Southern China" Sustainability 10, no. 6: 1874. https://doi.org/10.3390/su10061874
APA StyleXu, C., Xiang, W., Gou, M., Chen, L., Lei, P., Fang, X., Deng, X., & Ouyang, S. (2018). Effects of Forest Restoration on Soil Carbon, Nitrogen, Phosphorus, and Their Stoichiometry in Hunan, Southern China. Sustainability, 10(6), 1874. https://doi.org/10.3390/su10061874





