Reverse Logistics of Postconsumer Medicines: The Roles and Knowledge of Pharmacists in the Municipality of São Paulo, Brazil
Abstract
1. Introduction
2. Brazilian Legislation on Waste B
3. Materials and Methods
4. Results and Discussion
4.1. Characteristics of Pharmacies
4.2. Knowledge of RPs about RL of Medicines
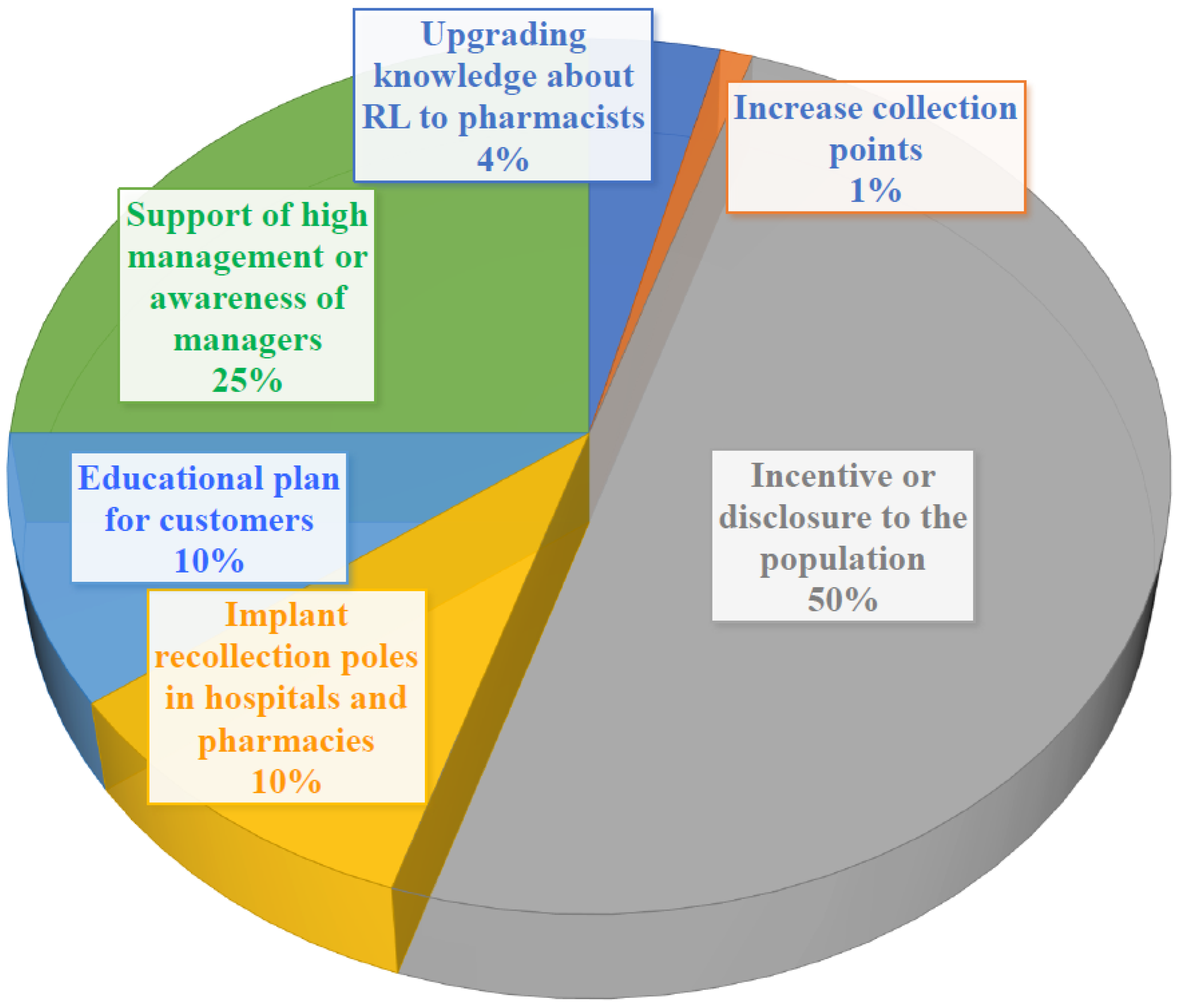
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Zajac, M.A.L.; Fernandes, R.O.; David, C.J.; Aquino, S. Logística reversa de resíduos classe D em ambiente hospitalar: Monitoramento e avaliação da reciclagem no hospital Infantil Cândido Fontoura. Revista de Gestão Ambiental e Sustentabilidade 2016, 5, 78–93. [Google Scholar] [CrossRef]
- Bento, D.G.; Costa, R.; Luz, J.H.; Klock, P. Waste management of healthcare services from the perspective of nursing professionals. Texto Context. Enferm. 2017, 26, e6680015. [Google Scholar] [CrossRef]
- Resolution of the National Environment Council n° 358, May 4, 2005. It Has the Treatment and the Final Disposition of the Health Services Waste. Available online: http://www.mma.gov.br/port/conama/legiabre.cfm?codlegi=462 (accessed on 23 September 2018).
- Resolution of the National Health Surveillance Agency n° 306, of 7 December 2004. It Has the Technical Regulation for Waste Management of Health Services. Available online: http://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2004/res0306_07_12_2004.html (accessed on 17 August 2018).
- Resolution of the Collegiate Board of the National Health Surveillance Agency n° 222, March 28th, 2018. Regulates Good Health-Care Waste Management Practices and Provides Other Measures. Available online: http://portal.anvisa.gov.br/documents/10181/3427425/RDC_222_2018_.pdf/c5d3081d-b331-4626-8448-c9aa426ec410 (accessed on 12 September 2018).
- Kongar, E.; Haznedaroglu, E.; Abdelghany, S.; Bahtiyar, M.O. A Novel IT Infrastructure for Reverse Logistics Operations of End-of-life Pharmaceutical Products. Inf. Technol. Manag. 2015, 16, 51–65. [Google Scholar] [CrossRef]
- Campos, E.A.R.; Paula, I.C.; Pagani, R.N.; Guarnieri, P. Reverse logistics for the end-of-life and end-of-use products in the pharmaceutical industry: A systematic literature review. Supply Chain. Manag. 2017, 22, 375–392. [Google Scholar] [CrossRef]
- Brazilian Industrial Development Agency. Reverse Logistics for the Drug Sector. 2013. Available online: www.abdi.com.br/Estudo/Logística%20Reversa%20de%20Medicamentos.pdf (accessed on 21 July 2018).
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical residues in environmental waters and wastewater: Current state of knowledge and future research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466–467, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Padhye, L.P.; Yao, H.; Kung’u, F.T.; Huang, C. Year-long evaluation on the occurrence and fate of pharmaceuticals, personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant. Water Res. 2014, 51, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.J.; Murby, E.J.; Costanzo, S.D. Removal of antibiotics in conventional and advanced wastewater treatment: Implications for environmental discharge and wastewater recyling. Water Res. 2007, 41, 4164–4176. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Promotion of the Rational Use of Medicines: Central Components. Who Drug Policy Perspectives; WHO: Genebra, Switzerland, 2002; pp. 1–6. [Google Scholar]
- Arkaravichien, W.; Ruchipiyarak, T.; Thawinwan, W.; Benjawilaikul, S. A Threat to the Environment from Practice of Drug Disposal in Thailand. EnvironmentAsia 2014, 7, 13–18. [Google Scholar]
- Melo, S.A.S.; Trovo, A.G.; Bautitz, I.R.; Nogueira, R.F.P. Degradation of residual pharmaceuticals by advanced oxidation processes. Quím. Nova 2009, 32, 188–197. [Google Scholar] [CrossRef]
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front. Microbiol. 2016, 7, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ying, T.; Wang, X.; Liu, J. Occurrence and preliminarily environmental risk assessment of selected pharmaceuticals in the urban rivers, China. Scient. Rep. 2016, 6, 34928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chang, V.W.; Giannis, A.; Wang, J.Y. Removal of cytostatic drugs from aquatic environment: A review. Sci. Total Environ. 2013, 445, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Gabet-Giraud, V.; Miège, C.; Choubert, J.M.; Ruel, S.M.; Coquery, M. Occurrence and removal of estrogens and beta blockers by various processes in wastewater treatment plants. Sci. Total Environ. 2010, 408, 4257–4269. [Google Scholar] [CrossRef] [PubMed]
- El-Hamamsy, M. Unused medications: How cost and how disposal of in Cairo, Egypt. Int. J. Pharm. Sci. Res. 2011, 2, 21–27. [Google Scholar]
- Akatu Institute. Disposal of Medications: A Very Serious Issue. 2008. Available online: https://www.akatu.org.br/noticia/mais-vida-para-o-transito-de-sao-paulo-2-2/ (accessed on 23 September 2018).
- Pro-Genéricos. Market. Available online: http://progenericos.org.br/mercado (accessed on 14 September 2018).
- Government of Brazil. Health: Generics Are Best-Selling Medicines in the Country. Available online: http://www.brasil.gov.br/saude/2017/12/genericos-sao-remedios-mais-vendidos-no-pais (accessed on 29 August 2018).
- Ueda, J.; Tavernaro, R.; Marostega, V.; Pavan, W. Environmental impact of drugs disposal and study of population awareness about the problem. Rev. Ciênc. Amb. 2009, 5, 9. [Google Scholar]
- Brazilian Federal Law nº 12,305, August 3, 2010. Has on the National Solid Waste Policy. Available online: http://www.planalto.gov.br/ccivil_03/_ato2007-2010/2010/lei/l12305.htm (accessed on 16 September 2018).
- Brazilian Federal Senate Portal. Remedies: Regulation Depends on Sector Agreement. Edição 551. 2016. Available online: https://www12.senado.leg.br/cidadania/edicoes/551/regulamentacao-depende-de-acordo-setorial (accessed on 27 July 2018).
- National Health Surveillance Agency. Health Waste Management Manual. 2010. Available online: www.anvisa.gov.br/servicosaude/manuais/manual_gerenciamento_residuos.pdf (accessed on 9 August 2018).
- Bellan, N.; Pinto, T.J.A.; Kaneko, T.M.; Moretto, L.D.; Santos Junior, N. Critical analysis of the regulations regarding the disposal of medication waste. Braz. J. Pharm. Sci. 2012, 48, 507–513. [Google Scholar] [CrossRef]
- Aurélio, C.J.; Pimenta, R.F.; Ueno, H.M. Reverse Logistics of medications: Structure in the pharmaceutical retailing. GEPROS 2015, 10, 1–15. [Google Scholar] [CrossRef]
- ABC Farma. ACBFarma Discusses the Pharmaceutical Scenario: The Disposal of Medications. 2015. Available online: http://abcfarma.org.br/midia/cbfarma-discute-o-cenario-farmaceutico.html (accessed on 4 September 2018).
- Conselho Regional de Farmácia do Estado de São Paulo (CRF-SP). Committee on Waste and Environmental Management. Waste and Environmental Management; Conselho Regional de Farmácia do Estado de São Paulo: São Paulo, Brazil, 2015; p. 44. [Google Scholar]
- Medeiros, M.S.G.; Moreira, L.M.F.; Lopes, C.C.G.O. Drug disposal: Recollection programs and new challenges. Rev. Ciênc. Farm. Básica Apl. 2014, 35, 651–662. [Google Scholar]
- Resolution of the National Environment Council CONAMA nº 5. Definition and Management of Solid Waste. Available online: http://www2.mma.gov.br/port/conama/res/res93/res0593.html (accessed on 24 July 2018).
- Resolution of the National Environment Council CONAMA n° 283, July 12, 2001. The Treatment and Final Destination of Health Services. Available online: http://www.mma.gov.br/port/conama/res/res01/res28301.html (accessed on 13 September 2018).
- Resolution of the Collegiate Board nº 44, of 17 August 2009. It Has Good Pharmaceutical Practices for the Sanitary Control of the Operation, the Dispensing and Marketing of Products and the Provision of Pharmaceutical Services in Pharmacies and Drugstores and Provides Other Measures. Available online: https://www20.anvisa.gov.br/segurancadopaciente/index.php/legislacao/item/rdc-44-2009 (accessed on 28 July 2018).
- Resolution of the Collegiate Board nº. 17, of 16 April 2010. Good Practice of Medicinal Products. Available online: http://portal.anvisa.gov.br/documents/33880/2568070/res0017_16_04_2010.pdf/b9a8a293-f04c-45d1-ad4c-19e3e8bee9fa (accessed on 12 September 2018).
- Resolution of the Collegiate Board nº 210, August 4, 2003. Determining to All Manufacturers of Medicinal Establishments, Compliance with the Guidelines Laid Down in the Technical Regulation of Good Practice for the Manufacture of Medicinal Products. Available online: http://www.cff.org.br/userfiles/file/resolucao_sanitaria/210.pdf (accessed on 13 July 2018).
- Demajorovic, J.; Augusto, E.E.F.; De Souza, M.T.S. Reverse logistics of WEEE in developing countries: Challenges and perspectives for the Brazilian model. Ambient. Soc. 2016, 9, 119–138. [Google Scholar]
- Daughton, C.G. Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. I. Rationale for and Avenues toward a Green Pharmacy. Environ. Health Perspect. 2003, 111, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.A.; Theóphilo, C.R. Methodology of Scientific Research for Applied Social Sciences, 2nd ed.; Editora Atlas: São Paulo, Brazil, 2009. [Google Scholar]
- Júnior, B.; Júnior, N.F. The use of the technique of the interview in scientific works. Evidência 2011, 7, 237–250. [Google Scholar]
- Gil, A.C. Methods and Techniques of Social Research, 5th ed.; Atlas: São Paulo, Brazil, 1999; p. 202. [Google Scholar]
- Triviños, A.N.S. Introduction to Research in Social Sciences: Qualitative Research in Education; Editora Atlas: São Paulo, Brazil, 1987. [Google Scholar]
- Bardin, L. Content Analysis; Almedina: São Paulo, Brazil, 2011. [Google Scholar]
- Yoshida, C. Competence and guidelines of PNMSW: Conflicts and criteria for harmonisation between other laws and standards. In Policy National, Management and Solid Waste; Philippi, A., Jr., Ed.; Manole: São Paulo, Brazil, 2012; pp. 3–38. [Google Scholar]
- Lambert, S.; Riopel, D.; Abdul-Kader, W. A reverse logistics decisions conceptual framework. Comp. Ind. Eng. 2011, 61, 561–581. [Google Scholar] [CrossRef]
- Shaik, M.N.; Abdul-Kader, W. Comprehensive performance measurement and causal-effect decision making model for reverse logistics enterprise. Comp. Ind. Eng. 2014, 68, 87–103. [Google Scholar] [CrossRef]
- Falqueto, E.; Cynamon, K.D.; Facchetti, A.R. Como realizar o correto descarte de resíduos de medicamentos? Ciênc. Saúde Col. 2010, 15, 3283–3293. [Google Scholar] [CrossRef]
- Regional Pharmacy Council of Rio Grande do Sul. Technical Guidance Informs: Drugstores and Pharmacies Should Keep Containers for the Collection of Expired Drugs. Available online: https://www.crfrs.org.br/portal/pagina/noticias-detalhes.php?idn=1519 (accessed on 16 August 2018).
- US Food & Drug Administration. Disposal of Unused Medicines: What You Should Know. Available online: https://www.fda.gov/drugs/resourcesforyou/consumers/buyingusingmedicinesafely/ensuringsafeuseofmedicine/safedisposalofmedicines/ucm186187.htm#Medicines_recommended (accessed on 15 September 2018).
- Khan, U.; Bloom, R.A.; Nicell, J.A.; Laurenson, J.P. Risks associated with the environmental release of pharmaceuticals on the U.S. Food and Drug Administration “flush list”. Sci Total Environ. 2017, 609, 1023–1040. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.; Tit, D.M.; Fodor, K.; Cioca, G.; Agop, M.; Iovan, C.; Cseppento, D.C.N.; Bumbu, A.; Bustea, C. Aspects Regarding the Pharmaceutical Waste Management in Romania. Sustainability 2018, 10, 2788. [Google Scholar] [CrossRef]
- Huang, H.; Li, Y.; Huang, B.; Pi, X. An Optimization Model for Expired Drug Recycling Logistics Networks and Government Subsidy Policy Design Based on Tri-level Programming. Int. J. Environ. Res. Public Health 2015, 12, 7738–7751. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Zhao, X.; Prahinski, C.; Li, Y. The impact of institutional pressures, top managers’ posture and reverse logistics on performance—Evidence from China. Int. J. Prod. Econ. 2013, 143, 132–143. [Google Scholar] [CrossRef]
- Sundaramoorthy, A.; Karimi, I.A. Planning in pharmaceutical supply chains with outsourcing and new product introductions. Ind. Eng. Chem. Res. 2004, 43, 8293–8306. [Google Scholar] [CrossRef]
- Narayana, S.A.; Pati, R.K.; Vrat, P. Managerial research on the pharmaceutical supply chain—A critical review and some insights for future directions. J. Purch. Supply Manag. 2014, 20, 18–40. [Google Scholar] [CrossRef]
- Guenchev, S. Reverse logistics program design: A company study. Bus. Horiz. 2009, 52, 139–148. [Google Scholar] [CrossRef]
- Alvarenga, L.S.V.; Nicoletti, M.A. Domestic discarding of medicines and some considerations about the current environment impact. Rev. Saúde 2010, 4, 34–39. [Google Scholar]
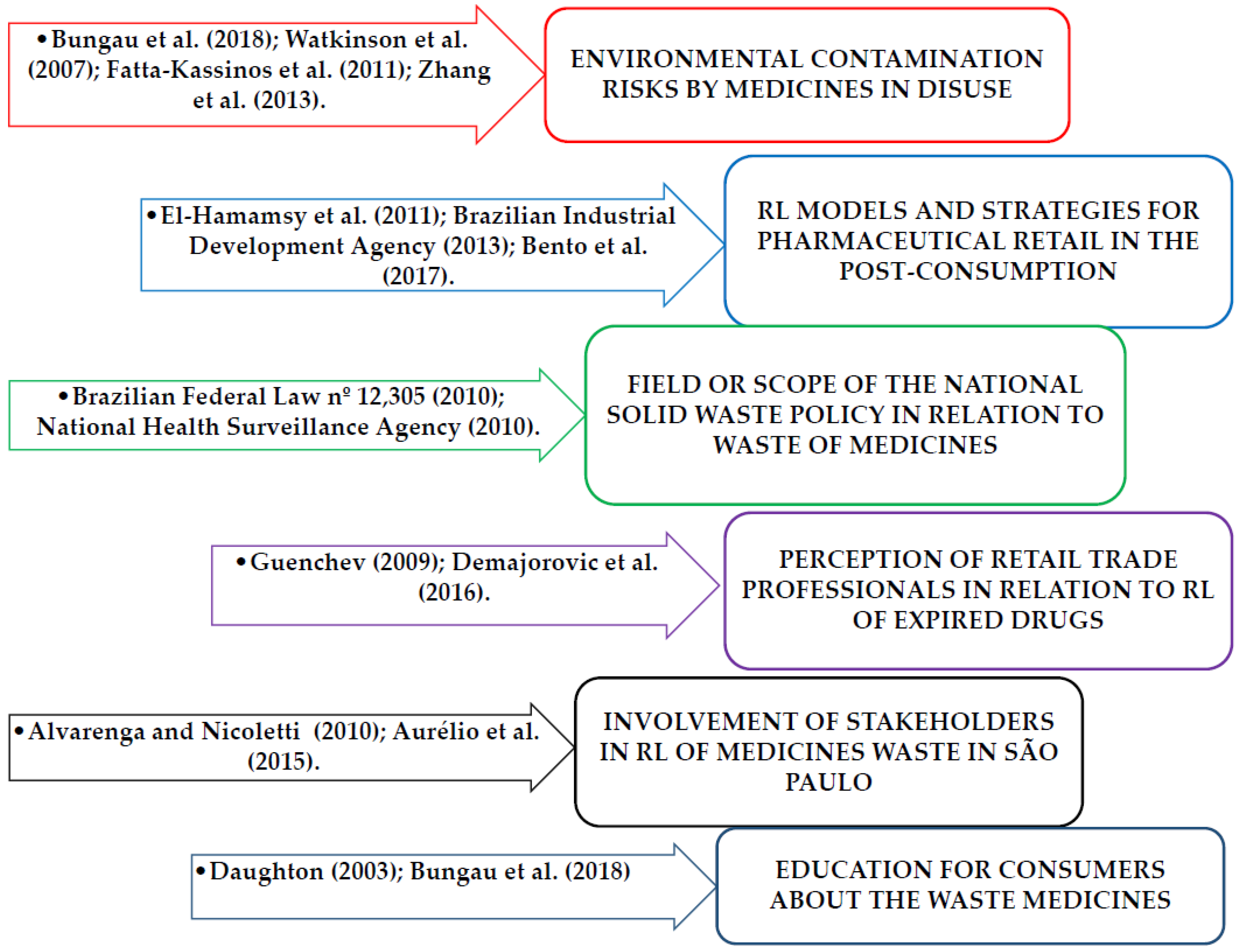


| Dimensions | Objectives | Indicators | References |
|---|---|---|---|
| Environmental contamination by medicines | Verify if the medications in disuse are being collected instead of being discarded in the environment | Harmful to the environment due to irregular disposal | Bungau et al. (2018); Watkinson et al. (2007); Fatta-Kassinos et al. (2011); Kosma et al. (2014); Zhang et al. (2013). |
| Knowledge related to RL in the company where the RP work. | |||
| Brazilian Federal Law No. 12,305 | National Solid Waste Policy (NSWP) | Field of NSWP to chemical residues | Brazilian Federal Law nº 12,305 (2010); El-Hamamsy (2011); Brazilian Industrial Development Agency (2013); Bento et al. (2017). |
| Scope of NSWP in relation to medicines | |||
| Reverse Logistic (RL) of medicines waste | |||
| Pharmaceutical role in postconsumption | Appropriate physical and organizational structure and procedures to guide consumers | Adequate physical and organizational structure | Medeiros et al. (2014); Regional Pharmacy Council of Rio Grande do Sul (2018); Kongar et al. (2015); Campos et al. (2017). |
| Existence of standard operating procedures | |||
| Employee training on the subject | |||
| Reverse Logistic to expired drugs | Collect medicines in post-consumption | Pharmacist’s perception about RL of medications after sale | Guenchev (2009); Demajorovic et al. (2016); Alvarenga and Nicolleti (2010); Aurélio et al. (2015). |
| Drug collection program in disuse | |||
| RL implanted in pharmacy for disposal of consumers | |||
| Education about the waste medicines | Provide clear and accurate information about RL of expired drugs. | Education for pharmacists about NSWP and RL of used medicines | National Health Surveillance Agency (2010); Daughton (2003); Bungau et al. (2018); CRF-SP (2015). |
| Stakeholder’ engagement at RL of expired or disused medications | |||
| Education for consumers about the waste medicines |
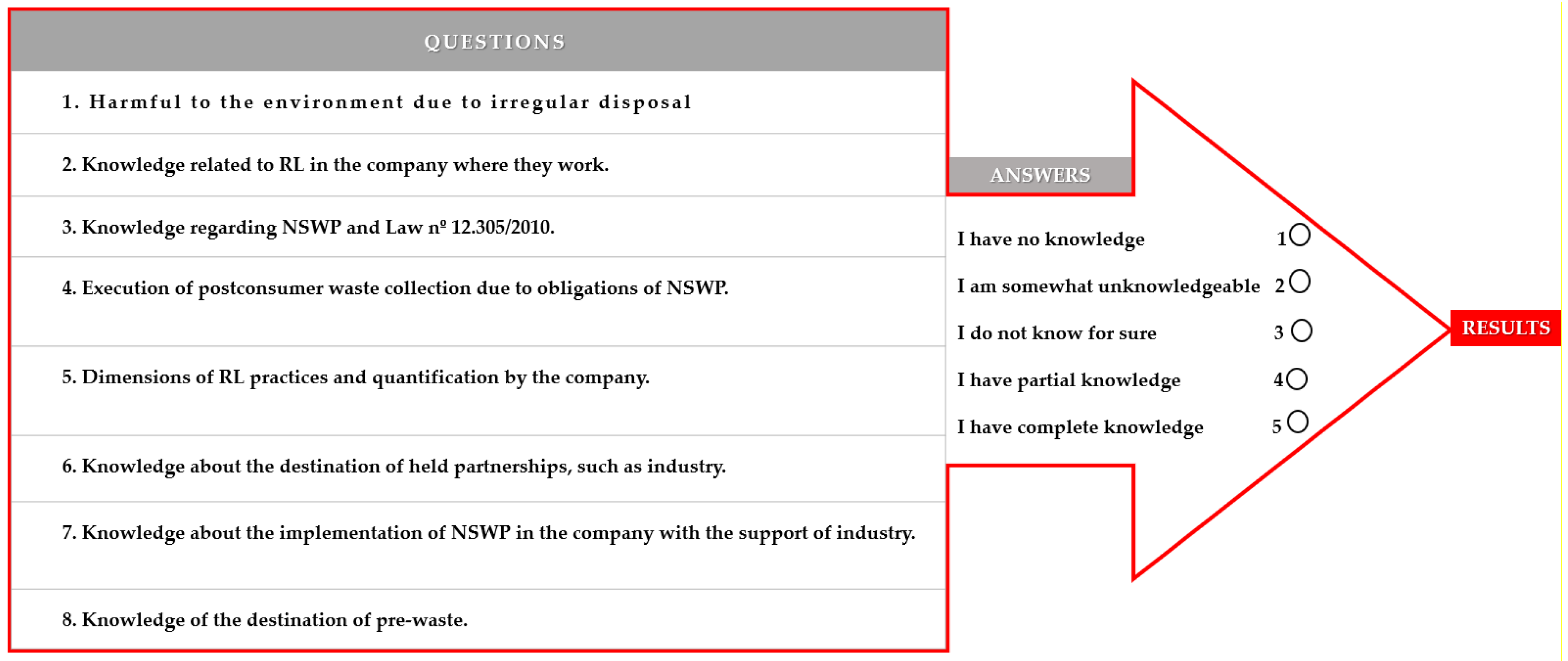
| 1. Harmful to the environment due to irregular disposal. | Answers in Likert Scale | Frequency | Valid Percent |
| I have no knowledge | 8 | 5 | |
| I am somewhat unknowledgeable | 5 | 3.1 | |
| I do not know for sure | 8 | 5 | |
| I have partial knowledge | 113 | 70.2 | |
| I have complete knowledge | 27 | 16.8 | |
| Total | 161 | 100 | |
| 2. Knowledge related to RL in the company where they work. | I have no knowledge | 20 | 12.4 |
| I am somewhat unknowledgeable | 9 | 5.6 | |
| I do not know for sure | 39 | 24.2 | |
| I have partial knowledge | 57 | 35.4 | |
| I have complete knowledge | 36 | 22.4 | |
| Total | 161 | 100 | |
| 3. Knowledge regarding NSWP and Law nº 12.305/2010. | I have no knowledge | 16 | 9.9 |
| I am somewhat unknowledgeable | 15 | 9.3 | |
| I do not know for sure | 47 | 29.2 | |
| I have partial knowledge | 71 | 44.1 | |
| I have complete knowledge | 12 | 7.5 | |
| Total | 161 | 100 | |
| 4. Execution of postconsumer waste collection due to obligations of NSWP. | I have no knowledge | 34 | 21.1 |
| I am somewhat unknowledgeable | 5 | 3.1 | |
| I do not know for sure | 69 | 42.9 | |
| I have partial knowledge | 29 | 18.0 | |
| I have complete knowledge | 24 | 14.9 | |
| Total | 161 | 100 | |
| 5. Dimensions of RL practices and quantification by the company. | I have no knowledge | 9 | 5.6 |
| I am somewhat unknowledgeable | 10 | 6.2 | |
| I do not know for sure | 43 | 26.7 | |
| I have partial knowledge | 83 | 51.6 | |
| I have complete knowledge | 16 | 9.9 | |
| Total | 161 | 100 | |
| 6. Knowledge about the destination of held partnerships, such as industry. | I have no knowledge | 17 | 10.6 |
| I am somewhat unknowledgeable | 24 | 14.9 | |
| I do not know for sure | 79 | 49.1 | |
| I have partial knowledge | 38 | 23.6 | |
| I have complete knowledge | 3 | 1.9 | |
| Total | 161 | 100 | |
| 7. Knowledge about the implementation of NSWP in the company with the support of industry. | I have no knowledge | 18 | 11.2 |
| I am somewhat unknowledgeable | 16 | 9.9 | |
| I do not know for sure | 69 | 42.9 | |
| I have partial knowledge | 41 | 25.5 | |
| I have complete knowledge | 17 | 10.6 | |
| Total | 161 | 100 | |
| 8. Knowledge of the destination of pre-waste. | I have no knowledge | 13 | 8.1 |
| I am somewhat unknowledgeable | 17 | 10.6 | |
| I do not know for sure | 52 | 32.3 | |
| I have partial knowledge | 64 | 39.8 | |
| I have complete knowledge | 15 | 9.3 | |
| Total | 161 | 100 |
| Structure for RL of Drugs | Drugstores | |||
|---|---|---|---|---|
| A | B | C | ||
| Studied Topics | Operations | Receives the medicines | Volunteer delivery post available | Receives the medicines |
| Education | Information available, but should be sought by the consumer | |||
| Economic issues | Partnerships with pharmaceutical laboratories | |||
| Institutional policies | Volunteer delivery post available | Annual sporting event | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquino, S.; Antonio Spina, G.; Leitão Zajac, M.A.; Luiz Lopes, E. Reverse Logistics of Postconsumer Medicines: The Roles and Knowledge of Pharmacists in the Municipality of São Paulo, Brazil. Sustainability 2018, 10, 4134. https://doi.org/10.3390/su10114134
Aquino S, Antonio Spina G, Leitão Zajac MA, Luiz Lopes E. Reverse Logistics of Postconsumer Medicines: The Roles and Knowledge of Pharmacists in the Municipality of São Paulo, Brazil. Sustainability. 2018; 10(11):4134. https://doi.org/10.3390/su10114134
Chicago/Turabian StyleAquino, Simone, Glauco Antonio Spina, Maria Antonietta Leitão Zajac, and Evandro Luiz Lopes. 2018. "Reverse Logistics of Postconsumer Medicines: The Roles and Knowledge of Pharmacists in the Municipality of São Paulo, Brazil" Sustainability 10, no. 11: 4134. https://doi.org/10.3390/su10114134
APA StyleAquino, S., Antonio Spina, G., Leitão Zajac, M. A., & Luiz Lopes, E. (2018). Reverse Logistics of Postconsumer Medicines: The Roles and Knowledge of Pharmacists in the Municipality of São Paulo, Brazil. Sustainability, 10(11), 4134. https://doi.org/10.3390/su10114134





