Production of Biosorbents from Waste Olive Cake and Its Adsorption Characteristics for Zn2+ Ion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
| Non activated (OC) | Activated with H2SO4 (OC-H) | Activated with NaOH (OC-OH) | ||||
| Total (T) | >2mm (P) | Total (T) | >2mm (P) | Total (T) | >2mm (P) | |
| Ash content (% dm) | 21 ± 2 | 14 ± 1 | 23 ± 3 | 18 ± 4 | 22 ± 1 | 15 ± 3 |
| pH | 6.5 ± 0.1 | 7.1 ± 0.3 | 2.4 ± 0.1 | 2.3 ± 0.0 | 8.0 ± 0.1 | 8.1 ± 0.1 |
| Iodine Number (mg/g) | 135 ± 10 | 64 ± 5 | 329 ± 13 | 247 ± 5 | 217 ± 39 | 64 ± 5 |
| C (% dm) | 43 ± 2 | 41 ± 1 | 40 ± 2 | 48 ± 2 | 37 ± 2 | 34 ± 2 |
| H (% dm) | 5.9 ± 0.8 | 6.4 ± 0.2 | 3.3 ± 0.6 | 2.9 ± 0.2 | 6.3 ± 0.3 | 6.6 ± 0.3 |
| N (% dm) | 1.10 ± 0.03 | 0.49 ± 0.08 | 0.90 ± 0.06 | 0.44 ± 0.06 | 0.98 ± 0.08 | 0.50 ± 0.09 |
| Non activated (OC) | Activated with H2SO4 (OC-H) | Activated with NaOH (OC-OH) | ||||
| Total (T) | >2mm (P) | Total (T) | >2mm (P) | Total (T) | >2mm (P) | |
| Ca (% am) | 1.8 ± 0.2 | 1.3 ± 0.2 | 0.37 ± 0.09 | 0.25 ± 0.09 | < 0.0003 | < 0.0003 |
| Mg (% am) | 0.52 ± 0.01 | 0.51 ± 0.04 | 0.09 ± 0.01 | 0.03 ± 0.02 | < 0.00006 | < 0.00006 |
| K (% am) | 2.8 ± 0.4 | 1.2 ± 0.1 | 1.4 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.12 ± 0.03 |
| Na (% am) | 1.1 ± 0.1 | 0.65 ± 0.08 | 3.3 ± 0.1 | 4.0 ± 0.9 | 0.9 ± 0.5 | 1.2 ± 0.3 |
| Fe (% am) | 2.8 ± 0.3 | 3.2 ± 0.2 | < 0.0012 | < 0.0012 | < 0.0012 | < 0.0012 |
| Mn (mg/kg am) | 79 ± 3 | 96 ± 7 | < 5.8 | < 5.8 | < 5.8 | < 5.8 |
| Zn (mg/kg am) | 214 ± 45 | 153 ± 7 | 19 ± 2 | 101 ± 4 | < 2.6 | < 2.6 |
| Cu (mg/kg am) | 53 ± 3 | 88 ± 17 | < 8.2 | < 8.2 | < 8.2 | < 8.2 |
2.2. Zinc Adsorption
2.2.1. Contact time
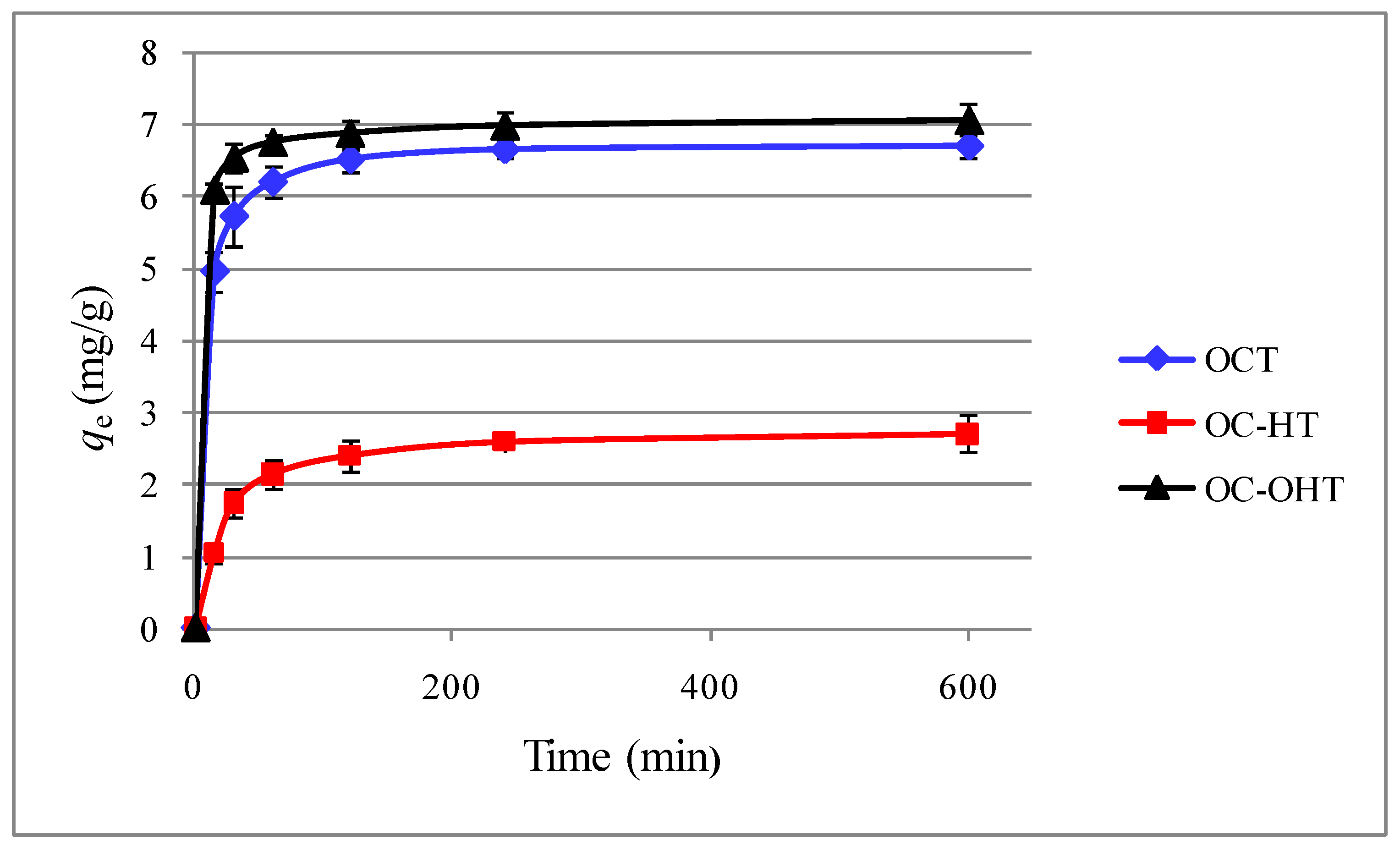
2.2.2. Kinetic modelling
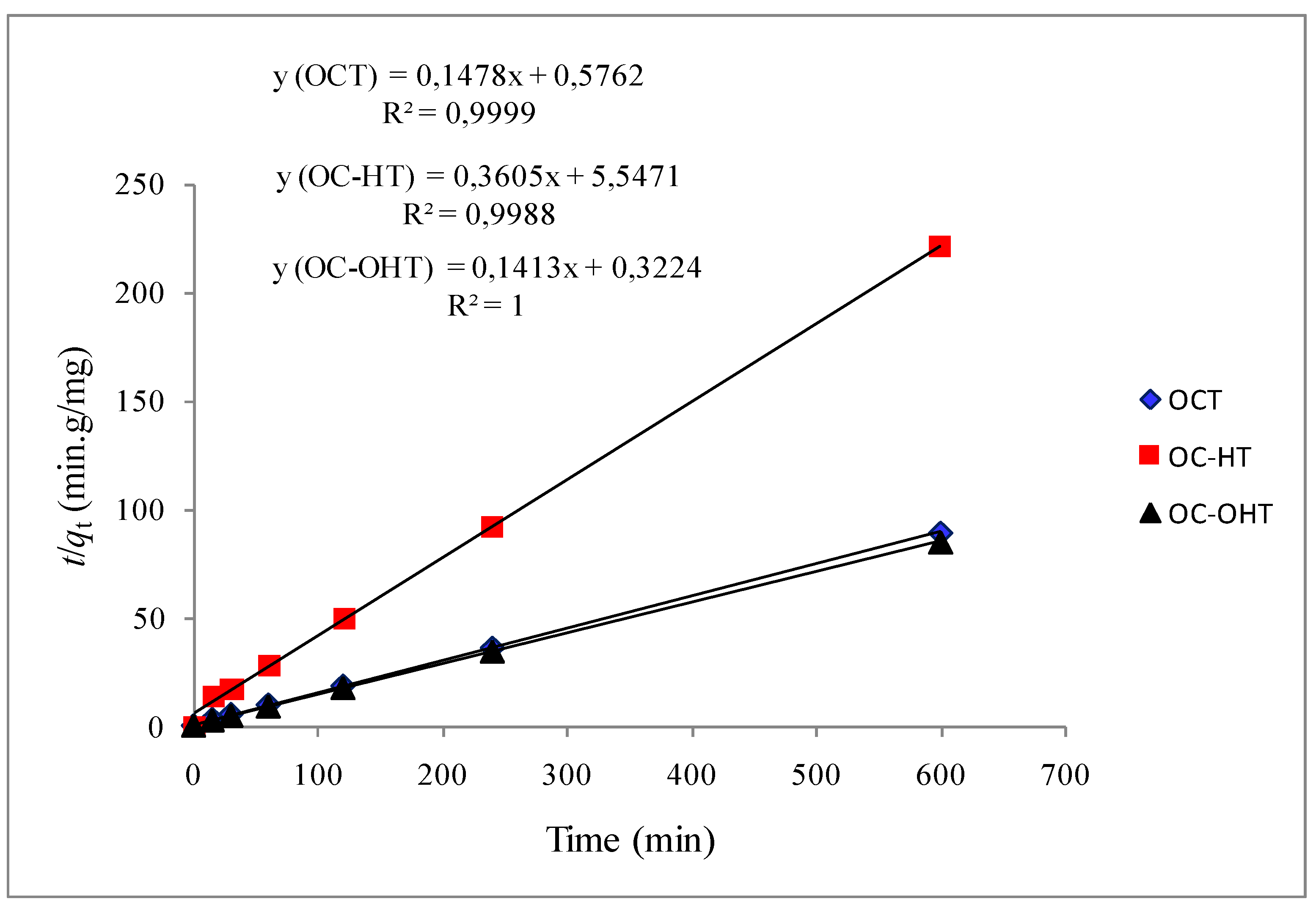
| First-order model | Pseudo-second-order model | ||||||
| qe experimental (mg/g) | qe calculated (mg/g) | k1 (min−1) | R2 | qe calculated (mg/g) | k2 (g·mg−1·min−1) | R2 | |
| OCT | 6.7 ± 0.2 | 2.6 | 0.021 | 0.923 | 6.8 | 0.038 | 1.000 |
| OC-HT | 2.7 ± 0.3 | 1.7 | 0.011 | 0.895 | 2.8 | 0.023 | 0.999 |
| OC-OHT | 7.1 ± 0.3 | 1.6 | 0.019 | 0.812 | 7.1 | 0.062 | 1.000 |
| Adsorbent | First-order model | Pseudo-second-order model | References |
| k1 (min−1) | k2 (g·mg−1·min−1) | ||
| Activated carbon derived from bagasse | 0.0079 | - | [2] |
| Solvent extracted olive pulp activated with steam and N2 gas mixture | 0.0037-0.0090 | - | [34] |
| Olive stones activated with steam and N2 gas mixture | 0.0035 | - | [34] |
| Sugar beet pulp | - | 0.102 | [37] |
| Coffee husks | - | 0.18-0.59 | [38] |
| OCT | 0.021 | 0.038 | This study |
| OC-HT | 0.011 | 0.023 | This study |
| OC-OHT | 0.019 | 0.062 | This study |
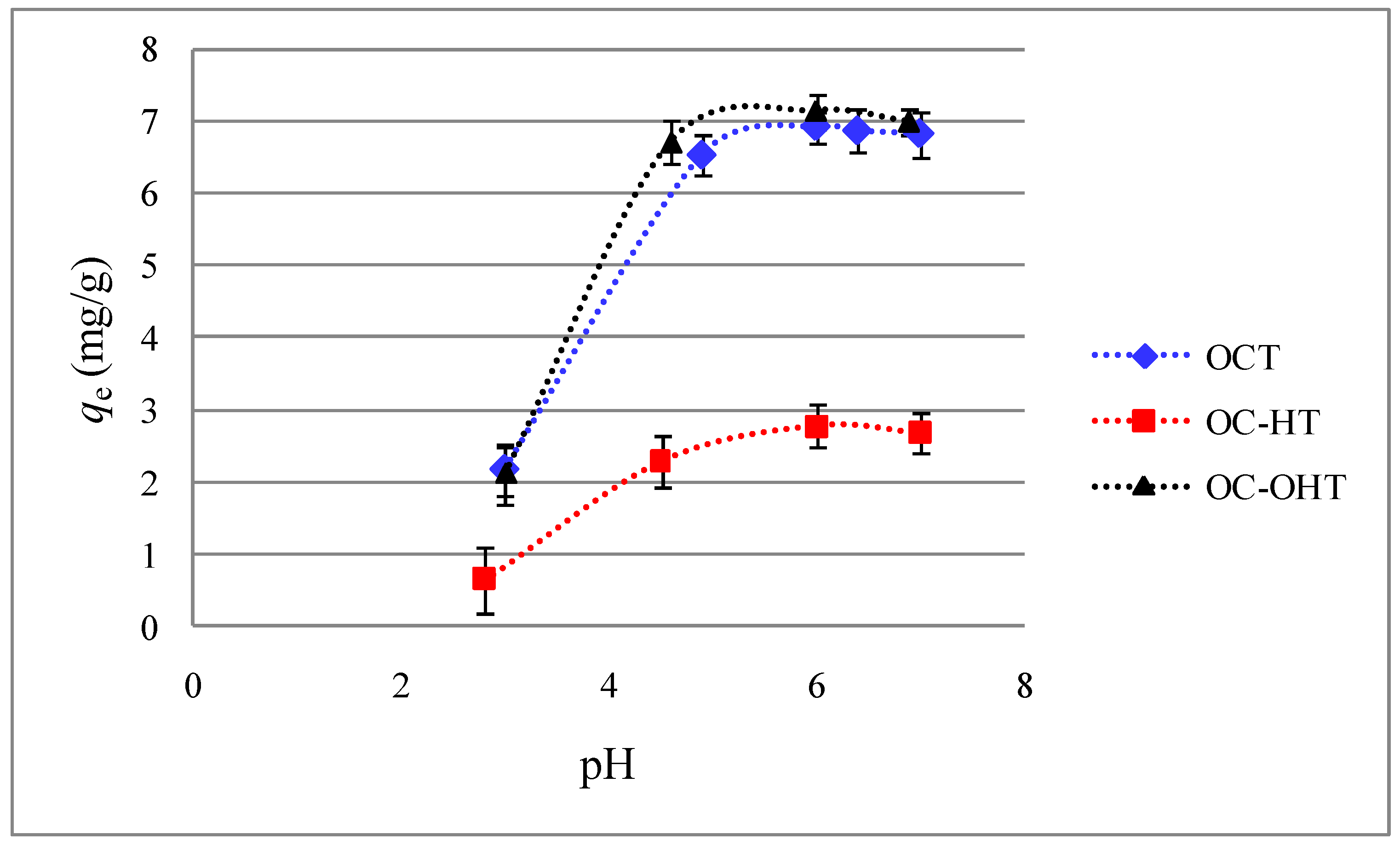
2.2.3. Effect of solution pH
2.2.4. Effect of initial zinc concentration
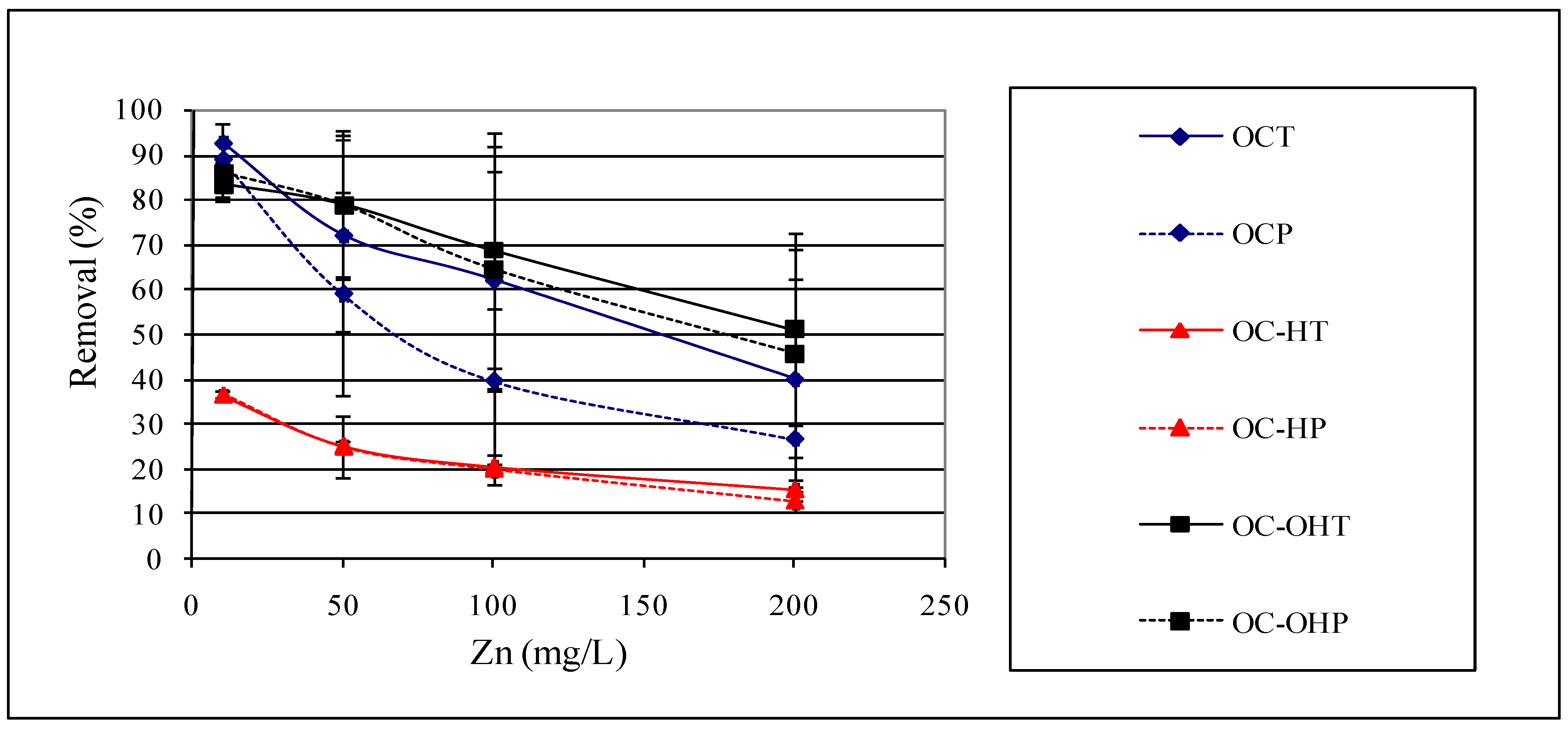
2.2.5. Effect of particle size
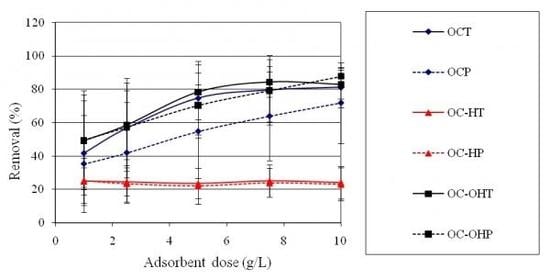
2.2.6. Effect of chemical treatment
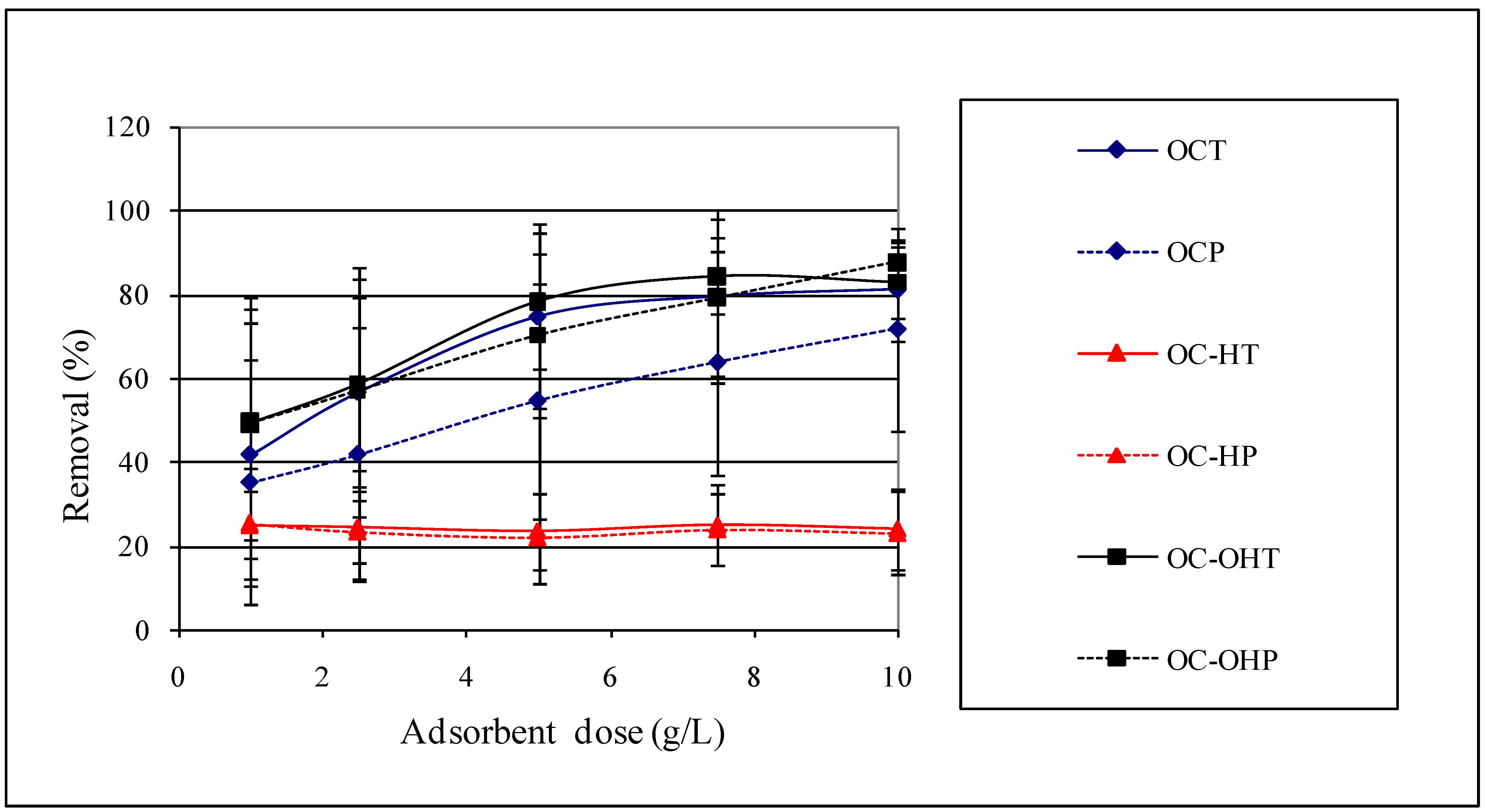
2.2.7. Adsorbent dose study
2.2.8. Sorption isotherms
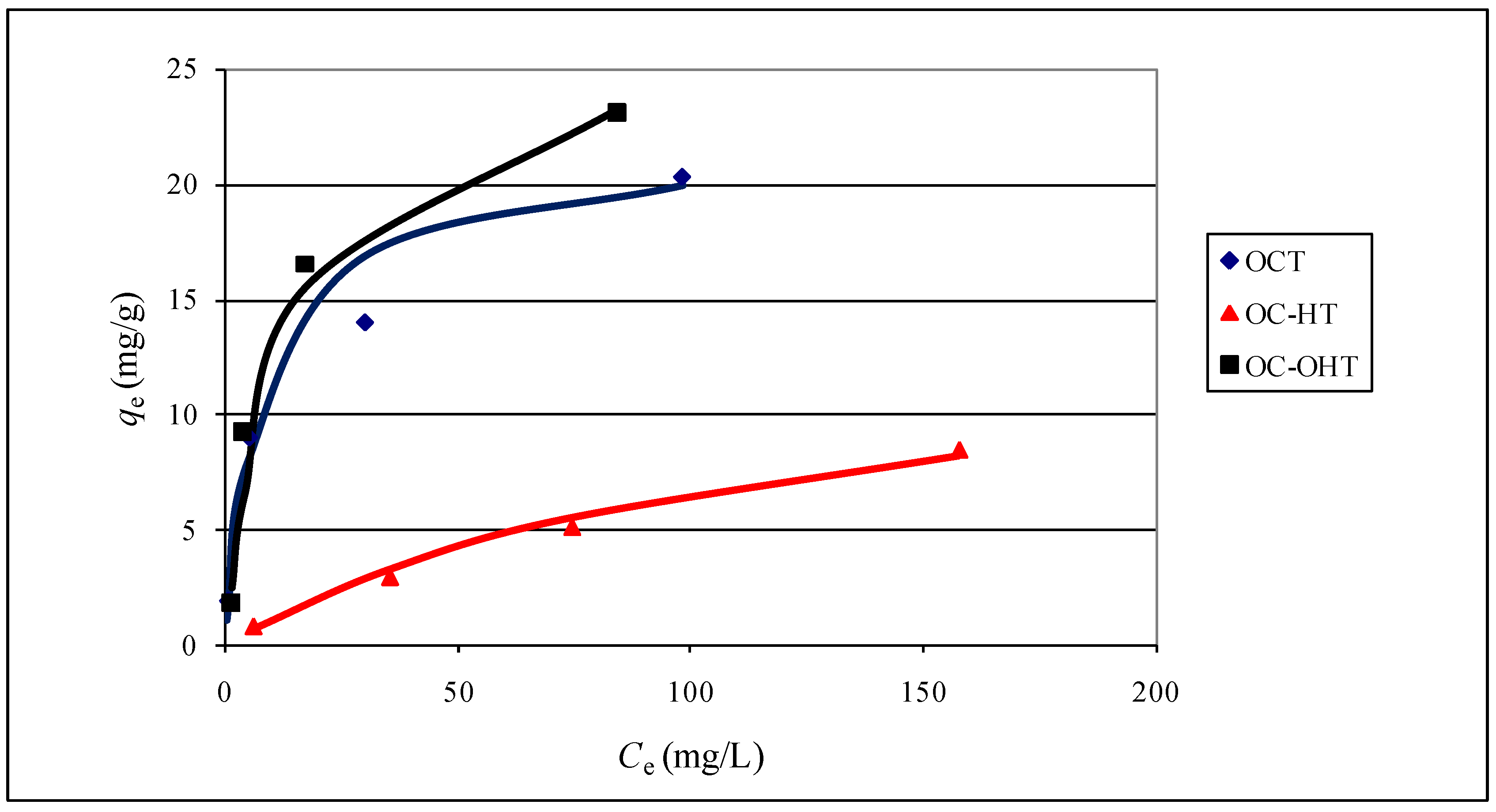
| Langmuir | Freundlich | |||||
| qmax (mg/g) | KL (L/mg) | R2 | KF | n | R2 | |
| OCT | 22 | 0.117 | 0.987 | 3.23 | 2.31 | 0.950 |
| OCP | 15 | 0.046 | 0.911 | 2.05 | 2.66 | 0.990 |
| OC-HT | 14 | 0.008 | 0.925 | 0.23 | 1.39 | 1.000 |
| OC-HP | 12 | 0.009 | 0.876 | 0.25 | 1.50 | 0.998 |
| OC-OHT | 27 | 0.081 | 0.987 | 2.45 | 1.74 | 0.830 |
| OC-OHP | 22 | 0.067 | 0.992 | 1.91 | 1.81 | 0.856 |
| Adsorbent | qmax (mg/g) | References |
| Waste olive cake extracted and roasted | 5.4 | [28] |
| Solvent extracted olive pulp activated with steam and N2 gas mixture | 31-33 | [34] |
| Olive stones activated with steam and N2 gas mixture | 16 | [34] |
| Peach stones activated with steam and N2 gas mixture | 6.4 | [34] |
| Apricot stones activated with steam and N2 gas mixture | 13 | [34] |
| Sugar beet pulp | 18 | [37] |
| Coffee husks | 5.6 | [38] |
| Coir | 8.6 | [48] |
| Papaya wood | 14 | [49] |
| Groundnut shells | 7.6 | [50] |
| Dye loaded groundnut shells | 9.6 | [50] |
| Teakwood Sawdust | 11 | [50] |
| Dye loaded teakwood sawdust | 17 | [50] |
| Rice husk alkali treated and autoclaved | 8.1 | [51] |
| Peanut hulls | 9.0 | [52] |
| Peanut hull pellets | 10 | [52] |
| Corncobs | 2.0 | [53] |
| Corncobs treated with citric acid | 7.8-35 | [53] |
| Corncobs treated with phosphoric acid | 32-35 | [53] |
| Cornstarch | 6.9 | [54] |
| Succinylated cornstarch | 13 | [54] |
| Oxidized cornstarch | 37 | [54] |
| OCT | 22 | This study |
| OCP | 15 | This study |
| OC-HT | 14 | This study |
| OC-HP | 12 | This study |
| OC-OHT | 27 | This study |
| OC-OHP | 22 | This study |
3. Experimental Section
3.1. Biosorbents Preparation and Characterization
3.2. Adsorption Tests
3.2.1. Effect of contact time
3.2.2. Effect of solution pH
3.2.3. Equilibrium isotherms
4. Conclusions
Acknowledgements
References
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.; Babel, S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Envir. 2006, 366, 409–426. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P. Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse – an agricultural waste. Wat. Res. 2002, 36, 2304–2318. [Google Scholar] [CrossRef]
- Reimann, C.; Caritat, P. Chemical Elements in the Environment: Factsheets for the Geochemist and Environmental Scientist; Springer-Verlag Berlin: Heidelberg, Germany, 1998. [Google Scholar]
- Kobya, M.; Demirbas, E.; Senturk, E.; Ince, M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresource Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Sahu, J.N.; Mahalik, K.K.; Mohanty, C.R.; Mohan, B.R.; Meikap, B.C. Studies on the removal of Pb (II) from wastewater by activated carbon developed from Tamarind wood activated with sulphuric acid. J. Hazard. Mater. 2008, 153, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Sohn, M.; Kim, D.; Sohn, S.; Kwon, Y. Production of granular activated carbon from waste walnut shell and its adsorption characteristics for Cu2+ ion. J. Hazard. Mater. 2001, 85, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Demiral, H.; Demiral, I.; Tümsek, F.; Karabacakoğlu, B. Adsorption of chromium (VI) from aqueous solution by activated carbon derived from olive bagasse and applicability of different adsorption models. Chem. Eng. J. 2008, 144, 188–196. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Radhika, M.; Vennilamani, N.; Pattabhi, S. Utilisation of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresource Technol. 2003, 87, 129–132. [Google Scholar] [CrossRef]
- Khan, N.A.; Ibrahim, S.; Subramaniam, P. Elimination of heavy metals from wastewater using agricultural wastes as adsorbents. Malaysian J. Sci. 2004, 23, 43–51. [Google Scholar]
- Pagnanelli, F.; Mainelli, S.; Vegliò, F.; Toro, L. Heavy metal removal by olive pomace: biosorbent characterization and equilibrium modeling. Chem. Eng. Sci. 2003, 58, 4709–4717. [Google Scholar] [CrossRef]
- Şensöz, S.; Demiral, İ.; Gerçel, H.F. Olive bagasse (Olea europea L.) pyrolysis. Bioresource Technol. 2006, 97, 429–436. [Google Scholar] [CrossRef]
- Sampedro, I.; Cajthaml, T.; Marinari, S.; Petruccioli, M.; Grego, S.; D’Annibale, A. Organic matter transformation and detoxification in dry olive mill residue by the saprophytic fungus Paecilomyces farinosus. Process Biochem. 2009, 44, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Hachicha, S.; Sellami, F.; Medhioub, K.; Hachicha, R.; Ammar, E. Quality assessment of composts prepared with olive mill wastewater and agricultural wastes. Waste Manage. 2008, 28, 2593–2603. [Google Scholar] [CrossRef]
- Sellami, F.; Hachicha, S.; Chtourou, M.; Medhioub, K.; Ammar, E. Maturity assessment of composted olive mill wastes using UV spectra and humification parameters. Bioresource Technol. 2008, 99, 6900–6907. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Yáñez-Ruiz, D.R. Potential use of olive by-products in ruminant feeding: a review. Anim. Feed Sci.Tech. 2008, 147, 247–264. [Google Scholar] [CrossRef]
- Ben-Salem, H.; Znaidi, I.A. Partial replacement of concentrate with tomato pulp and olive cake-based feed blocks as supplements for lambs fed wheat straw. Anim. Feed Sci.Tech. 2008, 147, 206–222. [Google Scholar] [CrossRef]
- Demiral, I.; Şensöz, S. The effects of different catalysts on the pyrolysis of industrial wastes (olive and hazelnut bagasse). Bioresource Technol. 2008, 99, 8002–8007. [Google Scholar] [CrossRef]
- Skoulou, V.; Swiderski, A.; Yang, W.; Zabaniotou, A. Process characteristics and products of olive kernel high temperature steam gasification (HTSG). Bioresource Technol. 2009, 100, 2444–2451. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Martínez, G.; González, J.M. Two stages catalytic pyrolisis of olive oil waste. Fuel Process. Technol. 2008, 89, 1448–1455. [Google Scholar] [CrossRef]
- Petrov, N.; Budinova, T.; Razvigorova, M.; Parra, J.; Galiatsatou, P. Conversion of olive wastes to volatiles and carbon adsorbents. Biomass and Bioenergy 2008, 32, 1303–1310. [Google Scholar] [CrossRef]
- Aboulkas, A.; El-Harfi, K.; El-Bouadili, A. Non-isothermal kinetic studies on co-processing of olive residue and polypropylene. Energ. Conv. Manage. 2008, 49, 3666–3671. [Google Scholar] [CrossRef]
- Aboulkas, A.; El-Harfi, K.; El-Bouadili, A. Pyrolysis of olive residue/Low density polyethylene mixture: Part I Thermogravimetric kinetics. J. Fuel Chem. Technol. 2008, 36, 672–678. [Google Scholar] [CrossRef]
- André, R.N.; Pinto, F.; Franco, C.; Dias, M.; Gulyurtlu, I.; Matos, M.A.A.; Cabrita, I. Fluidised bed co-gasification of coal and olive oil industry wastes. Fuel 2005, 84, 1635–1644. [Google Scholar]
- Pinto, F.; André, R.N.; Franco, C.; Lopes, H.; Gulyurtlu, I.; Cabrita, I. Co-gasification of coal and wastes in a pilot-scale installation 1: Effect of catalysts in syngas treatment to achieve tar abatement. Fuel 2009. [Google Scholar] [CrossRef]
- Aziz, A.; Ouali, M.S.; Elandaloussi, E.H. Chemically modified olive stone: a low cost sorbent for heavy metals and basic dyes removal from aqueous solutions. J. Hazard. Mater. 2009, 163, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Baccar, R.; Bouzid, J.; Feki, M.; Montiel, A. Preparation of activated carbon from Tunisian olive-waste cakes and its application for adsorption of heavy metal ions. J. Hazard. Mater. 2009, 162, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Gharaibeh, S.H.; Abu-El-Sha’r, W.Y.; Al-Kofahi, M.M. Removal of selected heavy metals from aqueous solutions using processed solid residue of olive mill products. Wat. Res. 1998, 32, 498–502. [Google Scholar] [CrossRef]
- Konstantinou, M.; Kolokassidou, K.; Pashalidis, I. Studies on the interaction of olive cake and its hydrophilic extracts with polyvalent metal ions (Cu(II), Eu(II)) in aqueous solutions. J. Hazard. Mater. 2009. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Hernáinz, F.; Calero, M.; Blázquez, G.; Tenorio, G. Surface chemistry evaluation of some solid wastes from olive-oil industry used for lead removal from aqueous solution. Biochem. Eng. J. 2009, 44, 151–159. [Google Scholar] [CrossRef]
- Nuhoglu, Y.; Malkoc, E. Thermodinamic and kinetic studies for environmentally friendly Ni (II) biosorption using waste pomace of olive oil factory. Bioresource Technol. 2009, 100, 2375–2380. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Pagnanelli, F.; Mainelli, S.; Calero, M.; Toro, L. Chemical treatment of olive pomace: Effect on acid-basic properties and metal biosorption capacity. J. Hazard. Mater. 2008, 156, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Pagnanelli, F.; Mainelli, S.; Toro, L. New biosorbent materials for heavy metal removal: Product development guided by active site characterization. Wat. Res. 2008, 42, 2953–2962. [Google Scholar] [CrossRef]
- Galiatsatou, P.; Metaxas, M.; Kasselouri-Rigopoulou, V. Adsorption of zinc by activated carbons prepared from solvent extracted olive pulp. J. Hazard. Mater. 2002, B91, 187–203. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Determination of Iodine Number of Activated Carbon; ASTM Committee on Standards, ASTM D 4607-94; ASTM: Philadelphia, PA, USA, 2006. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Reddad, Z.; Gerente, C.; Andres, Y.; Le-Cloirec, P. Adsorption of Several Metal Ions onto a Low-Cost Biosorbent: Kinetic and Equilibrium Studies. Environ. Sci. Technol. 2002, 36, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.E.; Franca, A.S.; Oliveira, L.S.; Rocha, S.D. Untreated coffee husks as biosorbents for the removal of heavy metals from aqueous solutions. J. Hazard. Mater. 2008, 152, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Carrott, P.J.M.; Carrott, M.M.L.R.; Nabais, J.M.V.; Ramalho, J.P.P. Influence of Surface ionization on the adsorption of aqueous zinc species by activated carbons. Carbon 1997, 35, 403–410. [Google Scholar] [CrossRef]
- Skodras, G.; Diamantopoulou, I.; Pantoleontos, G.; Sakellaropoulos, G.P. (2008) Kinetic studies of elemental mercury adsorption in activated carbon fixed bed reactor. J. Hazard. Mater. 2008, 158, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Wat. Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Bhargava, D.S.; Sheldarkar, S.B. Use of TNSAC in phosphate adsorption studies and relationships. Literature, experimental methodology, justification and effects of process variables. Wat. Res. 1993, 27, 303–312. [Google Scholar] [CrossRef]
- Tarley, C.R.T.; Arruda, M.A.Z. Biosorption of heavy metals using rice milling by-products. Characterisation and application for removal of metals from aqueous effluents. Chemosphere 2004, 54, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Vlyssides, A.G.; Loizidou, M.; Zorpas, A.A. Characteristics of solid residues from olive oil processing as bulking material for co-composting with industrial wastewaters. J. Environ. Sci. Health A 1999, 34, 737–748. [Google Scholar] [CrossRef]
- Kandaha, M.I.; Meunier, J.L. Removal of nickel ions from water by multi-walled carbon nanotubes. J. Hazard. Mater. 2007, 146, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Lata, H.; Garg, V.K.; Gupta, R.K. Sequestration of nickel from aqueous solution onto activated carbon prepared from Parthenium hysterophorus L. J. Hazard. Mater. 2008, 157, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Namane, A.; Mekarzia, A.; Benrachedi, K.; Belhaneche-Bensemra, N.; Hellal, A. Determination of the adsorption capacity of activated carbon made from coffee grounds by chemical activation with ZnCl2 and H3PO4. J. Hazard. Mater. 2005, B119, 189–194. [Google Scholar] [CrossRef]
- Conrad, K.; Hansen, H.C.B. Sorption of zinc and lead on coir. Bioresource Technol. 2007, 98, 89–97. [Google Scholar] [CrossRef]
- Saeed, A.; Akhter, M.W.; Iqba, M. Removal and recovery of heavy metals from aqueous solution using papaya wood as a new biosorbent. Separation and Purification Technology 2005, 45, 25–31. [Google Scholar] [CrossRef]
- Shukla, S.R.; Pai, R.S. Adsorption of Cu (II), Ni (II) and Zn(II) on dye loaded groundnut shells and sawdust. Sep. Purif. Technol. 2005, 43, 1–8. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Meng, X.; Christodoulatos, C.; Bodduc, V.M. Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J. Hazard. Mater. 2008, 153, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Jefcoat, I.A.; Parrish, D.; Gill, S.; Graham, E. Evaluation of the adsorptive capacity of peanut hull pellets for heavy metals in solution. Adv.Environ. Res. 2000, 4, 19–29. [Google Scholar] [CrossRef]
- Vaughan, T.; Seo, C.W.; Marshall, W.E. Removal of selected metal ions from aqueous solution using modified corncobs. Bioresource Technol. 2001, 78, 133–139. [Google Scholar] [CrossRef]
- Kweon, D.-K.; Choi, J.-K.; Kim, E.-K.; Lim, S.-T. Adsorption of divalent metal ions by succinylated and oxidized corn starches. Carbohyd. Polym. 2001, 46, 171–177. [Google Scholar] [CrossRef]
- Robinson, T.; Chandran, B.; Nigam, P. Effect of pretreatments of three waste residues, wheat straw, corncobs and barley husks on dye adsorption. Bioresource Technol. 2002, 85, 119–124. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. Agricultural Chemicals; Contaminants; Drugs. Volume I, 15th Ed. ed; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- ASTM. Standard Test Methods for Instrumental Determination of Carbon, Hydrogen, and Nitrogen in Laboratory Samples of Coal and Coke; ASTM Committee on Standards, ASTM D5373-93; ASTM: Philadelphia, PA, USA, 2002. [Google Scholar]
- Vandecasteele, C.; Block, C.B. Modern Methods for Trace Element Determination; John Wiley & Sons: Chichester, U.K., 1993. [Google Scholar]
- Rio, S.; Faur-Brasquet, C.; Le-Coq, L.; Courcoux, P.; Le-Cloirec, P. Experimental design methodology for the preparation of carbonaceous sorbents from sewage sludge by chemical activation––application to air and water treatments. Chemosphere 2005, 58, 423–437. [Google Scholar] [CrossRef] [PubMed]
© 2009 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fernando, A.; Monteiro, S.; Pinto, F.; Mendes, B. Production of Biosorbents from Waste Olive Cake and Its Adsorption Characteristics for Zn2+ Ion. Sustainability 2009, 1, 277-297. https://doi.org/10.3390/su1020277
Fernando A, Monteiro S, Pinto F, Mendes B. Production of Biosorbents from Waste Olive Cake and Its Adsorption Characteristics for Zn2+ Ion. Sustainability. 2009; 1(2):277-297. https://doi.org/10.3390/su1020277
Chicago/Turabian StyleFernando, Ana, Sofia Monteiro, Filomena Pinto, and Benilde Mendes. 2009. "Production of Biosorbents from Waste Olive Cake and Its Adsorption Characteristics for Zn2+ Ion" Sustainability 1, no. 2: 277-297. https://doi.org/10.3390/su1020277
APA StyleFernando, A., Monteiro, S., Pinto, F., & Mendes, B. (2009). Production of Biosorbents from Waste Olive Cake and Its Adsorption Characteristics for Zn2+ Ion. Sustainability, 1(2), 277-297. https://doi.org/10.3390/su1020277