Soil Biodiversity in Urban Forests as a Consequence of Litterfall Management: Implications for São Paulo’s Ecosystem Services
Abstract
1. Introduction
2. Material and Methods
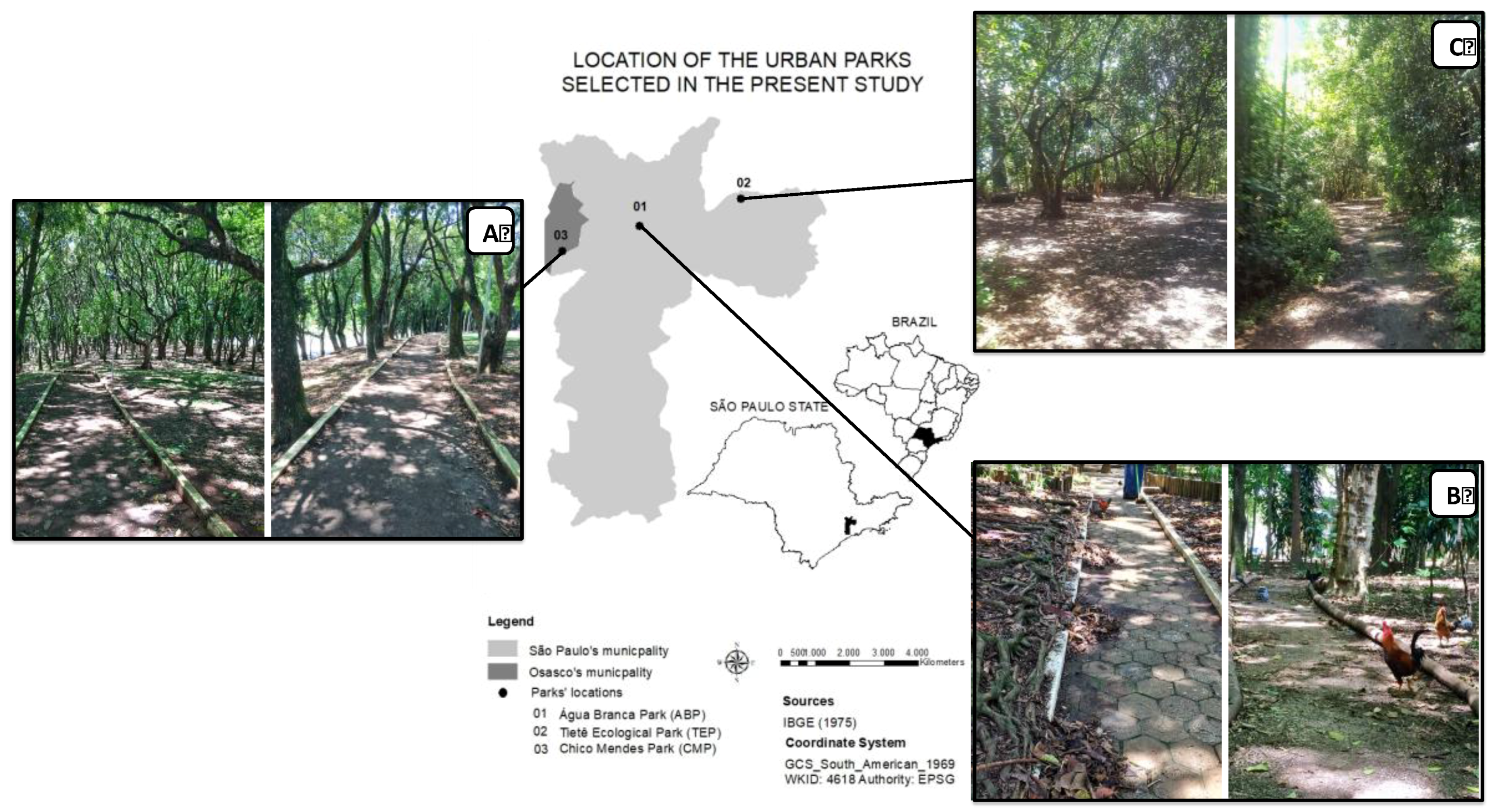
2.1. Study Areas
2.2. Litterfall Sampling
2.3. Screening of Litterfall Fractions
2.4. Screening and Identification of Arthropods
2.5. Data Analysis
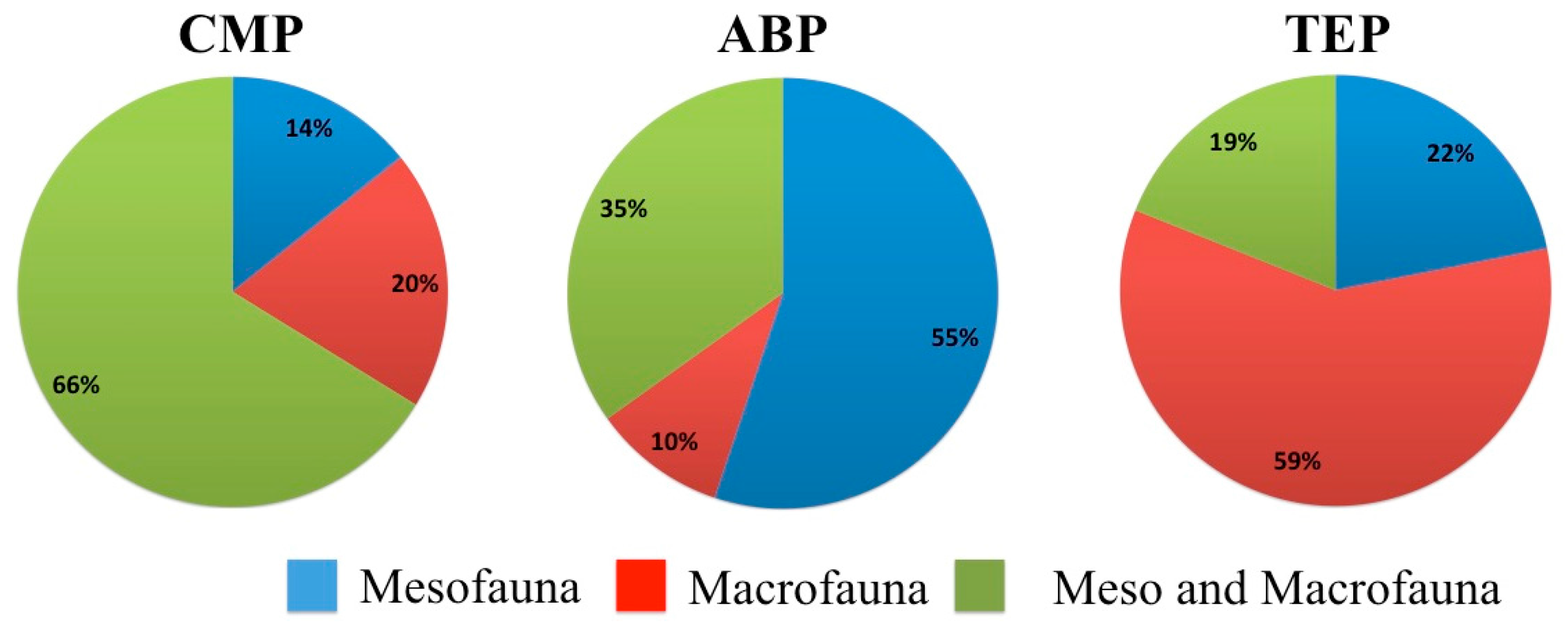
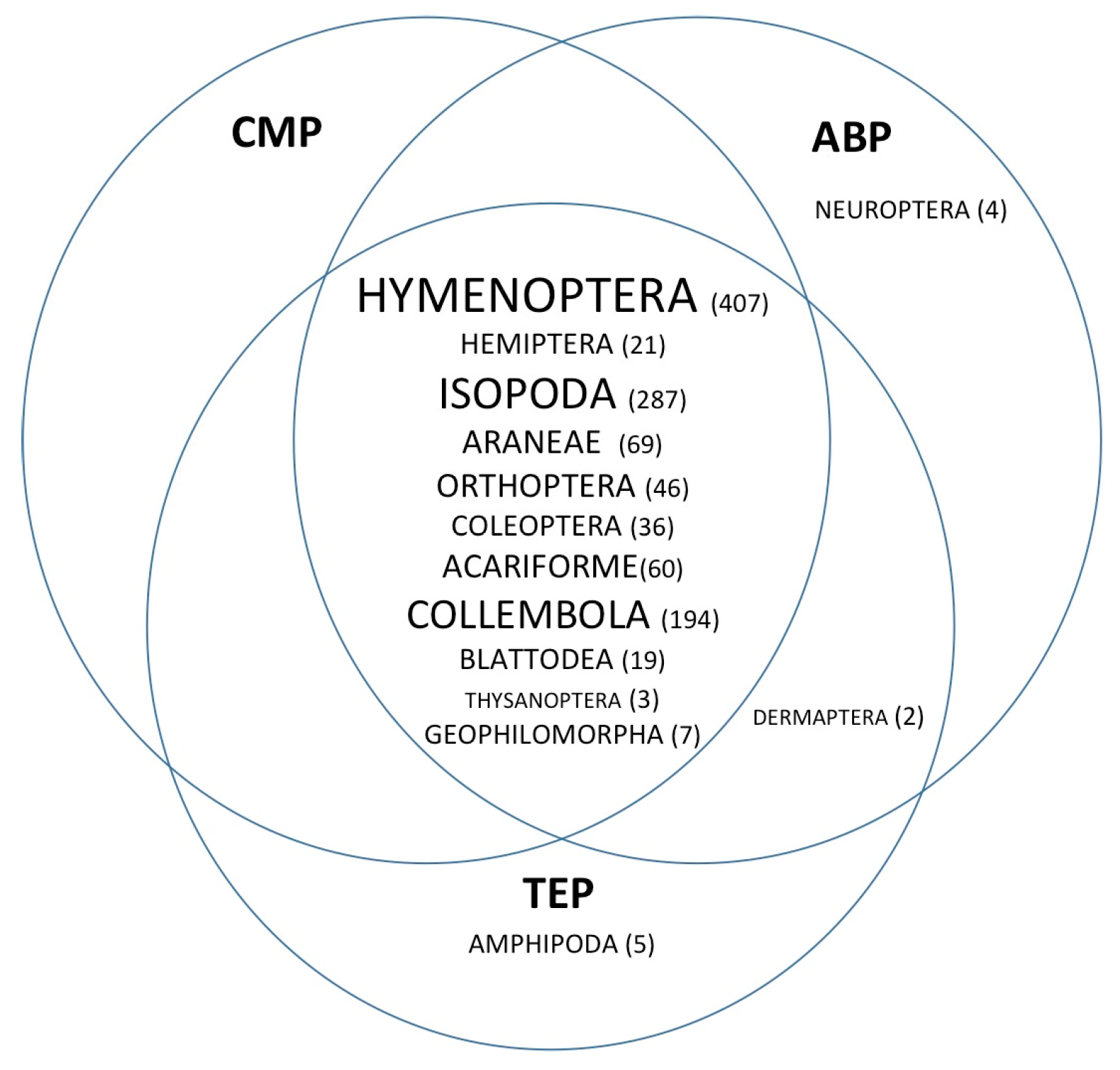
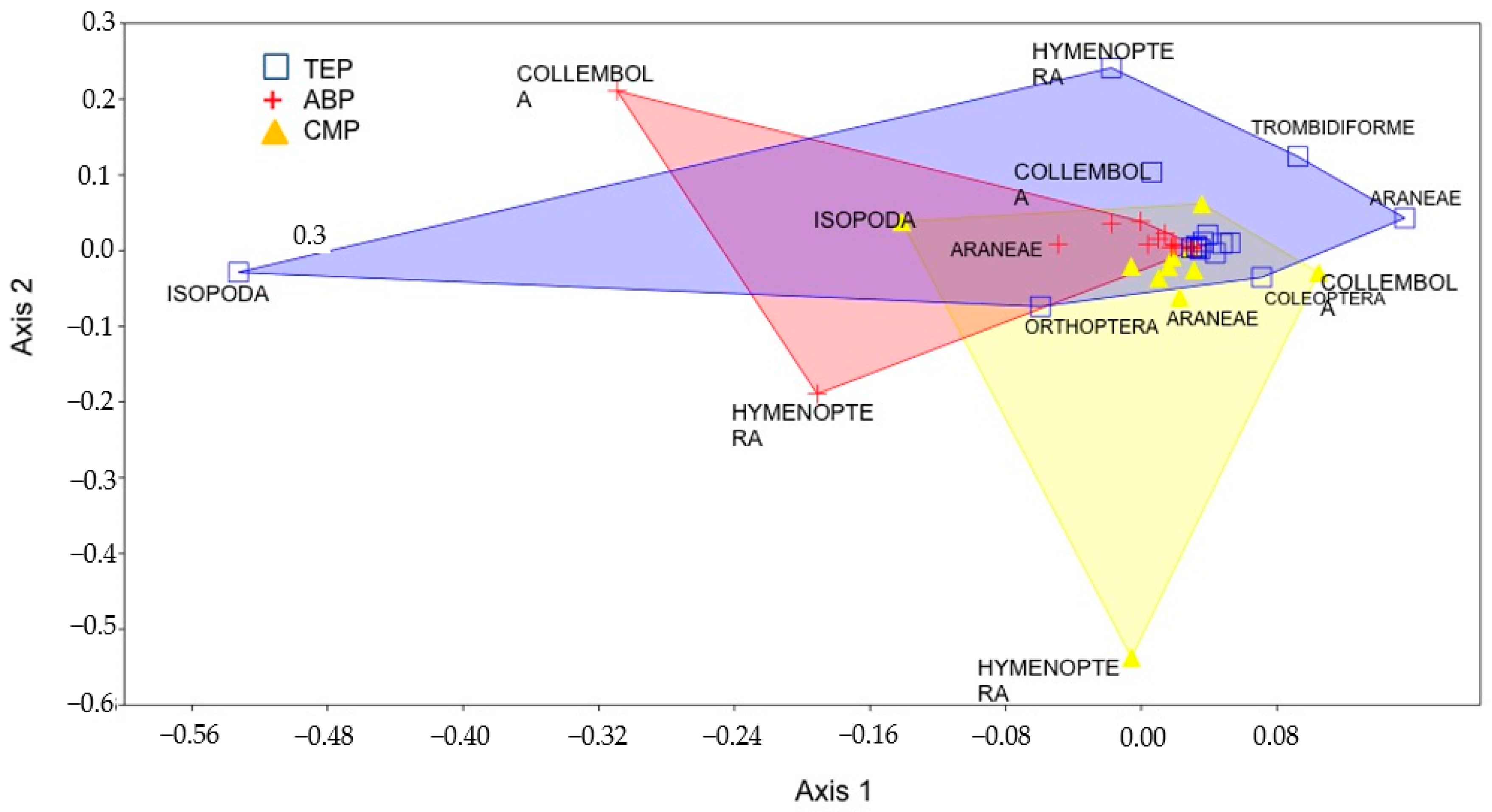
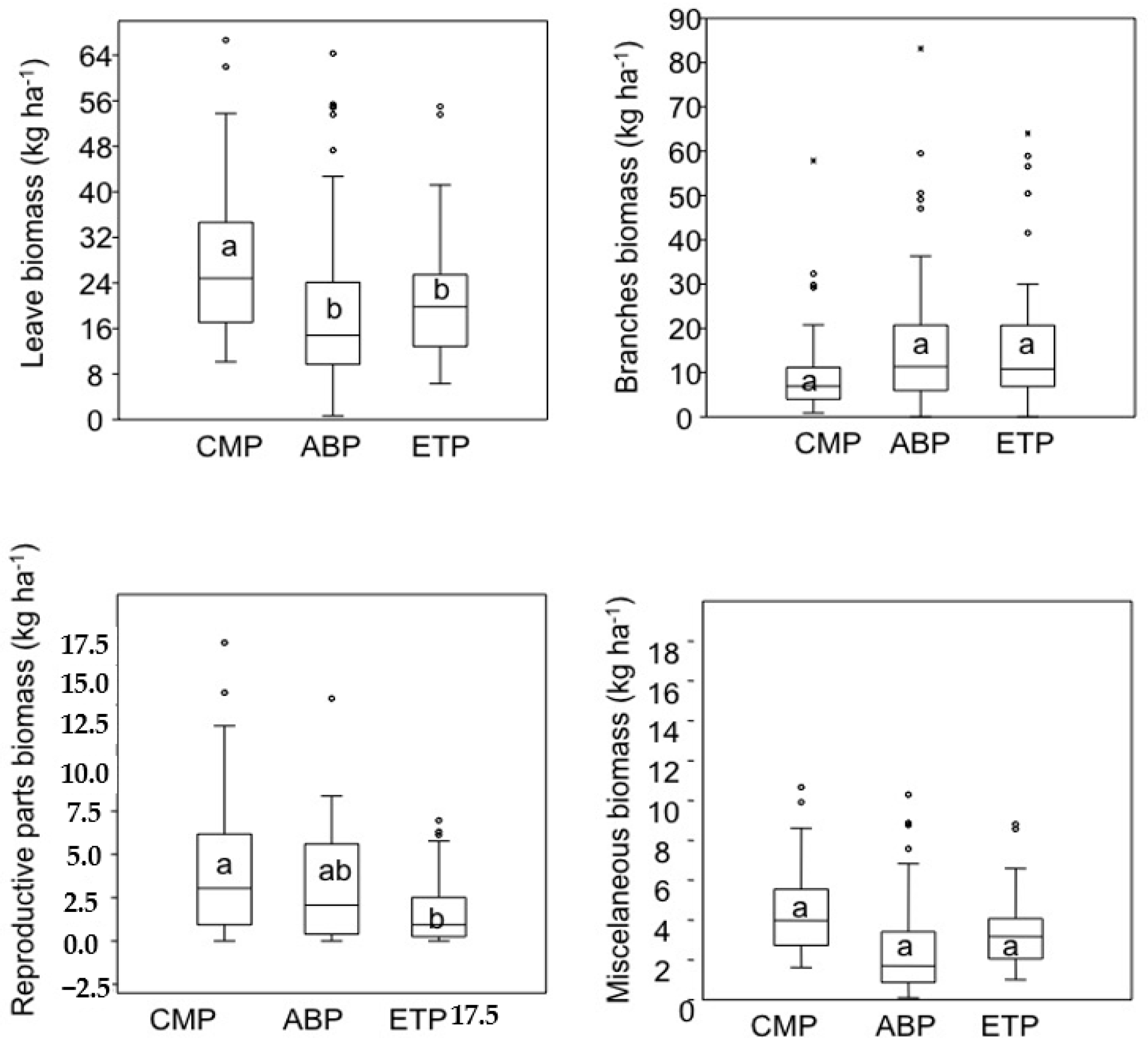
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seto, K.C.; Gueneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, B.H.S. The effect of urban green infrastructure on disaster mitigation in Korea. Sustainability 2017, 9, 26. [Google Scholar] [CrossRef]
- Nowak, D.J.; Noble, M.H.; Sisinni, S.M.; Dwyer, J.F. People and Trees: Assessing the US Urban Forest Resource. J. For. 2001, 99, 37–42. [Google Scholar]
- Li, H.; Li, Y.; Gao, Y.; Zou, C.; Yan, S.; Gao, J. Human impact on vegetation dynamics around Lhasa, southern Tibetan Plateau, China. Sustainability 2016, 8, 1146. [Google Scholar] [CrossRef]
- Vitousek, P.M. Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 1984, 79, 10–22. [Google Scholar] [CrossRef]
- Brown, K.S.; Freitas, A.V. Butterfly communities of urban forest fragments in Campinas, Sao Paulo, Brazil: Structure, instability, environmental correlates, and conservation. J. Insect Conserv. 2002, 6, 217–231. [Google Scholar] [CrossRef]
- Bai, S.H.; Xu, Z.; Blumfield, T.J.; Reverchon, F. Human footprints in urban forests: Implication of nitrogen deposition for nitrogen and carbon storage. J. Soils Sediments 2015, 15, 1927–1936. [Google Scholar]
- Bednova, O.V.; Kuznetsov, V.A.; Tarasova, N.P. Transformation of urban forest ecosystems: Indication and integral assessment. Dokl. Akad. Nauk 2015, 463, 713–718. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Uchiyama, E.A. Litterfall assessement in a fragment of secondary tropical forest, Ibiúna, SP, southeastern Brazil. Rev. Árvore 2015, 39, 791–799. [Google Scholar] [CrossRef]
- Ashford, O.S.; Foster, W.A.; Turner, B.L.; Sayer, E.J.; Sutcliffe, L.; Tanner, E.V.J. Litter manipulation and the soil arthropod community in a lowland tropical rainforest. Soil Biol. Biochem. 2013, 62, 5–12. [Google Scholar] [CrossRef]
- Panno, A.; Carrus, G.; Lafortezza, R.; Mariani, L.; Sanesi, G. Nature-based solutions to promote human resilience and wellbeing in cities during increasingly hot summers. Environ. Res. 2017, 159, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kreutzweiser, D.P.; Good, K.P.; Capell, S.S.; Holmes, S.B. Leaf-litter decomposition and macroinvertebrate communities in boreal forest streams linked to upland logging disturbance. J. N. Am. Benthol. Soc. 2008, 27, 1–15. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Matson, P.A.; Vitousek, P.; Chapin, M.C. Principles of Terrestrial Ecosystem Ecology, 2nd ed.; Springer: New York, NY, USA, 2011; p. 529. [Google Scholar]
- Livesley, S.J.; McPherson, E.G.; Calfapietra, C. The urban forest and ecosystem services: Impacts on urban water, heat, and pollution cycles at the tree, street, and city scale. J. Environ. Qual. 2016, 45, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Crowther, T.W.; Boddy, L.; Jones, T.H. Outcomes of fungal interactions are determined by soil invertebrate grazers. Ecol. Lett. 2011, 14, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.M.; Ostertag, R.; Cowie, R.H. Macro-invertebrates accelerate litter decomposition and nutrient release in a Hawaiian rainforest. Soil Biol. Biochem. 2011, 43, 206–211. [Google Scholar] [CrossRef]
- Siqueira, T.; Bini, L.M.; Roque, F.O.; Couceiro, S.R.M.; Trivinho-Strixino, S.; Cottenie, K. Common and rare species respond to similar niche processes in macroinvertebrate metacommunities. Ecography 2012, 35, 183–192. [Google Scholar] [CrossRef]
- Bottinelli, N.; Jouquet, P.; Capowiez, Y.; Podwojewski, P.; Grimaldi, M.; Peng, X. Why is the influence of soil macrofauna on soil structure only considered by soil ecologists? Soil Tillage Res. 2015, 146, 118–124. [Google Scholar] [CrossRef]
- Moço, M.K.S.; Rodrigues, E.F.G.; Rodrigues, A.C.G.; Correia, M.E.F. Caracterização da fauna edáfica em diferentes coberturas vegetais na região Norte Fluminense. Rev. Bras. Ciênc. Solo 2005, 29, 555–564. [Google Scholar] [CrossRef]
- Augusto, E.L. Morcegos do Parque Chico Mendes, Osasco, São Paulo, como dispersores de sementes. Rev. PIBIC 2004, 1, 15–19. [Google Scholar]
- Ferreira, M.L.; Silva, J.L.; Pereira, E.E.; Ferreira, A.P.N.L. Litter fall production and decomposition in a fragment of secondary Atlantic Forest of São Paulo, SP, Southeastern Brazil. Rev. Árvore 2014, 38, 591–600. [Google Scholar] [CrossRef]
- Jesus, P.R.; Filho, H.O.L.; Ferreira, M.L. Litterfall production in three different sites with different abundance of eucalyptus (Eucalyptus spp.) at the Piqueri Park, SP. Braz. J. Ecol. 2014, 1–2, 59–65. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Ash, J.S. A study of the Mallophaga of birds with particular reference to their ecology. IBIS 1960, 102, 93–110. [Google Scholar] [CrossRef]
- Evans, D.E.; Martins, J.R.; Guglielmone, A.A. A review of the ticks (Acari, Ixodida) of Brazil, their hosts and geographic distribution—1. The State of Rio Grande do Sul, Southern Brazil. Mem. Inst. Oswaldo Cruz 2000, 95, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.G.; Brigham, C.A.; Traut, B.H.; Schwartz, M.W. Rare species and ecosystem functioning. Conserv. Biol. 2005, 19, 1019–1023. [Google Scholar] [CrossRef]
- Lawrence, K.L.; Wise, D.H. Spider predation on forest-floor Collembola and evidence for indirect effects on decomposition. Pedobiologia 2000, 44, 33–39. [Google Scholar] [CrossRef]
- Wetterer, J.K. Geographic Distribution of Strumigenys lanuginosa (Hymenoptera: Formicidae). Trans. Am. Entomol. Soc. 2017, 143, 729–733. [Google Scholar] [CrossRef]
- Folgarait, P.J. Ant biodiversity and its relationship to ecosystem functioning: A review. Biodivers. Conserv. 1998, 7, 1221–1244. [Google Scholar] [CrossRef]
- Gippet, J.M.; Mondy, N.; Diallo-Dudek, J.; Bellec, A.; Dumet, A.; Mistler, L.; Kaufmann, B. I’m not like everybody else: Urbanization factors shaping spatial distribution of native and invasive ants are species-specific. Urban Ecosyst. 2017, 20, 157–169. [Google Scholar] [CrossRef]
- Brancher, D.; Roza-Gomes, M.F. Survey of edaphic fauna in forest fragment in the municipality of Anchieta (SC, Brazil). Biota Neotrop. 2012, 12, 94–98. [Google Scholar] [CrossRef]
- Boucher, P.; Hébert, C.; Francoeur, A.; Sirois, L. Postfire Succession of Ants (Hymenoptera: Formicidae) Nesting in Dead Wood of Northern Boreal Forest. Environ. Entomol. 2015, 44, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Barajas-Gusmán, G.; Alvarez-Sánchez, J. The relationships between litter fauna and rates of litter decomposition in a tropical rain forest. Appl. Soil Ecol. 2003, 24, 91–100. [Google Scholar] [CrossRef]
- Sayer, E.J.; Tanner, E.V.J.; Lacey, A.L. Effects of litter manipulation on early-stage decomposition and meso-arthropod abundance in a tropical moist forest. For. Ecol. Manag. 2006, 229, 285–293. [Google Scholar] [CrossRef]
- Sánchez, D.P.; Alexandra, S.M.; Parada, M.R.P. Poblaciones de acaros, colembolos y otra mesofauna en un inceptisol bajo diferentes. Rev. Fac. Nac. Agron. Medellin 2015, 68, 7411–7422. [Google Scholar] [CrossRef]
- Coleman, D.C.; Crossley, D.A., Jr. Fundamentals of Soil Ecology, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2004; p. 408. [Google Scholar]
- Ferguson, S.H. Changes in trophic abundance of soil arthropods along a grass–shrub–forest gradient. Can. J. Zool. 2001, 79, 457–464. [Google Scholar] [CrossRef]
- Agodi, A.; Conti, G.O.; Barchitta, M.; Quattrocchi, A.; Lombardo, B.M.; Montesanto, G.; Messina, G.; Fiore, M.; Ferrante, M. Validation of Armadillo officinalis Dumèril, 1816 (Crustacea, Isopoda, Oniscidea) as a bioindicator: In vivo study of air benzene exposure. Ecotoxicol. Environ. Saf. 2015, 114, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Bregenski, M.A. Variação Espacial e Temporal de Talitroides Topitotum (BURT, 1934) (Crustacea, Amphipoda, Talitridae), em um Remanescente de Floresta Ombrófila Mista, no Parque Municipal do Iguaçu, Curitiba, PR. Master’s Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2008; p. 49. [Google Scholar]
- Eutropio, F.B.; Krohling, W. First record of Amphipoda Talitroides topitotum (Burt, 1934) (Gammaridea, Talitridae) in the State of Espírito Santo, Brazil. Acta Sci. Biol. Sci. 2013, 35, 37–39. [Google Scholar] [CrossRef][Green Version]
- Saunders, D.A.; Hobbs, R.J.; Margules, C.R. Biological consequences of ecosystem fragmentation: A review. Conserv. Biol. 1991, 5, 18–32. [Google Scholar] [CrossRef]
- Hamberg, L.; Lehvävirta, S.; Malmivaara-Lämsä, M.; Rita, H.; Kotze, D.J. The effects of habitat edges and trampling on understorey vegetation in urban forests in Helsinki, Finland. Appl. Veg. Sci. 2008, 11, 83–98. [Google Scholar] [CrossRef]
- Schwarz, K.; Herrmann, D.L.; Mchale, M.R. Abiotic Drivers of Ecological Structure and Function in Urban Systems. In Urban Wildlife Conservation; Springer: New York, NY, USA, 2014; pp. 55–74. [Google Scholar]
- Ossola, A.; Hahs, A.K.; Nash, M.A.; Livesley, S.J. Habitat complexity enhances comminution and decomposition processes in urban ecosystems. Ecosystems 2016, 19, 927–941. [Google Scholar] [CrossRef]
- Chen, Y.A.; Forschler, B.T. Elemental concentrations in the frass of saproxylic insects suggest a role in micronutrient cycling. Ecosphere 2016, 7, e01300. [Google Scholar] [CrossRef]
- Sizer, N.C.; Tanner, E.V.J.; Ferraz, I.D.K. Edge effects on litterfall mass and nutrient concentrations in forest fragments in central Amazonia. J. Trop. Ecol. 2000, 16, 853–863. [Google Scholar] [CrossRef]
- Zhang, J.H.; Hou, X.; Shu, Q.S. Research on the hydrological effects of forest litter-fall. In Energy Science and Applied Technology: Proceedings of the 2nd International Conference on Energy Science and Applied Technology (ESAT 2015); CRC Press: Boca Raton, FL, USA, 2015; p. 85. [Google Scholar]
- Ferreira, M.L.; Ribeiro, A.P.; Albuquerque, C.R.; Lamano-Ferreira, A.P.N.; Figueira, R.C.L.; Lafortezza, R. Air contaminants and litter fall decomposition in urban forest areas: The case of São Paulo, SP, Brazil. Environ. Res. 2017, 155, 314–320. [Google Scholar] [CrossRef] [PubMed]

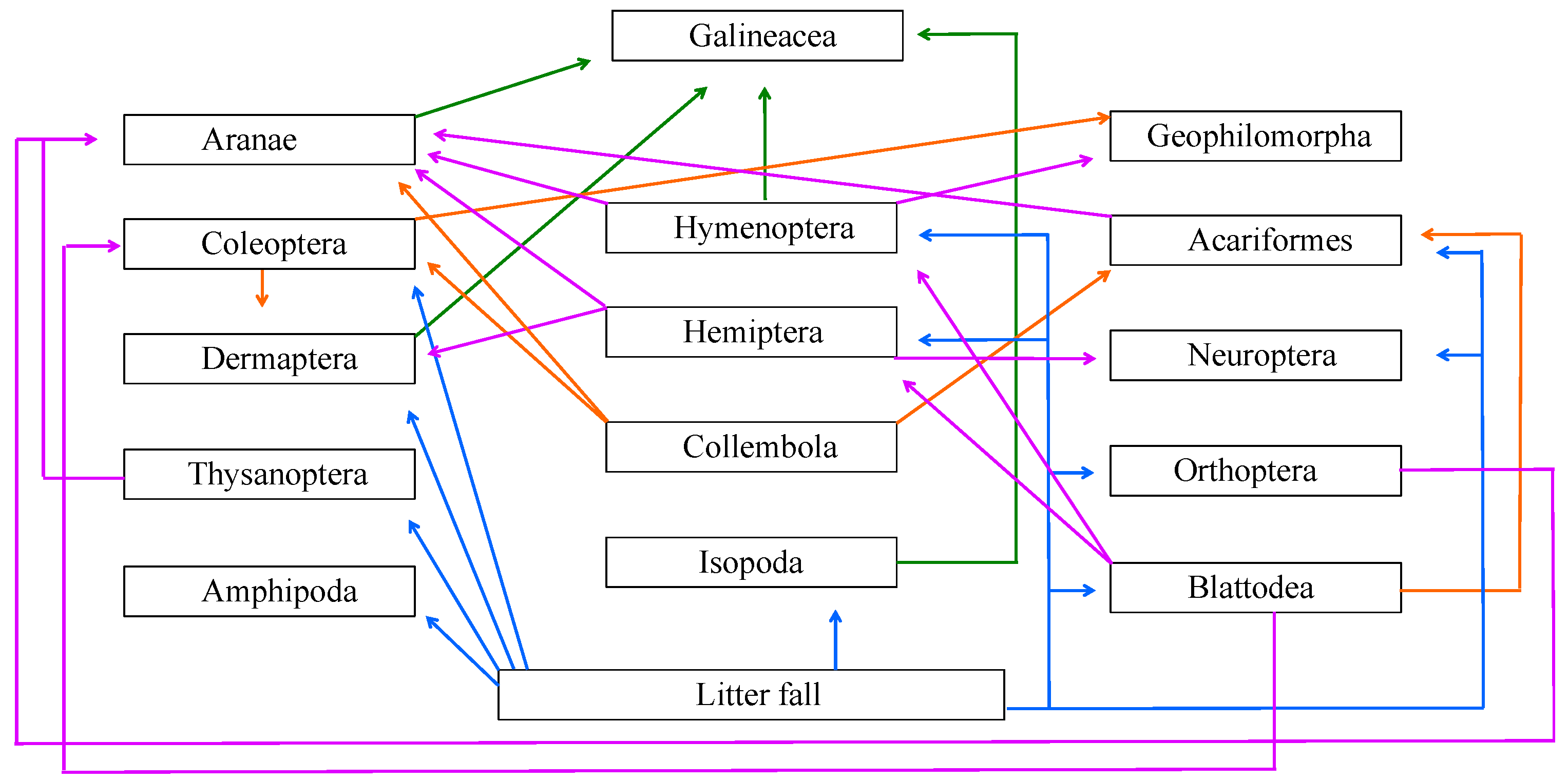
| Class | Order | CMP | ABP | TEP | Use of Litter Fall | Type of Fauna | Functional Aspects |
|---|---|---|---|---|---|---|---|
| Insecta | HYMENOPTERA | 232 | 100 | 75 | Leaves, Reproductive parts: Feeding; Leaves, Branches: Nesting | Macro and mesofauna | Herbivorous, Saprophyte-Predatory |
| HEMIPTERA | 7 | 8 | 6 | Leaves: Feeding | Mesofauna | Phytophagous | |
| ORTHOPTERA | 12 | 3 | 31 | Leaves, reproductive parts: Feeding; Leaves, braches: Shelter | Mesofauna | Phytophagous | |
| NEUROPTERA | 0 | 4 | 0 | Leaves, reproductive parts: Feeding; Leaves, branches: Shelter | Mesofauna | Saprophyte-Predatory | |
| BLATTODEA | 10 | 4 | 5 | Leaves, Reproductive parts: Feeding | Macrofauna | Saprophyte | |
| THYSANOPTERA | 1 | 1 | 1 | Leaves: Feeding | Mesofauna | Saprophyte-Predatory | |
| DERMAPTERA | 0 | 1 | 1 | Leaves, reproductive parts: Feeding; Leaves, branches: Shelter. | Macro and mesofauna | Saprophyte-Predatory | |
| COLEOPTERA | 9 | 12 | 15 | Leaves, reproductive parts: Feeding; Leaves and Branches: Shelter | Macro and mesofauna | Saprophyte-Predatory | |
| Entognata | COLLEMBOLA | 21 | 148 | 25 | Leaves, branches: Shelter | Mesofauna | Microphage |
| Arachnida | ACARIFORMES | 11 | 13 | 36 | Leaves, branches: Shelter | Mesofauna | Microphage |
| ARANEAE | 16 | 19 | 34 | Leaves, branches: Shelter | Macro and mesofauna | Predatory | |
| Malacostraca | ISOPODA | 44 | 7 | 236 | Leaves: Feeding | Macrofauna | Saprophyte |
| AMPHIPODA | 0 | 0 | 5 | Leaves, Reproductive parts: Feeding | Mesofauna | Saprophyte | |
| Chilopoda | GEOPHILOMORPHA | 1 | 1 | 5 | Leaves, branches: Shelter | Macrofauna | Predatory |
| Insecta | LEPIDOPTERA | 0 | 7 | 0 | Leaves, Branches: camouflage | Not applicable | Phytophagous |
| DIPTERA | 0 | 1 | 1 | Not applicable | Not applicable | Saprophyte | |
| SIPHONAPTERA | 0 | 0 | 3 | Not applicable | Not applicable | Parasitic | |
| MALLOPHAGA | 0 | 0 | 1 | Not applicable | Not applicable | Parasitic | |
| Arachnida | IXODIDA | 1 | 1 | 1 | Not applicable | Not applicable | Parasitic |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamano Ferreira, M.; De Souza, L.C.; Conti, D.D.M.; Capellani Quaresma, C.; Reis Tavares, A.; Gonçalves da Silva, K.; Terezinha Kniess, C.; De Camargo, P.B. Soil Biodiversity in Urban Forests as a Consequence of Litterfall Management: Implications for São Paulo’s Ecosystem Services. Sustainability 2018, 10, 684. https://doi.org/10.3390/su10030684
Lamano Ferreira M, De Souza LC, Conti DDM, Capellani Quaresma C, Reis Tavares A, Gonçalves da Silva K, Terezinha Kniess C, De Camargo PB. Soil Biodiversity in Urban Forests as a Consequence of Litterfall Management: Implications for São Paulo’s Ecosystem Services. Sustainability. 2018; 10(3):684. https://doi.org/10.3390/su10030684
Chicago/Turabian StyleLamano Ferreira, Maurício, Luciana Cantanhede De Souza, Diego De Melo Conti, Cristiano Capellani Quaresma, Armando Reis Tavares, Karina Gonçalves da Silva, Claudia Terezinha Kniess, and Plínio Barbosa De Camargo. 2018. "Soil Biodiversity in Urban Forests as a Consequence of Litterfall Management: Implications for São Paulo’s Ecosystem Services" Sustainability 10, no. 3: 684. https://doi.org/10.3390/su10030684
APA StyleLamano Ferreira, M., De Souza, L. C., Conti, D. D. M., Capellani Quaresma, C., Reis Tavares, A., Gonçalves da Silva, K., Terezinha Kniess, C., & De Camargo, P. B. (2018). Soil Biodiversity in Urban Forests as a Consequence of Litterfall Management: Implications for São Paulo’s Ecosystem Services. Sustainability, 10(3), 684. https://doi.org/10.3390/su10030684






