Analysis of Trends and Emerging Technologies in Water Electrolysis Research Based on a Computational Method: A Comparison with Fuel Cell Research
Abstract
:1. Introduction
2. Method
3. Water Electrolysis Research Perspective
3.1. Initial Classification into Main Research Areas
3.2. Brief Explanation of Each Electrolysis Type
3.2.1. Brief Explanations of AWE
3.2.2. Brief Explanations of PEME
3.2.3. Brief Explanations of SOEC
3.2.4. Brief Explanations of MEC
3.3. Trends of Main Studies
4. Perspective and Emerging Technologies of Each Water Electrolyzer Types
4.1. Cluster I “System and Cathode for AWE”
4.2. Cluster II “System for PEME”
4.3. Cluster III “Anode and Acid-Stable Cathode for AWE and PEME”
4.3.1. Perspective of Cluster III
4.3.2. Emerging Technologies in Cluster III
4.4. Cluster IV “SOEC”
4.5. Perspective and Emerging Technologies of Cluster V “MEC”
4.6. Cluster VI “Hydrogen Production Based on Renewable Energy”
5. Discussion
5.1. Overview of Overall Trends in Water Electrolysis Research
5.2. Comparison with Fuel Cell Technologies
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Anvari, M.; Lohmann, G.; Wachter, M.; Milan, P.; Lorenz, E.; Heinemann, D.; Tabar, M.R.R.; Peinke, J. Short term fluctuations of wind and solar power systems. New J. Phys. 2016, 18, 063027. [Google Scholar] [CrossRef]
- Hernandez, R.R.; Hoffacker, M.K.; Murphy-Mariscal, M.L.; Wu, G.C.; Allen, M.F. Solar energy development impacts on land cover change and protected areas. Proc. Natl. Acad. Sci. USA 2015, 112, 13579–13584. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, K.; Meibom, P. Wind power impacts and electricity storage—A time scale perspective. Renew. Energy 2012, 37, 318–324. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Gong, X.; Guo, Z. The intensification technologies to water electrolysis for hydrogen production—A review. Renew. Sustain. Energy Rev. 2014, 29, 573–588. [Google Scholar] [CrossRef]
- Bi, L.; Boulfrad, S.; Traversa, E. Steam electrolysis by solid oxide electrolysis cells (SOECs) with proton-conducting oxides. Chem. Soc. Rev. 2014, 43, 8255–8270. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.; Saw, E.C.; Lu, L.Y.Y.; Liu, J.S. Technological barriers and research trends in fuel cell technologies: A citation network analysis. Technol. Forecast. Soc. Chang. 2014, 82, 66–79. [Google Scholar] [CrossRef]
- Small, H. Tracking and predicting growth areas in science. Scientometrics 2006, 68, 595–610. [Google Scholar] [CrossRef]
- Wan, L.; Wei, Y.M.; Brown, M.A. Global transition to low-carbon electricity: A bibliometric analysis. Appl. Energy 2017, 205, 57–68. [Google Scholar]
- Suominen, A. Phases of growth in a green tech research network: A bibliometric evaluation of fuel cell technology from 1991 to 2010. Scientometrics 2014, 100, 51–72. [Google Scholar] [CrossRef]
- Kajikawa, Y.; Ohno, J.; Takeda, Y.; Matsushima, K.; Komiyama, H. Creating an academic landscape of sustainability science: An analysis of the citation network. Sustain. Sci. 2007, 2, 221–231. [Google Scholar] [CrossRef]
- Shibata, N.; Kajikawa, Y.; Takeda, Y.; Matsushima, K. Detecting emerging research fronts based on topological measures in citation networks of scientific publications. Technovation 2008, 28, 758–775. [Google Scholar] [CrossRef]
- Kajikawa, Y.; Yoshikawa, J.; Takeda, Y.; Matsushima, K. Tracking emerging technologies in energy research: Towardaroadmap for sustainableenergy. Technol. Forecast. Soc. Chang. 2008, 75, 771–782. [Google Scholar] [CrossRef]
- Ogawa, T.; Iyoki, K.; Fukushima, T.; Kajikawa, Y. Landscape of Research Areas for Zeolites and Metal-Organic Frameworks Using Computational Classification Based on Citation Networks. Materials 2017, 10, 1428. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Kajikawa, Y.; Sakata, I. Extracting the commercialization gap between science and technology case study of a solar cell. Technol. Forecast. Soc. Chang. 2010, 77, 1147–1155. [Google Scholar] [CrossRef]
- Ogawa, T.; Kajikawa, Y. Assessing the industrial opportunity of academic research with patent relatedness: A case study on polymer electrolyte fuel cells. Technol. Forecast. Soc. Chang. 2015, 90, 469–475. [Google Scholar] [CrossRef]
- Ogawa, T.; Kajikawa, Y. Generating novel research ideas using computational intelligence: A case study involving fuel cells and ammonia synthesis. Technol. Forecast. Soc. Chang. 2017, 120, 41–47. [Google Scholar] [CrossRef]
- Ogawa, T.; Takeuchi, M.; Kajikawa, Y. Comprehensive analysis of trends and emerging technologies in all types of fuel cells based on a computational method. Sustainability 2018, 10, 458. [Google Scholar] [CrossRef]
- Shibata, N.; Kajikawa, Y.; Takeda, Y.; Matsushima, K. Comparative study on methods of detecting research fronts using different types of citation. J. Am. Soc. Inf. Sci. Technol. 2009, 60, 571–580. [Google Scholar] [CrossRef]
- Newman, M. Fast algorithm for detecting community structure in networks. Phys. Rev. E 2004, 69, 066133. [Google Scholar] [CrossRef] [PubMed]
- Bass, F.M. A new product growth for model consumer durables. Manag. Sci. 1969, 15, 215–227. [Google Scholar] [CrossRef]
- Bass, F.M. Comments on “a new product growth for model consumer durables the bass model”. Manag. Sci. 2004, 50, 1833–1840. [Google Scholar] [CrossRef]
- Adai, A.T.; Date, S.V.; Wieland, S.; Marcotte, E.M. Lgl: Creating a map of protein function with an algorithm for visualizing very large biological networks. J. Mol. Biol. 2004, 340, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Carmo, M.; Fritz, D.L.; Merge, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Dönitz, W.; Erdle, E. High-temperature electrolysis of water vapor—Status of development and perspectives for application. Int. J. Hydrogen Energy 1985, 10, 291–295. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef] [Green Version]
- Ebbesen, S.D.; Jensen, S.H.; Hauch, A.; Mogensen, M.B. High temperature electrolysis in alkaline cells, solid proton conducting cells, and solid oxide cells. Chem. Rev. 2014, 114, 10697–10734. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically assisted microbial production of hydrogen from acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef] [PubMed]
- Miyake, J.; Miyake, M.; Asada, Y. Biotechnological hydrogen production: Research for efficient light energy conversion. J. Biotechnol. 1999, 70, 89–101. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions—A review. Int. J. Hydrogen Energy 2015, 40, 256–274. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.-C.; Uchimura, M.; Paulikas, A.P.; Stamenkovic, V.; Markovic, N.M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science 2011, 334, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Hwang, S.J.; Yoo, S.J.; Choi, I.; Kim, H.-J.; Jang, J.H.; Nam, S.W.; Lim, T.-H.; Lim, T.; Kim, S.-K.; et al. Electrodeposited Ni dendrites with high activity and durability for hydrogen evolution reaction in alkaline water electrolysis. J. Mater. Chem. 2012, 22, 15153–15159. [Google Scholar] [CrossRef]
- Burke, L.D.; Naser, N.S. Metastability and electrocatalytic activity of ruthenium dioxide cathodes used in water electrolysis cells. J. Appl. Electrochem. 2005, 35, 931–938. [Google Scholar] [CrossRef]
- Holmin, S.; Näslund, L.-Å.; Ingason, Á.S.; Rosen, J.; Zimmerman, E. Corrosion of ruthenium dioxide based cathodes in alkaline medium caused by reverse currents. Electrochim. Acta 2014, 146, 30–36. [Google Scholar] [CrossRef]
- Näslund, L.-Å.; Ingason, Á.S.; Holmin, S.; Rosen, J. Formation of RuO(OH)2 on RuO2-based electrodes for hydrogen production. J. Phys. Chem. C 2014, 118, 15315–15323. [Google Scholar] [CrossRef]
- Matsushima, H.; Nishida, T.; Konishi, Y.; Fukunaka, Y.; Ito, Y.; Kuribayashi, K. Water electrolysis under microgravity: Part 1. Experimental technique. Electrochim. Acta 2003, 48, 4119–4125. [Google Scholar] [CrossRef]
- Matsushima, H.; Fukunaka, Y.; Kuribayashi, K. Water electrolysis under microgravity: Part II. Description of gas bubble evolution phenomena. Electrochim. Acta 2006, 51, 4190–4198. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, S.; Pan, J.; Yang, C.; He, M.; Zhuang, L.; Lu, J. First implementation of alkaline polymer electrolyte water electrolysis working only with pure water. Energy Environ. Sci. 2012, 5, 7869–7871. [Google Scholar] [CrossRef]
- Leng, Y.; Chen, G.; Mendoza, A.J.; Tighe, T.B.; Hickner, M.A.; Wang, C.-Y. Solid-state water electrolysis with an alkaline membrane. J. Am. Chem. Soc. 2012, 134, 9054–9057. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, H.; Chen, G.; Zhang, Y. Study of IrxRu1−xO2 oxides as anodic electrocatalysts for solid polymer electrolyte water electrolysis. Electrochim. Acta 2009, 54, 6250–6256. [Google Scholar] [CrossRef]
- Marshall, A.; Børresen, B.; Hagen, G.; Tsypkin, M.; Tunold, R. Electrochemical characterisation of IrxSn1−xO2 powders as oxygen evolution electrocatalysts. Electrochim. Acta 2006, 51, 3161–3167. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Millet, P.; Dzhus, K.A.; Middleton, H.; Saetre, T.O.; Fateev, V.N. Design and characterization of bi-functional electrocatalytic layers for application in pem unitized regenerative fuel cells. Int. J. Hydrogen Energy 2010, 35, 5070–5076. [Google Scholar] [CrossRef]
- Pettersson, J.; Ramsey, B.; Harrison, D. A review of the latest developments in electrodes for unitised regenerative polymer electrolyte fuel cells. J. Power Sources 2006, 157, 28–34. [Google Scholar] [CrossRef]
- Gabbasa, M.; Sopian, K.; Fudholi, A.; Asim, N. A review of unitized regenerative fuel cell stack: Material, design and research achievements. Int. J. Hydrogen Energy 2014, 39, 17765–17778. [Google Scholar] [CrossRef]
- Nikiforov, A.V.; Petrushina, I.M.; Christensen, E.; Tomás-García, A.L.; Bjerrum, N.J. Corrosion behaviour of construction materials for high temperature steam electrolysers. Int. J. Hydrogen Energy 2011, 36, 111–119. [Google Scholar] [CrossRef]
- Aili, D.; Hansen, M.K.; Pan, C.; Li, Q.; Christensen, E.; Jensen, J.O.; Bjerrum, N.J. Phosphoric acid doped membranes based on Nafion®, PBI and their blends—Membrane preparation, characterization and steam electrolysis testing. Int. J. Hydrogen Energy 2011, 36, 6985–6993. [Google Scholar] [CrossRef]
- Hansen, M.K.; Aili, D.; Christensen, E.; Pan, C.; Eriksen, S.; Jensen, J.O.; von Barner, J.H.; Li, Q.; Bjerrum, N.J. PEM steam electrolysis at 130 °C using a phosphoric acid doped short side chain pfsa membrane. Int. J. Hydrogen Energy 2012, 37, 10992–11000. [Google Scholar] [CrossRef]
- Take, T.; Tsurutani, K.; Umeda, M. Hydrogen production by methanol–water solution electrolysis. J. Power Sources 2007, 164, 9–16. [Google Scholar] [CrossRef]
- Lamy, C.; Jaubert, T.; Baranton, S.; Coutanceau, C. Clean hydrogen generation through the electrocatalytic oxidation of ethanol in a proton exchange membrane electrolysis cell (PEMEc): Effect of the nature and structure of the catalytic anode. J. Power Sources 2014, 245, 927–936. [Google Scholar] [CrossRef]
- Marshall, A.T.; Haverkamp, R.G. Production of hydrogen by the electrochemical reforming of glycerol–water solutions in a PEM electrolysis cell. Int. J. Hydrogen Energy 2008, 33, 4649–4654. [Google Scholar] [CrossRef]
- Jiao, F.; Frei, H. Nanostructured cobalt oxide clusters in mesoporous silica as efficient oxygen-evolving catalysts. Angew. Chem. Int. Ed. 2009, 48, 1841–1844. [Google Scholar] [CrossRef] [PubMed]
- Trotochaud, L.; Ranney, J.K.; Williams, K.N.; Boettcher, S.W. Solution-cast metal oxide thin film electrocatalysts for oxygen evolution. J. Am. Chem. Soc. 2012, 134, 17253–17261. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Song, W.; Huang, H.; Ren, Z.; Chen, S.-Y.; Suib, S.L. Structure–property relationship of bifunctional MnO2 nanostructures: Highly efficient, ultra-stable electrochemical water oxidation and oxygen reduction reaction catalysts identified in alkaline media. J. Am. Chem. Soc. 2014, 136, 11452–11464. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jia, H.; Sun, Z.; Chen, H.; Xu, P.; Du, P. Nanostructured copper oxide electrodeposited from copper(II) complexes as an active catalyst for electrocatalytic oxygen evolution reaction. Electrochem. Commun. 2014, 46, 1–4. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.N.; Singh, J.P.; Lal, B.; Thomas, M.J.K.; Bera, S. New NiFe2−xCrxO4 spinel films for O2 evolution in alkaline solutions. Electrochim. Acta 2006, 51, 5515–5523. [Google Scholar] [CrossRef]
- Srirapu, V.K.V.P.; Sharma, C.S.; Awasthi, R.; Singh, R.N.; Sinha, A.S.K. Copper-iron-molybdenum mixed oxides as efficient oxygen evolution electrocatalysts. Phys. Chem. Chem. Phys. 2014, 16, 7385–7393. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Awasthi, R.; Pramanick, A.K.; Singh, R.N. New ternary mixed oxides of Fe, Ni and Mo for enhanced oxygen evolution. Int. J. Hydrogen Energy 2011, 36, 12698–12705. [Google Scholar] [CrossRef]
- Singh, R.N.; Lal, B. High surface area lanthanum cobaltate and its A and B sites substituted derivatives for electrocatalysis of O2 evolution in alkaline solution. Int. J. Hydrogen Energy 2002, 27, 45–55. [Google Scholar] [CrossRef]
- Singh, R.N.; Tiwari, S.K.; Singh, S.P.; Singh, N.K.; Poillerat, G.; Chartier, P. Synthesis of (La, Sr)CoO3 perovskite films via a sol-gel route and their physicochemical and electrochemical surface characterization for anode application in alkaline water electrolysis. J. Chem. Soc. Faraday Trans. 1996, 92, 2593–2597. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Cui, W.; Jiang, P.; Cheng, N.; Asiri, A.M.; Sun, X. Carbon nanotubes decorated with CoP nanocrystals: A highly active non-noble-metal nanohybrid electrocatalyst for hydrogen evolution. Angew. Chem. Int. Ed. 2014, 126, 6828–6832. [Google Scholar] [CrossRef]
- Voiry, D.; Yamaguchi, H.; Li, J.; Silva, R.; Alves, D.C.B.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G.; et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 2013, 12, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, H.; Lu, Z.; Cui, Y. CoSe2 nanoparticles grown on carbon fiber paper: An efficient and stable electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. [Google Scholar] [CrossRef] [PubMed]
- Merki, D.; Fierro, S.; Vrubel, H.; Hu, X. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water. Chem. Sci. 2011, 2, 1262–1267. [Google Scholar] [CrossRef]
- Chen, W.F.; Wang, C.H.; Sasaki, K.; Marinkovic, N.; Xu, W.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Highly active and durable nanostructured molybdenum carbide electrocatalysts for hydrogen production. Energy Environ. Sci. 2013, 6, 943–951. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, S.; Lei, F.; Liu, J.; Liang, L.; Xie, Y. Atomically-thin non-layered cobalt oxide porous sheets for highly efficient oxygen-evolving electrocatalysts. Chem. Sci. 2014, 5, 3976–3982. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.-Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Yu, Y.; Zhou, W.; Xu, X.; Zhu, Z. Highly defective CeO2 as a promoter for efficient and stable water oxidation. J. Mater. Chem. A 2015, 3, 634–640. [Google Scholar] [CrossRef]
- Kötz, R.; Stucki, S. Stabilization of RuO2 by IrO2 for anodic oxygen evolution in acid media. Electrochim. Acta 1986, 31, 1311–1316. [Google Scholar] [CrossRef]
- Louie, M.W.; Bell, A.T. An investigation of thin-film Ni–Fe oxide catalysts for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 2013, 135, 12329–12337. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Dai, H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Stern, L.-A.; Feng, L.; Song, F.; Hu, X. Ni2P as a janus catalyst for water splitting: The oxygen evolution activity of Ni2P nanoparticles. Energy Environ. Sci. 2015, 8, 2347–2351. [Google Scholar] [CrossRef]
- Laursen, A.B.; Patraju, K.R.; Whitaker, M.J.; Retuerto, M.; Sarkar, T.; Yao, N.; Ramanujachary, K.V.; Greenblatt, M.; Dismukes, G.C. Nanocrystalline ni5p4: A hydrogen evolution electrocatalyst of exceptional efficiency in both alkaline and acidic media. Energy Environ. Sci. 2015, 8, 1027–1034. [Google Scholar] [CrossRef]
- Ledendecker, M.; Krick Calderón, S.; Papp, C.; Steinrück, H.-P.; Antonietti, M.; Shalom, M. The synthesis of nanostructured Ni5P4 films and their use as a non-noble bifunctional electrocatalyst for full water splitting. Angew. Chem. Int. Ed. 2015, 54, 12361–12365. [Google Scholar] [CrossRef] [PubMed]
- El-Deab, M.S.; Awad, M.I.; Mohammad, A.M.; Ohsaka, T. Enhanced water electrolysis: Electrocatalytic generation of oxygen gas at manganese oxide nanorods modified electrodes. Electrochem. Commun. 2007, 9, 2082–2087. [Google Scholar] [CrossRef]
- Mette, K.; Bergmann, A.; Tessonnier, J.-P.; Hävecker, M.; Yao, L.; Ressler, T.; Schlögl, R.; Strasser, P.; Behrens, M. Nanostructured manganese oxide supported on carbon nanotubes for electrocatalytic water splitting. ChemCatChem 2012, 4, 851–862. [Google Scholar] [CrossRef]
- Faber, M.S.; Lukowski, M.A.; Ding, Q.; Kaiser, N.S.; Jin, S. Earth-abundant metal pyrites (FeS2, CoS2, NiS2, and their alloys) for highly efficient hydrogen evolution and polysulfide reduction electrocatalysis. J. Phys. Chem. C 2014, 118, 21347–21356. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xi, D.; Zhou, C.; Shi, Z.; Xia, H.; Liu, G.; Qiao, G. CoSe2 necklace-like nanowires supported by carbon fiber paper: A 3d integrated electrode for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 9415–9420. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Qiao, Q.; Yu, Y.; Peterson, D.; Zafar, A.; Kumar, R.; Curtarolo, S.; Hunte, F.; Shannon, S.; et al. All the catalytic active sites of MoS2 for hydrogen evolution. J. Am. Chem. Soc. 2016, 138, 16632–16638. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tsai, C.; Kong, D.; Chan, K.; Abild-Pedersen, F.; Nørskov, J.K.; Cui, Y. Transition-metal doped edge sites in vertically aligned MoS2 catalysts for enhanced hydrogen evolution. Nano Res. 2015, 8, 566–575. [Google Scholar] [CrossRef]
- Harnisch, F.; Sievers, G.; Schröder, U. Tungsten carbide as electrocatalyst for the hydrogen evolution reaction in pH neutral electrolyte solutions. Appl. Catal. B 2009, 89, 455–458. [Google Scholar] [CrossRef]
- Esposito, D.V.; Hunt, S.T.; Kimmel, Y.C.; Chen, J.G. A new class of electrocatalysts for hydrogen production from water electrolysis: Metal monolayers supported on low-cost transition metal carbides. J. Am. Chem. Soc. 2012, 134, 3025–3033. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.V.; Chen, J.G. Monolayer platinum supported on tungsten carbides as low-cost electrocatalysts: Opportunities and limitations. Energy Environ. Sci. 2011, 4, 3900–3912. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, C.; Liu, S.; Wang, J.-L.; Lin, W. A biomimetic copper water oxidation catalyst with low overpotential. J. Am. Chem. Soc. 2014, 136, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Du, J.; Su, X.-J.; Zhang, M.-T.; Xu, X.; Meyer, T.J.; Chen, Z. Cu(II) aliphatic diamine complexes for both heterogeneous and homogeneous water oxidation catalysis in basic and neutral solutions. ACS Catal. 2016, 6, 77–83. [Google Scholar] [CrossRef]
- Udagawa, J.; Aguiar, P.; Brandon, N.P. Hydrogen production through steam electrolysis: Model-based steady state performance of a cathode-supported intermediate temperature solid oxide electrolysis cell. J. Power Sources 2007, 166, 127–136. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. Mathematical modeling of the coupled transport and electrochemical reactions in solid oxide steam electrolyzer for hydrogen production. Electrochim. Acta 2007, 52, 6707–6718. [Google Scholar] [CrossRef]
- Graves, C.; Ebbesen, S.D.; Mogensen, M.; Lackner, K.S. Sustainable hydrocarbon fuels by recycling CO2 and H2O with renewable or nuclear energy. Renew. Sustain. Energy Rev. 2011, 15, 1–23. [Google Scholar] [CrossRef]
- Ebbesen, S.D.; Mogensen, M. Electrolysis of carbon dioxide in solid oxide electrolysis cells. J. Power Sources 2009, 193, 349–358. [Google Scholar] [CrossRef]
- Yildiz, B.; Kazimi, M. Efficiency of hydrogen production systems using alternative nuclear energy technologies. Int. J. Hydrogen Energy 2006, 31, 77–92. [Google Scholar] [CrossRef]
- O’Brien, J.E.; Stoots, C.M.; Herring, J.S.; Hartvigsen, J.J. Performance of planar high-temperature electrolysis stacks for hydrogen production from nuclear energy. Nucl. Technol. 2007, 158, 118–131. [Google Scholar] [CrossRef]
- O’Brien, J.E.; Stoots, C.M.; Herring, J.S.; Hartvigsen, J. Hydrogen production performance of a 10-cell planar solid-oxide electrolysis stack. J. Fuel Cell Sci. Technol. 2005, 3, 213–219. [Google Scholar] [CrossRef]
- Yang, X.; Irvine, J.T.S. (La0.75Sr0.25)0.95Mn0.5Cr0.5O3 as the cathode of solid oxide electrolysis cells for high temperature hydrogen production from steam. J. Mater. Chem. 2008, 18, 2349–2354. [Google Scholar] [CrossRef]
- Jin, C.; Yang, C.; Zhao, F.; Cui, D.; Chen, F. La0.75Sr0.25Cr0.5Mn0.5O3 as hydrogen electrode for solid oxide electrolysis cells. Int. J. Hydrogen Energy 2011, 36, 3340–3346. [Google Scholar] [CrossRef]
- Iwahara, H.; Asakura, Y.; Katahira, K.; Tanaka, M. Prospect of hydrogen technology using proton-conducting ceramics. Solid State Ion. 2004, 168, 299–310. [Google Scholar] [CrossRef]
- Ishihara, T.; Jirathiwathanakul, N.; Zhong, H. Intermediate temperature solid oxide electrolysis cell using LaGaO3 based perovskite electrolyte. Energy Environ. Sci. 2010, 3, 665–672. [Google Scholar] [CrossRef]
- Ishihara, T.; Kanno, T. Steam electrolysis using LaGaO3 based perovskite electrolyte for recovery of unused heat energy. ISIJ Int. 2010, 50, 1291–1295. [Google Scholar] [CrossRef]
- Virkar, A.V. Mechanism of oxygen electrode delamination in solid oxide electrolyzer cells. Int. J. Hydrogen Energy 2010, 35, 9527–9543. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, S.P. Failure mechanism of (La, Sr)MnO3 oxygen electrodes of solid oxide electrolysis cells. Int. J. Hydrogen Energy 2011, 36, 10541–10549. [Google Scholar] [CrossRef]
- Croese, E.; Pereira, M.A.; Euverink, G.-J.W.; Stams, A.J.M.; Geelhoed, J.S. Analysis of the microbial community of the biocathode of a hydrogen-producing microbial electrolysis cell. Appl. Microbiol. Biotechnol. 2011, 92, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Jeremiasse, A.W.; Hamelers, H.V.M.; Buisman, C.J.N. Microbial electrolysis cell with a microbial biocathode. Bioelectrochemistry 2010, 78, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Jeremiasse, A.W.; Hamelers, H.V.M.; Saakes, M.; Buisman, C.J.N. Ni foam cathode enables high volumetric H2 production in a microbial electrolysis cell. Int. J. Hydrogen Energy 2010, 35, 12716–12723. [Google Scholar] [CrossRef]
- Selembo, P.A.; Merrill, M.D.; Logan, B.E. The use of stainless steel and nickel alloys as low-cost cathodes in microbial electrolysis cells. J. Power Sources 2009, 190, 271–278. [Google Scholar] [CrossRef]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef] [PubMed]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Zhen, G.; Kobayashi, T.; Lu, X.; Xu, K. Understanding methane bioelectrosynthesis from carbon dioxide in a two-chamber microbial electrolysis cells (MECs) containing a carbon biocathode. Bioresour. Technol. 2015, 186, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Fan, Y.; Liu, H. Hydrogen production using single-chamber membrane-free microbial electrolysis cells. Water Res. 2008, 42, 4172–4178. [Google Scholar] [CrossRef] [PubMed]
- Tartakovsky, B.; Manuel, M.F.; Wang, H.; Guiot, S.R. High rate membrane-less microbial electrolysis cell for continuous hydrogen production. Int. J. Hydrogen Energy 2009, 34, 672–677. [Google Scholar] [CrossRef] [Green Version]
- Rozendal, R.A.; Sleutels, T.H.J.A.; Hamelers, H.V.M.; Buisman, C.J.N. Effect of the type of ion exchange membrane on performance, ion transport, and pH in biocatalyzed electrolysis of wastewater. Water Sci. Technol. 2008, 57, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Sleutels, T.H.J.A.; Hamelers, H.V.M.; Rozendal, R.A.; Buisman, C.J.N. Ion transport resistance in microbial electrolysis cells with anion and cation exchange membranes. Int. J. Hydrogen Energy 2009, 34, 3612–3620. [Google Scholar] [CrossRef]
- Junghare, M.; Subudhi, S.; Lal, B. Improvement of hydrogen production under decreased partial pressure by newly isolated alkaline tolerant anaerobe, clostridium butyricum TM-9A: Optimization of process parameters. Int. J. Hydrogen Energy 2012, 37, 3160–3168. [Google Scholar] [CrossRef]
- Ust’ak, S.; Havrland, B.; Muñoz, J.O.J.; Fernández, E.C.; Lachman, J. Experimental verification of various methods for biological hydrogen production. Int. J. Hydrogen Energy 2007, 32, 1736–1741. [Google Scholar] [CrossRef]
- Manish, S.; Banerjee, R. Comparison of biohydrogen production processes. Int. J. Hydrogen Energy 2008, 33, 279–286. [Google Scholar] [CrossRef]
- Lu, L.; Ren, N.; Xing, D.; Logan, B.E. Hydrogen production with effluent from an ethanol–H2-coproducing fermentation reactor using a single-chamber microbial electrolysis cell. Biosens. Bioelectron. 2009, 24, 3055–3060. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, S.; Zhou, A.; Zhou, G.; Ren, N.; Wang, A.; Zhuang, G. Hydrogen generation in microbial electrolysis cell feeding with fermentation liquid of waste activated sludge. Int. J. Hydrogen Energy 2012, 37, 13859–13864. [Google Scholar] [CrossRef]
- Ajayi, F.F.; Kim, K.-Y.; Chae, K.-J.; Choi, M.-J.; Kim, S.-Y.; Chang, I.-S.; Kim, I.S. Study of hydrogen production in light assisted microbial electrolysis cell operated with dye sensitized solar cell. Int. J. Hydrogen Energy 2009, 34, 9297–9304. [Google Scholar] [CrossRef]
- Chae, K.-J.; Choi, M.-J.; Kim, K.-Y.; Ajayi, F.F.; Chang, I.-S.; Kim, I.S. A solar-powered microbial electrolysis cell with a platinum catalyst-free cathode to produce hydrogen. Environ. Sci. Technol. 2009, 43, 9525–9530. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Pan, Y.; Huang, L.; Zhou, P.; Quan, X.; Chen, H. Complete separation of Cu(II), Co(II) and Li(I) using self-driven MFCs–MECs with stainless steel mesh cathodes under continuous flow conditions. Sep. Purif. Technol. 2015, 147, 114–124. [Google Scholar] [CrossRef]
- Mehanna, M.; Saito, T.; Yan, J.; Hickner, M.; Cao, X.; Huang, X.; Logan, B.E. Using microbial desalination cells to reduce water salinity prior to reverse osmosis. Energy Environ. Sci. 2010, 3, 1114–1120. [Google Scholar] [CrossRef]
- Sevda, S.; Yuan, H.; He, Z.; Abu-Reesh, I.M. Microbial desalination cells as a versatile technology: Functions, optimization and prospective. Desalination 2015, 371, 9–17. [Google Scholar] [CrossRef]
- Ipsakis, D.; Voutetakis, S.; Seferlis, P.; Stergiopoulos, F.; Elmasides, C. Power management strategies for a stand-alone power system using renewable energy sources and hydrogen storage. Int. J. Hydrogen Energy 2009, 34, 7081–7095. [Google Scholar] [CrossRef]
- Vosen, S.R.; Keller, J.O. Hybrid energy storage systems for stand-alone electric power systems: Optimization of system performance and cost through control strategies. Int. J. Hydrogen Energy 1999, 24, 1139–1156. [Google Scholar] [CrossRef]
- Zhou, T.; Francois, B. Modeling and control design of hydrogen production process for an active hydrogen/wind hybrid power system. Int. J. Hydrogen Energy 2009, 34, 21–30. [Google Scholar] [CrossRef]
- Beccali, M.; Brunone, S.; Finocchiaro, P.; Galletto, J.M. Method for size optimisation of large wind-hydrogen systems with high penetration on power grids. Appl. Energy 2013, 102, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Olateju, B.; Kumar, A.; Secanell, M. A techno-economic assessment of large scale wind-hydrogen production with energy storage in western canada. Int. J. Hydrogen Energy 2016, 41, 8755–8776. [Google Scholar] [CrossRef]
- Clarke, R.E.; Giddey, S.; Ciacchi, F.T.; Badwal, S.P.S.; Paul, B.; Andrews, J. Direct coupling of an electrolyser to a solar PV system for generating hydrogen. Int. J. Hydrogen Energy 2009, 34, 2531–2542. [Google Scholar] [CrossRef]
- García-Valverde, R.; Espinosa, N.; Urbina, A. Optimized method for photovoltaic-water electrolyser direct coupling. Int. J. Hydrogen Energy 2011, 36, 10574–10586. [Google Scholar] [CrossRef]
- Tolga Balta, M.; Dincer, I.; Hepbasli, A. Thermodynamic assessment of geothermal energy use in hydrogen production. Int. J. Hydrogen Energy 2009, 34, 2925–2939. [Google Scholar] [CrossRef]
- Joshi, A.S.; Dincer, I.; Reddy, B.V. Exergetic assessment of solar hydrogen production methods. Int. J. Hydrogen Energy 2010, 35, 4901–4908. [Google Scholar] [CrossRef]
- Balta, M.T.; Dincer, I.; Hepbasli, A. Potential methods for geothermal-based hydrogen production. Int. J. Hydrogen Energy 2010, 35, 4949–4961. [Google Scholar] [CrossRef]
- Aili, D.; Hansen, M.K.; Renzaho, R.F.; Li, Q.; Christensen, E.; Jensen, J.O.; Bjerrum, N.J. Heterogeneous anion conducting membranes based on linear and crosslinked KOH doped polybenzimidazole for alkaline water electrolysis. J. Membr. Sci. 2013, 447, 424–432. [Google Scholar] [CrossRef]
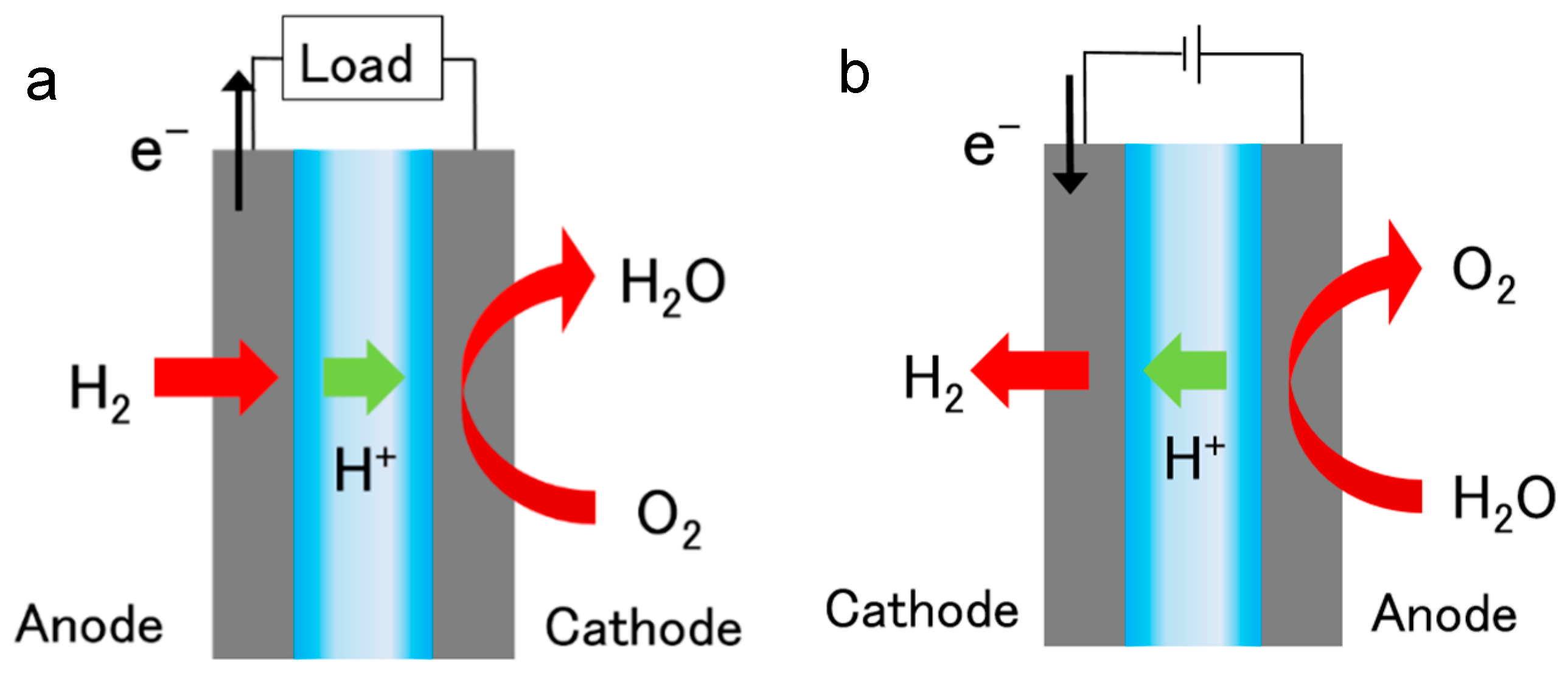
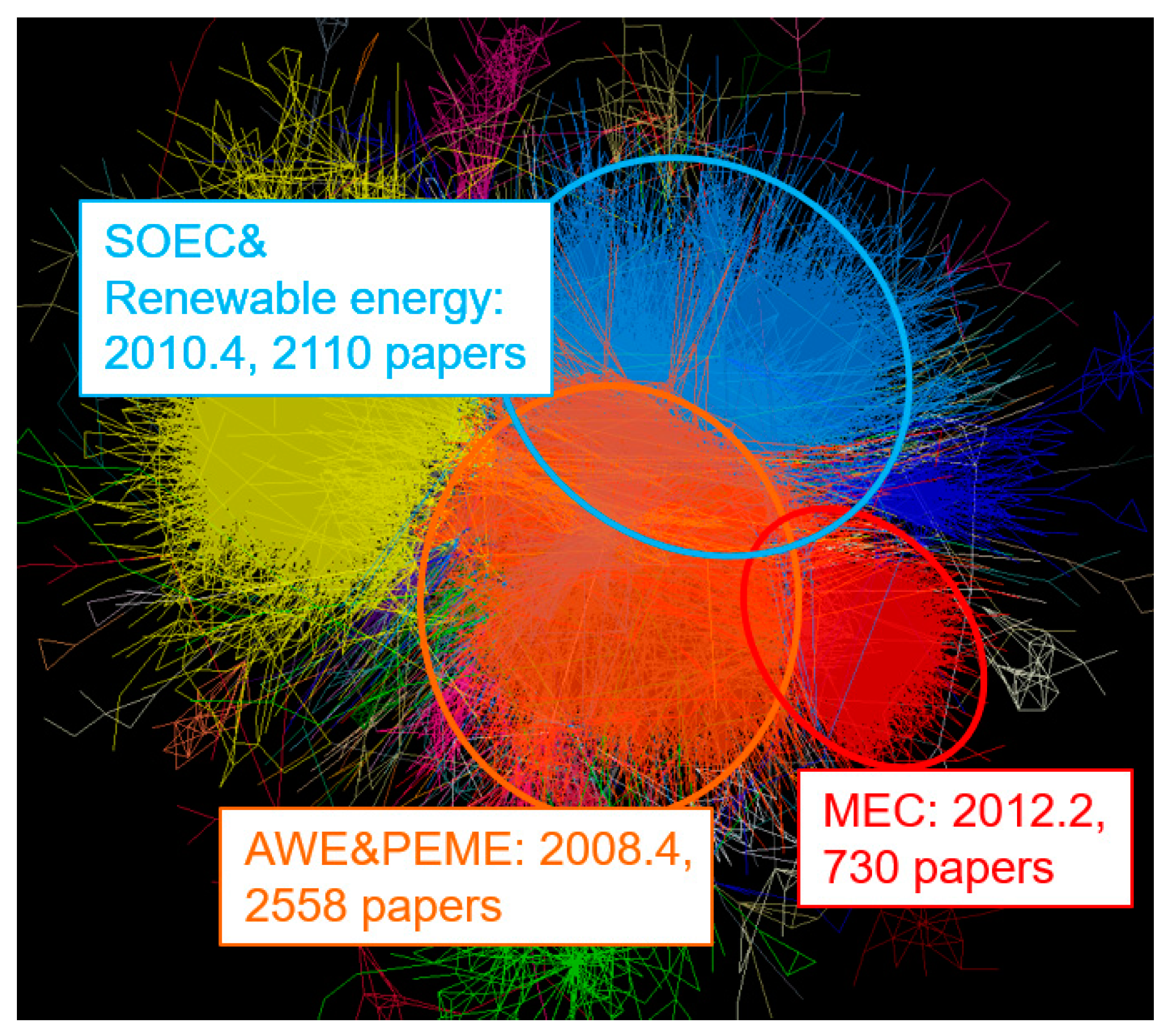
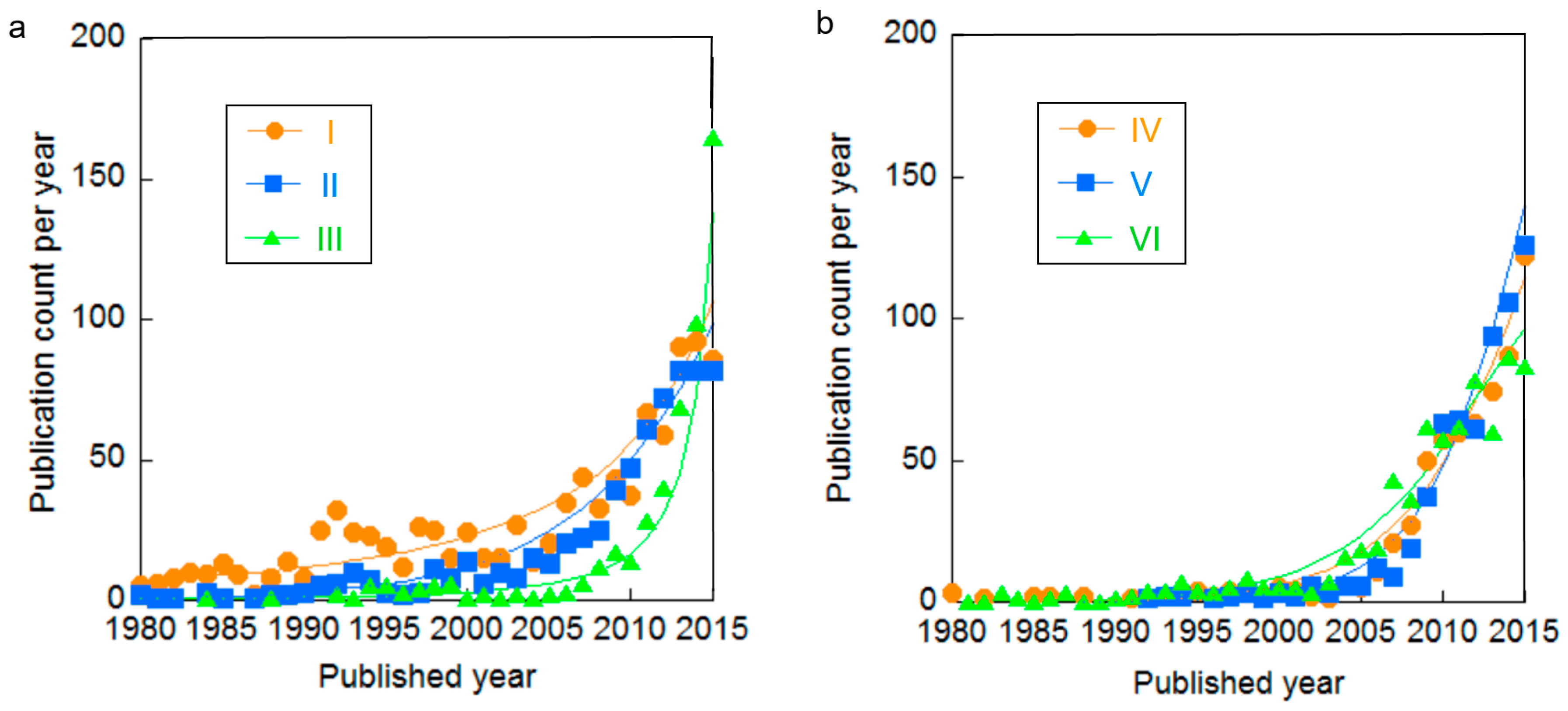
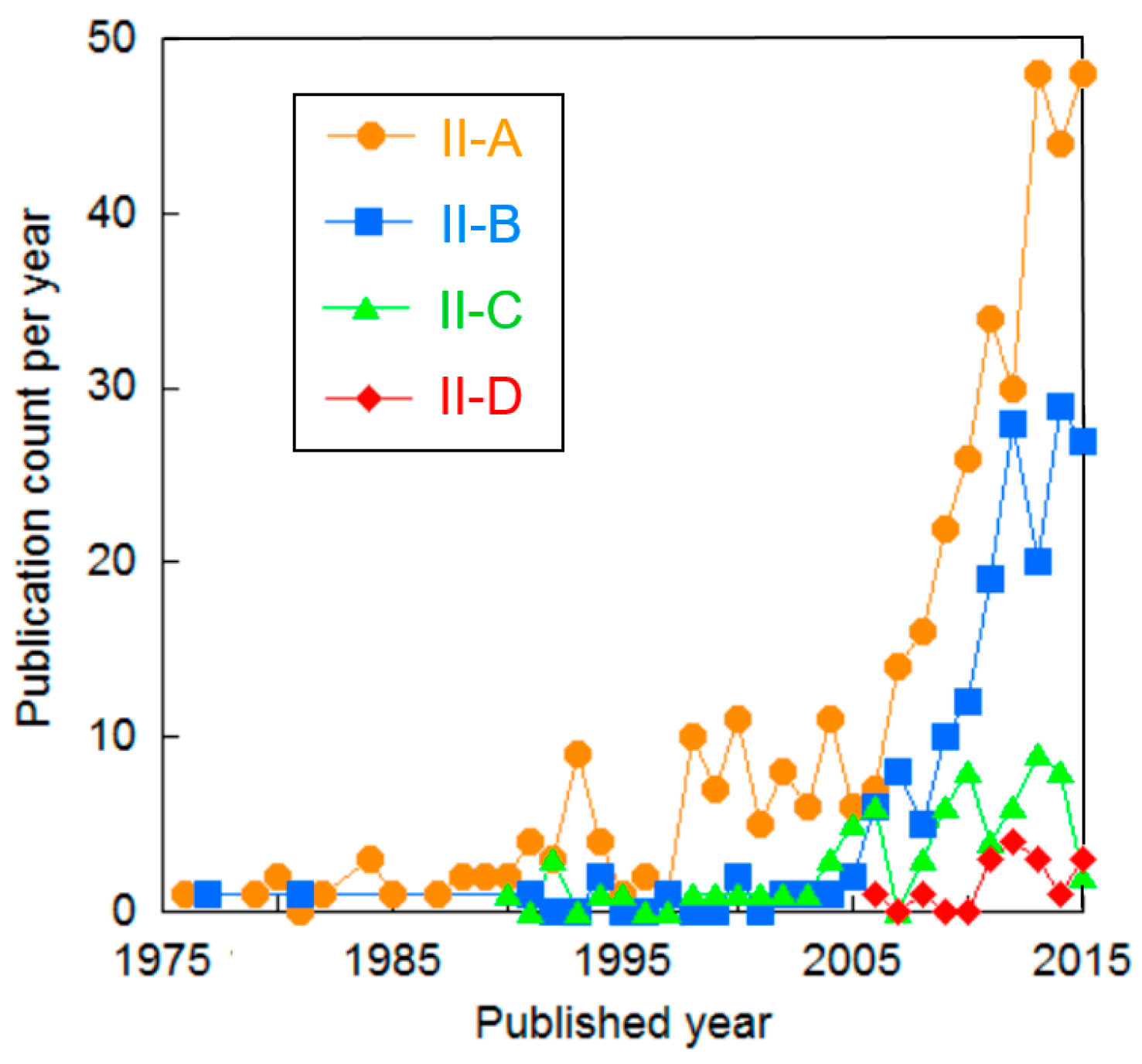
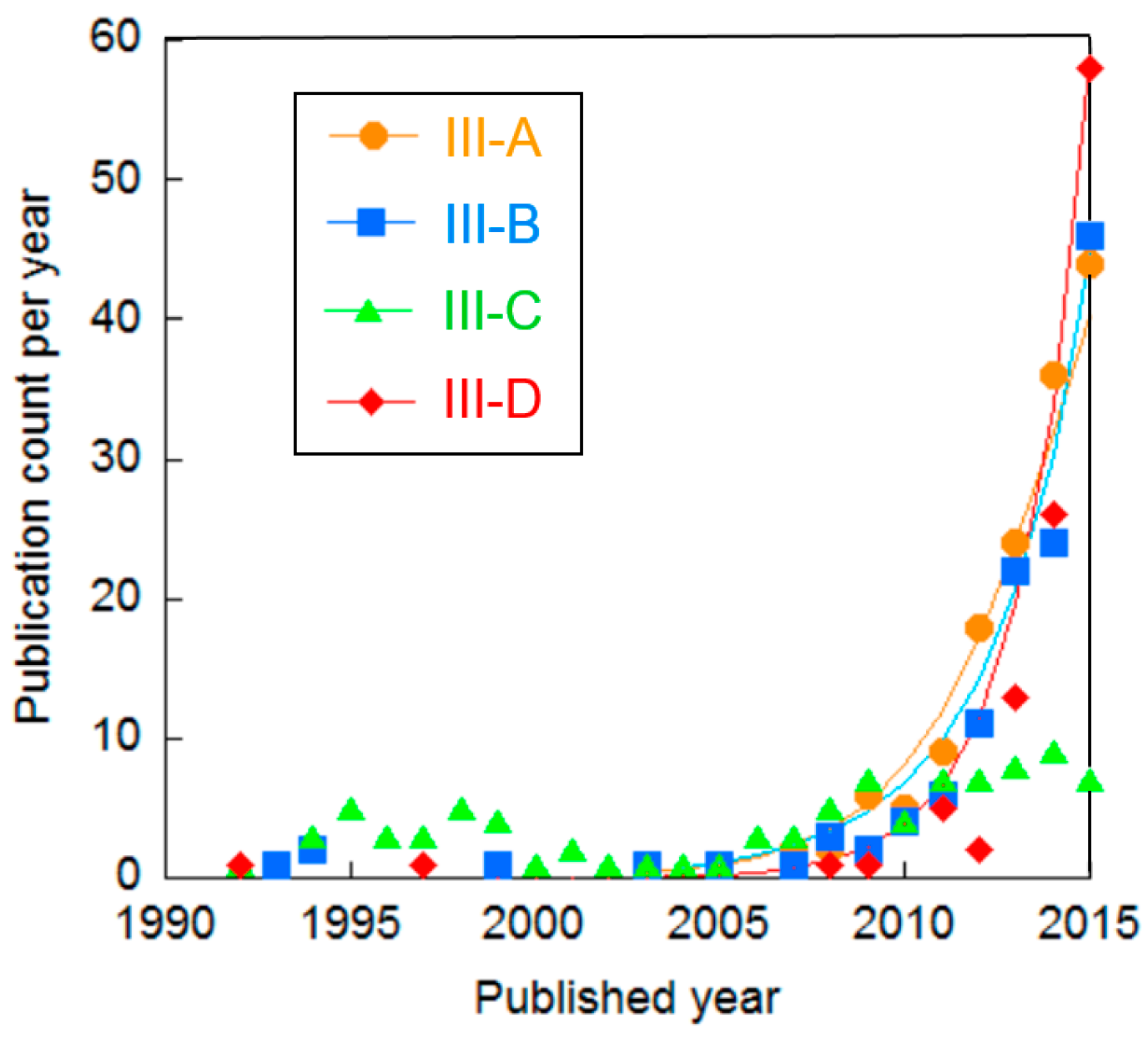
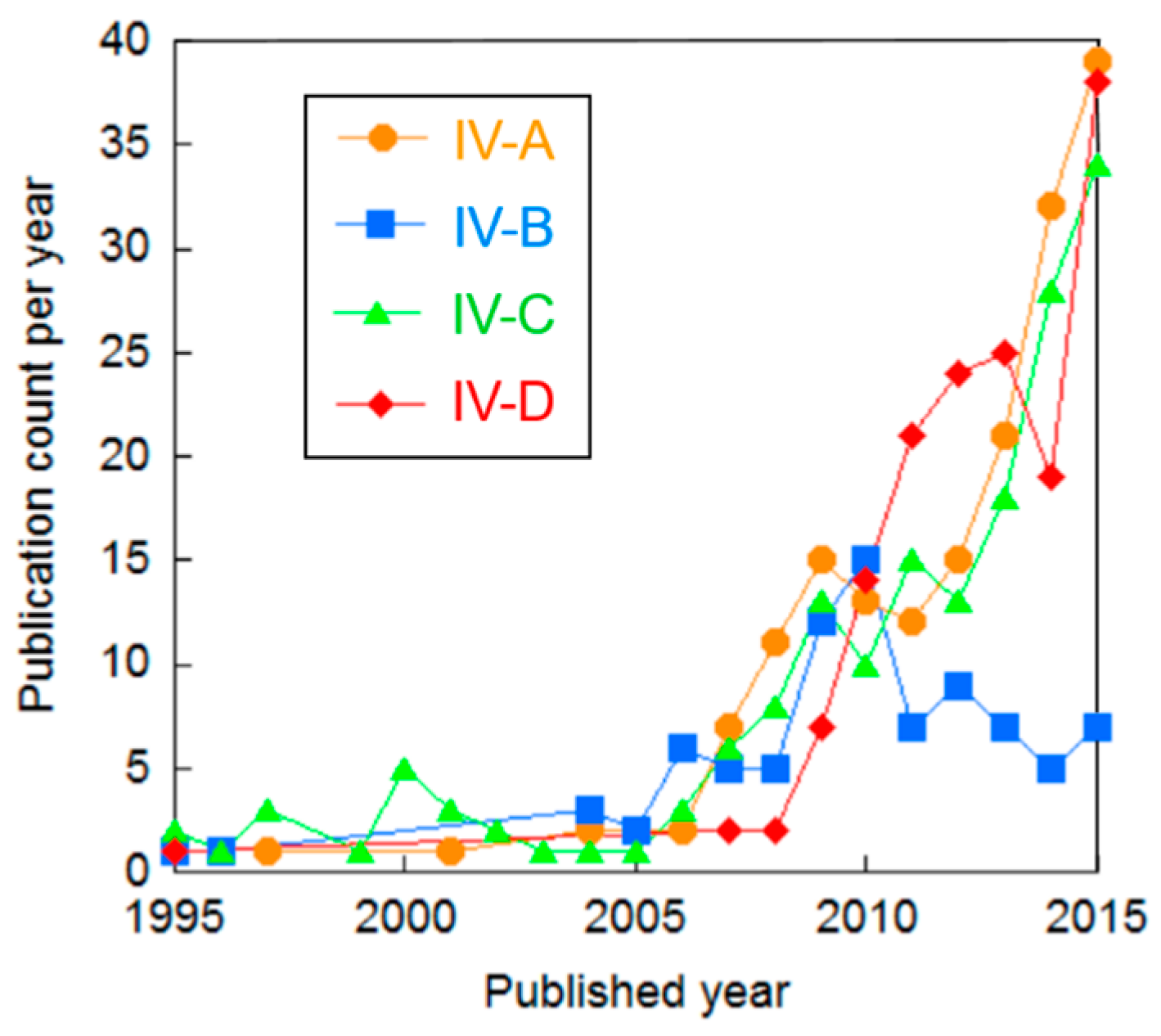
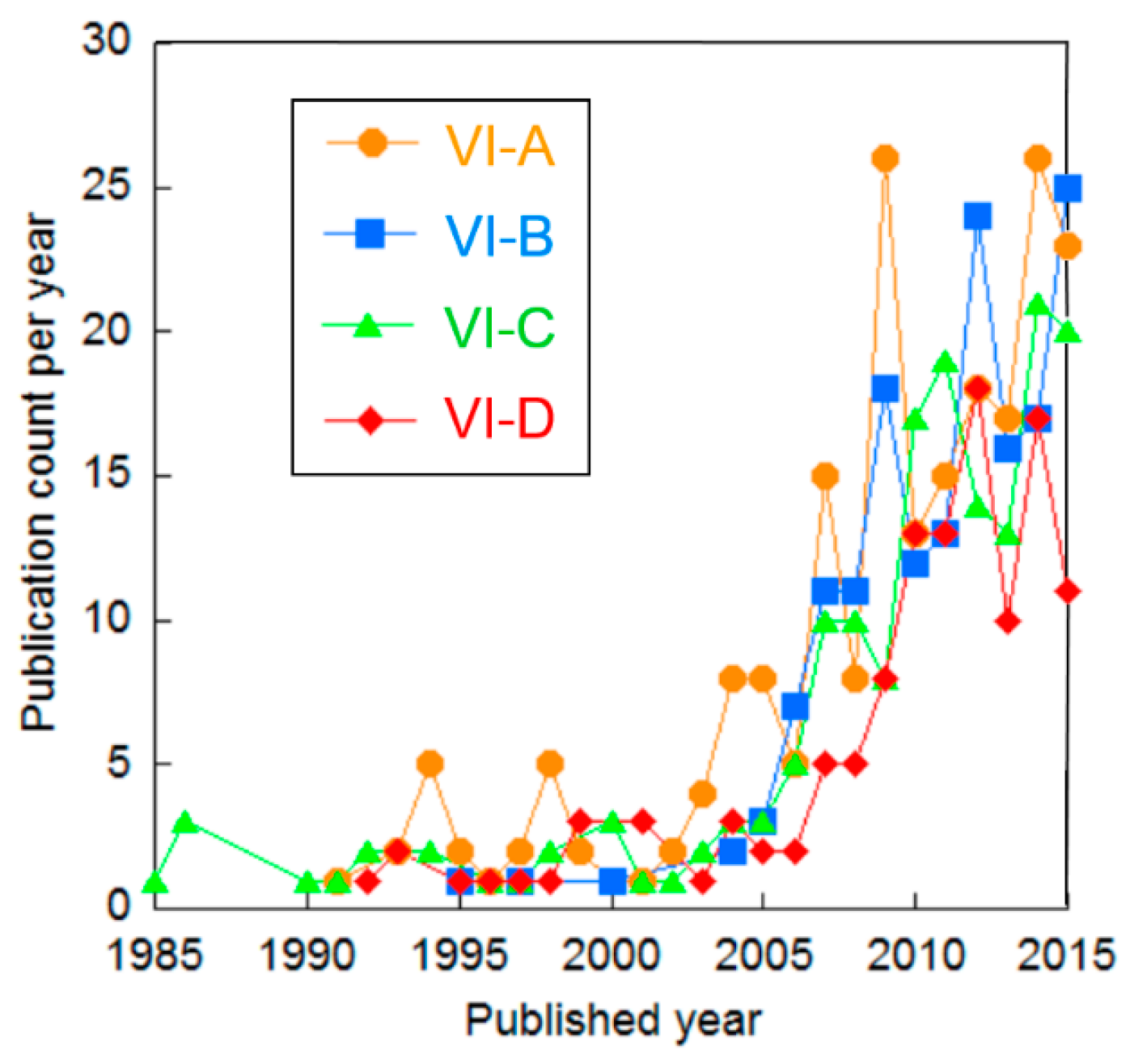
| Cluster | Research Topic | Number of Publications | Average Publication Year |
|---|---|---|---|
| I | System and cathode for AWE | 1088 | 2004.1 |
| II | System for PEME | 741 | 2009.4 |
| III | Anode and acid-stable cathode for AWE and PEME | 669 | 2012.9 |
| IV | SOEC | 741 | 2011.2 |
| V | MEC | 730 | 2012.2 |
| VI | Hydrogen production based on renewable energy | 750 | 2009.9 |
| Cluster | Research Topic | Number of Publications | Average Publication Year |
|---|---|---|---|
| I-A | AWE System | 310 | 2002.5 |
| I-B | Metal alloy and metal composite cathodes | 232 | 2001.4 |
| I-C | Fabrication, characterization of nickel alloy electrodes, and kinetic studies on HER | 232 | 2007.4 |
| I-D | Electrodeposition of hierarchically structured nickel composite electrodes | 33 | 2012.3 |
| I-E | Ruthenium dioxide-based cathode | 30 | 2007.1 |
| I-F | Gas bubble on electrode | 118 | 2005.9 |
| I-G | Anion Exchange Membrane Electrolysis Cell | 67 | 2012.0 |
| Cluster | Research Topic | Number of Publications | Average Publication Year |
|---|---|---|---|
| II-A | Fundamental studies and models on PEME | 430 | 2008.5 |
| II-B | Iridium-based oxide for OER in anode | 197 | 2011.3 |
| II-C | RFCs | 80 | 2008.7 |
| II-D | Material studies on components of PEME for high-temperature water (steam) electrolysis | 20 | 2012.9 |
| Cluster | Research Topic | Number of Publications | Average Publication Year |
|---|---|---|---|
| III-A | Metal oxides for OER | 188 | 2013.8 |
| III-B | Theoretical screening of metal oxides for OER | 173 | 2013.6 |
| III-C | Binary or ternary metal oxides for OER | 96 | 2006.5 |
| III-D | Carbide, phosphide, sulfide, and selenide for HER | 183 | 2014.7 |
| Cluster | Research Topic | Publication Number | Average Publication Year |
|---|---|---|---|
| III-1 | Nanostructured Co oxides for OER | 72 | 2013.9 |
| III-2 | RuO2 for OER in PEME | 61 | 2014.3 |
| III-3 | Ni-Fe for OER | 55 | 2014.4 |
| III-4 | Nickel and cobalt phosphide for OER and HER in acid condition | 52 | 2015.4 |
| III-5 | Mn oxides on conductive materials in alkaline condition | 42 | 2013.6 |
| III-6 | Metal disulfide or diselenide for HER | 50 | 2014.9 |
| III-7 | Mo2C for HER | 49 | 2014.0 |
| III-8 | Bioinspired Cu complex | 36 | 2014.0 |
| Cluster | Research Topic | Number of Publications | Average Publication Year |
|---|---|---|---|
| IV-A | Performance of SOEC | 193 | 2011.7 |
| IV-B | SOEC using power supplied by nuclear power plant | 92 | 2008.7 |
| IV-C | Materials of SOEC | 187 | 2010.5 |
| IV-D | Degradation of anode in SOEC | 168 | 2012.6 |
| Cluster | Research Topic | Number of Publications | Average Publication Year |
|---|---|---|---|
| V-1 | MEC biocathodes for HER | 33 | 2012.4 |
| V-2 | Nickel cathode for MEC | 57 | 2012.6 |
| V-3 | Methane production by MEC | 82 | 2013.1 |
| V-4 | Single chamber (membrane less) MEC | 79 | 2012.7 |
| V-5 | Polymer electrolyte membrane for MEC | 23 | 2013.6 |
| V-6 | Combining dark fermentation and MEC | 100 | 2012.1 |
| V-7 | Light-assisted H2 production with MEC | 28 | 2012.9 |
| V-8 | Metal recovery from aqueous mixtures with a hydrogen byproduct | 25 | 2012.9 |
| V-9 | Microbial electrolysis and desalination cell (MEDC) | 23 | 2013.4 |
| Cluster | Research Topic | Number of Publications | Average Publication Year |
|---|---|---|---|
| VI-A | Power management of a stand-alone system for storage of renewable energy as hydrogen | 228 | 2009.5 |
| VI-B | Hydrogen production based on wind power | 181 | 2011.4 |
| VI-C | Direct coupling of solar power and hydrogen generation system | 179 | 2008.9 |
| VI-D | Thermodynamics of renewable energy for hydrogen production | 130 | 2009.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, T.; Takeuchi, M.; Kajikawa, Y. Analysis of Trends and Emerging Technologies in Water Electrolysis Research Based on a Computational Method: A Comparison with Fuel Cell Research. Sustainability 2018, 10, 478. https://doi.org/10.3390/su10020478
Ogawa T, Takeuchi M, Kajikawa Y. Analysis of Trends and Emerging Technologies in Water Electrolysis Research Based on a Computational Method: A Comparison with Fuel Cell Research. Sustainability. 2018; 10(2):478. https://doi.org/10.3390/su10020478
Chicago/Turabian StyleOgawa, Takaya, Mizutomo Takeuchi, and Yuya Kajikawa. 2018. "Analysis of Trends and Emerging Technologies in Water Electrolysis Research Based on a Computational Method: A Comparison with Fuel Cell Research" Sustainability 10, no. 2: 478. https://doi.org/10.3390/su10020478
APA StyleOgawa, T., Takeuchi, M., & Kajikawa, Y. (2018). Analysis of Trends and Emerging Technologies in Water Electrolysis Research Based on a Computational Method: A Comparison with Fuel Cell Research. Sustainability, 10(2), 478. https://doi.org/10.3390/su10020478





