Phosphorus Availability in Wheat, in Volcanic Soils Inoculated with Phosphate-Solubilizing Bacillus thuringiensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Site I
2.3. Site II
2.4. Experimental Design
2.5. Pots
2.6. Origin of the Strain and Inoculum Preparation
Inoculation
2.7. Analysis of P in the Plants and Soil and pH Levels
2.8. Enzymatic Activity and Soil Microbial Biomass
2.9. Plant Yield
2.10. Statistical Analysis
3. Results
3.1. Effect of B. thuringiensis Inoculation in an Andisol in Different Growth Stages of Wheat Plants
3.1.1. Soil Chemical Properties
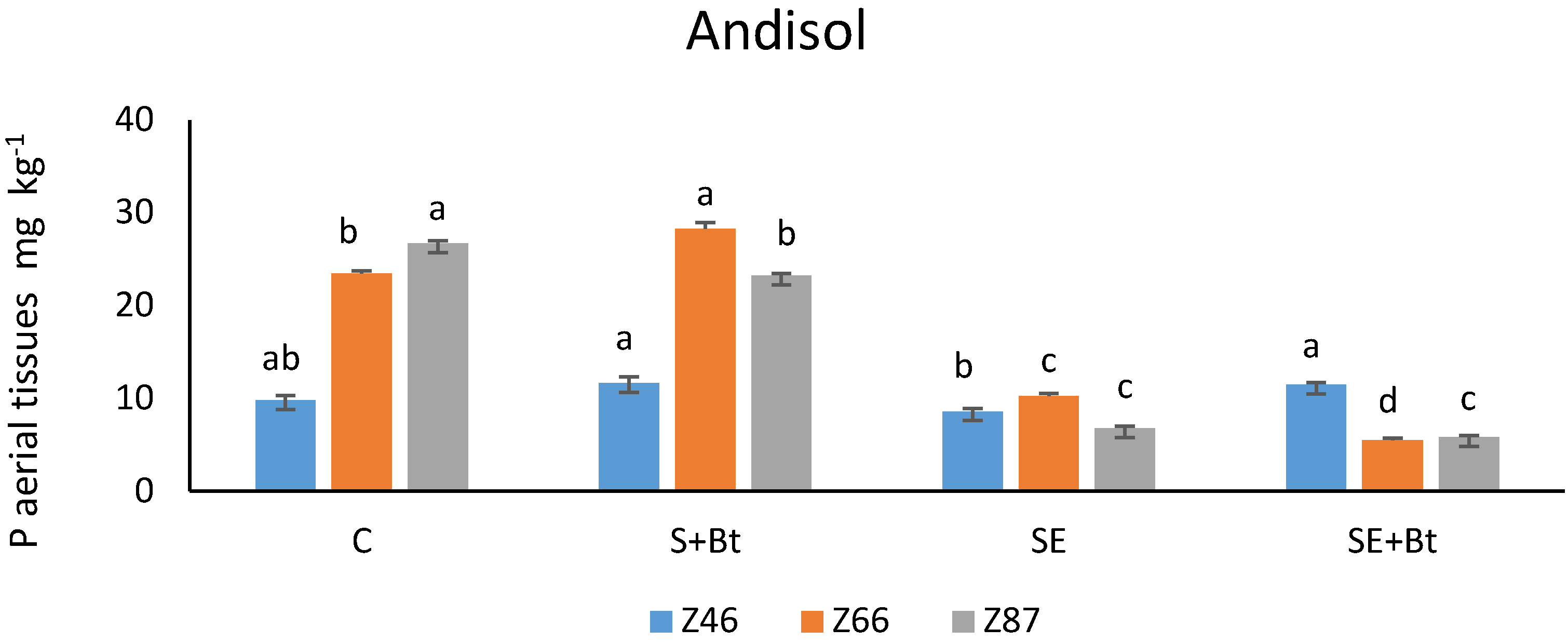
3.1.2. P in the Plant
3.1.3. Soil Biological Properties
3.1.4. Plant Biomass
3.2. Effect of the B. thuringiensis Inoculation in an Ultisol in Different Growth Stages of Wheat Plants
3.2.1. Soil Chemical Properties
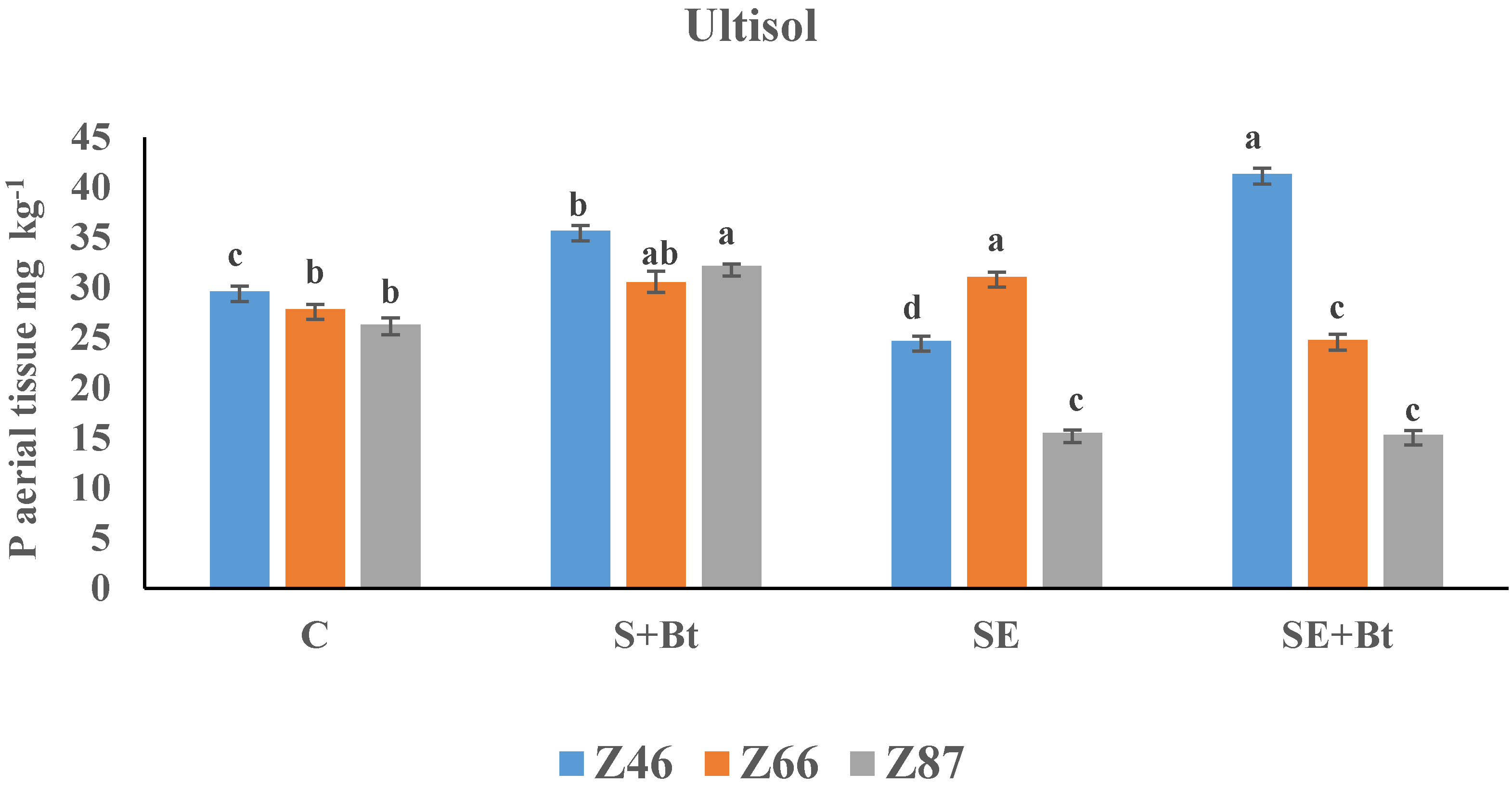
3.2.2. P in the Plant
3.2.3. Soil Biological Properties
3.2.4. Plant Biomass
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khan, M.S.; Zaidi, A.; Ahmad, E. Mechanism of phosphate solubilization and physiological functions of phosphate solubilizing microorganisms. In Phosphate Solubilizing Microorganisms: Principles and Application of Microphos Technology, 1st ed.; Khan, M.S., Zaidi, A., Musarrat, J., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 31–62. ISBN 978-3-319-34879-7. [Google Scholar]
- Redel, Y.; Rubio, R.; Godoy, R.; Borie, F. Phosphorus fractions and phosphatase activity in an Andisol under different forest ecosystems. Geoderma 2008, 145, 216–221. [Google Scholar] [CrossRef]
- Borie, F.; Rubio, R. Total and organic phosphorus in Chilean volcanic soils. Gayana Bot. 2003, 60, 69–78. [Google Scholar] [CrossRef]
- Stevenson, F.J.; Cole, M.A. Cycles of Soil: Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1999; p. 427. ISBN 978-0-471-32071-5. [Google Scholar]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; US Government Printing Office: Washington, DC, USA, 2006.
- Larraín, J.D.; Olfos, G.M.J. Boletín de Trigo: Producción, Precios y Comercio Exterior. Enero-Febrero 2012; Odepa: Santiago, Chile, 2012; 44p, Available online: http://www.odepa.gob.cl//odepaweb (accessed on 15 June 2016).
- Fundación Chile. Una Nueva Visión Para el Sector Triguero en Chile; Fundación Chile: Santiago, Chile, 2005; 99p, Available online: http://www.odepa.cl/odepaweb/servicios-informacion/publica/VisionTrigoFunChile.pdf (accessed on 5 January 2018).
- Römer, W.; Schilling, G. Phosphorus requirements of the wheat plant in various stages of its life cycle. Plant Soil 1986, 91, 221–229. [Google Scholar] [CrossRef]
- Batten, G.D.; Wardlaw, I.F.; Aston, M.J. Growth and the distribution of phosphorus in wheat developed under various phosphorus and temperature regimes. Aust. J. Agric. Res. 1986, 37, 459–469. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, I. Effects of phosphorus levels alone or in combination with phosphate-solubilizing bacteria and farmyard manure on growth, yield and nutrient up-take of wheat (Triticum aestivum L.). J. Agric. Soc. Sci. 2006, 2, 96–100. [Google Scholar]
- Dodd, I.C.; Ruiz-Lozano, J.M. Microbial enhancement of crop resource use efficiency. Curr. Opin. Biotechnol. 2012, 23, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, M.; Li, H. Inoculation of phosphatesolubilizing bacteria Bacillus thuringiensis B1 increases available phosphorus and growth of peanut in acidic soil. Acta Agric. Scand. BSP 2014, 64, 252–259. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant growth promotion by phosphate solubilizing fungi-current perspective. Arch. Agron. Soil Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Marra, L.M.; Soares, C.R.F.S.; de Oliveira, S.M.; Ferreira, P.A.A.A.; Soares, B.L.; Carvalho, R.F.; Lima, J.M.; Moreira, F.M. Biological nitrogen fixation and phosphate solubilization by bacteria isolated from tropical soils. Plant Soil 2012, 357, 289–307. [Google Scholar] [CrossRef]
- Scervino, J.M.; Mesa, M.P.; Mónica, I.D.; Recchi, M.; Moreno, N.S.; Godeas, A. Soil fungal isolates produce different organic acid patterns involved in phosphate salts solubilization. Biol. Fertil. Soils 2010, 46, 755–763. [Google Scholar] [CrossRef]
- Freitas, J.; Banerjee, M.R.; Germida, J.J. Phosphate-solubilizing rhizobacteria enhance the growth and yield but not phosphorus uptake of canola (Brassica napus L.). J. Biol. Fertil. Soils 1997, 24, 358–364. [Google Scholar] [CrossRef]
- Qiao, J.Q.; Wu, H.J.; Rong Huo, R.; Gao, X.W.; Borriss, R. Stimulation of plant growth and biocontrol by Bacillus amyloliquefaciens subsp. plantarum FZB42 engineered for improved action. Chem. Biol. Technol. Agric. 2014, 1, 12. [Google Scholar] [CrossRef]
- Cherif-Silini1, H.; Silini, A.; Yahiaoui, B.; Ouzari, I.; Boudabous, A. Phylogenetic and plant-growth-promoting characteristics of Bacillus isolated from the wheat rhizosphere. Ann. Microbiol. 2016, 66, 1087–1097. [Google Scholar] [CrossRef]
- Stolpe, N.B. Descripción de los Principales Suelos de la VIII Región de Chile; Universidad de Concepción: Chillán, Chile, 2006; 84p, ISBN 956-227-293-1. [Google Scholar]
- Sadzawka, M.A.R.; Carrasco, A.M.R.; Demanet, F.R.; Flores, P.H.; Grez, Z.R.; Mora, G.M.L.; Neaman, A. Métodos de Análisis Recomendados Para los Suelos de Chile. Revisión 2006; INIA Serie Actas Nº 34; Imprenta Salesianos S.A.: Santiago, Chile, 2006; 164p. [Google Scholar]
- Sandoval, E.M.; Dorner, J.F.; Seguel, O.S.; Cuevas, J.B.; Rivas, D.S. Métodos de Análisis Físicos de Suelos; Universidad de Concepción: Chillán, Chile, 2012; 80p. [Google Scholar]
- CIREN. Descripciones de Suelos, Materiales y Símbolos. Estudio Agrológico IX Región; Centro de Información de Recursos Naturales (CIREN): Santiago, Chile, 2002; 360p, ISBN 956-7153-35-3. [Google Scholar]
- Wolf, D.C.; Skipper, H.D. Soil sterilization. In Methods of Soil Analysis. Part 2. Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Bottonley, P.S., Eds.; SSSA Book Ser. 5; SSSA: Madison, WI, USA, 1994; pp. 41–51. ISBN 0-89118-810-X. [Google Scholar]
- Zadoks, J.C.; Changt, T.T.; Konzak, C.V. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Sepúlveda, C. Inoculación de Achicoria Industrial (Cichorium Intybus L.) con Rizobacterias Promotoras de Crecimiento; Tesis Ingeniero Agrónomo; Universidad de Concepción: Chillán, Chile, 2017. [Google Scholar]
- Slack, M.; Wheldon, D.B. A Simple and Safe Volumetric Alternative to the Method of Miles, Misra and Irwin for Counting Viable Bacteria. J. Med. Microbiol. 1978, 11, 5411. [Google Scholar] [CrossRef] [PubMed]
- Sadzawka, M.A.R.; Carrasco, A.M.R.; Demanet, F.R.; Flores, P.H.; Grez, Z.R.; Mora, G.M.L.; Neaman, A. Métodos de Análisis de Tejidos Vegetales, 2nd ed.; INIA Serie Actas Nº 40; Salesianos Impresores S.A.: Santiago, Chile, 2007; 140p. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Alef, K. Estimation of hydrolysis of fluerescein diacetate. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1995; pp. 232–233. ISBN 9780080527482. [Google Scholar]
- IBM Corp. Released. In IBM SPSS Statistics for macOS, version 23.0.; IBM Corp.: Armonk, NY, USA, 2015. [Google Scholar]
- Li, X.; Ding, X.; Xia, L.; Sun, Y.; Yuan, C.; Yin, J. Proteomic analysis of Bacillus thuringiensis strain 4.0718 at different growth phases. Sci. World J. 2012, 1–10. [Google Scholar] [CrossRef]
- Dinesh, R.; Anandaraj, M.; Kumar, A.; Srinivasan, V.; Bini, Y.K.; Subila, K.P.; Aravind, R.; Hamza, S. Effects of plant growth-promoting rhizobacteria and NPK fertilizers on biochemical and microbial properties of soils under ginger (Zingiber officinale) cultivation. Agric. Res. 2013, 2, 346–353. [Google Scholar] [CrossRef]
- Chen, Y.P.; Rekha, P.D.; Arun, A.B.; Shen, F.T.; Lai, W.A.; Young, C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Ogut, M.; Er, F. Mineral composition of field grown winter wheat inoculated with phosphorus solubilizingbacteria at different plant growth stages. J. Plant Nutr. 2016, 39, 479–490. [Google Scholar] [CrossRef]
- Ul Hassan, T.; Bano, A. The stimulatory effects of L-tryptophan and plant growth promoting rhizobacteria (PGPR) on soil health and physiology of wheat. J. Soil Sci. Plant Nutr. 2015, 15, 190–201. [Google Scholar] [CrossRef]
- Schoebitz, M.; Ceballos, C.; Ciamp, L. Effect of immobilized phosphate solubilizing bacteria on wheat growth and phosphate uptake. J. Soil Sci. Plant Nutr. 2013, 13, 1–10. [Google Scholar] [CrossRef]
- Morales, A.; Alvear1, M.; Valenzuela, E.; Rubio, R.; Borie, F. Effect of inoculation with Penicillium albidum, a phosphate-solubilizing fungus, on the growth of Trifolium pratense cropped in a volcanic soil. J. Basic. Microbiol. 2007, 47, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Turan, M.; Gulluce, M.; Wirén, N.; Sahin, F. Yield promotion and phosphorus solubilization by plant growth-promoting rhizobacteria in extensive wheat production in Turkey. J. Plant Nutr. Soil Sci. 2012, 175, 818–826. [Google Scholar] [CrossRef]
- Wani, P.A.; Khan, M.S.; Zaidi, A. Synergistic effects of the inoculation with nitrogen-fixing and phosphate-solubilizing rhizobacteria on the performance of field-grown chickpea. J. Plant Nutr. Soil Sci. 2007, 170, 283–287. [Google Scholar] [CrossRef]
- Schneider, K.; Turrion, M.; Grierson, P.; Gallardo, J. Phosphatase activity, microbial phosphorus, and fine root growth in forest soils in the Sierra de Gata, western central Spain. Biol. Fertil. Soils 2001, 34, 151–155. [Google Scholar] [CrossRef]
- Gianfreda, L. Enzymes of importance to rhizosphere processes. J. Soil Sci. Plant Nutr. 2015, 15, 283–306. [Google Scholar] [CrossRef]
- Salantur, A.; Ozturk, A.; Akten, S. Growth and yield response of spring wheat (Triticum aestivum L.) to inoculation with rhizobacteria. Plant Soil Environ. 2006, 52, 111–118. [Google Scholar] [CrossRef]
- Alphei, J.; Scheu, S. Effects of biocidal treatments on biological and nutritional properties of a mull-structured woodland soil. Geoderma 1993, 56, 435–448. [Google Scholar] [CrossRef]
- Williams-Linera, G.; Ewel, J.J. Effect of autoclave sterilization of a tropical andept on seed germination and seedling growth. Plant Soil 1984, 82, 263–268. [Google Scholar] [CrossRef]
- Mahomood, T.; Mehnaz, S.; Flaischmann, F.; Ali, R.; Hashmi, Z.H.; Iqbal, Z. Soil sterilization effects on root growth and formation of rhizosheaths in wheat seedlings. Pedobiologia 2014, 57, 123–130. [Google Scholar] [CrossRef]
- Gunjigake, N.; Wada, K. Effects of phosphorus concentration and pH on phosphate retention by active aluminium and iron of Andosols. Soil Sci. 1980, 132, 347–352. [Google Scholar] [CrossRef]
- Vistoso, E.; Theng, B.K.G.; Bolan, N.S.; Parfitt, R.L.; Mora, M.L. Competitive sorption of molybdate and phosphate in Andisols. J. Soil Sci. Plant Nutr. 2012, 12, 59–72. [Google Scholar] [CrossRef]
- Stutter, M.; Stand, C.; George, T.; Blackwell, M.; Dixon, L.; Bol, R.; MacKay, R.; Richardson, A.; Condron, L.; Haygarth, P. Land use and soil factors affecting accumulation of phosphorus species in temperate soils. Geoderma 2015, 257, 29–39. [Google Scholar] [CrossRef]
- Jorquera, M.A.; Hernández, M.T.; Rengel, Z.; Marschner, P.; Mora, M.L. Isolation of culturable phosphobacteria with both phytate-mineralization and phosphate-solubilization activity from the rhizosphere of plants grown in a volcanic soil. Biol. Fertil. Soils 2008, 44, 1025. [Google Scholar] [CrossRef]


| pH H2O | Olsen-P | Acid Phosphatase | Microbial Biomass | |
|---|---|---|---|---|
| mg kg−1 | μmoles pNF g ss−1 h−1 | μg F g ss−1 | ||
| Treatments | Z46 | |||
| C | 5.7 ± 0.05 b | 10.61 ± 0.23 a | 0.59 ± 0.01 a | 19.33 ± 0.69 b |
| S + Bt | 5.7 ± 0.00 b | 11.15 ± 0.10 a | 0.34 ± 0.01 b | 36.32 ± 1.27 a |
| SE | 6.2 ± 0.04 a | 7.86 ± 0.33 b | 0.30 ± 0.0 c | 11.24 ± 0.68 c |
| SE + Bt | 6.3 ± 0.02 a | 7.86 ± 0.51 b | 0.12 ± 0.01 d | 35.74 ± 0.64 a |
| Treatments | Z66 | |||
| C | 5.0 ± 0.0 b | 9.16 ± 0.37 a | 0.08 ± 0.0 b | 52.68 ± 3.27 a |
| S + Bt | 5.1 ± 0.02 b | 8.42 ± 0.30 a | 0.13 ± 0.01 a | 25.01 ± 0.48 b |
| SE | 6.2 ± 0.06 a | 6.35 ± 0.30 b | 0.01 ± 0.0 c | 55.62 ± 3.14 a |
| SE + Bt | 6.2 ± 0.05 a | 6.77 ± 0.53 b | 0.02 ± 0.0 c | 21.39 ± 1.10 b |
| Treatments | Z87 | |||
| C | 4.6 ± 0.02 b | 7.89 ± 0.29 a | 0.10 ± 0.0 b | 12.53 ± 1.30 b |
| S + Bt | 4.5 ± 0.02 b | 7.85 ± 0.06 a | 0.17 ± 0.01 a | 26.83 ± 3.09 a |
| SE | 5.7 ± 0.04 a | 5.47 ± 0.07 b | 0.05 ± 0.0 c | 10.70 ± 0.16 b |
| SE + Bt | 5.8 ± 0.05 a | 5.21 ± 0.32 b | 0.08 ± 0.0 b | 7.34 ± 0.61 b |
| Treatments | Z46 | Z66 | Z87 | |||
|---|---|---|---|---|---|---|
| Biomass | Biomass | Biomass | ||||
| Aerial | Root | Aerial | Root | Aerial | Root | |
| g Plant−1 | ||||||
| C | 0.42 ± 0.04 a | 0.23 ± 0.01 a | 1.83 ± 0.15 a | 0.06 ± 0.01 a | 1.93 ± 0.03 a | 0.21 ± 0.01 a |
| S + Bt | 0.37 ± 0.01 b | 0.24 ± 0.01 a | 1.29 ± 0.14 b | 0.07 ± 0.02 a | 1.35 ± 0.09 b | 0.21 ± 0.01 a |
| SE | 0.07 ± 0.01 c | 0.03 ± 0.0 b | 0.24 ± 0.03 c | 0.01 ± 0.0 b | 0.18 ± 0.02 c | 0.14 ± 0.0 b |
| SE + Bt | 0.08 ± 0.01 c | 0.03 ± 0.0 b | 0.07 ± 0.01 c | 0.01 ± 0.0 b | 0.23 ± 0.02 c | 0.01 ± 0.01 c |
| pH H2O | Olsen-P | Acid Phosphatase | Microbial Biomass | |
|---|---|---|---|---|
| mg kg−1 | μmols pNF g ss−1 h−1 | μg F g ss−1 | ||
| Treatments | Z46 | |||
| C | 5.8 ± 0.0 b | 14.92 ± 0.26 b | 0.49 ± 0.01 a | 11.88 ± 0.65 a |
| S + Bt | 5.9 ± 0.04 b | 12.89 ± 0.23 c | 0.49 ± 0.02 a | 10.91 ± 0.65 a |
| SE | 6.3 ± 0.04 a | 15.16 ± 0.47 b | 0.14 ± 0.01 b | 11.47 ± 0.76 a |
| SE + Bt | 6.4 ± 0.02 a | 16.80 ± 0.42 a | 0.18 ± 0.01 b | 6.60 ± 0.23 b |
| Treatments | Z66 | |||
| C | 5.2 ± 0.02 c | 10.91 ± 0.19 c | 0.43 ± 0.01 a | 2.20 ± 0.20 b |
| S + Bt | 5.6 ± 0.02 b | 10.19 ± 0.25 c | 0.41 ± 0.0 a | 20.84 ± 1.29 a |
| SE | 6.5 ± 0.02 a | 13.19 ± 0.33 a | 0.08 ± 0.0 b | 4.31 ± 0.46 b |
| SE + Bt | 6.6 ± 0.04 a | 11.74 ± 0.25 b | 0.07 ± 0.01 b | 21.62 ± 0.01 a |
| Treatments | Z87 | |||
| C | 5.5 ± 0.04 b | 10.42 ± 0.18 b | 0.43 ± 0.01 a | 17.05 ± 0.21 b |
| S + Bt | 5.5 ± 0.05 b | 9.62 ± 0.27 b | 0.36 ± 0.02 b | 17.92 ± 0.84 b |
| SE | 5.9 ± 0.02 a | 10.68 ± 0.14 ab | 0.07 ± 0.0 c | 12.19 ± 0.63 c |
| SE + Bt | 5.9 ± 0.05 a | 11.15 ± 0.36 a | 0.07 ± 0.04 c | 20.99 ± 0.67 a |
| Treatments | Z46 | Z66 | Z87 | |||
|---|---|---|---|---|---|---|
| Biomass | Biomass | Biomass | ||||
| Aerial | Root | Aerial | Root | Aerial | Root | |
| g Plant−1 | ||||||
| C | 1.76 ± 0.21 a | 0.42 ± 0.21 b | 3.11 ± 0.29 a | 0.68 ± 0.01 a | 3.50 ± 0.32 a | 0.30 ± 0.01 b |
| S+Bt | 1.76 ± 0.65 a | 0.59 ± 0.12 a | 3.14 ± 0.27 a | 0.63 ± 0.01 a | 3.99 ± 0.15 a | 0.35 ± 0.01 a |
| SE | 0.19 ± 0.76 b | 0.35 ± 0.02 c | 2.86 ± 0.27 a | 0.54 ± 0.02 b | 3.61 ± 0.12 a | 0.20 ± 0.0 c |
| SE+Bt | 0.10 ± 0.23 b | 0.43 ± 0.01 b | 2.42 ± 0.09 a | 0.48 ± 0.01 b | 2.54 ± 0.19 b | 0.06 ± 0.0 d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delfim, J.; Schoebitz, M.; Paulino, L.; Hirzel, J.; Zagal, E. Phosphorus Availability in Wheat, in Volcanic Soils Inoculated with Phosphate-Solubilizing Bacillus thuringiensis. Sustainability 2018, 10, 144. https://doi.org/10.3390/su10010144
Delfim J, Schoebitz M, Paulino L, Hirzel J, Zagal E. Phosphorus Availability in Wheat, in Volcanic Soils Inoculated with Phosphate-Solubilizing Bacillus thuringiensis. Sustainability. 2018; 10(1):144. https://doi.org/10.3390/su10010144
Chicago/Turabian StyleDelfim, Jorge, Mauricio Schoebitz, Leandro Paulino, Juan Hirzel, and Erick Zagal. 2018. "Phosphorus Availability in Wheat, in Volcanic Soils Inoculated with Phosphate-Solubilizing Bacillus thuringiensis" Sustainability 10, no. 1: 144. https://doi.org/10.3390/su10010144
APA StyleDelfim, J., Schoebitz, M., Paulino, L., Hirzel, J., & Zagal, E. (2018). Phosphorus Availability in Wheat, in Volcanic Soils Inoculated with Phosphate-Solubilizing Bacillus thuringiensis. Sustainability, 10(1), 144. https://doi.org/10.3390/su10010144






