Model-Based Evaluation of Urban River Restoration: Conflicts between Sensitive Fish Species and Recreational Users
Abstract
:1. Introduction
- Current evaluation procedures investigate ecological responses, but social evaluation remains lacking [9,36,41]. Few studies have performed parallel investigations into both social and biological aspects, e.g., water quality and project acceptance, but without the integration of both aspects into a single evaluation method, the resulting conclusions remain speculative.
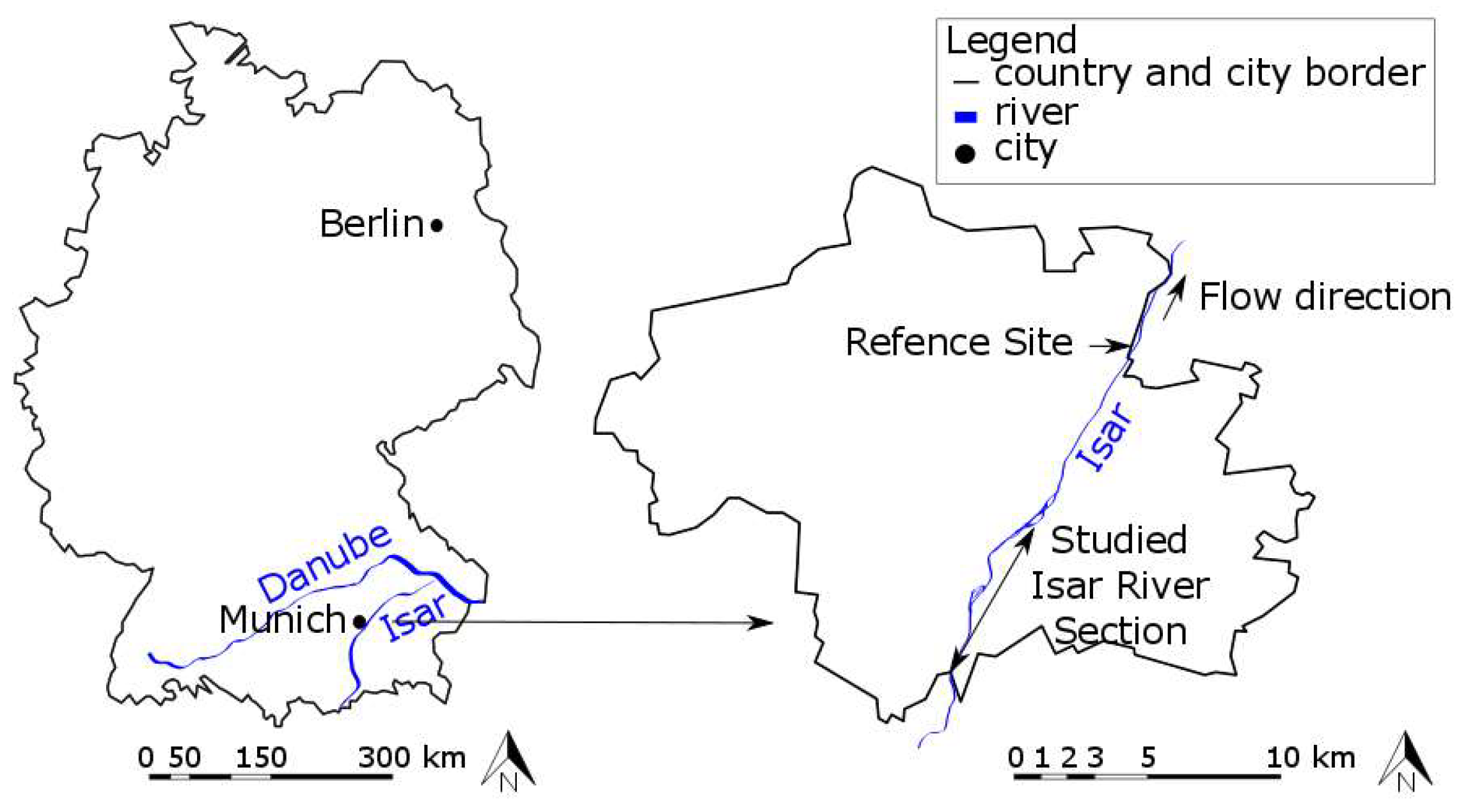
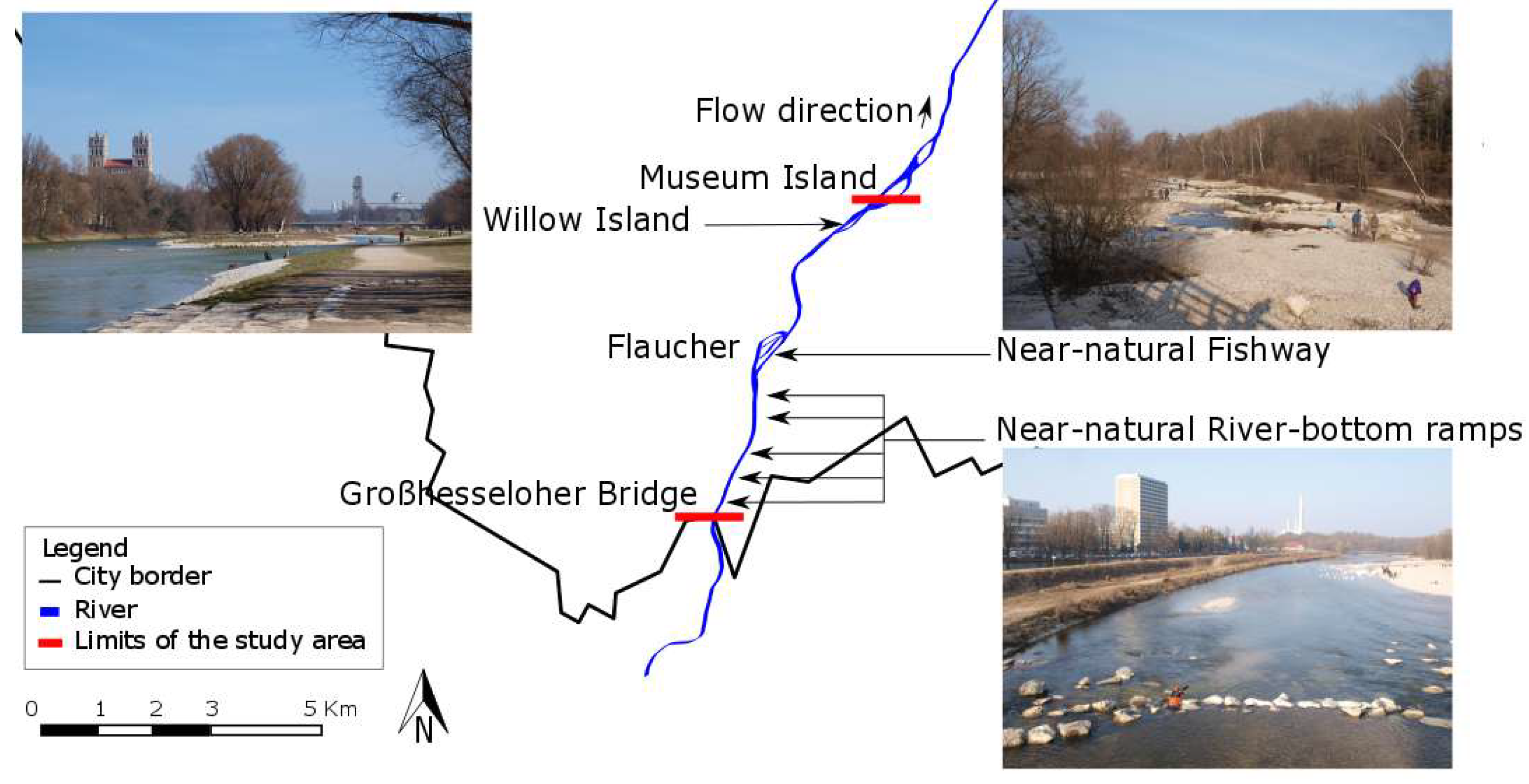
2. Study Area
2.1. Study Area
2.2. Biology of Chondrostoma nasus
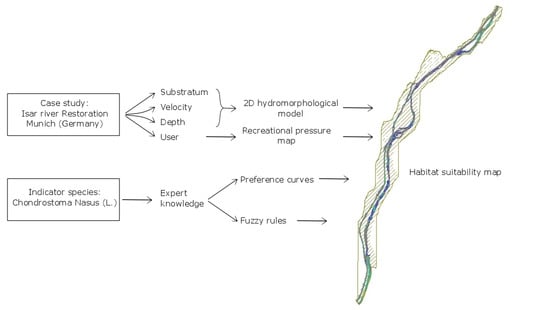
3. Materials and Methods
3.3. Physical Characteristics of the River
3.3.1. The Substrate
- Organic matter or detritus
- Silt, clay, or loam
- Sand (<2 mm)
- Fine gravel (2–6 mm)
- Medium gravel (6–20 mm)
- Large gravel (2–6 cm)
- Large stones (6–12 cm)
- Boulders (>20 cm)
- Rock or concrete.
3.3.2. The 2D Hydro-Morphological Model
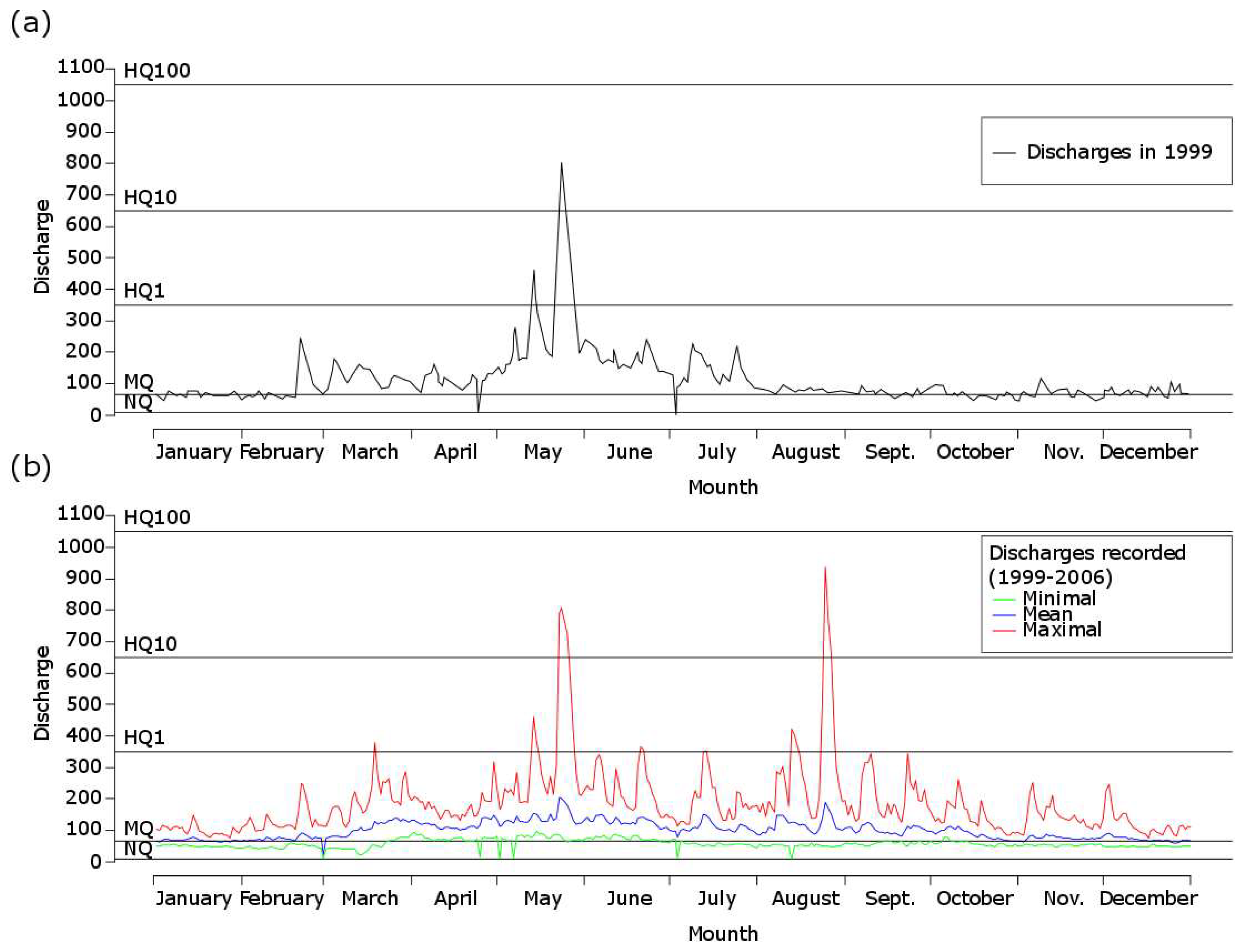
- Minimum discharge (NQ = 12 m³/s)
- Mean low discharge (MNQ = 16.5 m³/s)
- Annual mean discharge (MQ = 63.8 m³/s)
- Annual mean maximum discharge (HQ1 = 350 m³/s)
- Biennial mean maximum discharge (HQ2 = 405 m³/s)
- 5-year maximum discharge (HQ5 = 550 m³/s)
- 10-year maximum discharge (HQ10 = 650 m³/s)
- 50-year maximum discharge (HQ50 = 880 m³/s)
- 100-year maximum discharge (HQ100 = 1050 m³/s).
3.4. Recreational Pressure
3.5. Habitat Suitability Model
3.6. Model Analysis
4. Results
4.1. Physical Habitat Suitability
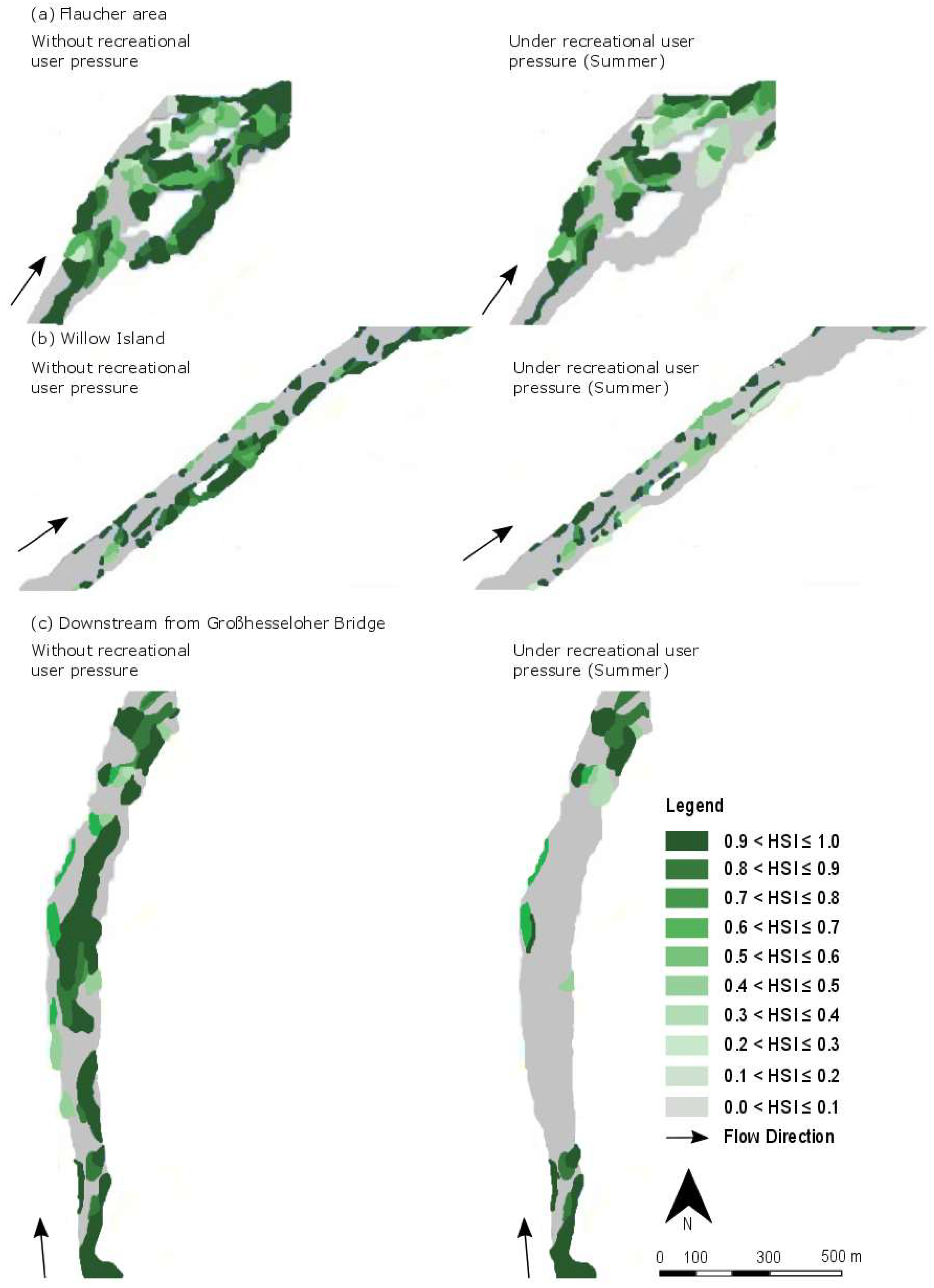
4.2. Spatial Distribution of Suitable Habitats
4.3. Spatial and Temporal Distribution of Recreational Pressure
4.4. Influence of Recreational Users on Availability of Habitats for C. nasus
5. Discussion
5.1. Physical Habitat Suitability
5.2. Conflicts between Wildlife and Recreational User Pressure
5.3. Benefits of Integrating User Pressure into the Physical Habitat Model
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The SER International Primer on Ecological Restoration (SER). Tucson: Society for Ecological Restoration International. 2004. Available online: www.ser.org (accessed on 15 May 2014).
- Wantzen, K.M.; Ballouche, A.; Longuet, I.; Bao, I.; Bocoum, H.; Cissé, L.; Chauhan, M.; Girard, P.; Gopal, B.; Kane, A.; et al. River Culture: An eco-social approach to mitigate the biological and cultural diversity crisis in riverscapes. Ecohydrol. Hydrobiol. 2016, 16, 7–18. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Current State and Trends; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Palmer, M.; Bernhardt, E.S. Hydroecology and river restoration: Ripe for research and synthesis. Water Resour. Res. 2006, 42, 1–4. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Palmer, M.A.; Allan, J.D.; Alexander, G.; Barnas, K.; Brooks, S.; Carr, J.; Clayton, S.; Dahm, C.; Follstad-Shah, J.; et al. Ecology-Synthesizing US river restoration efforts. Science 2005, 308, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.S.; Lake, P.S. River restoration in Victoria, Australia: Change is in the wind, and none too soon. Restor. Ecol. 2007, 15, 584–591. [Google Scholar] [CrossRef]
- Nakamura, K.; Tockner, K.; Amano, K. River and wetland restoration: Lessons from Japan. Bioscience 2006, 56, 419–429. [Google Scholar] [CrossRef]
- Morandi, B.; Piégay, H.; Lamouroux, N.; Vaudor, L. How is success or failure in river restoration projects evaluated? Feedback from French restoration projects. J. Environ. Manag. 2014, 137, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Leps, M.; Sundermann, A.; Tonkin, J.D.; Lorenz, A.W.; Haase, P. Time is no healer: Increasing restoration age does not lead to improved benthic invertebrate communities in restored river reaches. Sci. Total Environ. 2016, 557, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Zingraff-Hamed, A.; Greulich, S.; Pauleit, S.; Wantzen, K.M. Urban and rural river restoration in France: A typology. Restor. Ecol. 2017, 25, 994–1004. [Google Scholar] [CrossRef]
- Haase, P.; Hering, D.; Jähnig, S.C.; Lorenz, A.W.; Sundermann, A. The impact of hydromorphological restoration on river ecological status: A comparison of fish, benthic invertebrates, and macrophytes. Hydrobiologia 2013, 704, 475–488. [Google Scholar] [CrossRef]
- Kail, J.; Brabec, K.; Poppe, M.; Januschke, K. The effect of river restoration on fish, macroinvertebrates and aquatic macrophytes: A meta-analysis. Ecol. Indic. 2015, 58, 311–321. [Google Scholar] [CrossRef]
- Lepori, F.; Palm, D.; Brännäs, E.; Malmqvist, B. Does restoration of structural heterogeneity in streams enhance fish and macroinvertebrate diversity? Ecol. Appl. 2005, 15, 2060–2071. [Google Scholar] [CrossRef]
- Woolsey, S.; Capelli, F.; Gonser, T.; Hoehn, E.; Hostmann, M.; Junker, B.; Paetzold, A.; Roulier, C.; schweizer, S.; Tiegs, S.D.; et al. A strategy to assess river restoration success. Freshw. Biol. 2007, 52, 752–769. [Google Scholar] [CrossRef]
- Meyer, J.L.; Paul, M.J.; Taulbee, W.K. Stream ecosystem function in urbanizing landscapes. J. N. Am. Benthol. Soc. 2005, 24, 602–612. [Google Scholar] [CrossRef]
- Fletcher, T.D.; Andrieu, H.; Hamel, P. Understanding, management and modeling of urban hydrology and its consequences for receiving waters: A state of the art. Adv. Water Resour. 2013, 51, 261–279. [Google Scholar] [CrossRef]
- Walsh, C.J.; Fletcher, T.D.; Ladson, A.R. Stream restoration in urban catchments through redesigning stormwater systems: Looking to the catchment to save the stream. J. N. Am. Benthol. Soc. 2005, 24, 690–705. [Google Scholar] [CrossRef]
- Kondolf, G.M.; Pinto, P.J. The social connectivity of urban rivers. Geomorphology 2016, 277, 182–196. [Google Scholar] [CrossRef]
- Zingraff-Hamed, A.; Greulich, S.; Wantzen, K.M.; Pauleit, S. Societal Drivers of European Water Governance: A Comparison of Urban River Restoration Practices in France and Germany. Water 2017, 9, 206. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Palmer, M.A. Restoring streams in an urbanizing world. Freshw. Biol. 2007, 52, 738–751. [Google Scholar] [CrossRef]
- Vermaat, J.E.; Wagtendonk, A.J.; Brouwer, R.; Sheremet, O.; Ansink, E.; Brockhoff, T.; Plug, M.; Hellsten, S.; Aroviita, J.; Tylec, L.; et al. Assessing the societal benefits of river restoration using the ecosystem services approach. Hydrobiologia 2016, 769, 121–135. [Google Scholar] [CrossRef]
- Junker, B.; Buchecker, M. Aesthetic preferences versus ecological objectives in river restorations. Landsc. Urban Plan. 2008, 85, 141–154. [Google Scholar] [CrossRef]
- Flather, C.H.; Ken Cordell, H. Outdoor Recreation: Historical and Anticipated Trends. In Wildlife and Recreationists: Coexistence through Management and Research; Knight, R.L., Gutzwiller, K.J., Eds.; Island Press: Washington, DC, USA, 1995; pp. 3–17. [Google Scholar]
- Bennett, V.J.; Quinn, V.S.; Zollner, P.A. Exploring the implications of recreational disturbance on an endangered butterfly using a novel modelling approach. Biodivers. Conserv. 2013, 22, 1783–1798. [Google Scholar] [CrossRef]
- Huhta, E.; Sulkava, P. The Impact of Nature-Based Tourism on Bird Communities: A Case Study in Pallas-Yllastunturi National Park. Environ. Manag. 2014, 53, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Kangas, K.; Luoto, M.; Ihantola, A.; Tomppo, E.; Siikamaki, P. Recreation-induced changes in boreal bird communities in protected areas. Ecol. Appl. 2010, 20, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Pineiro, A.; Barja, I.; Silvan, G.; Illera, J.C. Effects of tourist pressure and reproduction on physiological stress response in wildcats: Management implications for species conservation. Wildl. Res. 2012, 39, 532–539. [Google Scholar] [CrossRef]
- Sarah, P.; Zhevelev, H.M. Effect of visitors’ pressure on soil and vegetation in several different micro-environments in urban parks in Tel Aviv. Landsc. Urban Plan. 2007, 83, 284–293. [Google Scholar] [CrossRef]
- Olive, N.D.; Marion, J.L. The influence of use-related, environmental, and managerial factors on soil loss from recreational trails. J. Environ. Manag. 2009, 90, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Wood, D. Resolving the conflicts of the impacts of recreation on a waterway. In IWA Technical Restoration Handbook; Trust, C.R., Ed.; Canal & River Trust: Milton Keynes, UK, 2015; p. 14. [Google Scholar]
- Aberg, E.U.; Tapsell, S. Revisiting the River Skerne: The long-term social benefits of river rehabilitation. Landsc. Urban Plan. 2013, 113, 94–103. [Google Scholar] [CrossRef]
- Bessa, F.; Scapini, F.; Cabrini, T.M.B.; Cardoso, R.S. Behavioural responses of talitrid amphipods to recreational pressures on oceanic tropical beaches with contrasting extension. J. Exp. Mar. Biol. Ecol. 2017, 486, 170–177. [Google Scholar] [CrossRef]
- Riera, R.; Menci, C.; Sanabria-Fernandez, J.A.; Becerro, M.A. Do recreational activities affect coastal biodiversity? Estuar. Coast. Shelf Sci. 2016, 178, 129–136. [Google Scholar] [CrossRef]
- Hayes, C.T.; Baumbach, D.S.; Juma, D.; Dunbar, S.G. Impacts of recreational diving on hawksbill sea turtle (Eretmochelys imbricata) behaviour in a marine protected area. J. Sustain. Tour. 2017, 25, 79–95. [Google Scholar] [CrossRef]
- Pander, J.; Geist, J. Ecological indicators for stream restoration success. Ecol. Indic. 2013, 30, 106–118. [Google Scholar] [CrossRef]
- Bouleau, G.; Pont, D. Did you say reference conditions? Ecological and socio-economic perspectives on the European Water Framework Directive. Environ. Sci. Policy 2015, 47, 32–41. [Google Scholar] [CrossRef]
- Friberg, N.; Bonada, N.; Bradley, D.C.; Dunbar, M.J.; Edwards, F.K.; Grey, J.; Hayes, R.B.; Hildrew, A.G.; Lamouroux, N.; Trimmer, M.; et al. Biomonitoring of Human Impacts in Freshwater Ecosystems: The Goof, the Bad and the Ugly; Woodward, G.: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Niemi, G.J.; McDonald, M.E. Application of ecological indicators. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 89–111. [Google Scholar] [CrossRef]
- Chiari, S.; Muhar, S.; Muhar, A. Linking ecological and social aspects of river restoration. In Proceedings of the 4th International River Restoration Conference of the European Centre for River Restoration (ECRR), Venice, Italy, 16–21 June 2008. [Google Scholar]
- Rogers, K.; Biggs, H. Integrating indicators, endpoints and value systems in strategic management of rivers of the Kruger National Park. Freshw. Biol. 1999, 41, 439–451. [Google Scholar] [CrossRef]
- Boavida, I.; Santos, J.M.; Ferreira, T.; Pinheiro, A. Barbel habitat alterations due to hydropeaking. J. Hydro-Environ. Res. 2015, 9, 237–247. [Google Scholar] [CrossRef]
- Pisaturo, G.R.; Righetti, M.; Dumbser, M.; Noack, M.; Schneider, M.; Cavedon, V. The role of 3D-hydraulics in habitat modelling of hydropeaking events. Sci. Total Environ. 2017, 575, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, P.; Leitner, P.; Habersack, H.; Graf, W.; Hauer, C. Evaluation of hydropeaking impacts on the food web in alpine streams based on modelling of fish- and macroinvertebrate habitats. Sci. Total Environ. 2017, 575, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Im, D.; Kang, H.; Kim, K.-H.; Choi, S.-U. Changes of river morphology and physical fish habitat following weir removal. Ecol. Eng. 2011, 37, 883–892. [Google Scholar] [CrossRef]
- Shih, S.-S.; Lee, H.-Y.; Chen, C.-C. Model-based evaluations of spur dikes for fish habitat improvement: A case study of endemic species Varicorhinus barbatulus (Cyprinidae) and Hemimyzon formosanum (Homalopteridae) in Lanyang River, Taiwan. Ecol. Eng. 2008, 34, 127–136. [Google Scholar] [CrossRef]
- Lange, C.; Schneider, M.; Mutz, M.; Haustein, M.; Halle, M.; Seidel, M.; Sieker, H.; Wolter, C.; Hinkelmann, R. Model-based design for restoration of a small urban river. J. Hydro-Environ. Res. 2015, 9, 226–236. [Google Scholar] [CrossRef]
- Bovee, K.D. A Guide to Stream Habitat Analysis Using the Instream Flow Invremental Methodology; National Technical Information Service: Springfield, VA, USA, 1982; Volume 273.
- Jorde, K.; Schneider, M.; Zoellner, F. Analysis of instream habitat quality—Preference functions and fuzzy models. In Stochastic Hydraulics; Wang, Z., Hu, S., Eds.; CRC Press: Rotterdam, The Netherland, 2000; pp. 671–680. [Google Scholar]
- Schneider, M. Habitat- und Abflussmodellierung für Fließgewässer mit unscharfen Berechnungsansätzen. In Hydraulic Engineering; University of Stuttgart: Stuttgart, Germany, 2001; p. 158. [Google Scholar]
- Adriaenssens, V.; Baets, B.D.; Goethals, P.L.M.; Pauw, N.D. Fuzzy rule-based models for decision support in ecosystem management. Sci. Total Environ. 2004, 319, 1–12. [Google Scholar] [CrossRef]
- Mouton, A.M.; De Baets, B.; Goethals, P.L.M. Knowledge-based versus data-driven fuzzy habitat suitability models for river management. Environ. Model. Softw. 2009, 24, 982–993. [Google Scholar] [CrossRef]
- Boavida, I.; Dias, V.; Ferreira, M.T.; Santos, J.M. Univariate functions versus fuzzy logic: Implications for fish habitat modeling. Ecol. Eng. 2014, 71, 533–538. [Google Scholar] [CrossRef]
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.; van de Bund, W.; Zampoukas, N.; Hering, D. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- Feld, C.K.; da Silva, P.M.; Sousa, J.P.; de Bello, F.; Bugter, R.; Grandin, U.; Hering, D.; Lavorel, S.; Mountford, O.; Pardo, I.; et al. Indicators of biodiversity and ecosystem services: A synthesis across ecosystems and spatial scales. Oikos 2009, 118, 1862–1871. [Google Scholar] [CrossRef]
- Mouton, A.; Schneider, M.; Depestele, J.; Goethals, P.; DePauw, N. Fish habitat modelling as a tool for river management. Ecol. Eng. 2007, 29, 305–315. [Google Scholar] [CrossRef]
- Mouton, A.M.; Schneider, M.; Peter, A.; Holzer, G.; Müller, R.; Goethals, P.L.M.; De Pauw, N. Optimisation of a fuzzy physical habitat model for spawning European grayling (Thymallus thymallus L.) in the Aare river (Thun, Switzerland). Ecol. Model. 2008, 215, 122–132. [Google Scholar] [CrossRef]
- Bagocius, D. Piling underwater noise impact on migrating salmon fish during Lithuanian LNG terminal construction (Curonian Lagoon, Eastern Baltic Sea Coast). Mar. Pollut. Bull. 2015, 92, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.E.; Johnston, C.E. Traffic noise masks acoustic signals of freshwater stream fish. Biol. Conserv. 2015, 187, 27–33. [Google Scholar] [CrossRef]
- Roberts, L.; Cheesman, S.; Hawkins, A.D. Effects of Sound on the Behavior of Wild, Unrestrained Fish Schools. In Effects of Noise on Aquatic Life; Popper, A.N., Hawkins, A., Eds.; Springer: Berlin, Germnay, 2016; pp. 917–924. [Google Scholar]
- Nedelec, S.L.; Simpson, S.D.; Morley, E.L.; Nedelec, B.; Radford, A.N. Impacts of regular and random noise on the behaviour, growth and development of larval Atlantic cod (Gadus morhua). Proc. R. Soc. B Biol. Sci. 2015, 282, 7. [Google Scholar] [CrossRef] [PubMed]
- Laub, B.G.; Baker, D.W.; Bledsoe, B.P.; Palmer, M.A. Range of variability of channel complexity in urban, restored and forested reference streams. Freshw. Biol. 2012, 57, 1076–1095. [Google Scholar] [CrossRef]
- Pottgiesser, T.; Rehfeld-Klein, M. Gewässerentwicklungskonzept für ein urbanes Gewässer zur Zielerreichung der Wasserrahmenrichtlinie—Das Pilotprojekt Panke in Berlin. Limnol. Aktuell 2011, 13, 59–81. [Google Scholar]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat, Cornol and Freyhof: Berlin, Germnay, 2007. [Google Scholar]
- Reinartz, R. Untersuchungen zur Gefährdungssituation der Fishart Nase (Chondrostoma nasus L.) in bayerischen gewäsern. In Institut für Tierwissenschaften; Technical University of Munich: Munich, Germnay, 1997; p. 379. [Google Scholar]
- Pander, J.; Mueller, M.; Geist, J. Succession of fish diversity after reconnecting a large floodplain to the upper Danube River. Ecol. Eng. 2015, 75, 41–50. [Google Scholar] [CrossRef]
- Bruslé, J.; Quignard, J.-P. Biologie des Poissons D’eau Douce Européens; Lavoisier Tec & Doc: Cachan, France, 2001. [Google Scholar]
- Hauer, C.; Unfer, G.; Schmutz, S.; Habersack, H. Morphodynamic effects on the habitat of juvenile cyprinids (Chondrostoma nasus) in a restored Austrian lowland river. Environ. Manag. 2008, 42, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Hennel, R. Untersuchungen zur Bestandssituation der Fischfauna der Mittleren Isar; TU Munich: Munich, Germany, 1991; p. 222. [Google Scholar]
- Keckeis, H.; Winkler, G.; Flore, L.; Reckendorfer, W.; Schiemer, F. Spatial and seasonal characteristics of O+ fish nursery habitats of nase, Chondrostoma nasus in the River Danube, Austria. Folia Zool. 1997, 46, 133–150. [Google Scholar]
- Keith, P.; Persat, H.; Feunteun, E.; Allardi, J. Les Poissons D’eau Douce en France; Publication Scientifique du Musée: Paris, France, 2011. [Google Scholar]
- Lelek, A.; Peñáz, M. Spawning of Chondrostoma nasus (L.) in the Brumovka River. Folia Zool. 1963, 12, 121–134. [Google Scholar]
- Maier, K.J. On the nase, Chondrostoma nasus spawning area situation in Switzerland. Folia Zool. 1997, 46, 79–88. [Google Scholar]
- Żarski, D.; Targońska, K.; Ratajski, S.; Kaczkowski, Z.; Kucharczy, D. Reproduction of Nase, Chondrostoma Nasus (L.), Under Controlled Conditions. Arch. Pol. Fish. 2008, 16, 355–362. [Google Scholar]
- Freyhof, J. Chondrostoma nasus. In The IUCN Red List of Threatened Species 2011; IUCN: Gland VD, Switzerland, 2011. [Google Scholar]
- European Commission (EC). Convention on the Conservation of European Wildlife and Natural Habitats; European Commission (EC): Brussel, Belgium, 1979. [Google Scholar]
- Schubert, M.; Klein, M.; Leuner, E.; Kraus, G.; Wendt, P.; Born, O.; Hoch, J.; Ring, T.; Silkenat, W.; Speierl, T.; et al. Fischzustandsbericht 2012; LfL-Information: Freising, Germnay, 2012; Volume 54. [Google Scholar]
- Chovanec, A.; Schiemer, F.; Waidbacher, H.; Spolwind, R. Rehabilitation of a Heavily Modified River Section of the Danube in Vienna (Austria): Biological Assessment of Landscape Linkages on Different Scales. Int. Rev. Hydrobiol. 2002, 87, 183–195. [Google Scholar] [CrossRef]
- Dung, N.V.; Merz, B.; Bárdossy, A.; Thang, T.D.; Apel, H. Multi-objective automatic calibration of hydrodynamic models utilizing inundation maps and gauge data. Hydrol. Earth Syst. Sci. 2011, 15, 1339–1354. [Google Scholar] [CrossRef] [Green Version]
- Stalnaker, C.B.; Lamb, B.L.; Henriksen, J.; Bovee, K.; Bartholow, J. The Instream Flow Incremental Methodology: A Primer for IFIM; National Biological Service Midcontinent Ecological Science Center: Fort Collins, CO, USA, 1995; pp. 1–53.
- Lupp, G.; Förster, B.; Kantelberg, V.; Markmann, T.; Naumann, J.; Honert, C.; Koch, M.; Pauleit, S. Assessing the Recreation Value of Urban Woodland Using the Ecosystem Service Approach in Two Forests in the Munich Metropolitan Region. Sustainability 2016, 8, 1156. [Google Scholar] [CrossRef]
- Clivaz, C.; Rupf, R.; Siegrist, D. VISIMAN: Beiträge zu Besuchermonitoring und Besuchermanagement in Pärken und Naturnahen Erholungsgebieten; HSR Hochschule für Technik Rapperswil: Rapperswil, Switzerland, 2013. [Google Scholar]
- Rupf, R.; Wernli, M. Besuchermonitoring—Ein überblick über methoden und anwendungsbereiche. In VISIMAN. Beiträge zu Besuchermonitoring und Besuchermanagement in Pärken und Naturnahen Erholungsgebieten; Clivaz, C., Rupf, R., Siegrist, D., Eds.; HSR Hochschule für Technik Rapperswil: Rapperswil, Switzerland, 2013; pp. 27–36. [Google Scholar]
- Zingraff-Hamed, A. Comment articuler les enjeux écologiques et paysagers dans le projet urbain de réaménagement des zones fluviales urbaines ? Étude de cas Munich et Angers. In Éco-Ingénieurie des Zones Humides, Biodiversité et Bioindicateurs; Université d’Angers, Agrocampus Ouest, Fachhoschule Weihenstephan Triesdorf: Angers, France, 2011; p. 347. [Google Scholar]
- Eagles, P.F.; McCool, S.F. Tourism in National Parks and Protected Areas—Planning and Management; CABI Publishing: Oxon, NY, USA, 2002. [Google Scholar]
- Heggenes, J.; Traaen, T. Downstream migration and critical water velocities in stream channels for fry of four salmonid species. J. Fish. Biol. 1988, 32, 717–727. [Google Scholar] [CrossRef]
- Jorde, K. Ökologisch Begründete, Dynamische Mindestwasserregelungen bei Ausleitungskraftwerken; University of Stuttgart: Stuttgart, Germany, 1996. [Google Scholar]
- Salski, A. Fuzzy-Sets-Anwendungen in der Umweltforschung. In Fuzzy Logic: Theorie und Praxis, 3. Dortmunder Fuzzy-Tage Dortmund, 7–9 Juni 1993; Reusch, B., Ed.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 13–21. [Google Scholar]
- Goeller, B.; Wolter, C. Performance of bottom ramps to mitigate gravel habitat bottlenecks in a channelized lowland river. Restor. Ecol. 2015, 23, 595–606. [Google Scholar] [CrossRef]
- Sterl, P.; Brandenburg, C.; Arnberger, A. Visitors’ awareness and assessment of recreational disturbance of wildlife in the Donau-Auen National Park. J. Nat. Conserv. 2008, 16, 135–145. [Google Scholar] [CrossRef]
- Manfredo, M.J.; Vaske, J.J.; Decker, D.J. Human Dimensions of Wildlife Management: Basic Concepts. In Wildlife and Recreationists: Coexistence through Management and Research; Knight, R.L., Gutzwiller, K.J., Eds.; Island Press: Washington, DC, USA, 1995; pp. 17–33. [Google Scholar]
- Cottet, M.; Piégay, H.; Bornette, G. Does human perception of wetland aesthetics and healthiness relate to ecological functioning? J. Environ. Manag. 2013, 128, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Bulut, Z.; Karahan, F.; Sezen, I. Determining visual beauties of natural waterscapes: A case study for Tortum Valley (Erzurum/Turkey). Sci. Res. Essays 2010, 5, 170–182. [Google Scholar]
- Bulut, Z.; Yilmaz, H. Determination of landscape beauties through visual quality assessment method: A case study for Kemaliye (Erzincan/Turkey). Environ. Monit. Assess. 2008, 141, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Bulut, Z.; Yilmaz, H. Determination of waterscape beauties through visual quality assessment method. Environ. Monit. Assess. 2009, 154, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.; Messenger, B. Using an Activity and Place-Based Typology to Explain Visitor Motivations. J. For. 2009, 107, 44–49. [Google Scholar]
- Feio, M.J.; Calapez, A.R.; Elias, C.L.; Cortes, R.M.V.; Grata, M.A.S.; Pinto, P.; Almeida, S.F.P. The paradox of expert judgment in rivers ecological monitoring. J. Environ. Manag. 2016, 184, 609–616. [Google Scholar] [CrossRef] [PubMed]


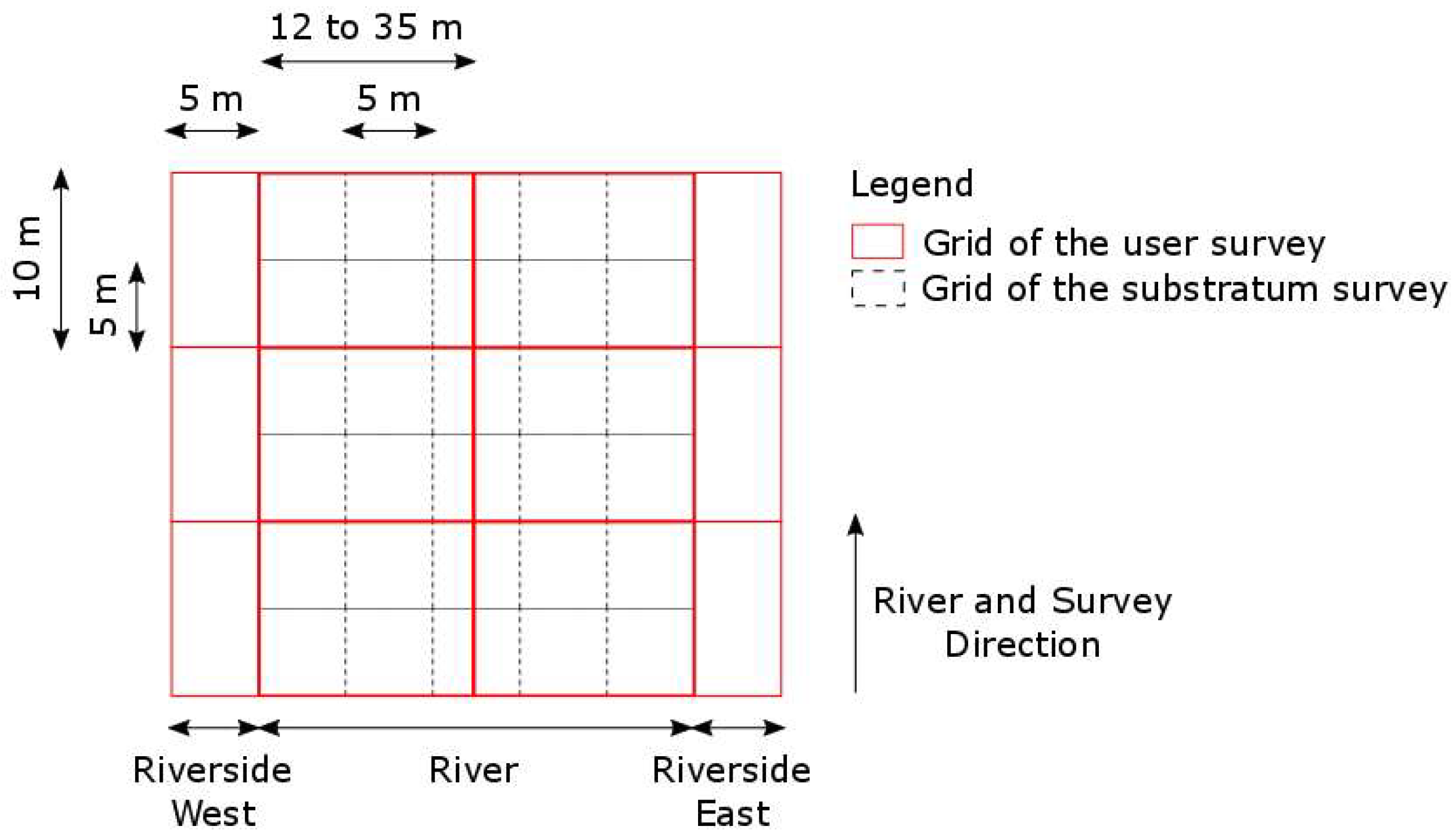

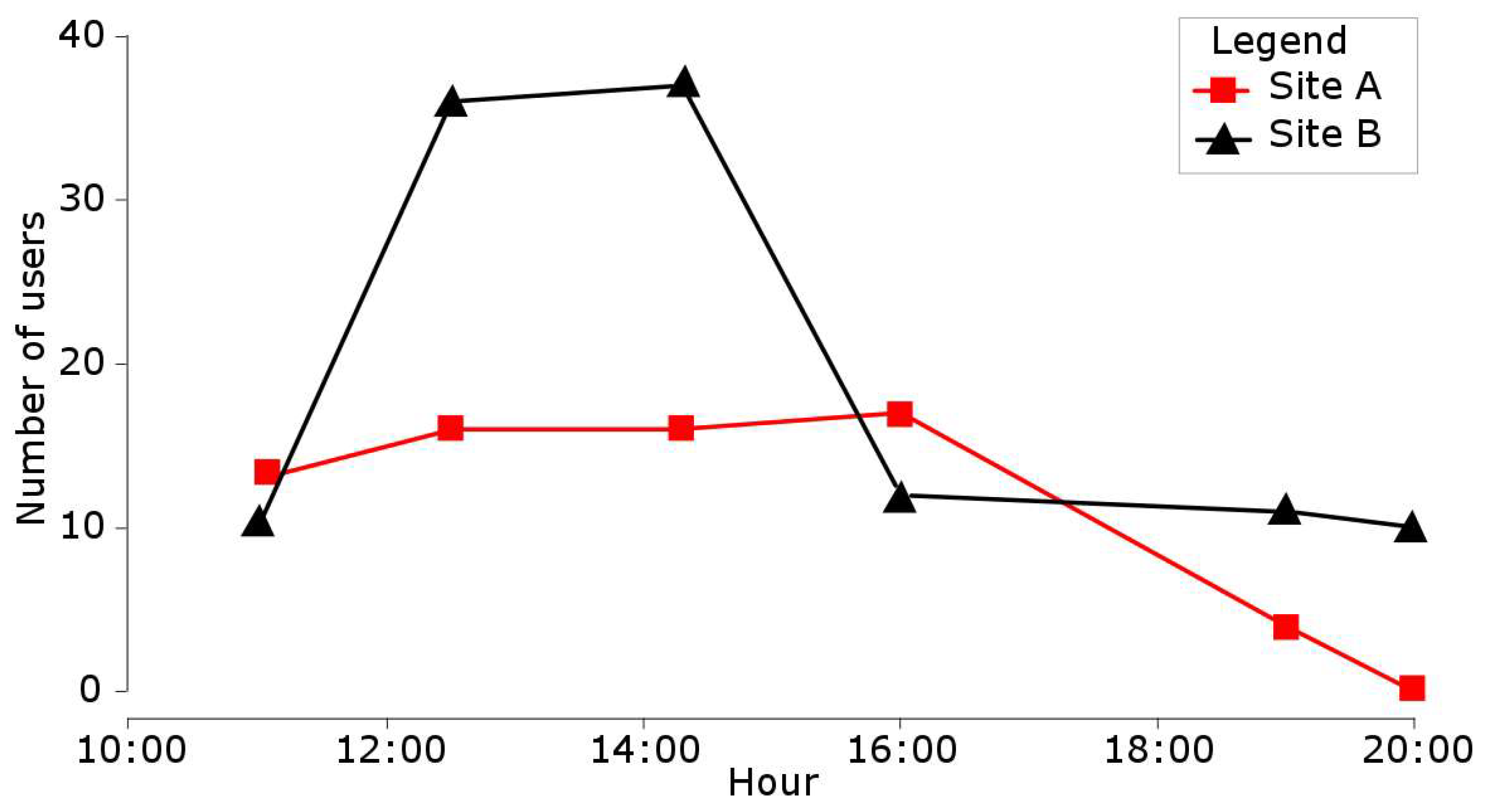
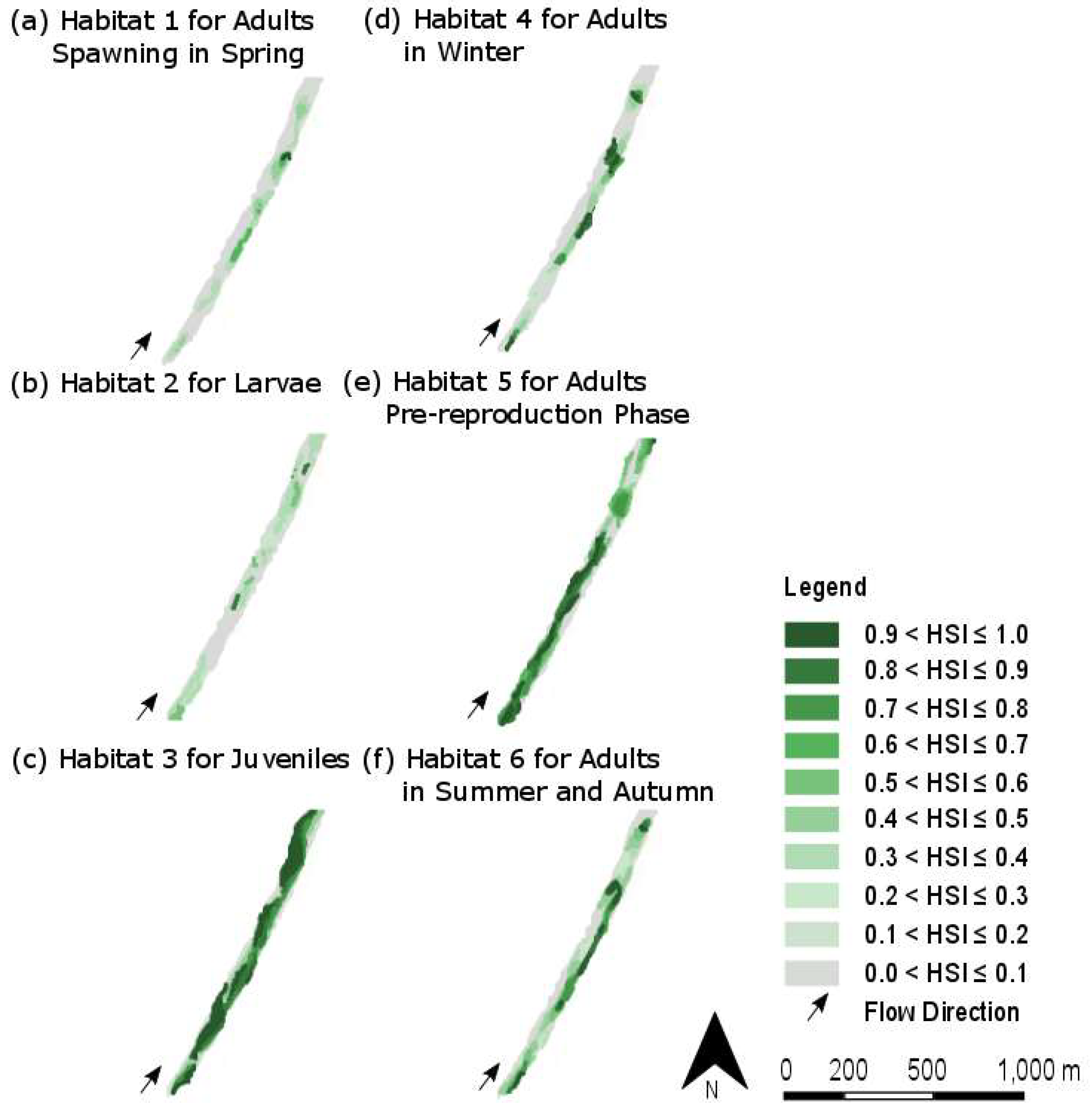
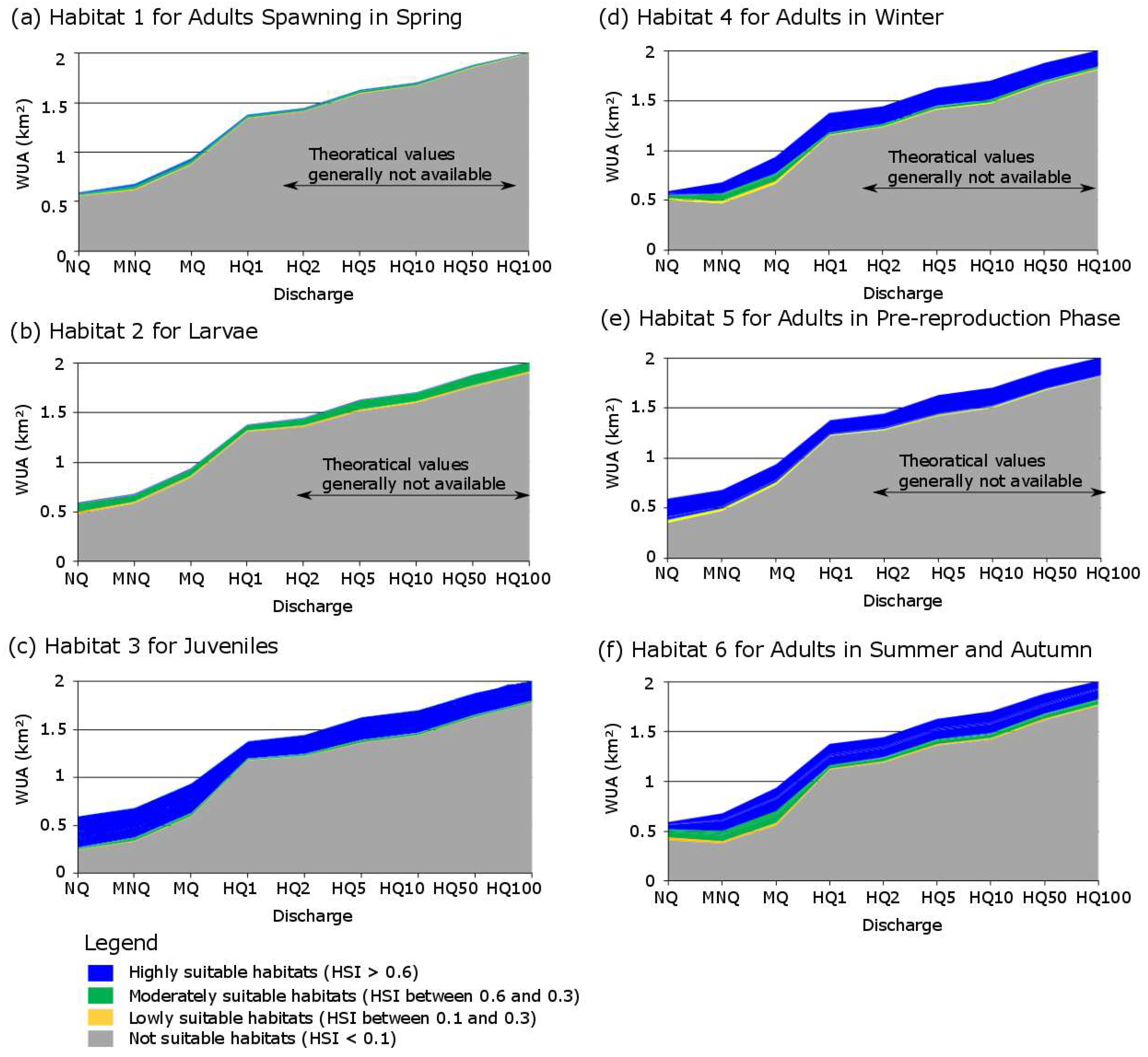
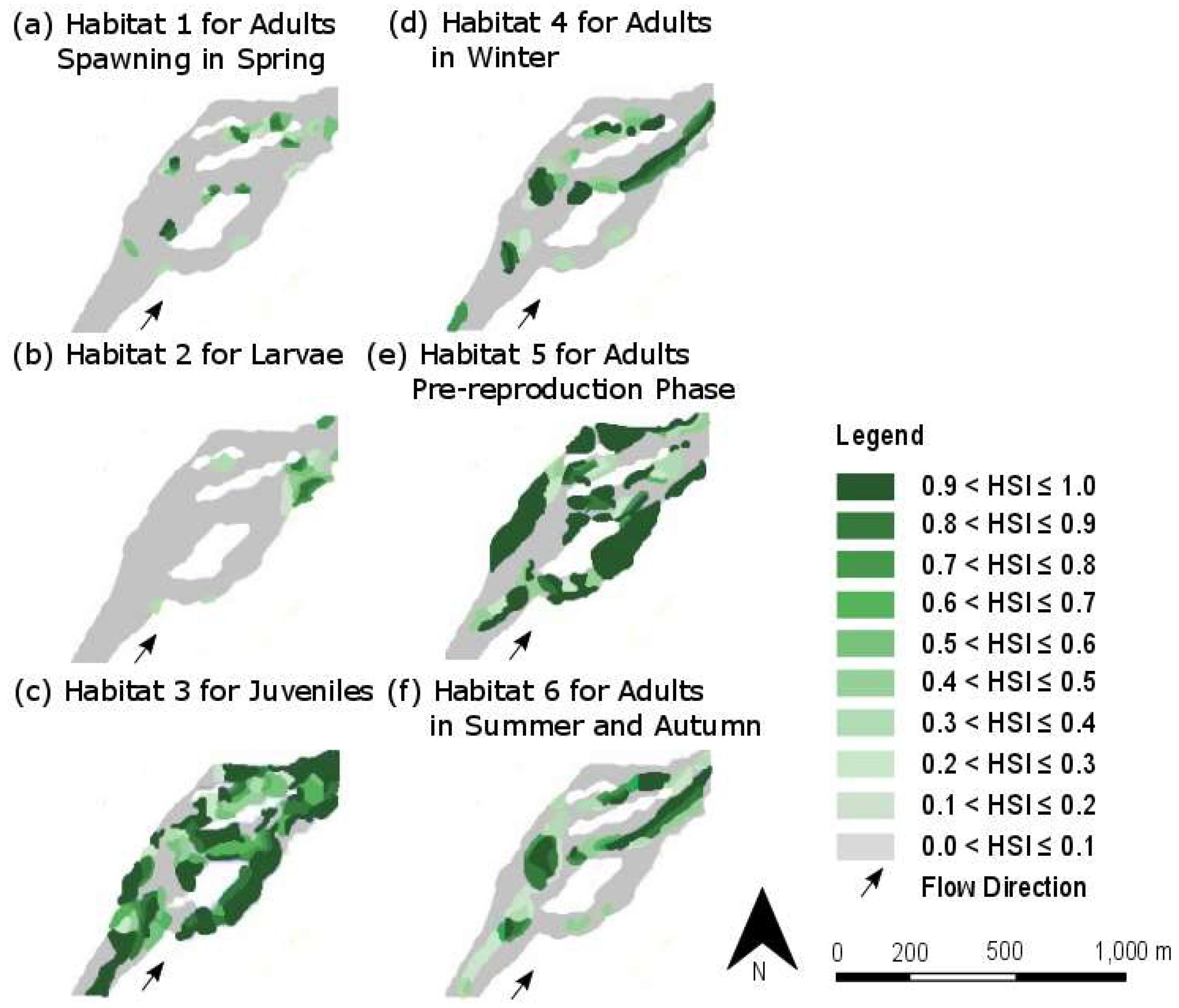
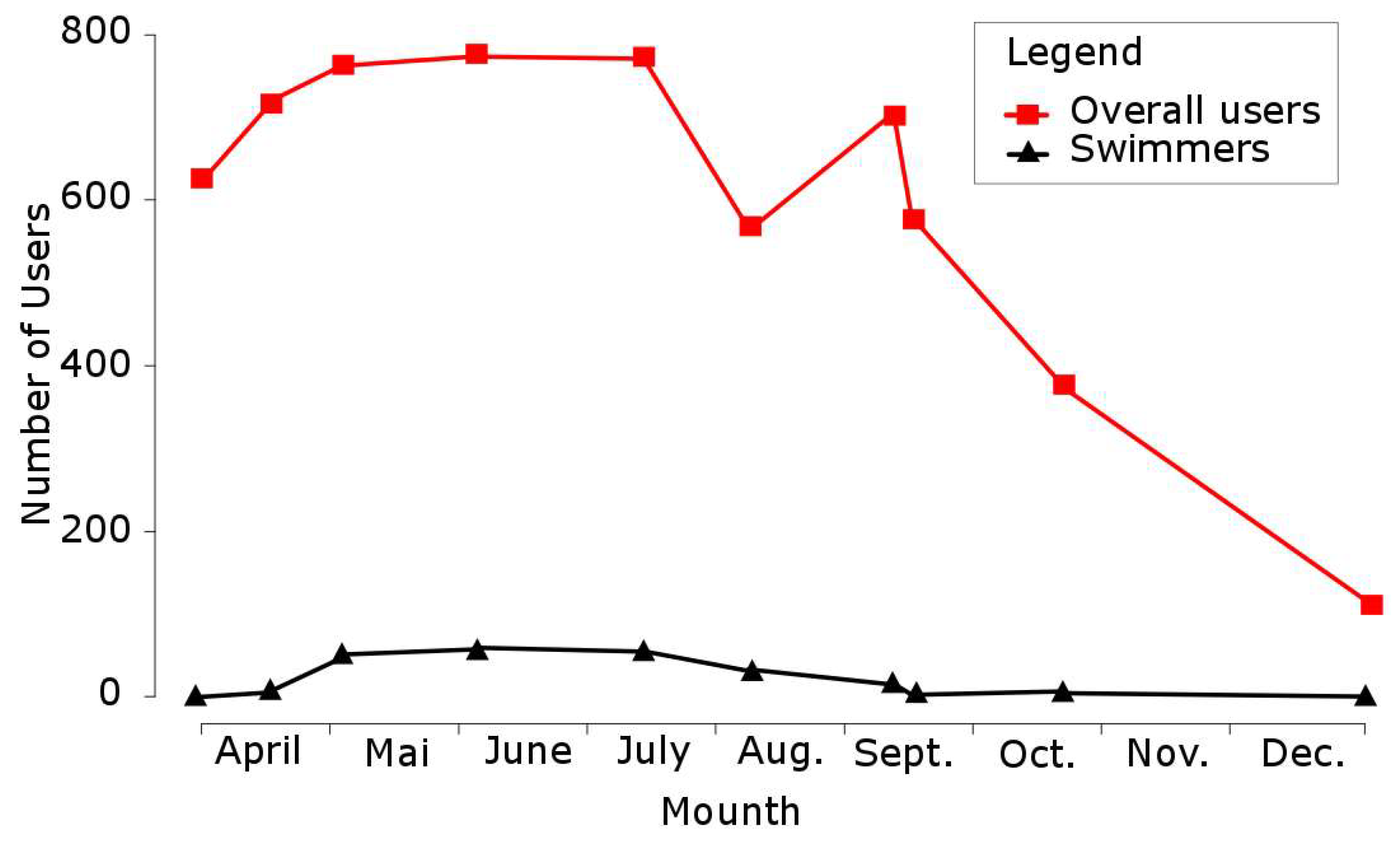
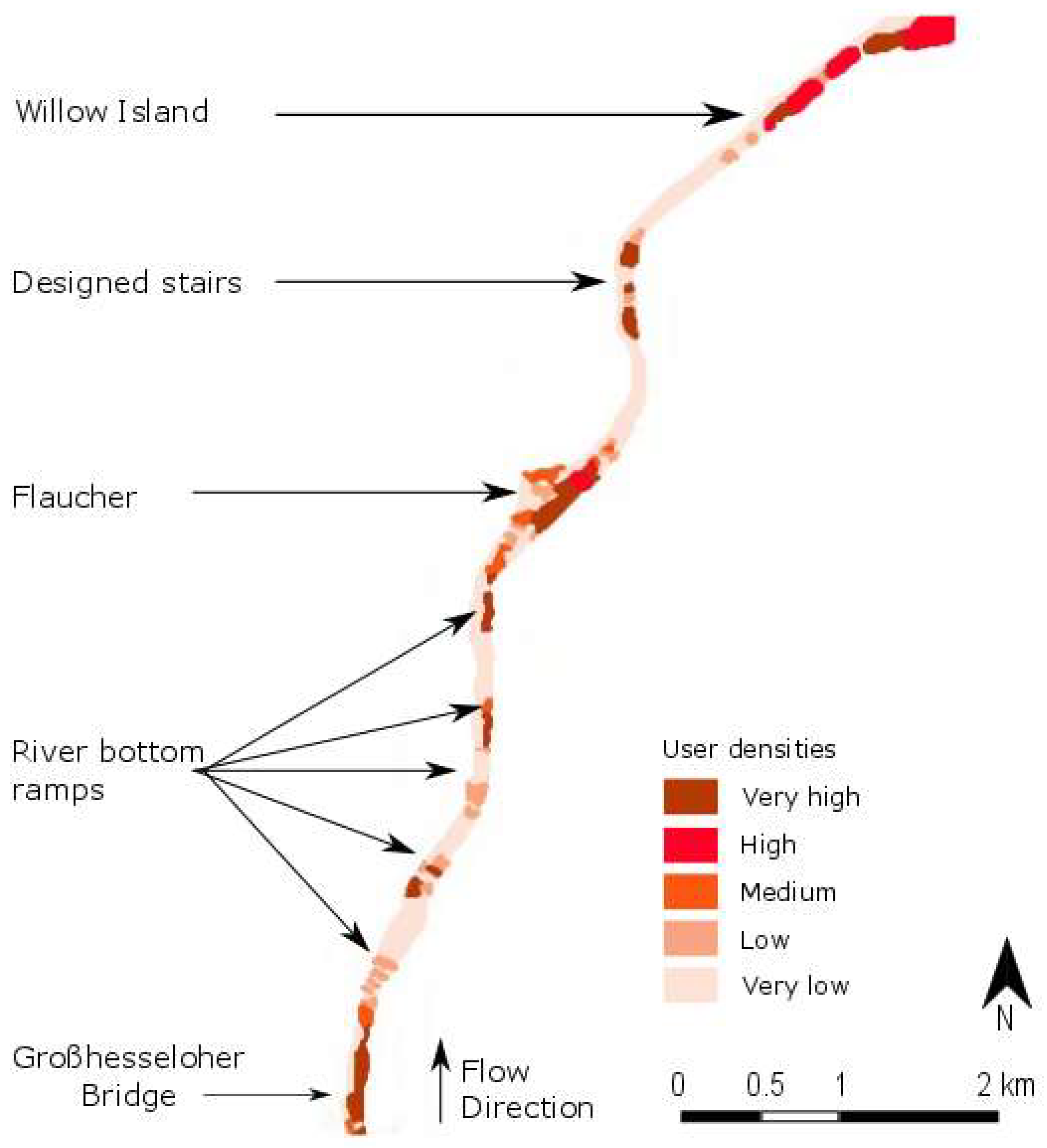
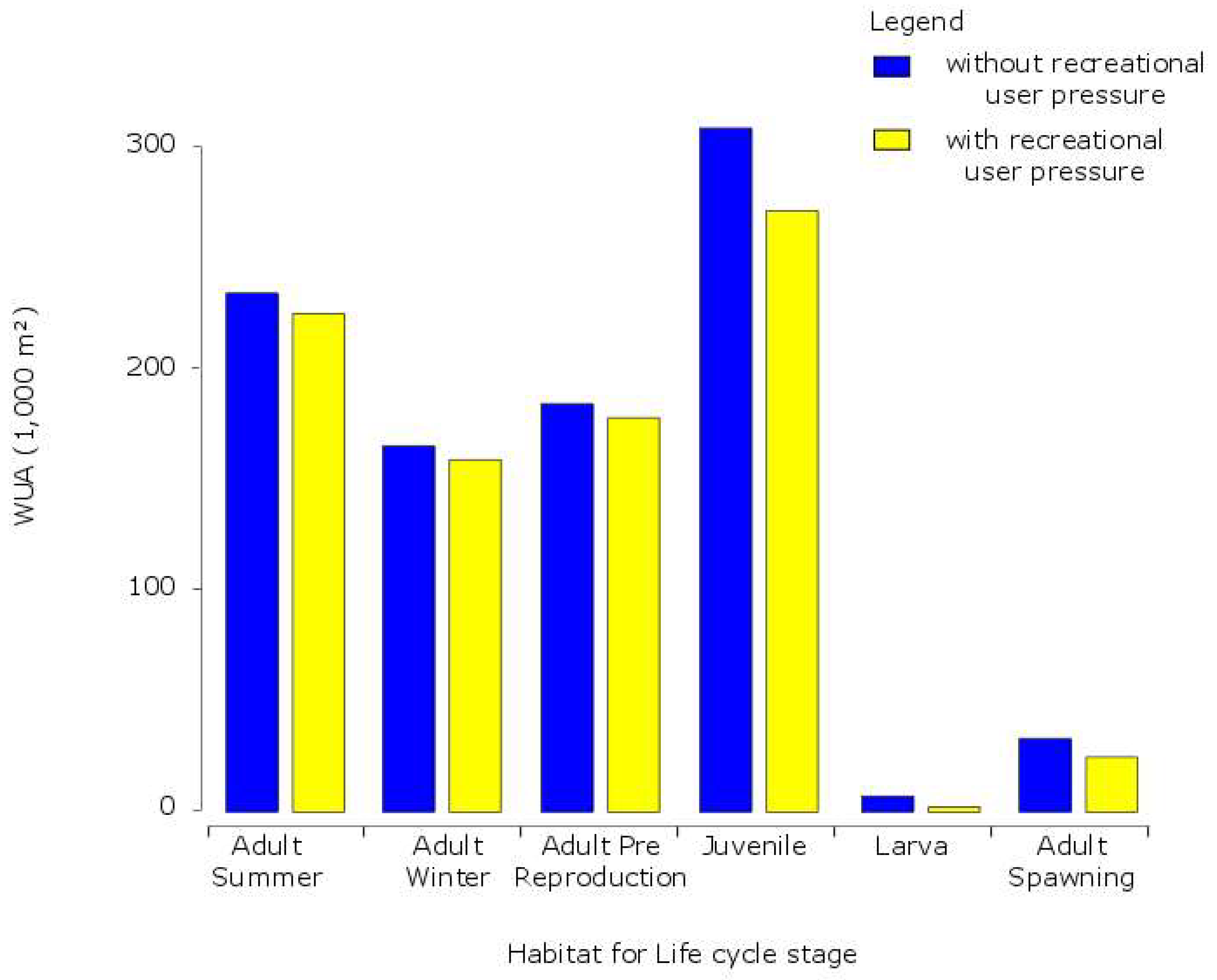
| Habitat Number | Lifecycle Stage | Season | Water Velocity (in m/s) | Water Depth (in cm) | Substratum |
|---|---|---|---|---|---|
| 1 | Adults spawning | Spring | High (1.0 to 1.5) | Moderate (20 to 40) | Gravel bars |
| 2 | Larvae | Spring | Low (0.5 to 0.7) | Low (5 to 10) | Fine-grained substratum |
| 3 | Juveniles | All | Very low (under 0.6) | Low (5 to 20) | Coarse substratum |
| 4 | Adults | Winter | High (1.0 to 1.5) | High (100 to 200) | Variable substratum |
| 5 | Adults (pre-reproduction) | Spring | Low to very low (less than 0.7) | Moderate (20 to 40) | Medium gravel to large stones |
| 6 | Adults | Summer and Autumn | Moderate to high (0.7 to 1.5) | Moderate (20 to 50) | Rock to gravel |
| Variable | Linguistic Category | Quantitative Fuzzy Meaning of the Physical Property |
|---|---|---|
| Water depth | very low | 0 to 0.1 m (±5 cm) |
| low | 0.1 m (±5 cm) to 0.2 m (±5 cm) | |
| medium | 0.2 m ±5 cm) to 0.5 m (±10 cm) | |
| high | 0.5 m (±10 cm) to 1.15 m (±25 cm) | |
| very high | Above 1.25 m (±25 cm) | |
| Velocity | very low | 0 to 0.4 m/s (±0.1 m/s) |
| low | 0.5 m/s (±0.1 m/s) to 0.7 m/s (±0.1 m/s) | |
| medium | 0.75 m/s (±0.1 m/s) to 0.9 m/s (±0.1 m/s) | |
| high | 1 m/s (±0.15 m/s) to 1.5 m/s (±0.25 m/s) | |
| very high | Above 1.75 m/s (±0.25 m/s) | |
| Substratum grain size | low | Organic material, detritus, silt, clay, loam, sand (<6 mm) |
| medium | Gravel from 6 mm to 12 cm | |
| high | Large stones (12–20 cm) | |
| very high | Boulders (>20 cm), rock | |
| User pressure (per 10-m long section) | very low | No users |
| low | One user on the riverbank and no users in/on the water | |
| medium | Two or more users on the riverbank or one user in/on the water | |
| high | One user in/on the water and >1 users on the riverbank | |
| very high | More than two users in the water and more than two users on the riverbank | |
| HSI | Very low | <0.1 |
| Low | [0.1 to 0.3] | |
| Medium | [0.3 to 0.6] | |
| High | [0.6 to 0.9] | |
| Very high | >0.9 |
| Velocity | Depth | Substrate | Recreational User Pressure | Habitat Suitability Index (HSI) | Example |
|---|---|---|---|---|---|
| VH | VH | VH | VH | VL | Rule 1: IF all input variables “Very high” THEN HSI “Very low” |
| VH | H | L | M | H | Rule 2: IF velocity “Very high” AND depth “High” AND substratum “Low” AND recreational pressure “Medium” THEN HSI “High” |
| H | H | L | L | VH | Rule 3: IF velocity “High” AND depth “High” AND substratum “Low” AND recreational pressure “Low” THEN HSI “Very high” |
| VL | M | VH | M | L | Rule 4: IF velocity “Very low” AND depth “Medium” AND substratum “Very high” AND recreational pressure “Medium” THEN HSI “Low” |
| Lifecycle Stage | Indicators | Suitability | |||
|---|---|---|---|---|---|
| Not Suitable | Low | Medium | High | ||
| Adults Spawning | Wetted area (m²) | 559,100 | 6975 | 13,350 | 16,000 |
| % of the wetted area | 94 | 1 | 2 | 3 | |
| Larvae | Wetted area (m²) | 481,400 | 20,550 | 86,350 | 7125 |
| % of the wetted area | 81 | 3 | 14 | 1 | |
| Juveniles | Wetted area (m²) | 245,425 | 4125 | 20,300 | 325,575 |
| % of the wetted area | 41 | 1 | 3 | 55 | |
| Adults in Winter | Wetted area (m²) | 506,350 | 16,725 | 36,425 | 35,925 |
| % of the wetted area | 85 | 3 | 6 | 6 | |
| Adults (pre-reproduction) | Wetted area (m²) | 354,125 | 12,225 | 21,350 | 207,725 |
| % of the wetted area | 59 | 2 | 4 | 35 | |
| Adults in Summer and Autumn | Wetted area (m²) | 412,375 | 28,150 | 83,875 | 71,025 |
| % of the wetted area | 69 | 5 | 14 | 12 | |
| Discharges | Scenarios | ||||||
|---|---|---|---|---|---|---|---|
| Without User | With User | ||||||
| NQ | MQ | HQ | NQ | MQ | HQ | ||
| Adults spawning | WUA (1000 m²) | 27 | 32 | 18 | 21 | 24 | 12 |
| HHS | 0.03 | 0.03 | 0.01 | 0.03 | 0.03 | 0.01 | |
| Mean HSI | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | |
| Larvae | WUA (1000 m²) | 59 | 33 | 26 | 51 | 30 | 31 |
| HHS | 0.07 | 0.04 | 0.02 | 0.06 | 0.03 | 0.02 | |
| Mean HSI | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | |
| Juveniles | WUA (1000 m²) | 330 | 269 | 145 | 291 | 232 | 167 |
| HHS | 0.41 | 0.28 | 0.10 | 0.36 | 0.24 | 0.12 | |
| Mean HSI | 0.5 | 0.5 | 0.2 | 0.4 | 0.3 | 0.2 | |
| Adults in Winter | WUA (1000 m²) | 100 | 183 | 199 | 94 | 170 | 176 |
| HHS | 0.12 | 0.19 | 0.14 | 0.12 | 0.18 | 0.13 | |
| Mean HSI | 0.3 | 0.4 | 0.3 | 0.1 | 0.2 | 0.2 | |
| Adults Pre-reproduction | WUA (1000 m²) | 248 | 178 | 145 | 243 | 176 | 183 |
| HHS | 0.30 | 0.19 | 0.11 | 0.30 | 0.19 | 0.13 | |
| Mean HSI | 0.4 | 0.3 | 0.2 | 0.2 | 0.2 | 0.1 | |
| Adults in Summer and Autumn | WUA (1000 m²) | 167 | 238 | 195 | 156 | 217 | 173 |
| HHS | 0.21 | 0.25 | 0.14 | 0.19 | 0.22 | 0.13 | |
| Mean HSI | 0.3 | 0.3 | 0.2 | 0.2 | 0.4 | 0.3 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zingraff-Hamed, A.; Noack, M.; Greulich, S.; Schwarzwälder, K.; Wantzen, K.M.; Pauleit, S. Model-Based Evaluation of Urban River Restoration: Conflicts between Sensitive Fish Species and Recreational Users. Sustainability 2018, 10, 1747. https://doi.org/10.3390/su10061747
Zingraff-Hamed A, Noack M, Greulich S, Schwarzwälder K, Wantzen KM, Pauleit S. Model-Based Evaluation of Urban River Restoration: Conflicts between Sensitive Fish Species and Recreational Users. Sustainability. 2018; 10(6):1747. https://doi.org/10.3390/su10061747
Chicago/Turabian StyleZingraff-Hamed, Aude, Markus Noack, Sabine Greulich, Kordula Schwarzwälder, Karl Matthias Wantzen, and Stephan Pauleit. 2018. "Model-Based Evaluation of Urban River Restoration: Conflicts between Sensitive Fish Species and Recreational Users" Sustainability 10, no. 6: 1747. https://doi.org/10.3390/su10061747
APA StyleZingraff-Hamed, A., Noack, M., Greulich, S., Schwarzwälder, K., Wantzen, K. M., & Pauleit, S. (2018). Model-Based Evaluation of Urban River Restoration: Conflicts between Sensitive Fish Species and Recreational Users. Sustainability, 10(6), 1747. https://doi.org/10.3390/su10061747