Some Influences of Underground Coal Gasification on the Environment
Abstract
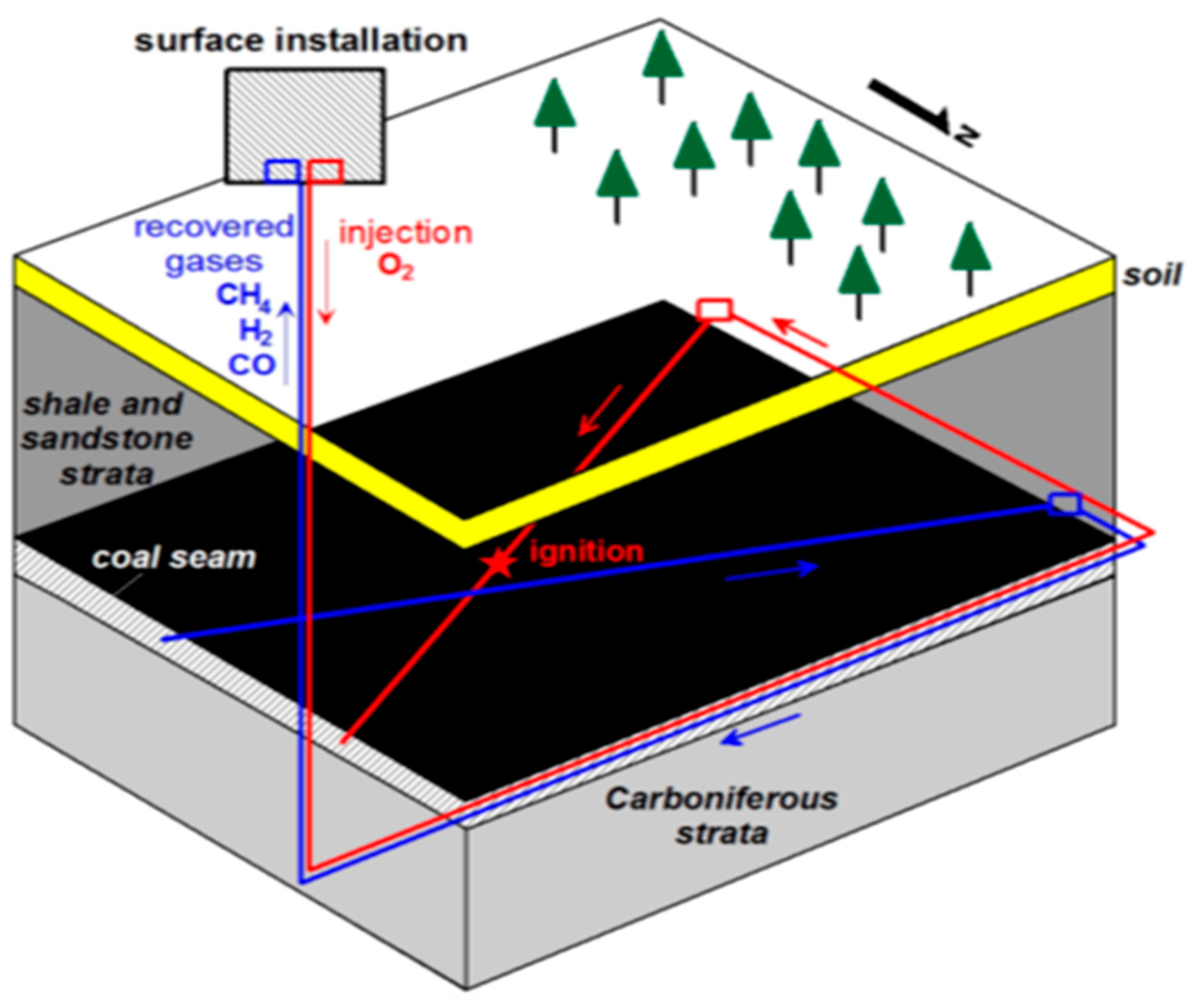
1. Introduction
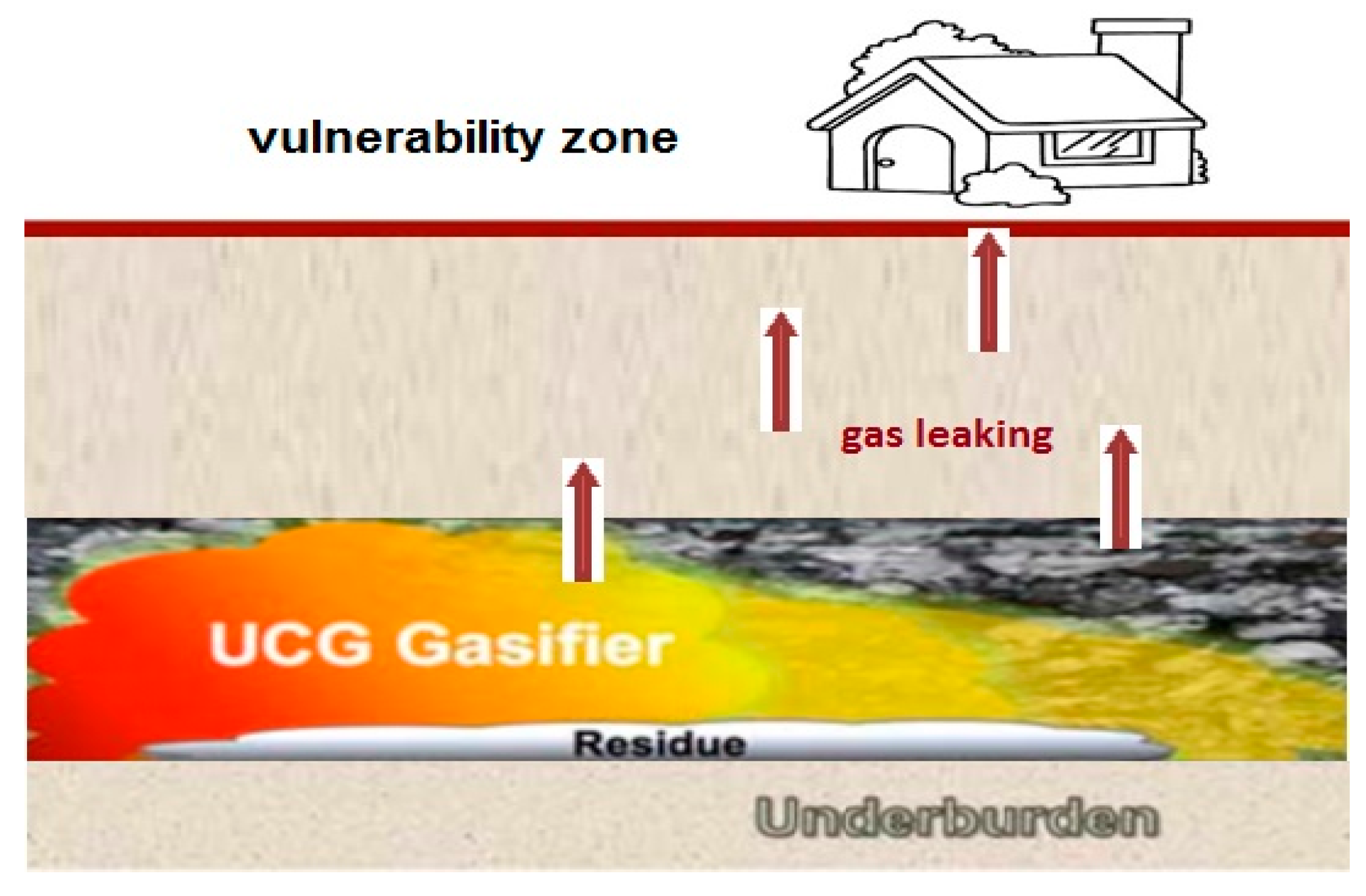
1.1. Groundwater Contamination
1.2. Air Pollution
1.3. The Heat Impact
2. Materials and Methods
2.1. The UCG Experiments
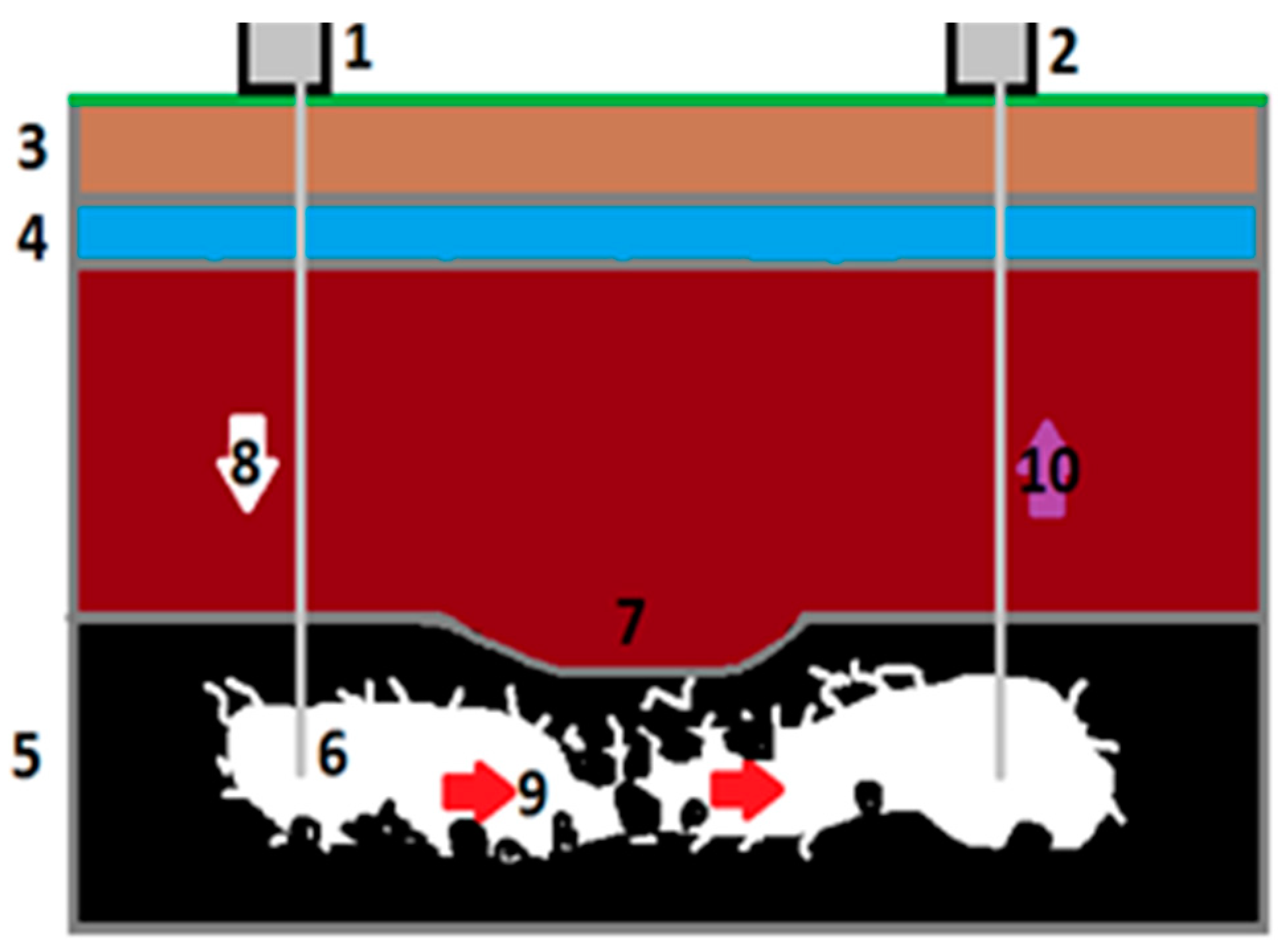
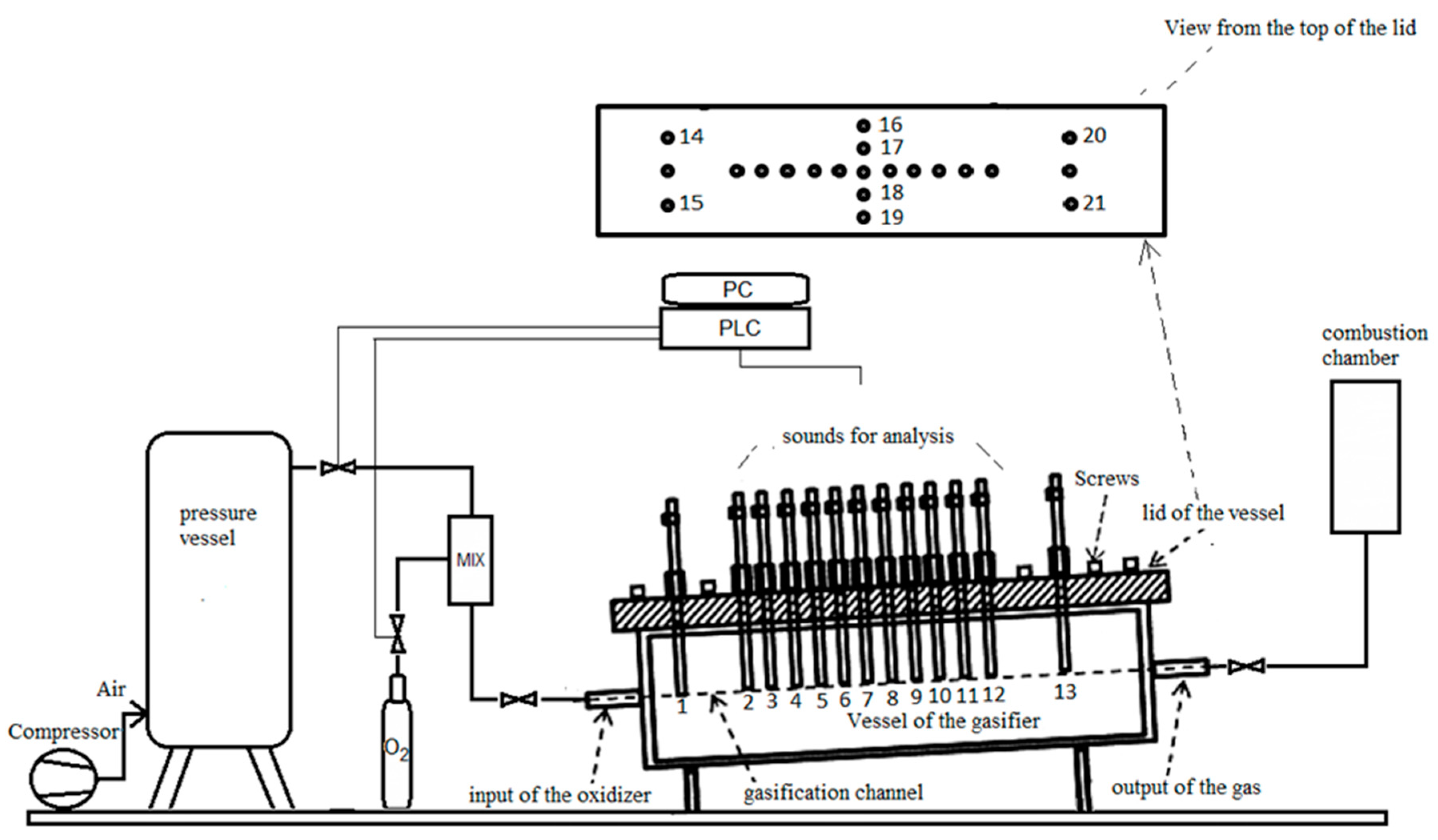
2.1.1. Description of Geo-Reactor and Process Gasification in Laboratory Condition
2.1.2. Characterization of Parameters of Geo-Reactor In Situ
2.2. Mathematical Modelling
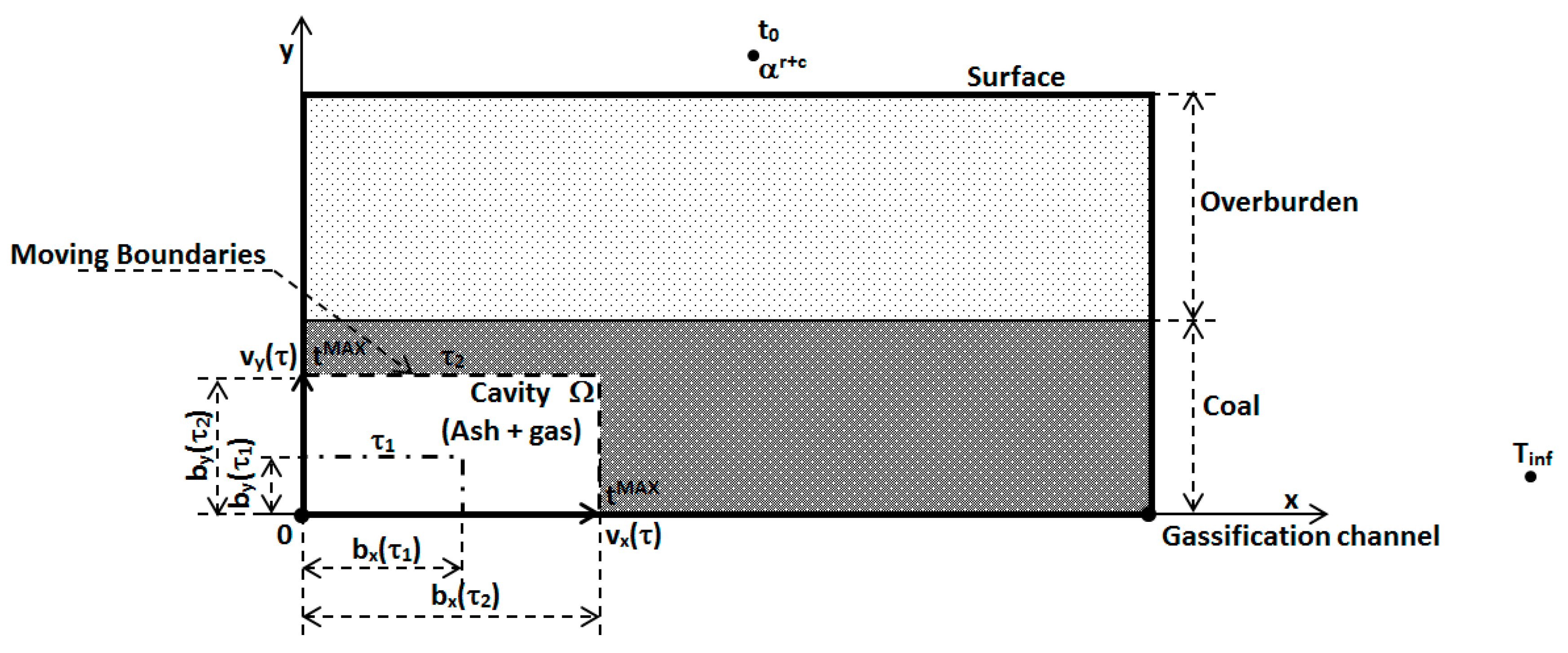
2.2.1. Mathematical Model for Two-Dimensional (2D) Temperature Fields
- by conduction,
- by radiation,
- by convection.
- Heat transfer by convection in the y axis direction is very low because the permeability of rocks underground is too small for gas streaming. Its heat capacity is very small in comparison with heat capacity of overburden.
- Heat transfer by radiation and convection in the “” axis direction has meaning only in the gasification channel. Mathematical model is solved by using first boundary condition which is defined by measured temperature on interface gasification channel—coal.
- Thermo-physical parameters are considered as average values in assumed temperature interval.
- Therefore, heat transfer around coal gasifier is limited to conduction.
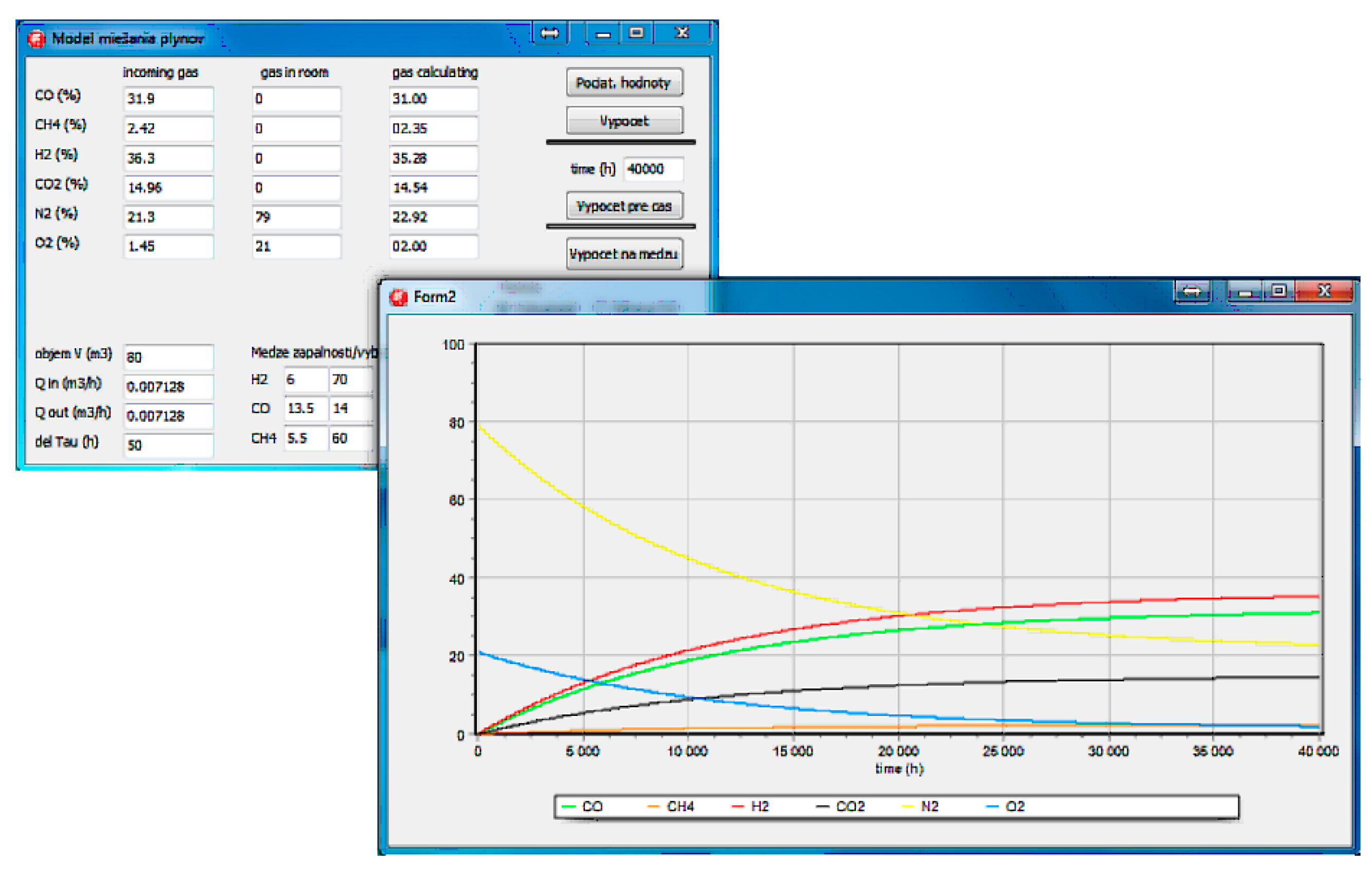
2.2.2. Mathematical Model for Mixing of gases
3. Results
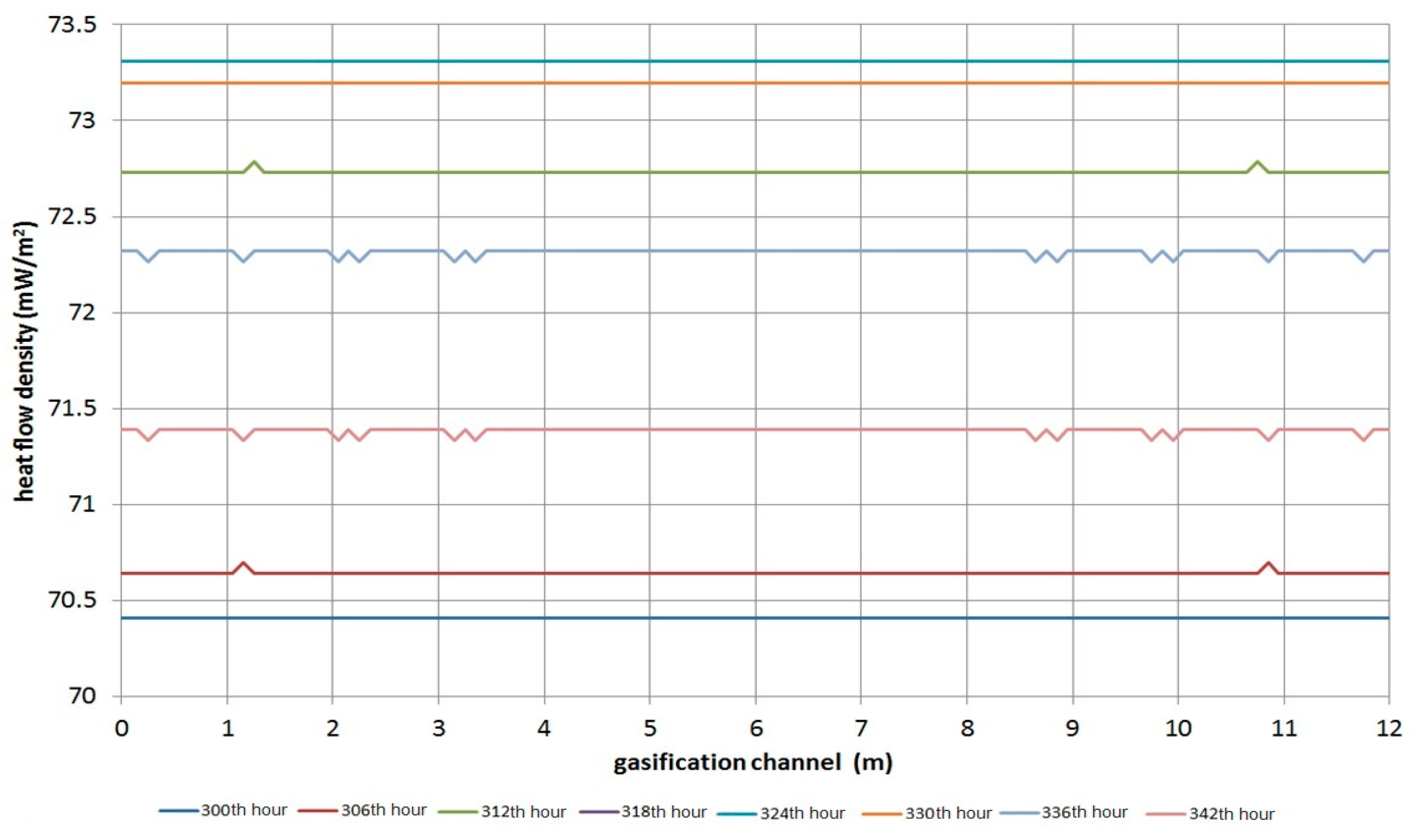
3.1. The Heat Influence of UCG on Overburden
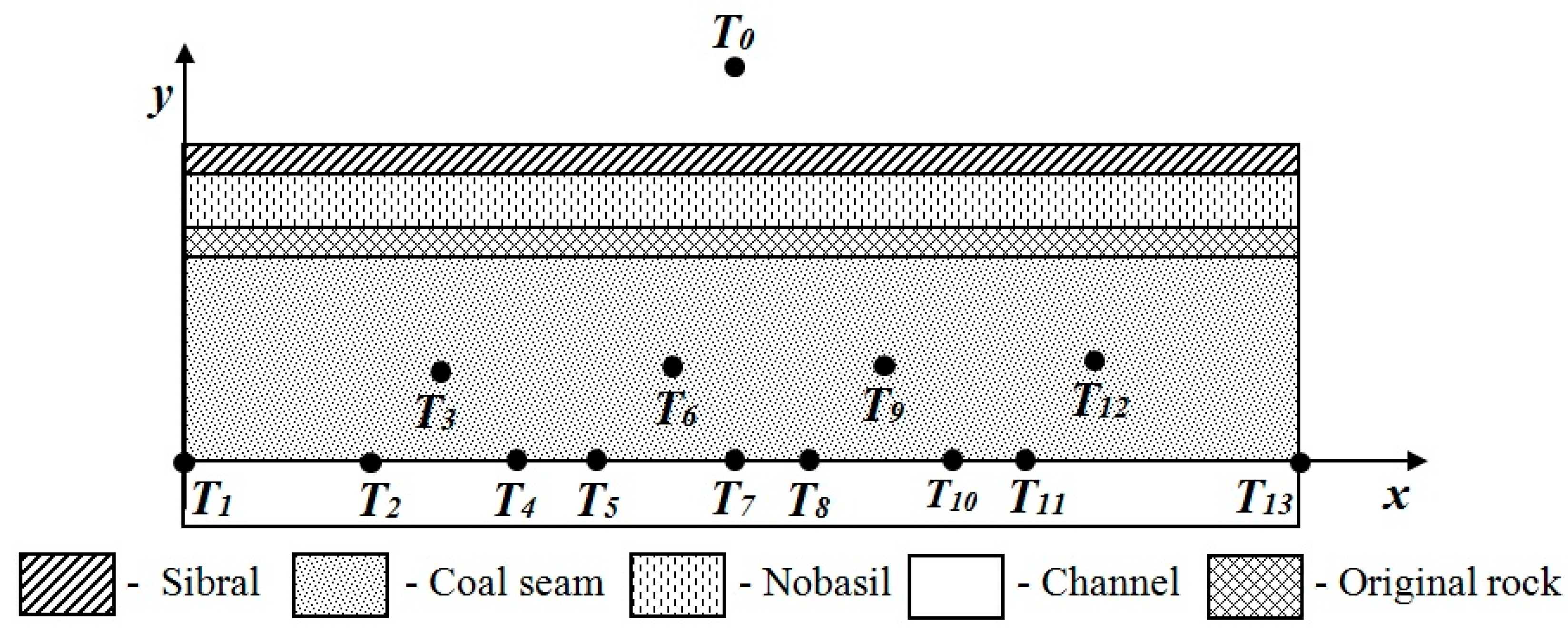
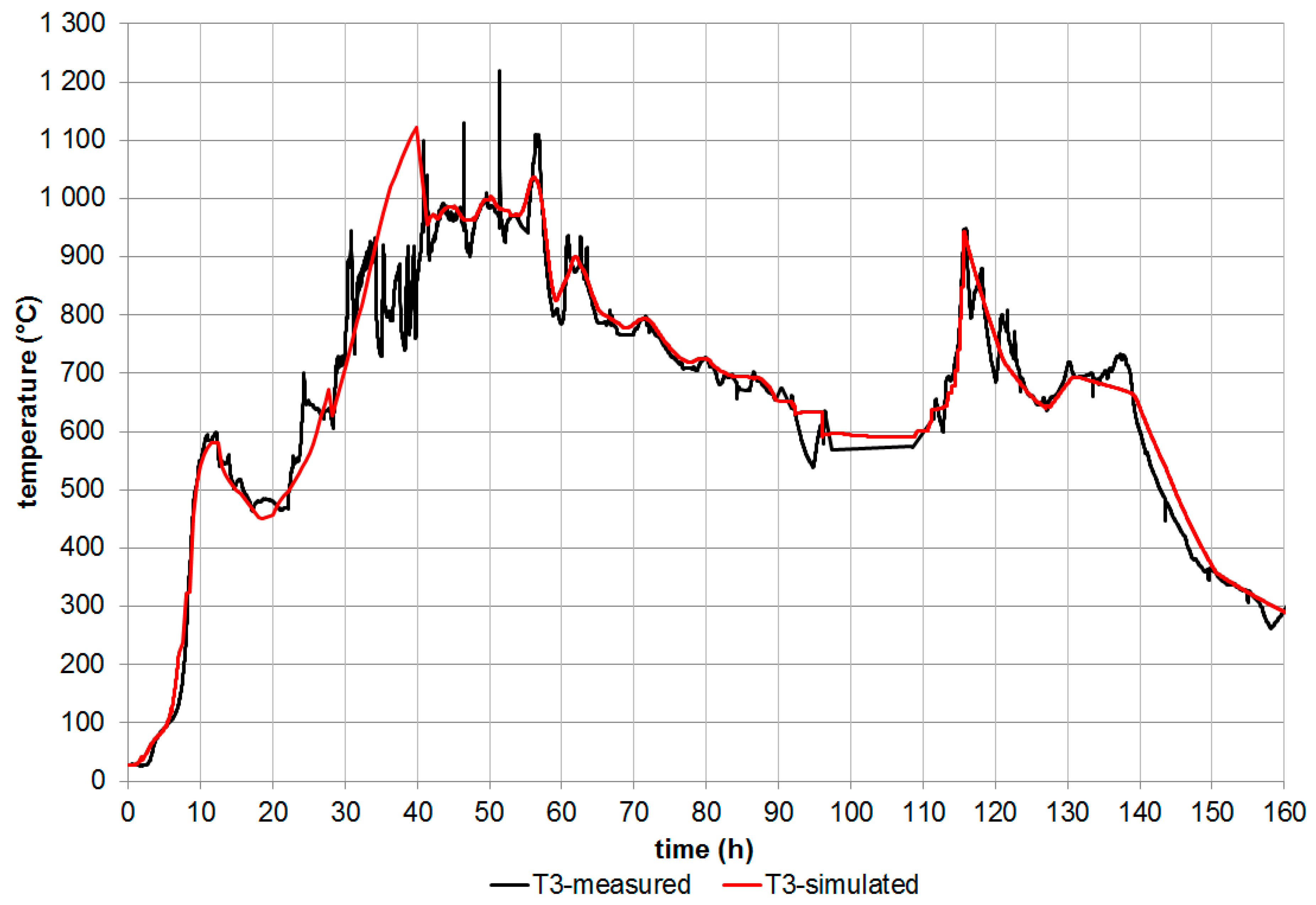
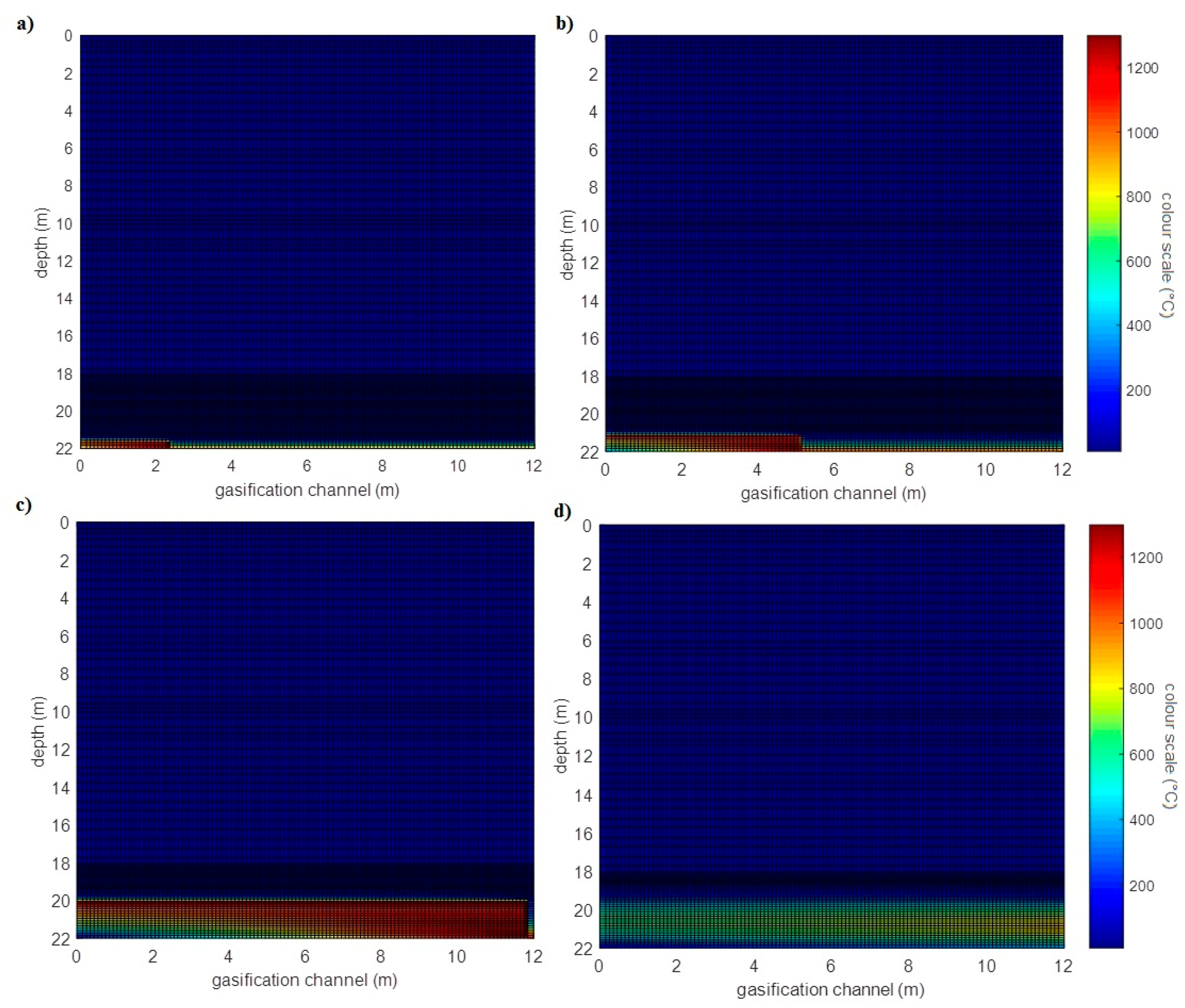
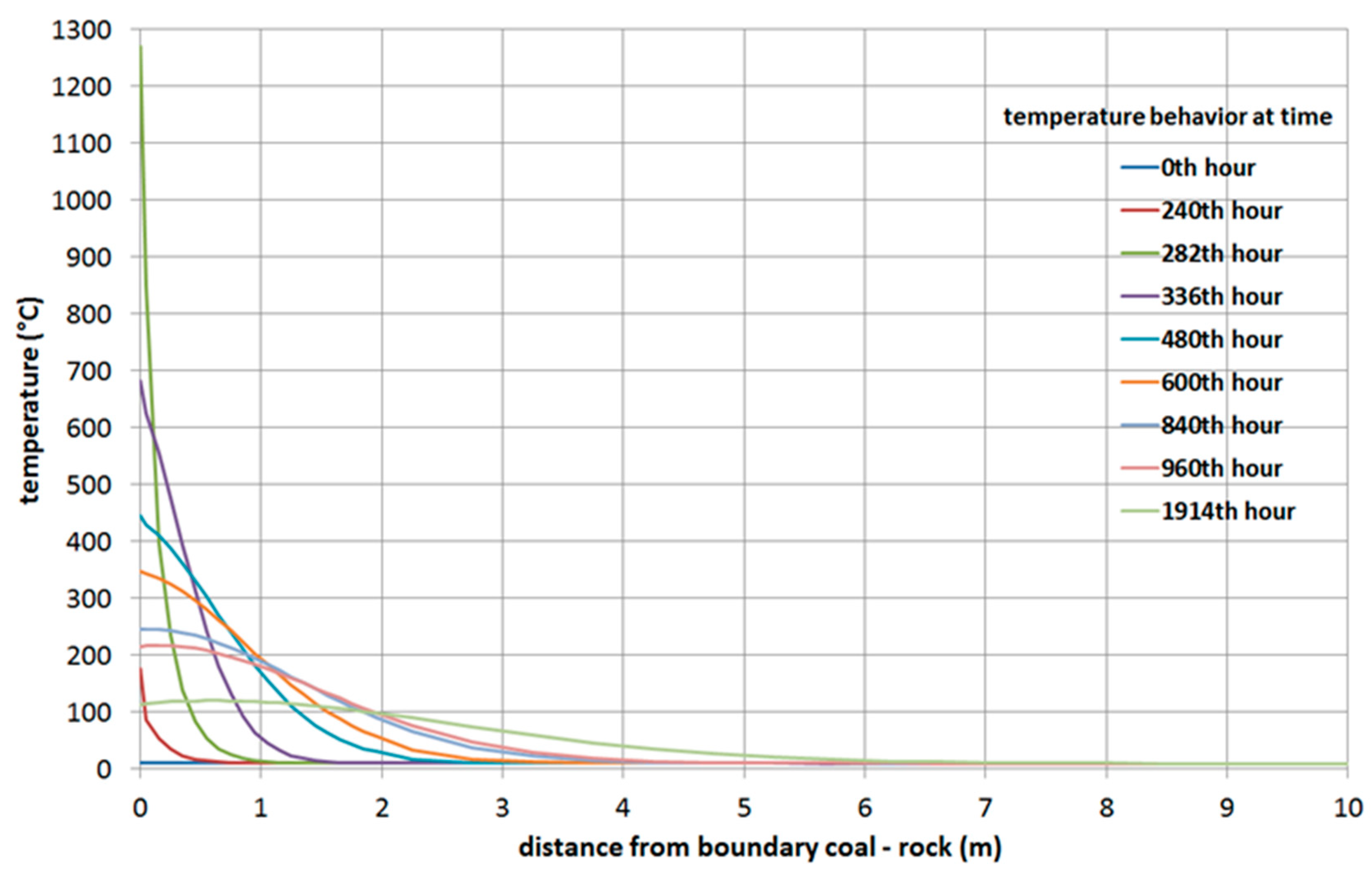
3.1.1. Simulation of the Barbara Experimental Mine
- The velocity of the movement of the gasification front was determined from individual measured temperatures on the coal seam for the x and y axes (The velocity in the x axis direction was larger than the velocity in the y axis direction) using laboratory measurement.
- The ratio between velocity in the y axis and in the x axis direction was calculated.
- The calculated ratio of the calculation of the velocity of the movement of the gasification front in the direction of the depth (y axis) in the real coal seam simulation was used. The velocity of the movement gasification front in direction in the y axis depth was created by multiplying the velocity of the movement of the gasification front in a direction on the x axis (gasification channel m/day) and the ratio calculated. The resulting value of the calculation was m/day. These velocities were used for the first simulation.
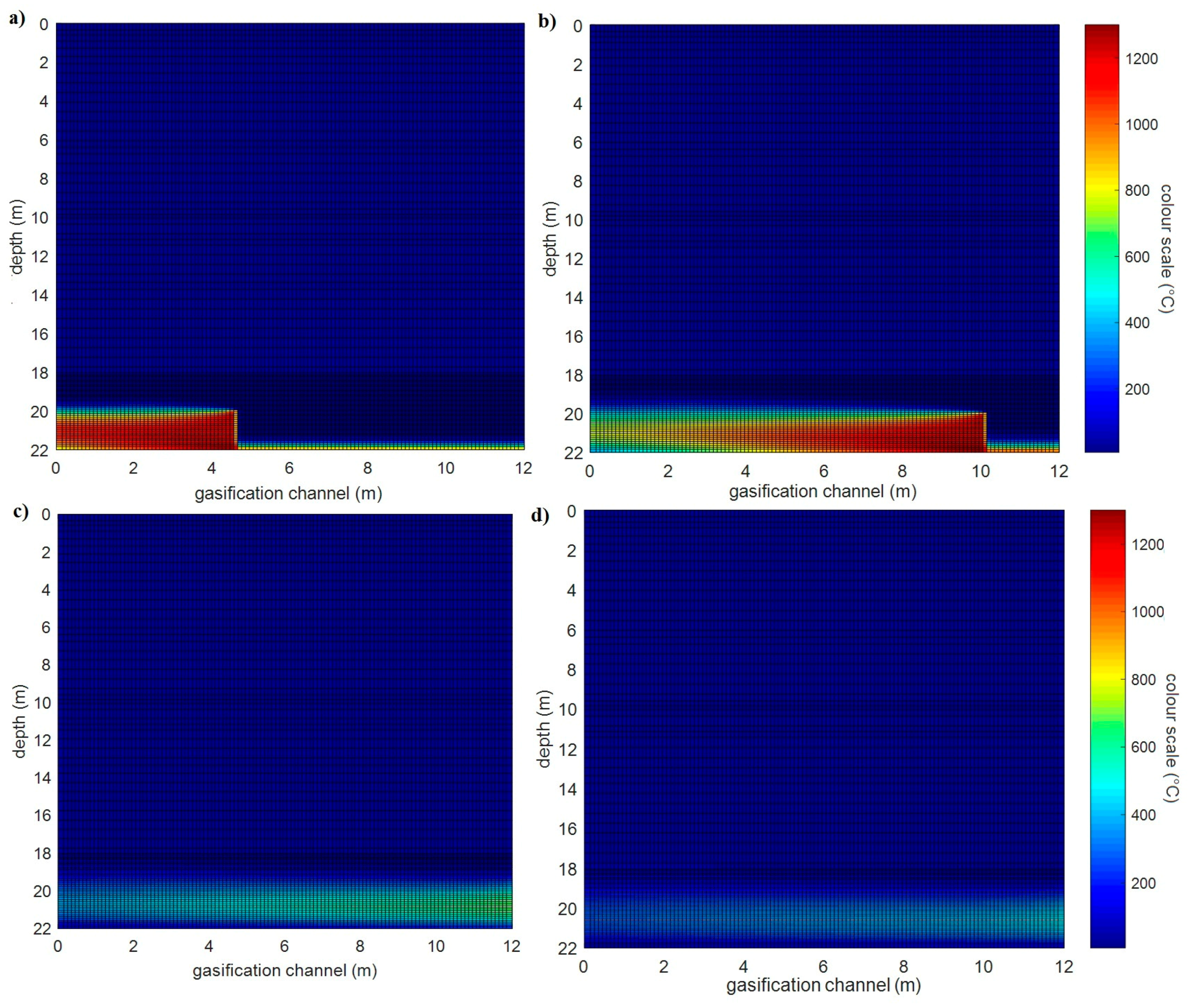
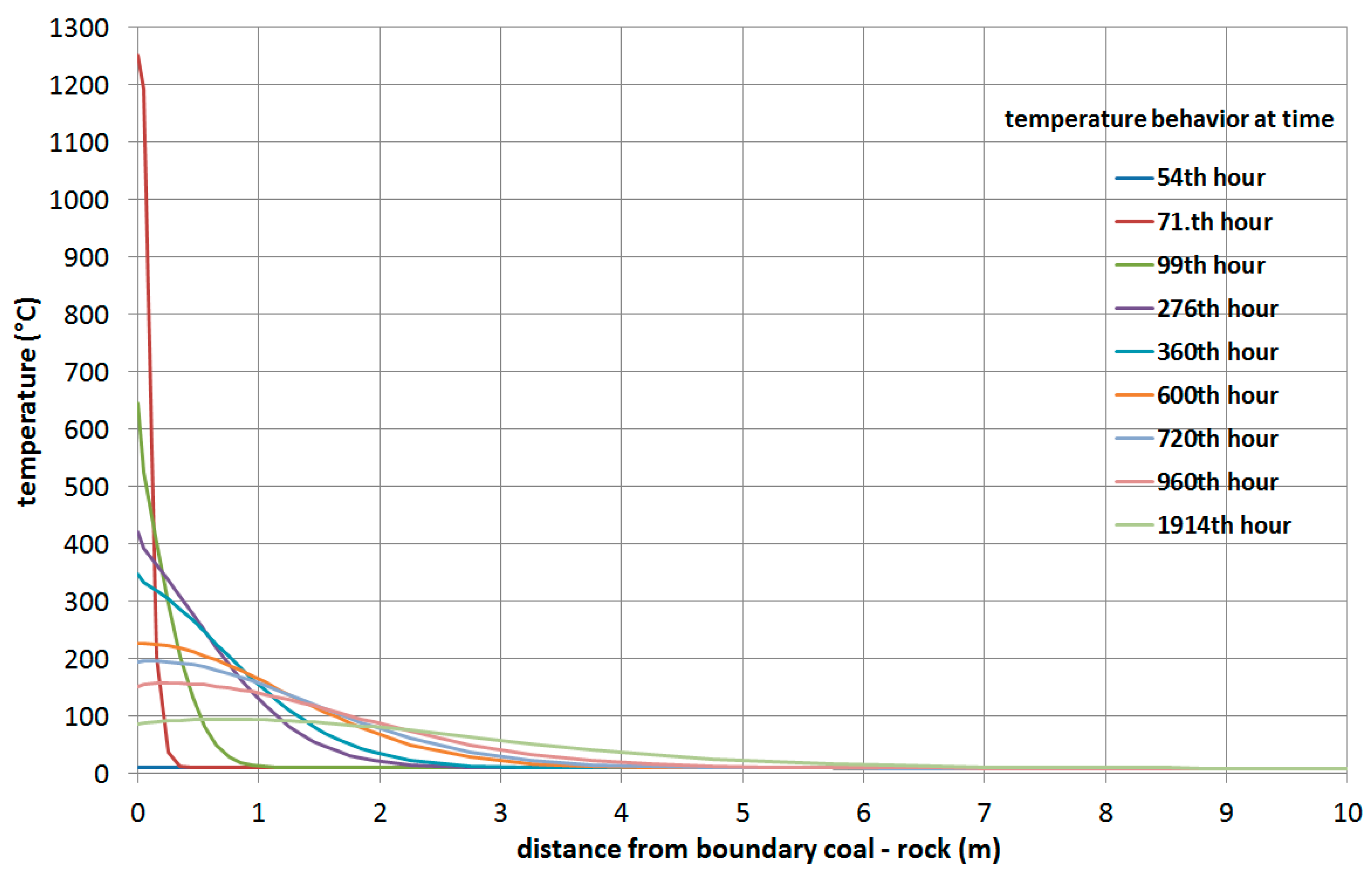
3.1.2. Simulation of the Wieczorek Experimental Mine
3.2. Analysis of a Dangerous Explosion and a Poisoning by Syngas
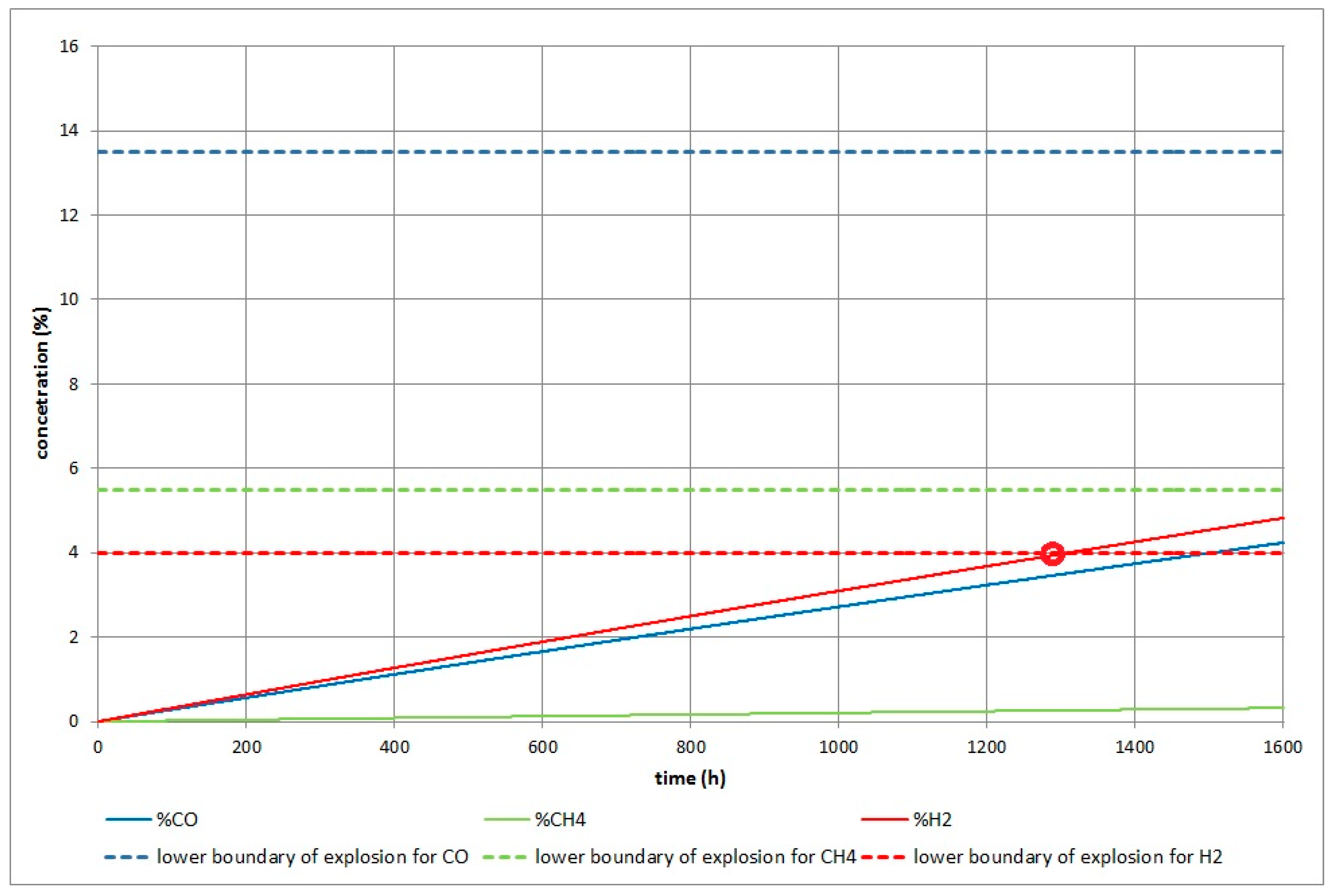
3.2.1. The Results of the Simulation for Dangerous Explosion
3.2.2. Results of the Simulation for CO Poisoning
3.2.3. The Results of the Simulation on the Model with Fresh Air Intake
4. Discussion
5. Conclusions
- heat impact on temperature of the surface and surroundings of the UCG cavity,
- the possibility of explosion and CO poisoning by syngas leaking from UCG through the overburden into vulnerability zone on surface.
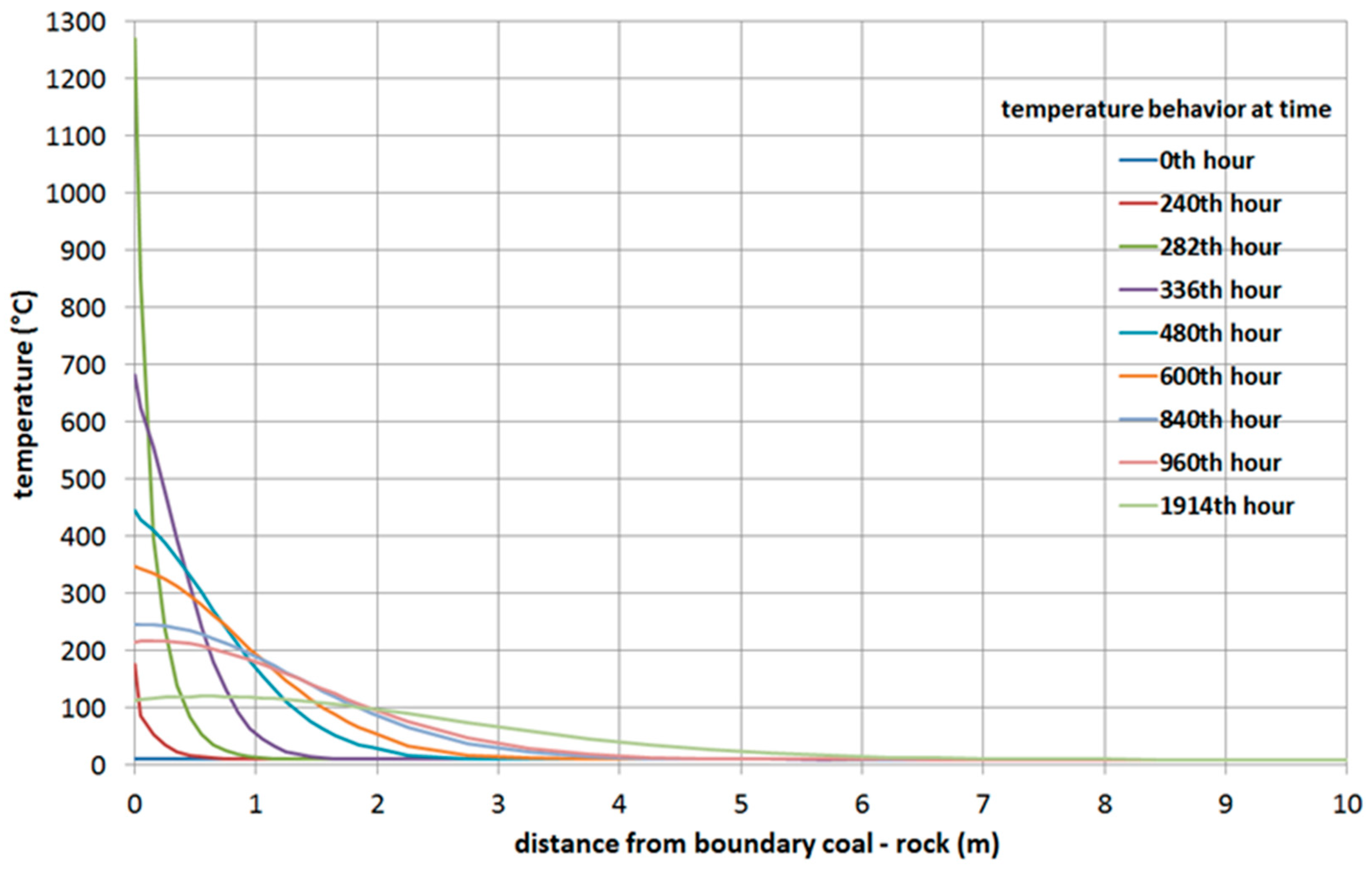
- The high-temperature heat source of the flame working face moves directionally during the process of UCG, which makes the boundary temperature of surrounding rock to be a velocity function in the horizontal and vertical directions. The temperature in the roof increases at first and then decreases with time; the peak value of the temperature curve decreases gradually, and the position of the peak value moves into the interior of the roof.
- In this paper, for simulations of temperature fields a simplified approach was used for a mathematical model. Heat transfer model (1–9) is possible to improve by exchange (1) by a model which describes the heat exchange between streaming water and overburden [84]. Of course, water physical parameters must be replaced by syngas leaking parameters. This access to the improving of the heat transfer model by heat transfer by convection and mass of syngas leaking is our aim in future.
- The greater the thickness of the coal seams, the higher the temperature of the rock at the same distance from the UCG cavity. This note is clear from comparing simulations of the heat fields of gasification of deposits in the Barbara and Wieczorek mines.
- From analysis of the influence of velocity of movement of the gasification front in both directions, the following was observed: at greater velocities of movement of the gasification front in both directions, the rock temperatures are lower at equivalent distances from the UCG cavity.
- In small unventilated areas a much more toxic and explosive atmosphere is produced than in large areas (e.g., halls, warehouses). Table 9 and Table 10 compare critical poisoning and explosion times after initialization for different volumes of enclosed working or living spaces. This critical time for poisoning or explosion with increasing volume of a room is prolonged linearly.
- The danger of explosion and poisoning with syngas is higher for shallow gasified coal deposits (Barbara) compared with deposits found at greater depths (Cigel and Wieczorek).
- Depending on the permeability of the overburden, over-pressure or under-pressure of the underground gasification regime should be used. If the UCG technology enables operation at greater permeability, it allows operation with under-pressure, then this method of controlling the pressure ratios needs to be applied.
- Syngas leakage will also increase the concentration of greenhouse gases in the air. However, carbon dioxide escapes into the air even from closed coal mines. An analysis of this negative environmental impact has shown that significantly less CO2 is released in the evaluated UCG experiments than in a closed coal mine [83]. This amount of leaked CO2 during UCG will be even lower if the recommendations in point 6 are implemented.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lyu, X.; Shi, A. Research on the Renewable Energy Industry Financing Efficiency Assessment and Mode Selection. Sustainability 2018, 10, 222. [Google Scholar] [CrossRef]
- Sajjad, M.; Rasul, M.G. Review on the Existing and Developing Underground Coal Gasification Techniques in Abandoned Coal Seam Gas Blocks: Australia and Global Context. In Proceedings of the 1st International e–Conference on Energies, Roma, Italy, 14–31 March 2014; pp. 1–16. Available online: http://sciforum.net/conference/ece-1 (accessed on 10 April 2018).
- Bhutto, A.W.; Bazmi, A.A.; Zahedi, G. Underground coal gasification: From fundamentals to applications. Prog. Energy Combust. Sci. 2013, 39, 189–214. [Google Scholar] [CrossRef]
- Couch, G.R. Underground Coal Gasification; Report No.: Contract No.: CCC/151IEA; Clean Coal Centre: London, UK, 2009. [Google Scholar]
- Hammond, G.P. Energy, environment and sustainable development: A UK perspective. Process Saf. Environ. Protect. 2000, 78, 304–323. [Google Scholar] [CrossRef]
- Royal Commission on Environmental Pollution. Twenty-Second Report: Energy—The Changing Climate, Cm 4749; The Stationery Office: London, UK, 2000; pp. 33–38. Available online: http://www.rcep.org.uk/reports/22-energy/22-nergyreport.pdf (accessed on 1 January 2011).
- Intergovernmental Panel on Climate Change (IPCC). IPCC Special Report on Carbon Dioxide and Storage; Cambridge University Press: Cambridge, UK, 2005; Available online: http://www.ipcc.ch/pdf/special-reports/srccs/srccs_wholereport.pdf (accessed on 1 January 2011).
- Hammond, G.P.; Waldron, R. Risk assessment of UK electricity supply in a rapidly evolving energy sector. Proc. Inst. Mech. Eng. A J. Power. Energy 2008, 222, 623–642. [Google Scholar] [CrossRef]
- Lendle, A.; Schaus, M. Sustainability Criteria in the EU Renewable. Energy Directive: Consistent with WTO Rules? ICTSD Information Note No. 2; International Centre for Trade and Sustainable Development: San Francisco, CA, USA, 2010. [Google Scholar]
- Fabra, N.; Matthes, F.C.; Newbery, D.; Colombier, M. The Energy Transition in Europe: Initial Lessons from Germany, the UK and France; Towards a Low Carbon European Power Sector; CERRE (Centre on Regulation in Europe): Brussels, Belgium, 2015. [Google Scholar]
- Deloitte, C. Energy Market Reform in Europe. European. Energy and Climate Policies: Achievements and Challenges to 2020 and Beyond; Deloitte Conseil: Paris, France, 2015. [Google Scholar]
- Onea, F.; Rusu, E. Sustainability of the Reanalysis Databases in Predicting the Wind and Wave Power along the European Coasts. Sustainability 2018, 10, 193. [Google Scholar] [CrossRef]
- EEA (European Environment Agency). Renewable Energy in Europe 2017. Recent Growthand Knock-On Effects; EEA Report No. 3/2017; EEA: Copenhagen, Denmark, 2017; ISBN 978-92-9213-848-6.
- Świądrowski, J.; Stańczyk, K. Environmental aspects of underground coal gasification. Chemik 2016, 70, 239–246. [Google Scholar]
- Mocek, P.; Gil, I. Analiza procesowa podziemnego zgazowania węgla w istniejącej i zlikwidowanej kopalni—Process analysis of underground coal gasification in existing and abandoned mine. Chemik 2015, 69, 827–839. [Google Scholar]
- Brown, K.M. In situ coal gasification: An emerging technology. J. Am. Soc. Min. Reclam. 2012, 1, 103–122. [Google Scholar] [CrossRef]
- Prabu, V.; Jayanti, S. Underground coal-air gasification based solid oxide fuel cell system. Appl. Energy 2012, 94, 406–414. [Google Scholar] [CrossRef]
- Prabu, V.; Jayanti, S. Integration of underground coal gasification with a solid oxide fuel cell system for clean coal utilization. Int. J. Hydrogen Energy 2012, 37, 1677–1688. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Liu, S.; Yu, L.; Zhang, W. Field test of large-scale hydrogen manufacturing from underground coal gasification (UCG). Int. J. Hydrogen Energy 2008, 33, 1275–1285. [Google Scholar] [CrossRef]
- Stanczyk, K.; Smolinski, A.; Kapusta, K.; Wiatowski, M.; Swiadrowski, J.; Kotyrba, A.; Rogut, J. Dynamic experimental simulation of hydrogen oriented underground gasification of lignite. Fuel 2010, 89, 3307–3314. [Google Scholar] [CrossRef]
- Stanczyk, K.; Kapusta, K.; Wiatowski, M.; Swiadrowski, J.; Smolinski, A.; Rogut, J.; Kotyrba, A. Experimental simulation of hard coal underground gasification for hydrogen production. Fuel 2012, 91, 40–50. [Google Scholar] [CrossRef]
- Seifi, M.; Abedi, J.; Chen, Z. Application of porous medium approach to simulate UCG process. Fuel 2014, 116, 191–200. [Google Scholar] [CrossRef]
- Stanczyk, K.; Howaniec, N.; Smolinski, A.; Swiadrowski, J.; Kapusta, K.; Wiatowski, M.; Grabowski, J.; Rogut, J. Gasification of lignite and hard coal with air and oxygen enriched air in a pilot scale ex situ reactor for underground gasification. Fuel 2011, 90, 1953–1962. [Google Scholar] [CrossRef]
- Laciak, M.; Kačur, J.; Kostúr, K. The verification of thermodynamic model for ucg process. In Proceeding of the IEEE 17th International Carpathian Control Conference (ICCC), Tatranska Lomnica, Slovakia, 29 May–1 June 2016; pp. 424–428. [Google Scholar]
- Friedmann, S.J.; Upadhye, R.; Kong, F.M. Prospects for underground coal gasification in carbon-constrained world. Energy Procedia 2009, 1, 4551–4557. [Google Scholar] [CrossRef]
- Laciak, M.; Kostúr, K.; Durdán, M.; Kačur, J.; Flegner, P. The analysis of the underground coal gasification in experimental equipment. Energy 2016, 114, 332–343. [Google Scholar] [CrossRef]
- Gür, M.; Eskin, N.; Okutan, H.; Arısoy, A.; Böke, E.; Altıntaş, Ü.; Yıldız, A.; Büyükşirin, O.; Yıldırım, O.; Canbaz, E.D. Experimental Results of Underground Coal Gasification of Turkish Lignite in an Ex-Situ Reactor. Fuel 2017, 203, 997–1006. [Google Scholar] [CrossRef]
- Yang, D.; Koukouzas, N.; Green, M.; Sheng, Y. Recent development on underground coal gasification and subsequent CO2 storage. J. Energy Inst. 2016, 89, 469–484. [Google Scholar] [CrossRef]
- Mao, F. Underground coal gasification (UCG): A new trend of the supply-side economics of fossil fuels. Nat. Gas Ind. B 2016, 3, 312–322. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Yu, L. Environmental benefits of underground coal gasification. J. Environ. Sci. 2002, 14, 284–288. [Google Scholar]
- Khadse, A.; Qayyumi, M.; Mahajani, S.; Aghalayam, P. Underground coal gasification: A new clean coal utilization technique for India. Energy 2007, 32, 2061–2071. [Google Scholar] [CrossRef]
- Lloyd-Smith, M. Underground Coal Gasification (UCG), Report NTN (National Toxics Network) 2015, Bangalow, Australia. Available online: www.ntn.org.au (accessed on 7 May 2018).
- Smoliński, A.; Stańczyk, K.; Kapusta, K.; Howaniec, N. Analysis of the organic contaminants in the condensate produced in the in situ underground coal gasification process. Water Sci. Technol. 2013, 67, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.D.; Boyd, R.M.; Schrider, L.A. Environmental Impact Studies Related to Underground Coal Gasification; Energy Research and Development Administration Laramie Energy Research Center, University Station Laramie: Laramie, WY, USA, 1975; p. 15. [Google Scholar]
- Imran, M.; Kumar, D.; Kumar, N.; Qayyum, A.; Saeed, A.; Bhatti, M.S. Environmental concerns of underground coal gasification. Renew. Sustain. Energy Rev. 2014, 31, 600–610. [Google Scholar] [CrossRef]
- Xu, B.; Chen, L.; Xing, B.; Li, L.; Zhang, L.; Wang, X.; Chen, H.; Yi, G.; Huang, G. The environmental effect of underground coal gasification semi-coke on confined groundwater Environmental Progress and Sustainable. Energy 2016, 35, 1584–1589. [Google Scholar]
- Sury, M.; White, M.; Kirton, J.; Carr, P.; Woodbridge, R.; Mostade, M.; Chappell, R.; Hartwell, D.; Hunt, D.; Rendell, N. Review of Environmental Issues of Underground Coal Gasification Report No. COAL R272 DTI/Pub URN 04/1880 2004, 24; FWS Consultants Ltd.: Spennymoor, UK, 2004; Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwjvjsTkv_faAhULpJQKHel2CkoQFgguMAA&url=http%3A%2F%2Flarge.stanford.edu%2Fcourses%2F2014%2Fph240%2Fcui2%2Fdocs%2Ffile19154.pdf&usg=AOvVaw0Kpor-fCFMSp2fofCw7Xf_ (accessed on 7 May 2018).
- Xu, B.; Chen, L.; Li, L.; Xing, B.; Huang, G.; Wang, X.; Yi, G.; Su, Y.; Zhan, L. Removal Properties of Hexavalent Chromium and Hazardous Trace Elements in UCG Condensate Water by Semi-Cokes. Coke Chem. 2015, 58, 188–195. [Google Scholar] [CrossRef]
- Van den Hove, S.; Le Menestrel, M.; de Bettignies, H.-C. The oil industry and climate change: Strategies and ethical dilemmas. Clim. Policy 2002, 2, 3–18. [Google Scholar] [CrossRef]
- Skjaerseth, J.B.; Skodvin, T. Climate Change and the Oil Industry: Common Problems, Different Strategies. Glob. Environ. Politics 2001, 1, 43–64. [Google Scholar] [CrossRef]
- Wang, D.; Li, T. Carbon Emission Performance of Independent Oil and Natural Gas Producers in the United States. Sustainability 2018, 10, 110. [Google Scholar] [CrossRef]
- Hiatt, S.R.; Grandy, J.B.; Lee, B.H. Organizational Responses to Public and Private Politics: An Analysis of Climate Change Activists and U.S. Oil and Gas Firms. Organ. Sci. 2015, 26, 1769–1786. [Google Scholar] [CrossRef]
- Ding, T.; Ning, Y.; Zhang, Y. The Contribution of China’s Outward Foreign Direct Investment (OFDI) to the Reduction of Global CO2 Emissions. Sustainability 2017, 9, 741. [Google Scholar] [CrossRef]
- Burchart-Korol, D.; Krawczyk, P.; Czaplicka, K.; Smoliński, A. Eco-efficiency of underground coal gasification (UCG) for electricity production. Fuel 2016, 173, 239–246. [Google Scholar] [CrossRef]
- Roddy, D.J.; Younger, P.L. Underground coal gasification with CCS: A pathway to decarbonising industry. Energy Environ. Sci. 2010, 3, 400–407. [Google Scholar] [CrossRef]
- Kempka, T.; Fernández-Steeger, T.; Li, D.Y.; Schulten, M.; Schlüter, R.; Krooss, B.M. Carbon dioxide sorption capacities of coal gasification residues. Environ. Sci. Technol. 2011, 45, 1719–1723. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.P.; Ondo Akwea, S.S.; Williams, S. Techno-economic appraisal of fossil-fueled power generation systems with carbon dioxide capture and storage. Energy 2011, 36, 975–984. [Google Scholar] [CrossRef]
- Kostur, K. Modeling of flowing underground waters and leakage of syngas from Underground Coal Gasification. In Proceedings of the 17th International Carpathian Control Conference (ICCC), Tatranska Lomnica, Slovakia, 29 May–1 June 2016; pp. 352–357. [Google Scholar]
- Kuo, H.F.; Tsou, K.W. Modeling and Simulation of the Future Impacts of Urban Land Use Change on the Natural Environment by SLEUTH and Cluster Analysis. Sustainability 2018, 10, 72. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Lu, X.; Hu, J.; Pan, X.; Zhu, Q.; Su, W. Effects of Building Design Elements on Residential Thermal Environment. Sustainability 2018, 10, 57. [Google Scholar] [CrossRef]
- Skelhorn, C.; Lindley, S.; Levermore, G. The impact of vegetation types on air and surface temperatures in a temperatecity: A fine scale assessment in Manchester, UK. Landsc. Urban Plan. 2014, 121, 129–140. [Google Scholar] [CrossRef]
- Su, W.; Zhang, Y.; Yang, Y.; Ye, G. Examining the Impact of Greenspace Patternson Land Surface Temperature by Coupling LiDAR Data with a CFD Model. Sustainability 2014, 6, 6799–6814. [Google Scholar] [CrossRef]
- Kazak, D.N.I.; Pristash, V.N. Changes in rocks at high temperatures. In Geotechnological Methods; Plenum Publishing Corporation: Berlin, Germany, 1994; pp. 472–777. [Google Scholar]
- Kinoshita, N.; Yasuhara, H. Thermally induced behavior of the openings in rock mass affected by high temperatures. ASCE Int. J. Geomech. 2011, 11, 124–130. [Google Scholar] [CrossRef]
- Bing, L.; Hong, G.; Yong-wei, L. Theoretical analysis and experimental study on relation between rock permeability and temperature. Chin. J. Rock Mech. Eng. 2005, 24, 176–188. [Google Scholar]
- Lin, X.; Zuotang, W.; Wengang, H.; Guojun, K.; Xuefeng, L.; Peng, Z.; Jianhua, W. Temperature field distribution of burnt surrounding rock in UCG stope. Int. J. Min. Sci. Technol. 2014, 4, 573–580. [Google Scholar]
- Chen, L.; Chaohu, H.; Chen, J.; Jiting, X. A back analysis of the temperature field in the combustion volume space during underground coal gasification. Min. Sci. Technol. 2009, 21, 581–585. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S. Numerical simulation on heat transfer in the process of underground coal gasification. Numerical Heat Transfer Part A: Applications. Int. J. Comput. Methodol. 2003, 44, 537–557. [Google Scholar]
- Wrana, A.; Rodriguez, A.; Schlueter, R.; Farret, R.; Syrek, B.; Kostúr, K. Underground Coal Gasification in Operating Mines and Areas of High Vulnerability, Midterm 2015 and Final 2017 Report; European Commission, RFCS: Brussels, Belgium, 2017. [Google Scholar]
- Kotyrba, A.; Kortas, Ł.; Stańczyk, K. Imaging the Underground Coal Gasification Zone with Microgravity Surveys. Acta Geophys. 2015, 63, 634–651. [Google Scholar] [CrossRef][Green Version]
- Kaloustian, N.; Diab, Y. Effects of urbanization on the urban heat island in Beirut. Urban Clim. 2015, 14, 154–165. [Google Scholar] [CrossRef]
- Kikon, N.; Singh, P.; Singh, S.K.; Vyas, A. Assessment of urban heat islands (UHI) of Noida City, India using multi-temporal satellite data. Sustain. Cities Soc. 2016, 22, 19–28. [Google Scholar] [CrossRef]
- D’Alfano, F.R.; Olesen, B.W.; Palella, B.I. Povl Ole Fanger’s Impact Ten Years Later. Energy Build. 2017, 152, 243–249. [Google Scholar]
- Parsons, K. Human Thermal Environments: The Effects of Hot, Moderate and Cold Environments on Human Health, Comfort and Performance; CRC Press, Inc.: Boca Raton, FL, USA, 2014; pp. 67–68. [Google Scholar]
- Gilani, S.I.U.H.; Khan, M.H.; Pao, W. Thermal Comfort Analysis of PMV Model Prediction in Air Conditioned and Naturally Ventilated Buildings. Energy Procedia 2015, 75, 1373–1379. [Google Scholar] [CrossRef]
- Lu, Y.I.; Wang, L.G.; Tang, F.R.; He, Y. Fracture evolution of overlying strata over combustion cavity under thermal-mechanical interaction during underground coal gasification. J. Chin. Coal Soc. 2012, 37, 1292–1298. [Google Scholar]
- Lewis, T.; Villinger, H.; Davis, E. Thermal conductivity measurement of rock fragments using a pulsed needle probe. Can. J. Earth Sci. 1993, 30, 480–485. [Google Scholar] [CrossRef]
- Daggupati, S.; Mandapati, R.N.; Mahajani, S.; Ganesh, A.; Pal, A.K.; Sharma, R.K.; Aghalayam, P. A study on the temperature profile and heat transfer coefficients in underground coal gasification cavities. In Proceedings of the 27th Annual International Pittsburgh Coal Conference 2010, Istanbul, Turkey, 11 October 2010; Volume 3, pp. 2243–2256. [Google Scholar]
- Kostur, K. The Possibilities of Improving Underground Coal Gasification Processes. J. Energy Power Eng. 2011, 51041–51052. [Google Scholar]
- Karwasiecka, M. Porównanie oerodowiska geotermicznego w obrêbie basenów wêglonooenych lubelskiego i górnooel¥skiego zag£êbia wêglowego (The comparison of geothermal environment in the Upper Silesian Coal Basin and Lublin Coal Basin). Geologia 2008, 34, 335–357. [Google Scholar]
- Fuchs, S.; Schütz, F.; Förster, H.-J.; Förster, A. Evaluation of common mixing models for calculating bulk thermal conductivity of sedimentary rocks: Correction charts and new conversion equations. Geothermics 2013, 47, 40–52. [Google Scholar] [CrossRef]
- Najafi, M.; Jalali, S.M.E.; KhaloKakaie, R. Thermal-Mechanical Numerical Analysis of Stress Distribution in the vicinity of Underground Coal Gasification (UCG) Panels. Int. J. Coal Geol. 2014, 134, 1–16. [Google Scholar] [CrossRef]
- Wolf, K.H.; Bruining, H. Modelling the interaction between underground coal fires and their roof rocks. Fuel 2007, 86, 2761–2777. [Google Scholar] [CrossRef]
- Christopher, O.; Kempka, T. Thermo-Mechanical Simulations of Rock Behavior in Underground Coal Gasification Show Negligible Impact of Temperature-Dependent Parameters on Permeability Changes. Energies 2015, 8, 5800–5827. [Google Scholar]
- Wrana, A. Additional Data for Project COGAR. Internal Information, Katowice, 2016. Available online: http://meteo.gig.eu (accessed on 8 May 2018).
- Janásek, D.; Potoček, T.; Svetlík, J. Dangerous Substances, 1st ed.; University of Zilina: Zilina, Slovakia, 2004; pp. 13–24. ISBN 80-8070-243-8. [Google Scholar]
- Nassimi, A.M.; Jafari, M.; Farrokhpour, H.; Keshavarz, M.H. Constants of explosive limits. Chem. Eng. Sci. 2017, 73, 384–389. [Google Scholar] [CrossRef]
- Struttmann, T.; Scheerer, A.; Prince, T.S.; Goldstein, L.A. Unintentional Carbon Monoxide Poisoning From an Unlikely Source. J. Am. Board Fam. Med. 1998, 11, 481–484. [Google Scholar] [CrossRef]
- Struttmann, T.; Scheerer, A.; Prince, T.S.; Goldstein, L.A. Carbon Monoxide Poisoning. J. Emerg. Nurs. 2008, 34, 538–542. [Google Scholar] [CrossRef]
- Wiatowski, M.; Stańczyk, K.; Świadrowski, J.; Kapusta, K.; Cibulski, K.; Krause, E.; Grabovwki, J.; Rogut, J.; Howaniec, N.; Smolinski, A. Semi-technical underground coal gasification (UCG) using the shaft method in experimental mine Barbara. Fuel 2012, 99, 170–179. [Google Scholar] [CrossRef]
- Mocek, P.; Pieszczek, M.; Świadrowski, J.; Kapusta, K.; Wiatowski, M.; Stańczyk, K. Pilot-scale underground coal gasification (UCG) experiment in an operating Mine “Wieczorek” in Poland. Energy 2016, 111, 313–321. [Google Scholar] [CrossRef]
- Kapusta, K.; Wiatowski, M.; Stańczyk, K. An experimental ex-situ study of the suitability of a high moisture ortho-lignite for underground coal gasification (UCG) process. Fuel 2016, 179, 150–155. [Google Scholar] [CrossRef]
- Wrona, P.; Różański, Z.; Pach, G.; Suponik, T.; Popczyk, M. Closed coal mine shaft as a source of carbon dioxide emissions. Environ. Earth Sci. 2016, 75, 1–12. [Google Scholar] [CrossRef][Green Version]
- Kostúr, K. Mathematical modeling temperature’s fields in overburden during underground coal gasification. In Proceeding of the IEEE 15th International Carpathian Control Conference (ICCC), Velke Karlovice, Czech Republic, 28–30 May 2014; pp. 248–253. [Google Scholar]
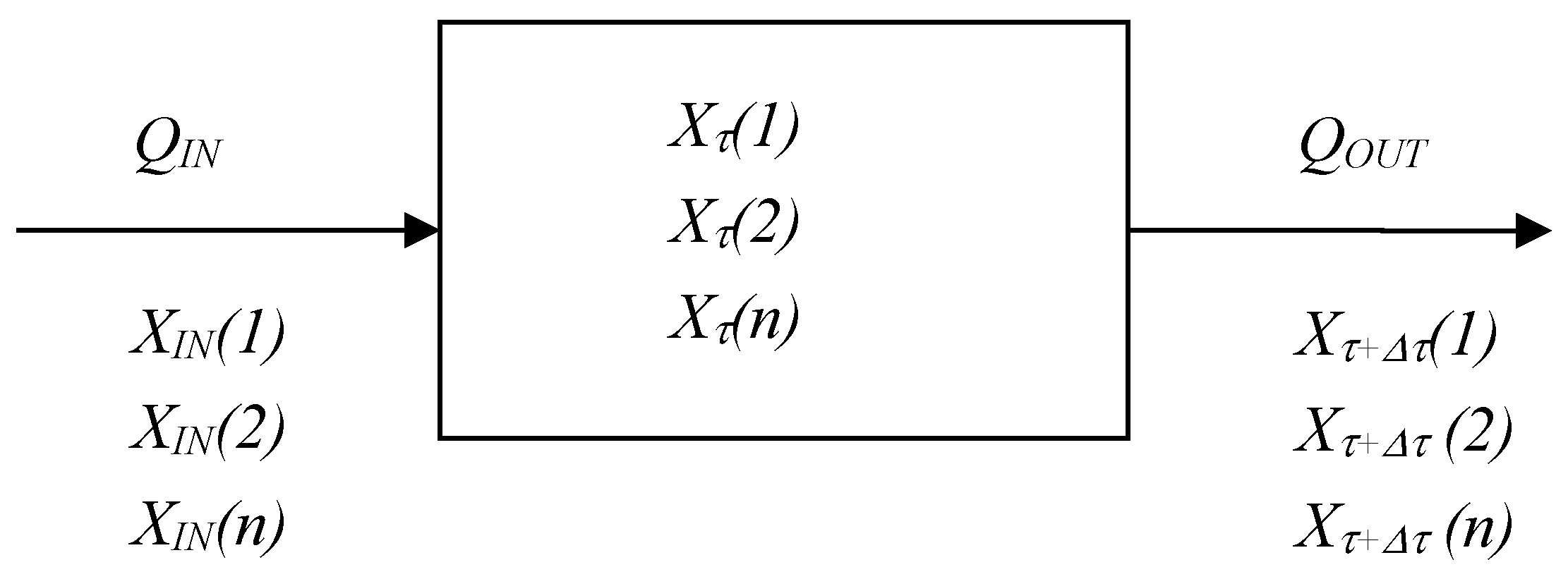




| Proximate Analysis | Elementary Analysis | ||
|---|---|---|---|
| Total moisture Wr | 38.2% | Cdaf | 76.5% |
| Volatiles Vdaf | 50.1% | Hdaf | 3.95% |
| Ash Ad | 9.4% | Ndaf | 1.48% |
| Heat value | 16.1 MJ/kg | Total Sulphur S | 1.62% |
| Moisture wt. (%) | 6.39 |
| Ash At (%) | 16.52 |
| Volatile matter Vt (%) | 29.84 |
| S total St (%) | 0.51 |
| Elementary analysis | |
| C (%) | 57.95 |
| H (%) | 3.70 |
| N (%) | 0.87 |
| O (%) | 14.03 |
| Layer | Heat Conductivity (W·m−1·K−1) | Specific Heat Capacity (J·kg−1·K−1) | Density (kg·m−3) |
|---|---|---|---|
| Coal | 0.4 | 1000 | 1500 |
| Shale | 0.9 | 800 | 2400 |
| Sandstone | 1 | 800 | 2400 |
| Clay | 1.25 | 920 | 1600 |
| Sand | 0.26 | 830 | 1900 |
| Sandstone fine-grained | 4.303 | 757 | 2183 |
| Sandstone coarse-grained | 1.73 | 917 | 1868 |
| Sand and Shale | 0.58 | 815 | 2150 |
| Clay and Shale | 1.075 | 860 | 2000 |
| Grey sandstone | 3.018 | 806 | 1994 |
| Simulation | Velocity in Direction | Velocity in Direction | Experiment Duration | |||
|---|---|---|---|---|---|---|
| (m/h) | (m/Day) | (m/h) | (m/Day) | (h) | (Day) | |
| 1 | 0.0417 | 1 | 0.0073 | 0.1757 | 288 | 12 |
| 2 | 0.0208 | 0.5 | 0.0208 | 0.5 | 576 | 24 |
| 3 | 0.0833 | 2 | 0.0833 | 2 | 144 | 6 |
| Temperature (°C) | Distance from Boundary Coal—Overburden (m) | ||
|---|---|---|---|
| The First Simulation | The Second Simulation | The Third Simulation | |
| 300 | 0.55 | 0.5 | 0.4 |
| 500 | 0.23 | 0.15 | 0.1 |
| Component | LEL (%) | UEL (%) |
|---|---|---|
| H2 | 4.0 | 70.0 |
| CH4 | 5.5 | 60.0 |
| CO | 13.5 | 14.0 |
| Effect | vol % CO |
|---|---|
| no symptoms of poisoning after a long time | 0.01 |
| headache after 2–3 h | 0.02 |
| a distinct poisoning after 2–3 h | 0.03 |
| a distinct poisoning after 1–2 h | 0.04 |
| unconsciousness after 2–3 h | 0.043 |
| hallucinations after 30–120 min | 0.05 |
| unconsciousness after 1.5 h, death after 6 h | 0.087 |
| death after 2 h | 0.1 |
| death after 1 h | 0.15 |
| death after 30 min | 0.3 |
| death a few minutes | 0.44 |
| immediate death | 0.79 |
| Experiment | %H2 | %CO | %CH4 | %CO2 | %N2 | %O2 |
|---|---|---|---|---|---|---|
| Cigel | 14.4 | 9.1 | 14.7 | 18.4 | 40.1 | 3.3 |
| Barbara 1 | 36.3 | 31.9 | 2.4 | 14.9 | 21.3 | 1.5 |
| Barbara 2 | 14.2 | 13.4 | 1.5 | 16.4 | 52.4 | 1.9 |
| Wieczorek | 11.0 | 14.4 | 1.8 | 9.3 | 63.2 | 0.1 |
| Case | Volume of Room (m3) | Critical Time (h) | Critical Time (Day) |
|---|---|---|---|
| Cigel | 80 | 88,732 | 3697 |
| 1600 | 1,774,628 | 73,943 | |
| Barbara 1 | 80 | 1310 | 54 |
| 1600 | 26,206 | 1092 | |
| Barbara 2 | 80 | 3714 | 155 |
| 1600 | 74,266 | 3094 | |
| Wieczorek | 80 | 123,383 | 5140 |
| 1600 | 2,467,650 | 102,818 |
| Case | Volume of Room (m3) | Critical Time (%CO = 0.1) (h) | Critical Time (%CO = 0.01) (h) |
|---|---|---|---|
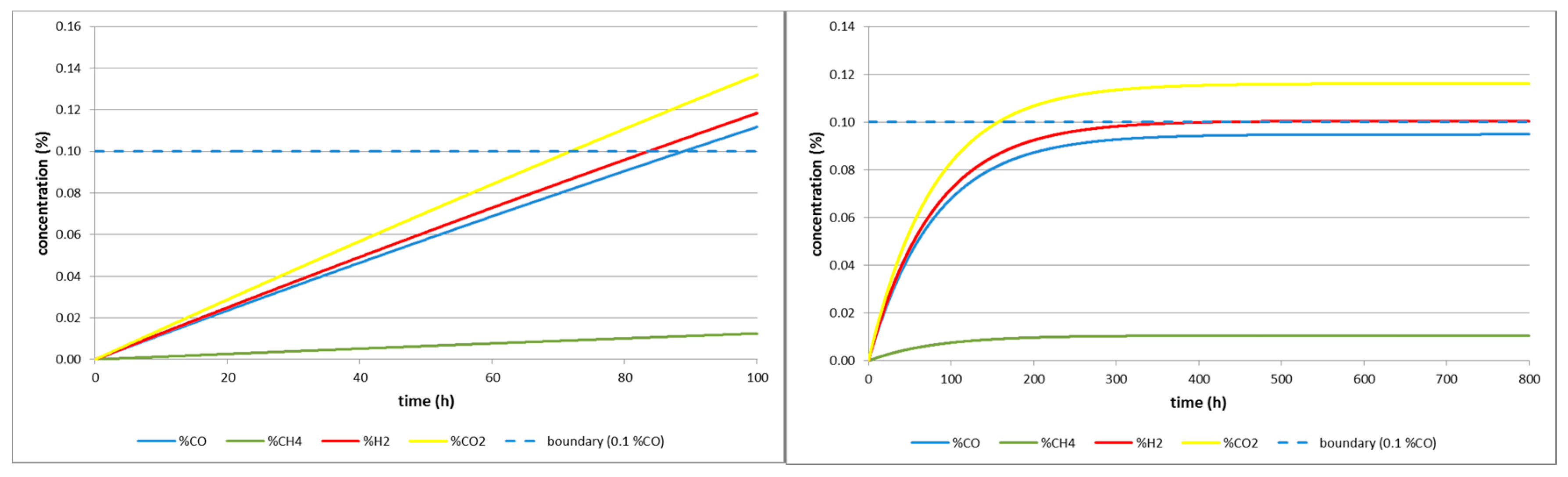
| Cigel | 80 | 3013 | 300 |
| 1600 | 60,259 | 5996 | |
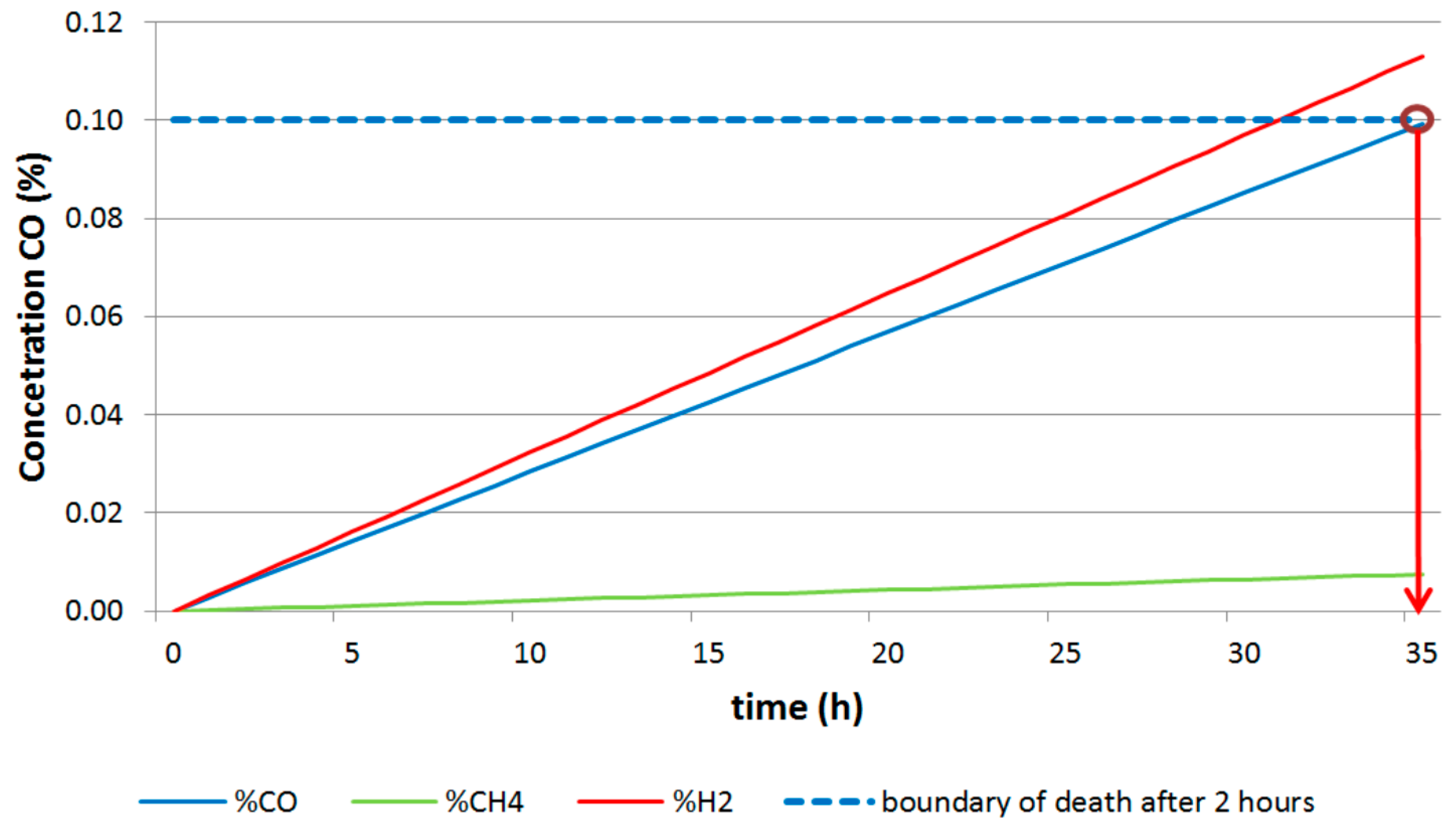
| Barbara 1 | 80 | 35 | 3.6 |
| 1600 | 704 | 70.4 | |
| Barbara 2 | 80 | 84 | 8.4 |
| 1600 | 1681 | 167.6 | |
| Wieczorek | 80 | 1910 | 189 |
| 1600 | 38,100 | 3788 |
| Critical Time (h) | ||||
|---|---|---|---|---|
| Volume Flow of Fresh Air (m3/h) | Explosion (exp. Barbara 1) | CO Poisoning (exp. Barbara 1) | Explosion (exp. Barbara 2) | CO Poisoning (exp. Barbara 2) |
| 0 | 2027 | 35.3 | 6162 | 84 |
| 0.01 | 2364 | 35.4 | - | 84.6 |
| 0.03 | 4251 | 35.5 | - | 85.5 |
| 0.1 | - | 36.1 | - | 88.9 |
| 1 | - | 46.5 | - | |
| Emission (×10−7 m3/s) | |||
|---|---|---|---|
| Experiment | CO2 | CO | CH4 |
| Barbara 1 | 2.95 | 6.32 | 0.47 |
| Barbara 2 | 3.25 | 2.65 | 0.29 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostúr, K.; Laciak, M.; Durdan, M. Some Influences of Underground Coal Gasification on the Environment. Sustainability 2018, 10, 1512. https://doi.org/10.3390/su10051512
Kostúr K, Laciak M, Durdan M. Some Influences of Underground Coal Gasification on the Environment. Sustainability. 2018; 10(5):1512. https://doi.org/10.3390/su10051512
Chicago/Turabian StyleKostúr, Karol, Marek Laciak, and Milan Durdan. 2018. "Some Influences of Underground Coal Gasification on the Environment" Sustainability 10, no. 5: 1512. https://doi.org/10.3390/su10051512
APA StyleKostúr, K., Laciak, M., & Durdan, M. (2018). Some Influences of Underground Coal Gasification on the Environment. Sustainability, 10(5), 1512. https://doi.org/10.3390/su10051512






