The Research on International Development Path of China’s Marine Biopharmaceutical Industry
Abstract
1. Introduction
2. Identification of Factors that Influence the Marine Biopharmaceutical Industry
- (1)
- Resources are the material basis for the development of the marine biopharmaceutical industry. Different from single resource-driven industries such as the steel and textile industry, the marine biopharmaceutical industry needs diverse types of marine bioresources. Marine bioresources are the main source for bioactive compounds in R&D, and the main raw materials for medicine production. The ideal marine medicinal bioresources renewable capacity, supply capacity and availability are regarded as the greatest material guarantee for sustainable development of the industry.
- (2)
- Technologies refer to the methods, approaches, means, production techniques and instruments for the research and development of marine biopharmaceutical products. Technologies are the driving force of the marine biopharmaceutical industry. The essential characteristic of the marine biopharmaceutical industry determines technology as a very important element. A wealth of marine medicinal bioresources without advanced technology cannot achieve the rapid development of the industry, which is an inspiration we have learned from ASEAN countries. Resources are the foundation, while bioactive substance screening, drug R&D, clinical experiments, pilot production, scale production and practical application are closely correlated to technological innovation and maturity.
- (3)
- Talents are the people who possess professional skills, technical proficiency and specific ability in marine biopharmaceutical field. Talents are the backbone of the marine biopharmaceutical industry. As one of its advanced factors, skilled talents play a crucial role in the development of the marine biopharmaceutical industry. The development of the marine biopharmaceutical industry depends heavily on technologies, while technological progress needs vast skilled talents, including professionals and management personnel equipped with basis knowledge, and versatile talents with command of skills and market. For example, in the aspect of the drug R&D call for R&D talents, drug production and business operation need management personnel, and its scientific application depends on advanced versatile talents. Merely high-quality versatile talents can effectively link all aspects of development, from raw materials collection, extraction, purification, processing and detection to marketing, in order to form a complete industrial chain.
- (4)
- Investments are the guarantee of the marine biopharmaceutical industry. As a strategic emerging industry in the 21st century, the marine biopharmaceutical industry calls for support with a great deal of investments. Marine resource exploitation, talent training developed by research institutions, and infrastructure construction are all inseparable from these vast funds. For example, scientific and technological progress and talent training cannot be achieved overnight, and needs long-term funds for achievement.
- (5)
- Policies are a necessity for the development of the marine biopharmaceutical industry. Policies formulated by the national government exert an overall macroeconomic control on the development of the marine biopharmaceutical industry. In order to enhance the international competitiveness and transform marine medicinal bioresources into practical economic advantages, reasonable and effective policies should be carried out, including policy orientation, implementation and regulation, in order guide the rational development and utilization of marine medicinal bioresources, and upgrade the industrial structure of the marine medical industry, promoting the internationalization of the industry.
3. Empirical Analysis
3.1. Construction of the Hierarchical Structure Model
3.2. Analytic Hierarchy Process Computation
4. Necessity Analysis of the Going Global of China’s Marine Biopharmaceutical Industry
4.1. Analysis of Comparative Differences in the Marine Biopharmaceutical Industry between China and the ASEAN
4.1.1. Strengths and Needs of China’s Marine Biopharmaceutical Industry
4.1.2. Strengths and Needs of the Marine Biopharmaceutical Industry in Southeast Asia
4.2. Game Theory Analysis of International Cooperation of the Marine Biopharmaceutical Industry
- (1)
- Party A is China and Party B stands for countries along the Maritime Silk Road. Both parties are rational economies. Revenues gained not through the cooperation between the two sides are Rc and Rm, and additional revenues gained through the cooperation are Ec and Em, respectively.
- (2)
- The method of cooperation between China and ASEAN countries is: China reveals its information and technology to ASEAN countries, and ASEAN countries open up their rich marine bioresources to China. Thus, two kinds of cooperation risks may arise: ① China is willing to cooperate with ASEAN countries, while ASEAN countries may be not willing to cooperate with China (that is, China tends to be open to share information and technology to ASEAN countries, but ASEAN countries do not want to do common exploitation of resources with China). At this situation, the absolute value of technology losses of China’s technology disclosure with the non-openness of resources in ASEAN countries and the absolute value of profit of ASEAN countries are labeled as Tc and Tm, respectively; ② ASEAN countries want to cooperate with China, while China does not (that is, ASEAN countries share marine bioresources with China, but China does not want to share information and technologies to ASEAN countries). At this status, the absolute values of China’s additional revenues with no information and technology disclosure combined with the resources sharing of ASEAN countries and resources losses of ASEAN countries are marked as rc and rm, respectively.
- (3)
- Both parties necessarily sign a contract stating that they will abide by the cooperative strategic agreement. If one party breaches the contract, the defaulting party will pay a fine or accept other penalties. If China fulfills the contract for cooperation and countries in Southeast Asia do not, these Southeast Asian countries will have to pay Pm liquidated damages to China. On the contrary, if China defaults, China will have to pay Pc liquidated damages to the Southeast Asian countries.
- (4)
- Pm = Pc; Pc > rc; Pm > Tm; Pm > Tc; Pc > rm.
- (1)
- When China makes a choice:
- (2)
- When Southeast Asian countries make their choices:
5. Design of the International Factor-Allocation Cooperation Path
5.1. Resources Allocation Path
- (1)
- By putting disputes over territorial sovereignty aside at present, China and Southeast Asian countries could work together to exploit marine medicinal bioresources in the South China Sea and provide raw materials for marine medicinal production.
- (2)
- China’s advanced marine expedition vessels could be dispatched to the waters along the Maritime Silk Road to obtain biopharmaceutical resources in ways that complete resource gathering and screening experiments for biochemical substances at the same time, and conduct basic research efficiently.
5.2. Technologies Allocation Path
- (1)
- The government and private investment would help build R&D institutions in countries along the Maritime Silk Road and explore more biopharmaceutical resources, in order to provide techniques for new drug R&D and product innovation.
- (2)
- Accumulated processing experience over years would provide technological guidance for the production and processing of marine medicinal products in alongside countries, and accelerate cooperation.
- (3)
- In addition to key technology and R&D institutions, China should give priority to intellectual property protection for marine medicinal products in a way that provides quality product basis for sustainable development.
5.3. Talents Allocation Path
- (1)
- Necessary personnel assistance program to transfer professionals to marine drug research institutions in alongside countries, and achieve new drug research and development combined with local resources.
- (2)
- Establishment of overseas personnel training institutions to localize the training of R&D and corporate management talents in ways that would optimize the overall skills of countries along the Maritime Silk Road.
5.4. Investments Allocation Path
- (1)
- China works to expand foreign investment, utilize marine medicinal bioresources in alongside countries, and strengthen the construction of R&D institutions and production infrastructures, in order to promote overseas marine biopharmaceutical production as soon as possible.
- (2)
- Except for necessary R&D funds, capital should be used for the industrialization and transformation of scientific and technological achievements to production in ways that would benefit mankind with marine drugs.
5.5. Policies Allocation Path
- (1)
- China and neighboring countries introduce marine biopharmaceutical industry-related policy systems, and strengthen policy implementation with effective policy regulation.
- (2)
- Policy orientation interconnects marine drug production and sales channels within the cooperated countries along the Maritime Silk Road, and eliminates technical trade barriers. The recognition of China’s marine biomedicine production technology regulations, standards and certification systems, including health, quarantine, safety, environmental protection, product quality and certification, through alongside countries would help smoothly carry out international cooperation in production.
6. Strategies for the Development of China’s Marine Biopharmaceutical Industry
- (1)
- Promotion of R&D of core technologies
- (2)
- Value talent training
- (3)
- Protection of marine bioresources
- (4)
- Strengthening policy support
- (5)
- Expanding investments
- (6)
- Deepening international cooperation
7. Conclusions
- (1)
- Through analyzing the status quo of China’s marine biopharmaceutical industry, the identified main factors were investments, technologies, talents, resources and policies.
- (2)
- The analysis with AHP revealed the importance ranking of these factors: technologies > talents > resources > policies > investments. These results revealed that technologies, talents and resources are the top three factors that affect marine biopharmaceutical industrial development. Simultaneously, although the proportion of policies and investments is relatively small compared with the former three, the development of the marine biopharmaceutical industry should be guided by policies and supported by funds.
- (3)
- Based on the comparative advantage and game theory, according to our studies, as China and Southeastern countries are interested in the partnership, international cooperation should be considered as the best choice for developing the marine biopharmaceutical industry. Supported by the 21st Century Maritime Silk Road Initiative, the international development of China’s marine biopharmaceutical industry will necessarily lay a win-win foundation for the rapid development of the industry in neighboring countries.
- (4)
- According to the results of the empirical analysis, the path of factor–allocation cooperation in terms of resources, technology, talents, investments and policies were designed and the factor–allocation cooperation path chart for the international development of marine biopharmaceutical industry was planned.
- (5)
- The strategy and pathway for the going global of China’s marine biopharmaceutical industry were proposed, as follows: to focus on R&D of core technologies, to value talents training, to protect marine bioresources, to introduce supporting policies, and to invest more and strengthen international cooperation.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, C.Z.; Zhang, K.L.; Zou, W.; Li, B.; Qin, X.H. Assessment and Evolution of the Sustainable Development Ability of Human–Ocean Systems in Coastal Regions of China. Sustainability 2015, 7, 10399–10427. [Google Scholar] [CrossRef]
- Wang, C.Y.; Shao, C.L.; Fu, X.M.; Lan, D.; Lan, K.X.; Li, G.Q.; Wu, Y.F.; Qian, S.B.; Guan, H.S. Investigation on the Status of Marine Materia Medica Resources and Research in China. Period. Ocean Univ. China 2009, 4, 669–675. [Google Scholar]
- Huang, S.; Zhou, J.Y. Study on the cluster development of marine biopharmaceutical industry in China. Econ. Rev. 2015, 44–47. (In Chinese) [Google Scholar] [CrossRef]
- Southcott, R.V. The neurologic effects of noxious marine creatures. Contemp. Neurol. Ser. 1975, 12, 165–258. [Google Scholar] [PubMed]
- Grant, P.T. Organic Resources of the Sea. Philos. Trans. R. Soc. Lond. 1982, 307, 351–362. [Google Scholar] [CrossRef]
- Silvestre, F.; Tosti, E. Impact of marine drugs on cytoskeleton-mediated reproductive events. Mar. Drugs 2010, 8, 881–915. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Gobe, G.; Masci, P.; Osborne, S.A. Marine bioactive compounds and health promoting perspectives; innovation pathways for drug discovery. Trends Food Sci. Technol. 2016, 50, 44–55. [Google Scholar] [CrossRef]
- Thomas, T.R.A.; Kavlekar, D.P.; Loka Bharathi, P.A. Marine drugs from sponge-microbe association—A review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.T.; Law, Y.C.; Wong, F.C.; Kim, S.K. Enzyme-Assisted Discovery of Antioxidant Peptides from Edible Marine Invertebrates: A Review. Mar. Drugs 2017, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the Marine Environment with Pharmacological, Cosmeceutical and Nutraceutical Potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2008, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Nastrucci, C.; Cesario, A.; Russo, P. Anticancer drug discovery from the marine environment. Recent Patents Anti-Cancer Drug Discov. 2012, 7, 218–232. [Google Scholar] [CrossRef]
- Mudit, M.; El Sayed, K.A. Cancer control potential of marine natural product scaffolds through inhibition of tumor cell migration and invasion. Drug Discov. Today 2016, 21, 1745–1760. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Choi, B.K.; Trinh, P.T.H.; Lee, H.S.; Kang, J.S.; Van, T.T.T.; Lee, H.S.; Lee, J.S.; Lee, Y.J.; Lee, J. Suppression of RANKL-Induced Osteoclastogenesis by the Metabolites from the Marine Fungus Aspergillus flocculosus Isolated from a Sponge Stylissa sp. Mar. Drugs 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Liu, Y.; Balasis, M.E.; Garner, T.P.; Li, J.; Simmons, N.L.; Berndt, N.; Song, H.; Pan, L.L.; Qin, Y.; et al. Marinopyrrole Derivatives with Sulfide Spacers as Selective Disruptors of Mcl-1 Binding to Pro-Apoptotic Protein Bim. Mar. Drugs 2014, 12, 4311. [Google Scholar] [CrossRef] [PubMed]
- García, P.A.; Valles, E.; Díez, D.; Castro, M.Á. Marine Alkylpurines: A Promising Group of Bioactive Marine Natural Products. Mar. Drugs 2018, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Bian, J. Progress and Prospect of marine drugs at home and abroad. J. Navy Med. 2007, 1, 84–87. (In Chinese) [Google Scholar]
- Fu, X.M.; Zhang, M.Q.; Shao, C.L.; Li, G.Q.; Bai, H.; Dai, G.L.; Chen, Q.W.; Kong, W.; Fu, X.J.; Wang, C.Y. Chinese marine materia medica resources: Status and potential. Mar. Drugs 2016, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Study on International Cooperation Model of Marine Drugs Ecological Industry. Master’s Thesis, Ocean University of China, Qingdao, China, 2014. [Google Scholar]
- Si, M.; Zhan, X.T. Research progress of marine bioactive substances. Chin. J. Mar. Drugs 2003, 6, 46–50. (In Chinese) [Google Scholar]
- Zhang, S.; Wu, J.; Qi, S.H.; Huang, J.S.; Xiao, Z.H.; Li, Q.X. Studies on marine bioactive compounds and chemical ecological mechanisms. Proceedings of Chinese Marine Biochemistry Conference, Wuhan, China; 5–6 June 2005. (In Chinese). [Google Scholar]
- Han, L.M.; Zhou, L.P. Study on the development of marine bio-pharmaceutical industry chain in Qingdao. Chin. Fish. Econ. 2013, 31, 109–116. [Google Scholar]
- Fu, X.M.; Chen, Q.W.; Wang, D.Y.; Wang, N. A Study on the International Cooperation Mechanism of Industrialization of Research Results of China’s Marine Biomedicine Research. Pac. J. 2015, 12, 011. [Google Scholar] [CrossRef]
- Gong, G.A.; Hu, G.A. Efficiency of Resource Allocation and Manufacturing Total Factor Productivity in China. Econ. Res. J. 2013, 4, 4–15, 29. [Google Scholar]
- Lin, X.H.; Zhou, T.; Gao, J. Marine Economy in Indonesia. Mar. Econ. 2014, 4, 46–54. [Google Scholar]
- Soopat. Available online: http://www.soopat.com/ (accessed on 20 July 2017). (In Chinese).
- Shen, J.; Chen, W.; Wu, L. Application of membrane separation technology in separation and purification of marine biomaterials. J. Zhejiang Univ. Technol. 2007, 35, 595. [Google Scholar]
- State Oceanic Administration of the People’s Republic of China. China Ocean Yearbook 2015; China Ocean Press: Beijing, China, 2016.
- State Oceanic Administration of the People’s Republic of China. China Ocean Yearbook 2010; China Ocean Press: Beijing, China, 2011.
- Fujikura, K.; Lindsay, D.; Kitazato, H.; Nishida, S.; Shirayama, Y. Marine biodiversity in Japanese waters. PLoS ONE 2010, 5, e11836. [Google Scholar] [CrossRef] [PubMed]
- Tittensor, D.P.; Mora, C.; Jetz, W.; Lotze, H.K.; Ricard, D.; Berghe, E.V.; Worm, B. Global patterns and predictors of marine biodiversity across taxa. Nature 2010, 466, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Sanciangco, J.C.; Carpenter, K.E.; Etnoyer, P.J.; Moretzsohn, F. Habitat availability and heterogeneity and the Indo-Pacific warm pool as predictors of marine species richness in the tropical Indo-Pacific. PLoS ONE 2013, 8, e56245. [Google Scholar] [CrossRef] [PubMed]
- Yamakita, T.; Sudo, K.; Jintsu-Uchifune, Y.; Yamamoto, H.; Shirayama, Y. Identification of important marine areas using ecologically or biologically significant areas (EBSAs) criteria in the East to Southeast Asia region and comparison with existing registered areas for the purpose of conservation. Mar. Policy 2017, 81, 273–284. [Google Scholar] [CrossRef]
- Selig, E.R.; Longo, C.; Halpern, B.S.; Best, B.D.; Hardy, D.; Elfes, C.T.; Scarborough, C.; Kleisner, K.M.; Katona, S.K. Assessing global marine biodiversity status within a coupled socio-ecological perspective. PLoS ONE 2013, 8, e60284. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.R. Conservation hotspots of biodiversity and endemism for Indo-Pacific coral reef fishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 541–556. [Google Scholar] [CrossRef]
- Roberts, C.M.; McClean, C.J.; Veron, J.E.N.; Hawkins, J.P.; Allen, G.R.; McAllister, D.E.; Mittermeier, C.G.; Schueler, F.W.; Spalding, M.; Wells, F.; et al. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 2002, 295, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Kamil, K.A.; Hailu, A.; Rogers, A.; Pandit, R. An assessment of marine protected areas as a marine management strategy in Southeast Asia: A literature review. Ocean Coast. Manag. 2017, 145, 72–81. [Google Scholar] [CrossRef]
- Burke, L.; Reytar, K.; Spalding, M.; Perry, A.L. Reefs at Risk Revisited in the Coral Triangle. Ethics Med. 2011, 22, 2008–2010. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Y.J. Analysis of international patent competition in the field of marine medicine. Chin. J. Mar. Drugs 2010, 29, 60–66. [Google Scholar] [CrossRef]
- Harshad, M. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83. [Google Scholar] [CrossRef]
- Chakraborty, C.; Agoramoorthy, G. A special report on India’s biotech scenario: Advancement in biopharmaceutical and health care sectors. Biotechnol. Adv. 2010, 28, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xue, C. The establishment of Asian Infrastructure Investment Bank (AIIB) on opportunities and adjustments of China. Heilongjiang Sci. 2016, 7, 140–141. [Google Scholar]
- Callaghan, M.; Hubbard, P. The Asian infrastructure investment bank: Multilateralism on the silk road. China Econ. J. 2016, 9, 116–139. [Google Scholar] [CrossRef]
- Shen, J.; Yang, G.; Zhang, J. The Research of Contribution Measure and Configuration Countermeasures of Marine Biological Pharmaceutical Development Factors. Ecol. Econ. 2013, 2, 056. [Google Scholar]
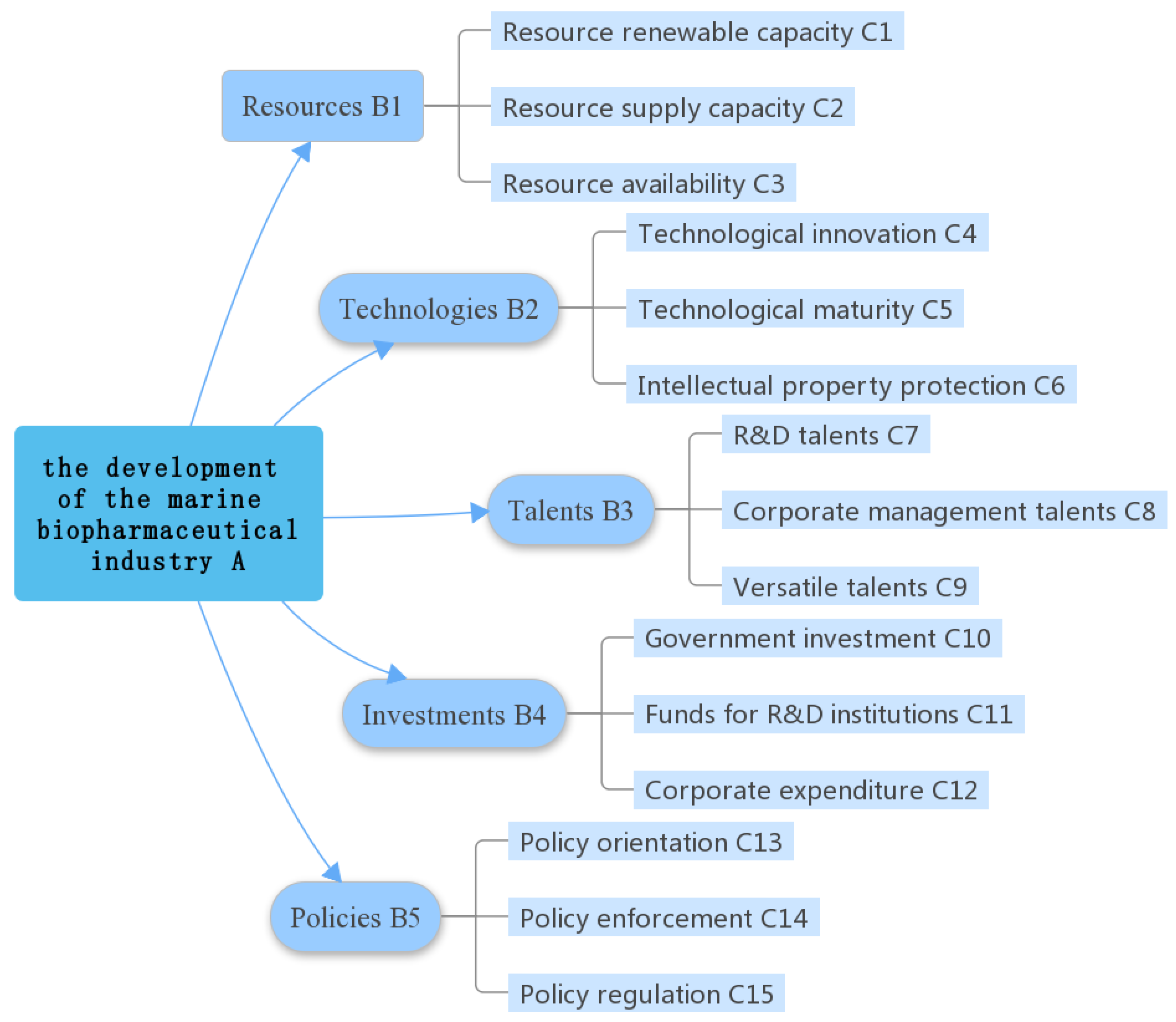
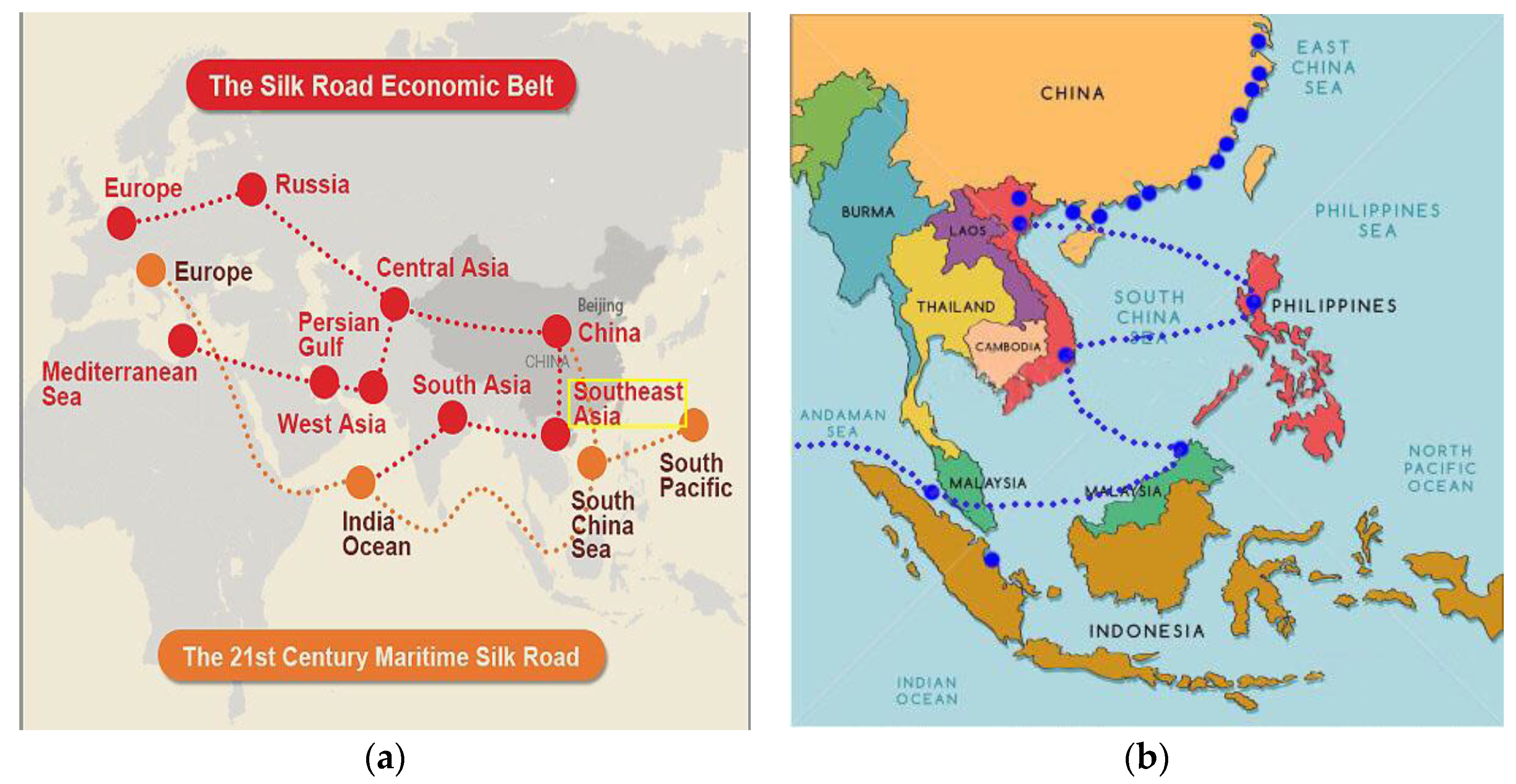
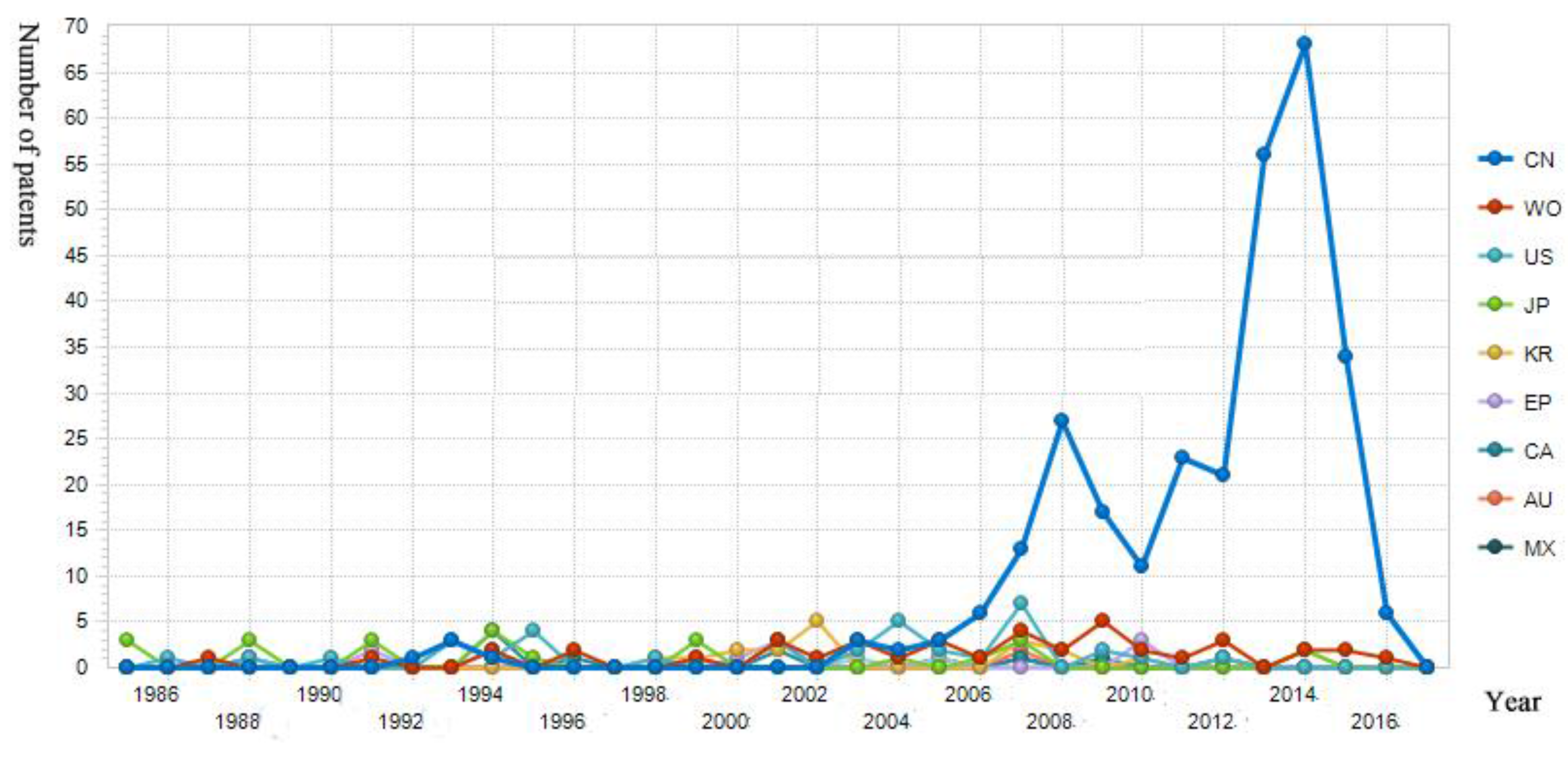
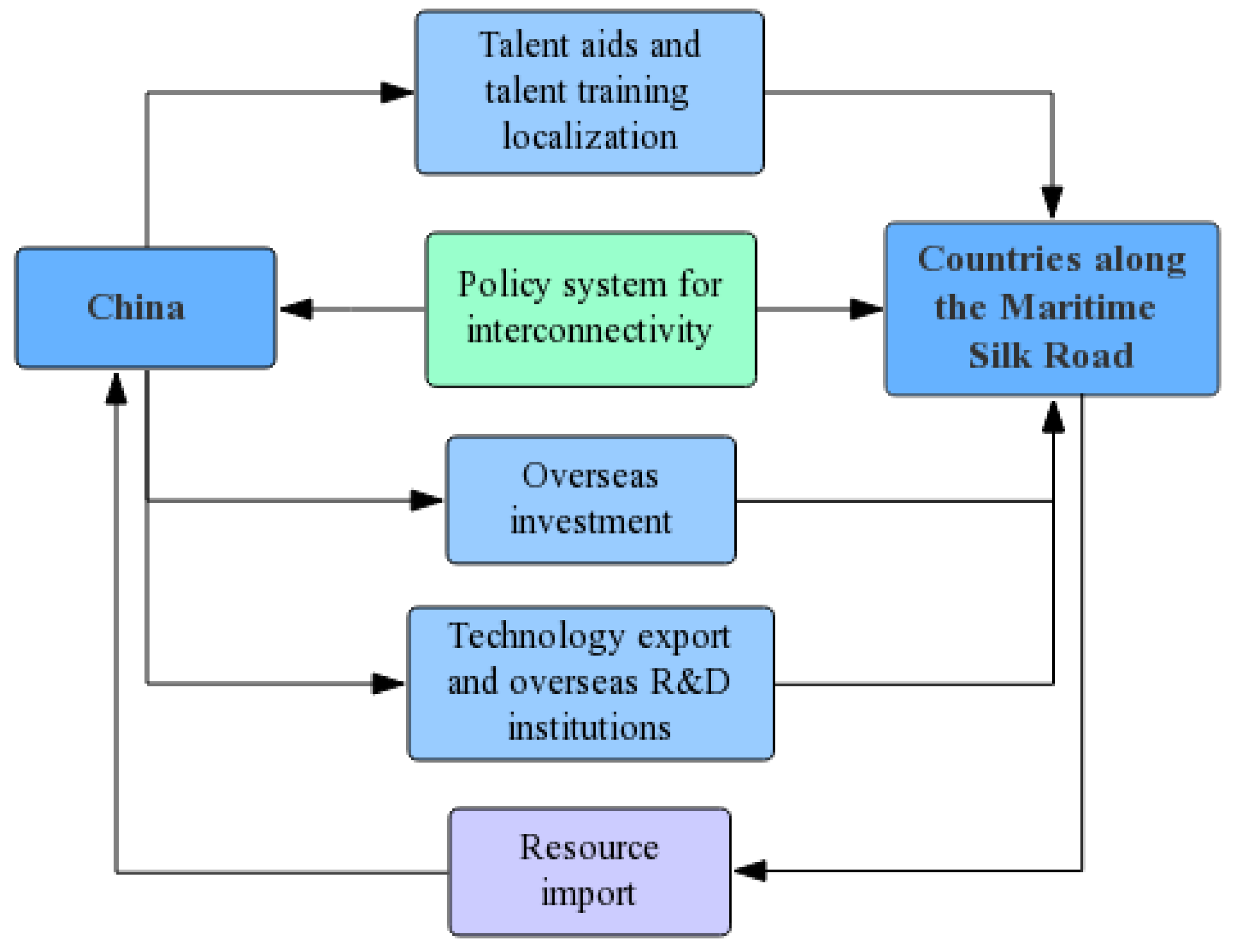
| Target Layer | Dimension Layer | Index Layer | Weight | C.W. | C.R. |
|---|---|---|---|---|---|
| Development of the marine biopharmaceutical industry | Resources (0.133) | Resource renewable capacity | 0.239 | 0.032 | 0.017 |
| Resource supply capacity | 0.625 | 0.083 | |||
| Resource availability | 0.136 | 0.018 | |||
| Technology (0.452) | Technological innovation | 0.558 | 0.252 | 0.017 | |
| Technological maturity | 0.122 | 0.055 | |||
| Intellectual property protection | 0.320 | 0.086 | |||
| Talents (0.270) | R&D talents | 0.218 | 0.059 | 0.054 | |
| Corporate management talents | 0.091 | 0.025 | |||
| Versatile talents | 0.691 | 0.189 | |||
| Investments (0.053) | Government investments | 0.683 | 0.036 | 0.025 | |
| Funds for R&D institutions | 0.200 | 0.011 | |||
| Corporate expenditure | 0.117 | 0.006 | |||
| Policies (0.092) | Policy orientation | 0.717 | 0.066 | 0.092 | |
| Policy enforcement | 0.195 | 0.018 | |||
| Policy regulation | 0.088 | 0.008 |
| Party A | Cooperation | Non-Cooperation | |
|---|---|---|---|
| Party B | |||
| Cooperation | (Rc + Ec, Rm + Em) | (Rc − Tc + Pm, Rm + Tm − Pm) | |
| Non-cooperation | (Rc + rc − Pc, Rm − rm + Pc) | (Rc, Rm) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.-M.; Jiang, S.-S.; Wang, N.; Wang, S.-Q.; Wang, C.-Y. The Research on International Development Path of China’s Marine Biopharmaceutical Industry. Sustainability 2018, 10, 399. https://doi.org/10.3390/su10020399
Fu X-M, Jiang S-S, Wang N, Wang S-Q, Wang C-Y. The Research on International Development Path of China’s Marine Biopharmaceutical Industry. Sustainability. 2018; 10(2):399. https://doi.org/10.3390/su10020399
Chicago/Turabian StyleFu, Xiu-Mei, Shan-Shan Jiang, Na Wang, Shi-Qi Wang, and Chang-Yun Wang. 2018. "The Research on International Development Path of China’s Marine Biopharmaceutical Industry" Sustainability 10, no. 2: 399. https://doi.org/10.3390/su10020399
APA StyleFu, X.-M., Jiang, S.-S., Wang, N., Wang, S.-Q., & Wang, C.-Y. (2018). The Research on International Development Path of China’s Marine Biopharmaceutical Industry. Sustainability, 10(2), 399. https://doi.org/10.3390/su10020399






