Microwave-Assisted Synthesis of rGO-ZnO/CuO Nanocomposites for Photocatalytic Degradation of Organic Pollutants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Synthesis of GO
2.2.2. Synthesis of rGO
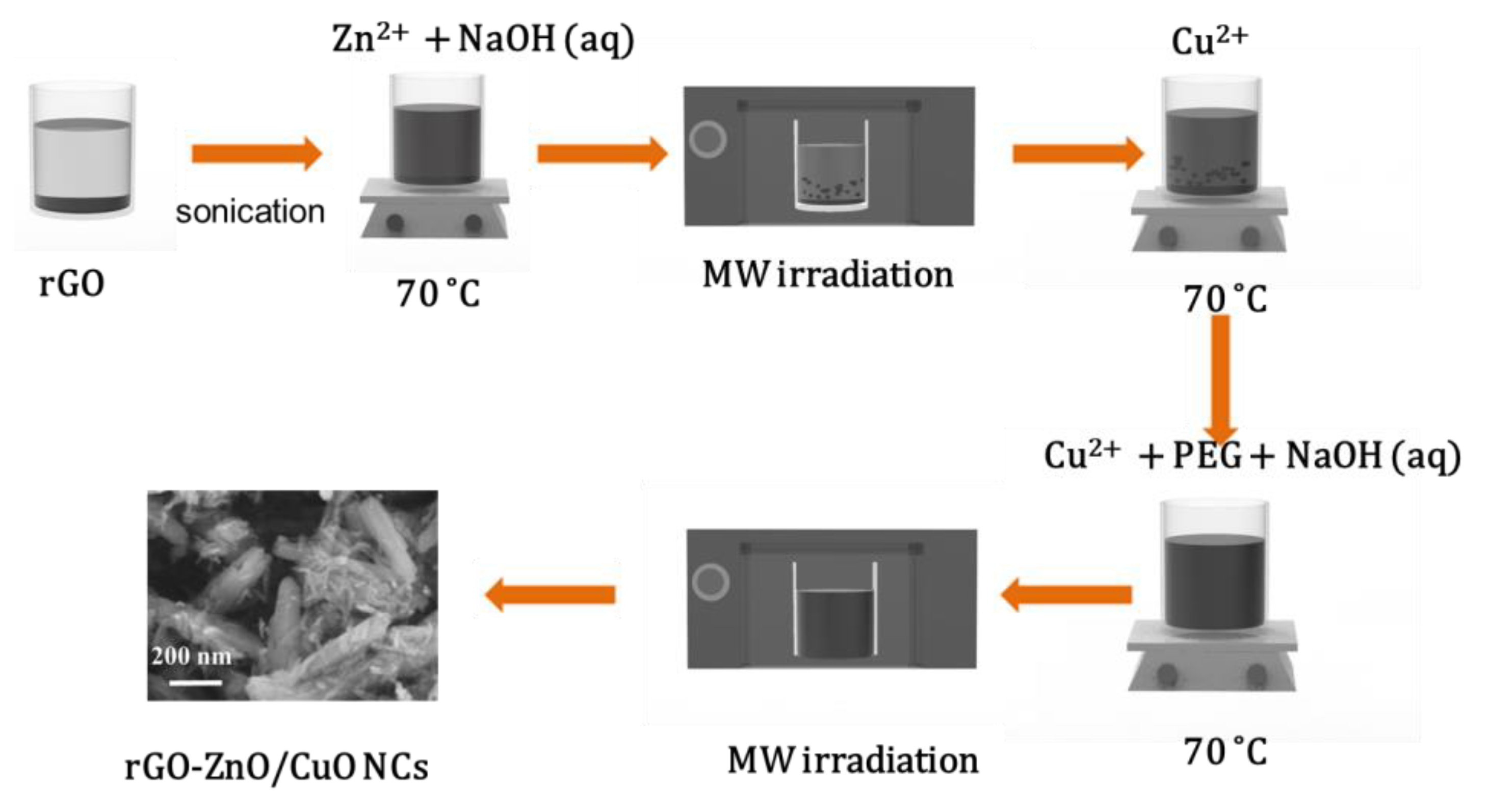
2.2.3. Synthesis of (rGO-ZnO)/CuO NCs
2.3. Characterizations
2.4. Performance Evaluation
3. Results and Discussion
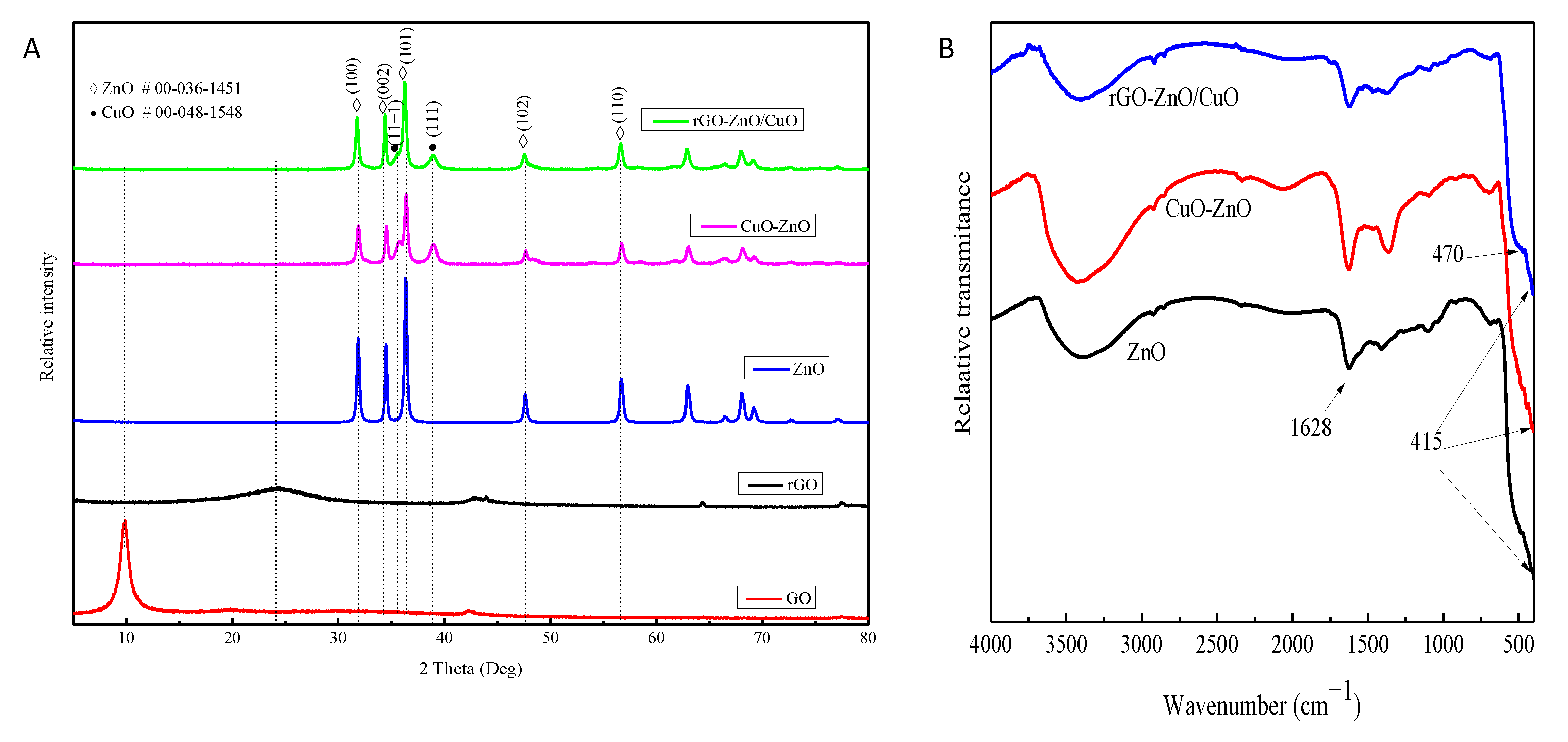
3.1. X-ray Diffraction Analysis
3.2. FTIR Analysis
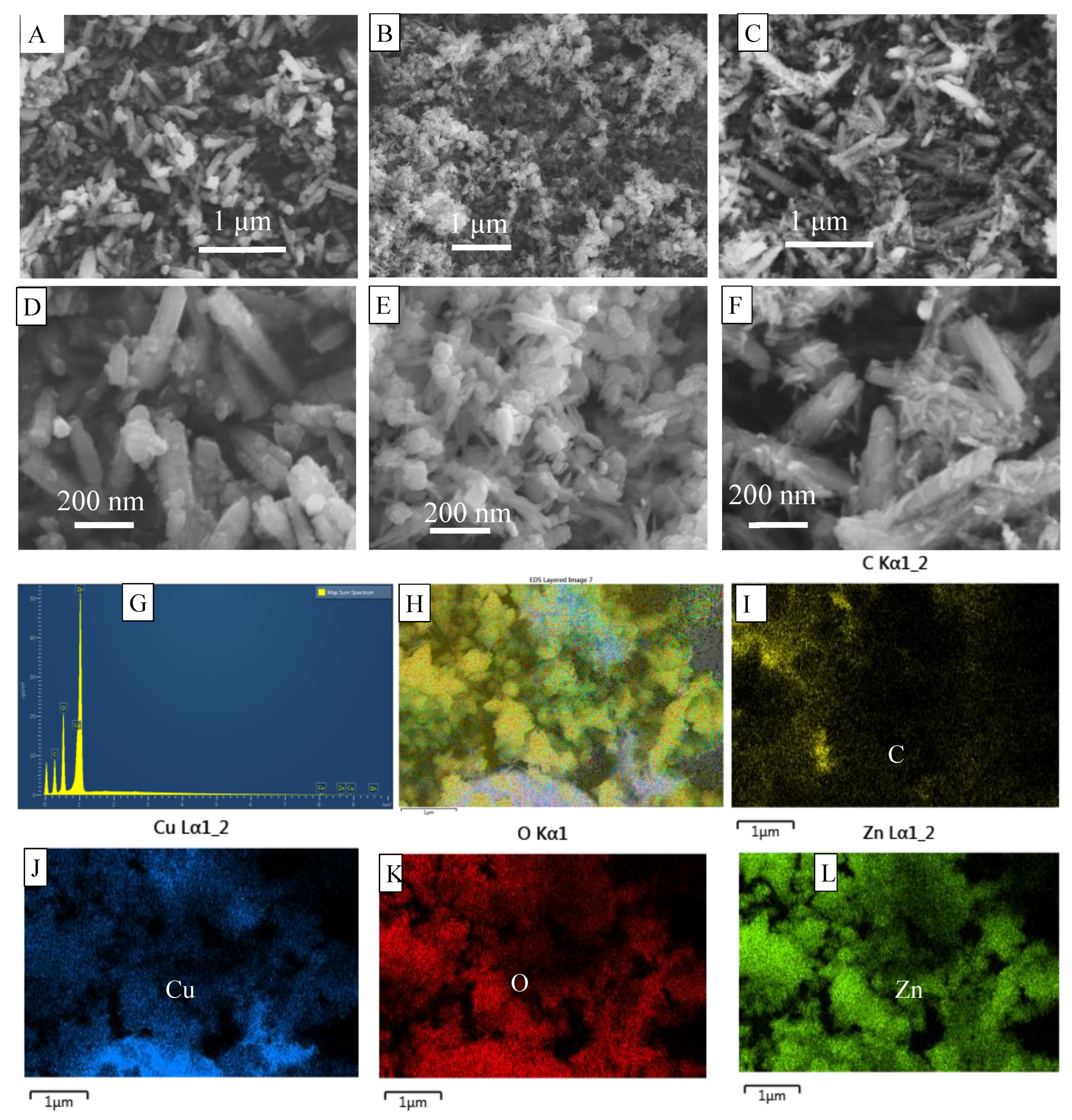
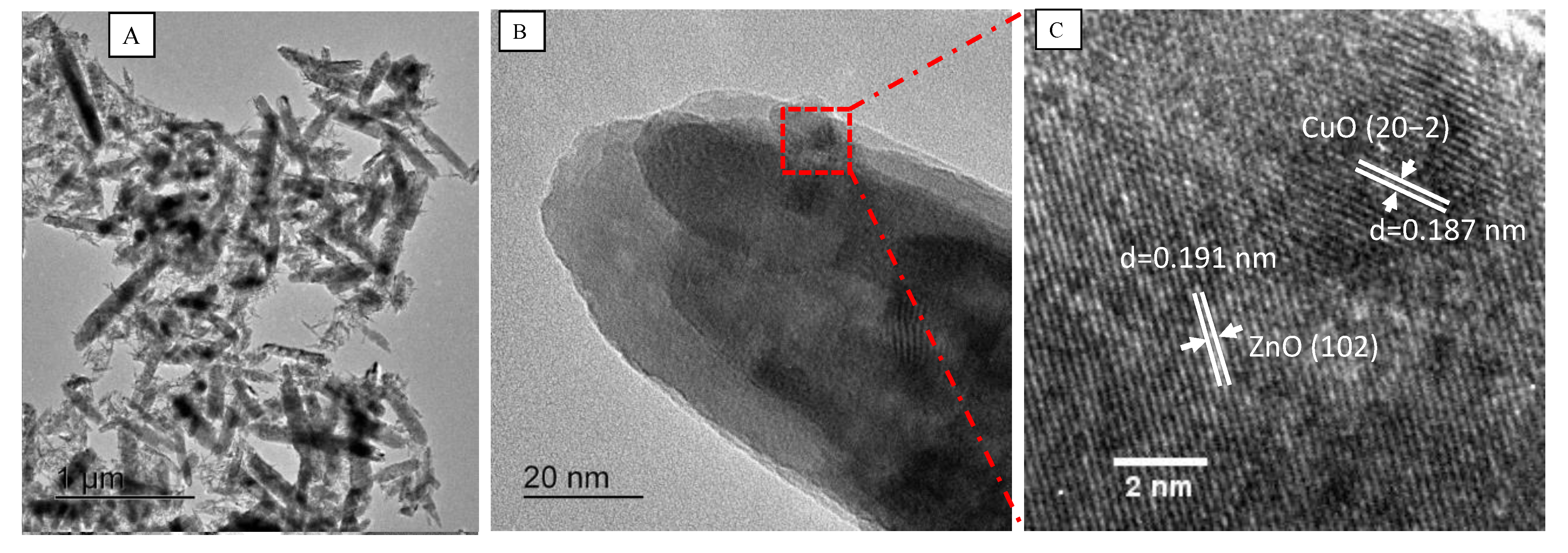
3.3. Morphology Characterization
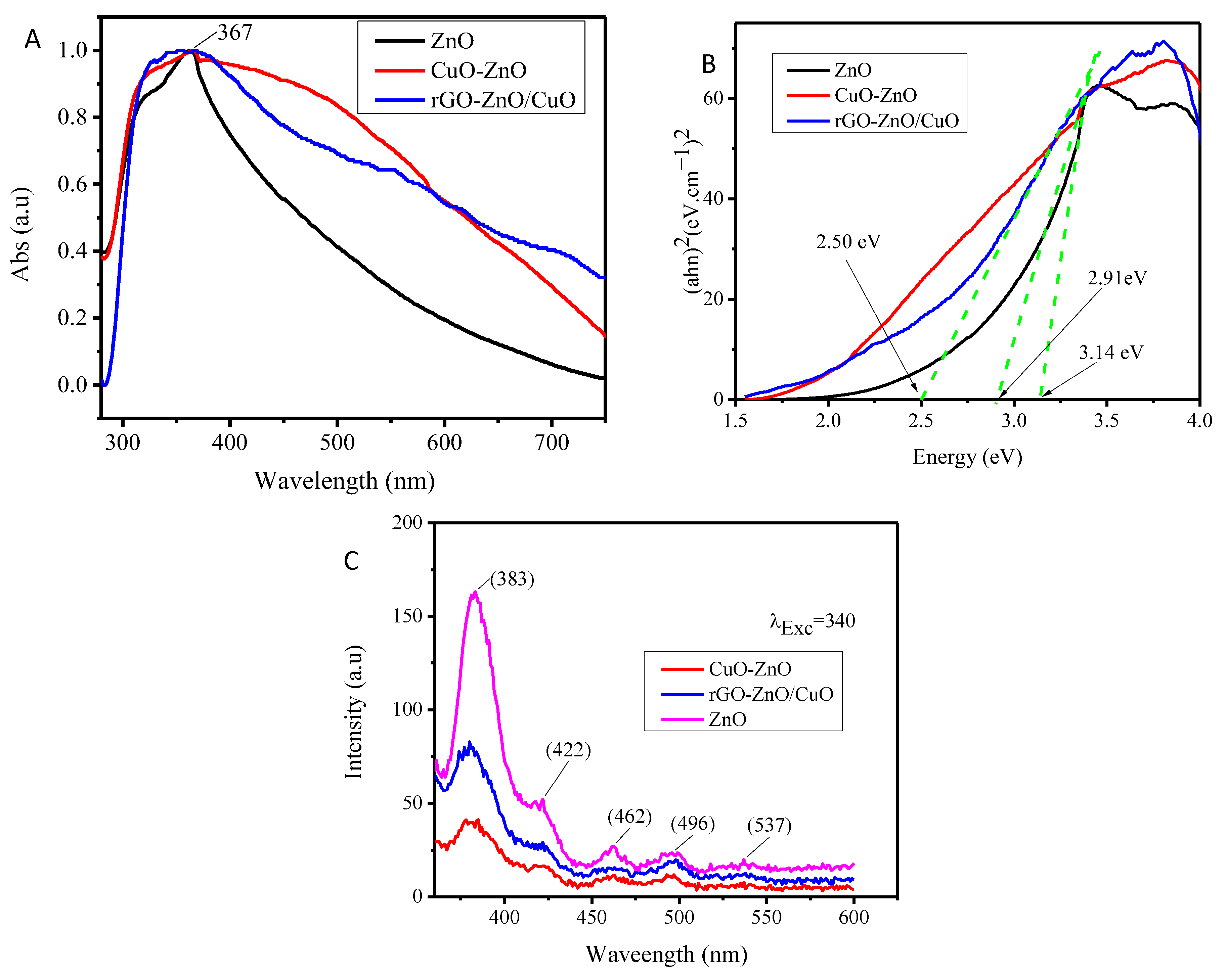
3.4. UV–vis and PL Analysis
3.5. Electrochemical Analysis
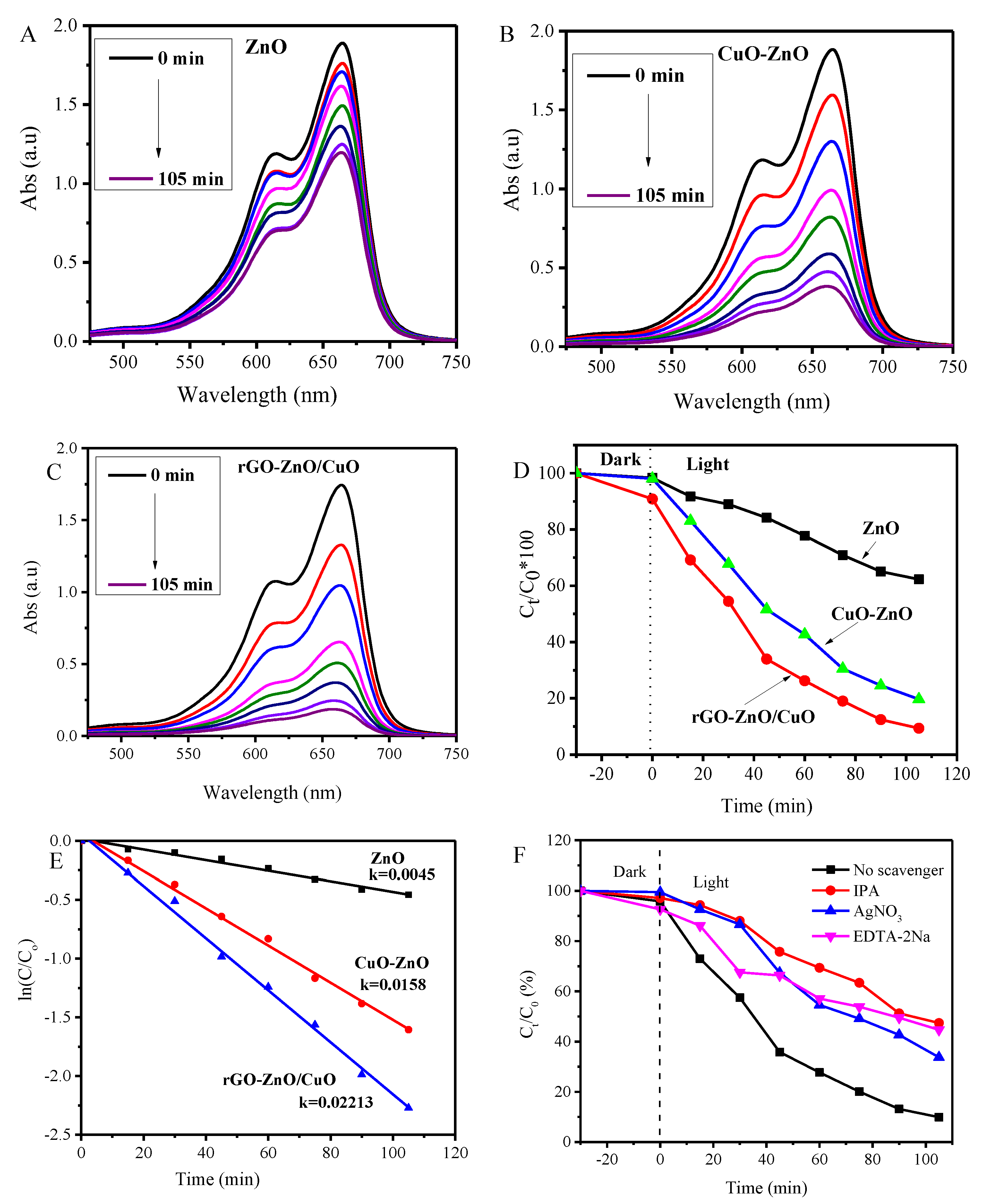
3.6. Photocatalytic Activity and Mechanism
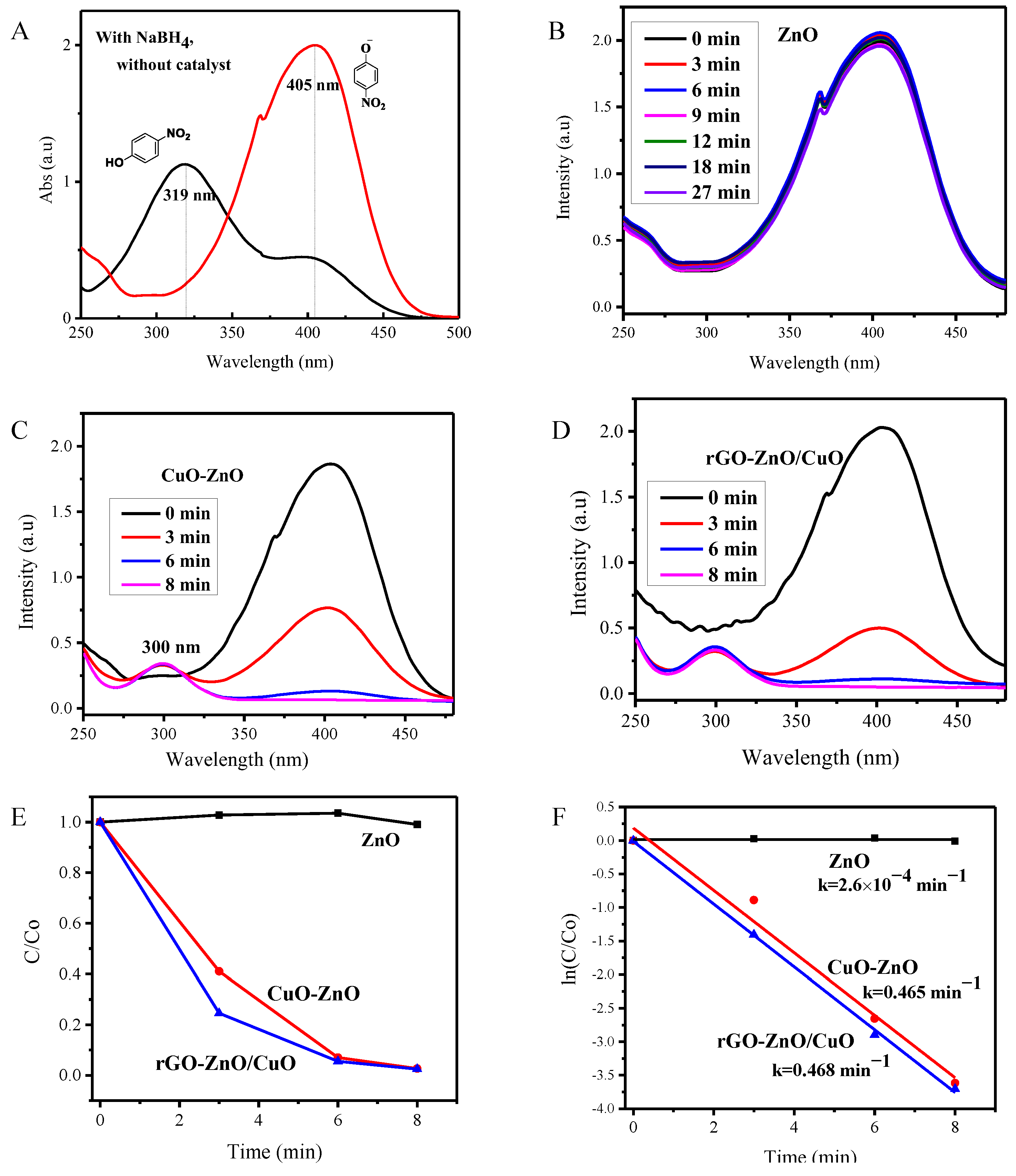
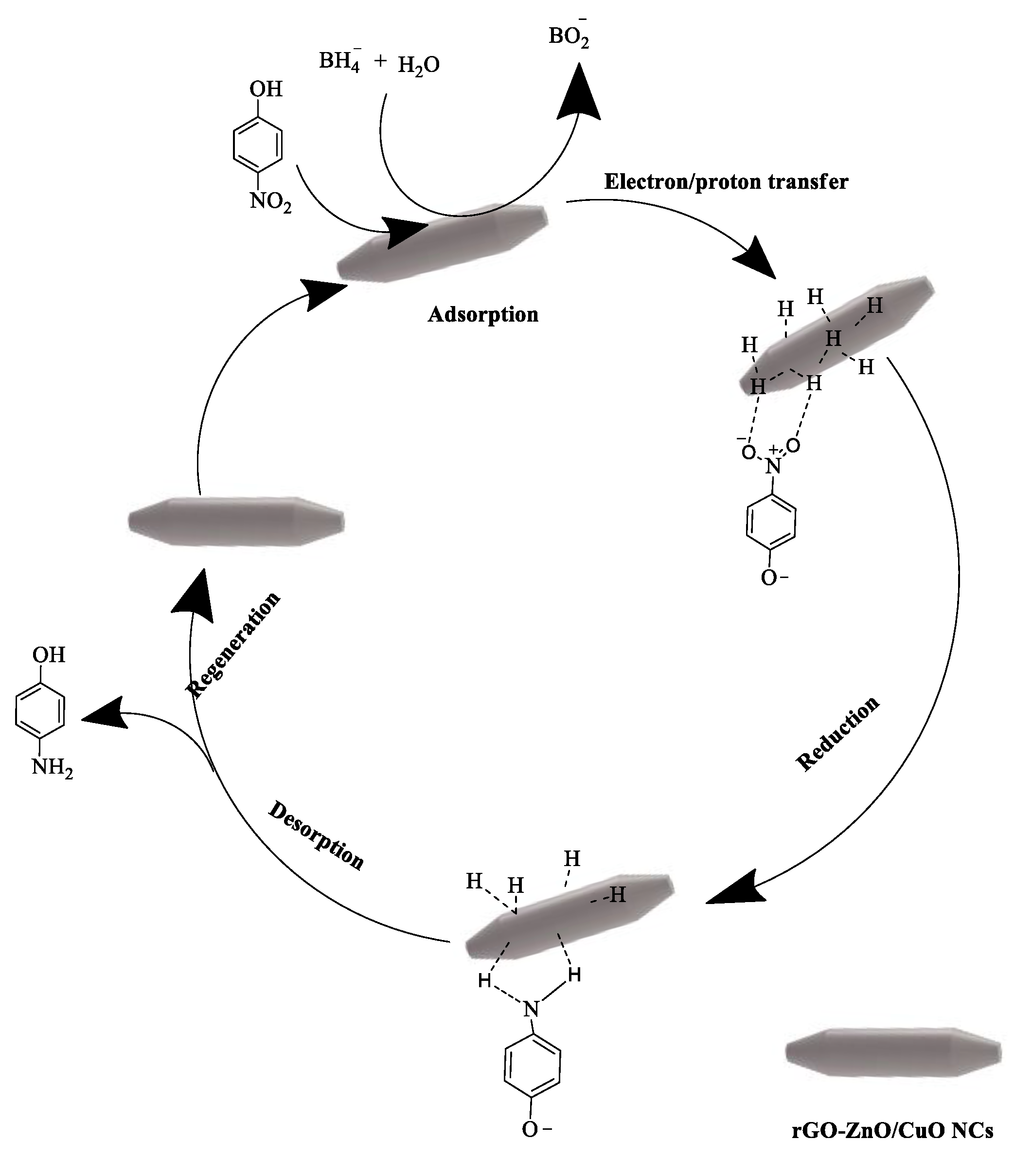
3.7. Catalytic Reduction Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalid, Z.B.; Nasrullah, M.; Nayeem, A.; Wahid, Z.; Singh, L.; Krishnan, S. Application of 2D Graphene-Based Nanomaterials for Pollutant Removal from Advanced Water and Wastewater Treatment Processes. In ACS Symposium Series; American Chemical Society: New York, NY, USA, 2020; pp. 191–217. [Google Scholar]
- Lu, F.; Astruc, D. Nanomaterials for removal of toxic elements from water. Coord. Chem. Rev. 2018, 356, 147–164. [Google Scholar] [CrossRef]
- Ibrahim, R.; Hayyan, M.; AlSaadi, M.; Hayyan, A.; Ibrahim, S. Environmental application of nanotechnology: Air, soil, and water. Environ. Sci. Pollut. Res. 2016, 23, 13754–13788. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Xu, H.; Liu, H.; Wang, M.; He, Y.; Pan, B. Nanomaterials-enabled water and wastewater treatment. NanoImpact 2016, 3, 22–39. [Google Scholar] [CrossRef]
- Silva, C.; Faria, J. Effect of key operational parameters on the photocatalytic oxidation of phenol by nanocrystalline sol-gel TiO2 under UV irradiation. J. Mol. Catal. A Chem. 2009, 305, 147–154. [Google Scholar] [CrossRef]
- Mandeep; Gupta, G.; Liu, H.; Shukla, P. Pulp and paper industry–based pollutants, their health hazards and environmental risks. Curr. Opin. Environ. Sci. Health 2019, 12, 48–56. [Google Scholar] [CrossRef]
- Sivaram, N.; Gopal, P.; Barik, D. Toxic waste from textile industries. In Energy from Toxic Organic Waste for Heat and Power Generation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 43–54. [Google Scholar]
- Ahmad, M.; Mushtaq, S.; Al Qahtani, H.; Sedky, A.; Alam, M. Investigation of TiO2 Nanoparticles Synthesized by Sol-Gel Method for Effectual Photodegradation, Oxidation and Reduction Reaction. Crystals 2021, 11, 1456. [Google Scholar] [CrossRef]
- Alam, M.; Al Qahtani, H.; Aamir, M.; Abuzir, A.; Khan, M.; Albuhulayqah, M.; Mushtaq, S.; Zaidi, N.; Ramya, A. Phyto Synthesis of Manganese-Doped Zinc Nanoparticles Using Carica papaya Leaves: Structural Properties and Its Evaluation for Catalytic, Antibacterial and Antioxidant Activities. Polymers 2022, 14, 1827. [Google Scholar] [CrossRef]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.; Park, J.; Yip, A. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Gadipelly, C.; Mannepalli, L. Nano-metal oxides for organic transformations. Curr. Opin. Green Sustain. Chem. 2019, 15, 20–26. [Google Scholar] [CrossRef]
- Das, T.; Remanan, S.; Ghosh, S.; Das, N. An environment friendly free-standing cellulose membrane derived for catalytic reduction of 4-nitrophenol: A sustainable approach. J. Environ. Chem. Eng. 2021, 9, 104596. [Google Scholar] [CrossRef]
- Das, T.; Das, N. Advances on catalytic reduction of 4-nitrophenol by nanostructured materials as benchmark reaction. Int. Nano Lett. 2022, 12, 223–242. [Google Scholar] [CrossRef]
- Nagajyothi, P.; Vattikuti, S.V.P.; Devarayapalli, K.; Yoo, K.; Shim, J.; Sreekanth, T. Green synthesis: Photocatalytic degradation of textile dyes using metal and metal oxide nanoparticles-latest trends and advancements. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2617–2723. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Ullah, H.; Mushtaq, L.; Ullah, Z.; Fazal, A.; Khan, A. Effect of vegetable waste extract on microstructure, morphology, and photocatalytic efficiency of ZnO–CuO nanocomposites. Inorg. Nano-Metal Chem. 2021, 51, 963–975. [Google Scholar] [CrossRef]
- Ong, C.; Ng, L.; Mohammad, A. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Sahu, K.; Bisht, A.; Kuriakose, S.; Mohapatra, S. Two-dimensional CuO-ZnO nanohybrids with enhanced photocatalytic performance for removal of pollutants. J. Phys. Chem. Solids 2020, 137, 109223. [Google Scholar] [CrossRef]
- Liu, C.; Meng, F.; Zhang, L.; Zhang, D.; Wei, S.; Qi, K.; Fan, J.; Zhang, H.; Cui, X. CuO/ZnO heterojunction nanoarrays for enhanced photoelectrochemical water oxidation. Appl. Surf. Sci. 2019, 469, 276–282. [Google Scholar] [CrossRef]
- Taraka, T.Y.; Gautam, A.; Jain, S.; Bojja, S.; Pal, U. Controlled addition of Cu/Zn in hierarchical CuO/ZnO p-n heterojunction photocatalyst for high photoreduction of CO2 to MeOH. J. CO2 Util. 2019, 31, 207–214. [Google Scholar] [CrossRef]
- Kumaresan, N.; Sinthiya, M.; Ramamurthi, K.; Babu, R.R.; Sethuraman, K. Visible light driven photocatalytic activity of ZnO/CuO nanocomposites coupled with rGO heterostructures synthesized by solid-state method for RhB dye degradation. Arab. J. Chem. 2020, 13, 3910–3928. [Google Scholar] [CrossRef]
- Li, X.; Shen, R.; Ma, S.; Chen, X.; Xie, J. Graphene-based heterojunction photocatalysts. Appl. Surf. Sci. 2018, 430, 53–107. [Google Scholar] [CrossRef]
- Liu, F.; Che, Y.; Chai, Q.; Zhao, M.; Lv, Y.; Sun, H.; Wang, Y.; Sun, J.; Zhao, C. Construction of rGO wrapping Cu2O/ZnO heterostructure photocatalyst for PNP and PAM degradation. Environ. Sci. Pollut. Res. 2019, 26, 25286–25300. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Zhu, H. Structure and properties of graphene. In Graphene: Fabrication, Characterizations, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–12. [Google Scholar]
- Zhang, G.; Sun, S. Advantages and Challenges of One-Dimensional Nanostructures for Fuel Cell Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Ramos, P.; Sánchez, L.; Rodriguez, J. A review on improving the efficiency of photocatalytic water decontamination using ZnO nanorods. J. Sol-Gel Sci. Technol. 2022, 102, 105–124. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, X.; Zhao, D.; Gao, M.; Xie, S. ZnO nanorods: Morphology control, optical properties, and nanodevice applications. Sci. China Phys. Mech. Astron. 2013, 56, 2243–2265. [Google Scholar] [CrossRef]
- Aspoukeh, P.; Barzinjy, A.; Hamad, S. Synthesis, properties and uses of ZnO nanorods: A mini review. Int. Nano Lett. 2022, 12, 153–168. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Q.; Jiang, P. Morphology-dependent photo-catalysis of bare zinc oxide nanocrystals. RSC Adv. 2013, 3, 12662–12670. [Google Scholar] [CrossRef]
- Man, H.; Wen, C.; Luo, W.; Bian, J.; Wang, W.; Li, C. Simultaneous deSOx and deNOx of marine vessels flue gas on ZnO-CuO/rGO: Photocatalytic oxidation kinetics. J. Ind. Eng. Chem. 2020, 92, 77–87. [Google Scholar] [CrossRef]
- Tantubay, K.; Das, P.; Sen, M.B. Ternary reduced graphene oxide–CuO/ZnO nanocomposite as a recyclable catalyst with enhanced reducing capability. J. Environ. Chem. Eng. 2020, 8, 103818. [Google Scholar] [CrossRef]
- Firoozi, S.; Hosseini-Sarvari, M. Visible-Light-Induced C-P-Bond Formation Using Reduced Graphene Oxide Decorated with Copper Oxide/Zinc Oxide (rGO/CuO/ZnO) as Ternary Recyclable Nanophotocatalyst. ChemistrySelect 2021, 6, 1764–1771. [Google Scholar] [CrossRef]
- Maity, C.; Hatui, G.; Verma, K.; Udayabhanu, G.; Pathak, D.; Nayak, G. Single pot fabrication of N doped reduced GO (N-rGO) /ZnO-CuO nanocomposite as an efficient electrode material for supercapacitor application. Vacuum 2018, 157, 145–154. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, J.; Liang, S.; Bi, H.; Han, Q.; Liu, X.; Wang, X. Reduced graphene oxide decorated with CuO-ZnO hetero-junctions: Towards high selective gas-sensing property to acetone. J. Mater. Chem. A 2014, 2, 18635–18643. [Google Scholar] [CrossRef]
- Jyoti; Varma, G.D. Synthesis of CuO-ZnO/rGO ternary composites for superior NO2 gas sensor at room temperature. Mater. Res. Express 2019, 6, 035011. [Google Scholar] [CrossRef]
- Jyoti; Srivastava, A.; Varma, G. Highly selective and efficient room temperature NO2 gas sensors based on Zn-doped CuO nanostructure-rGO hybrid. J. Mater. Sci. Mater. Electron. 2018, 29, 10640–10655. [Google Scholar] [CrossRef]
- Asgar, H.; Deen, K.; Haider, W. Estimation of electrochemical charge storage capability of ZnO/CuO/reduced graphene oxide nanocomposites. Int. J. Energy Res. 2020, 44, 1580–1593. [Google Scholar] [CrossRef]
- Schütz, M.; Xiao, L.; Lehnen, T.; Fischer, T.; Mathur, S. Microwave-assisted synthesis of nanocrystalline binary and ternary metal oxides. Int. Mater. Rev. 2018, 63, 341–374. [Google Scholar] [CrossRef]
- Kumar, A.; Kuang, Y.; Liang, Z.; Sun, X. Microwave chemistry, recent advancements, and eco-friendly microwave-assisted synthesis of nanoarchitectures and their applications: A review. Mater. Today Nano 2020, 11, 100076. [Google Scholar] [CrossRef]
- Zaaba, N.; Foo, K.; Hashim, U.; Tan, S.; Liu, W.; Voon, C. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- De Silva, K.; Huang, H.; Yoshimura, M. Progress of reduction of graphene oxide by ascorbic acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Prakash, A.; Sahu, N.; Bahadur, D. Enhanced photocatalytic performance of ZnO-reduced graphene oxide hybrid synthesized via ultrasonic probe-assisted study. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2013; Volume 1512, pp. 312–313. [Google Scholar]
- Zelekew, O.; Kuo, D. Facile synthesis of SiO2@CuxO@TiO2 heterostructures for catalytic reductions of 4-nitrophenol and 2-nitroaniline organic pollutants. Appl. Surf. Sci. 2017, 393, 110–118. [Google Scholar] [CrossRef]
- Bolaghi, Z.K.; Masoudpanah, S.; Hasheminiasari, M. Photocatalytic activity of ZnO/RGO composite synthesized by one-pot solution combustion method. Mater. Res. Bull. 2019, 115, 191–195. [Google Scholar] [CrossRef]
- Alam, S.; Sharma, N.; Kumar, L. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (rGO)*. Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Green reduction of graphene oxide by aqueous phytoextracts. Carbon N. Y. 2012, 50, 5331–5339. [Google Scholar] [CrossRef]
- Omar, F.; Ming, H.N.; Hafiz, S.; Ngee, L. Microwave synthesis of zinc oxide/reduced graphene oxide hybrid for adsorption-photocatalysis application. Int. J. Photoenergy 2014, 2014, 176835. [Google Scholar] [CrossRef]
- Priyadharsan, A.; Shanavas, S.; Vasanthakumar, V.; Balamuralikrishnan, B.; Anbarasan, P. Synthesis and investigation on synergetic effect of rGO-ZnO decorated MoS2 microflowers with enhanced photocatalytic and antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 43–53. [Google Scholar]
- Harish, S.; Archana, J.; Sabarinathan, M.; Navaneethan, M.; Nisha, K.; Ponnusamy, S.; Muthamizhchelvan, C.; Ikeda, H.; Aswal, D.K.; Hayakawa, Y. Controlled structural and compositional characteristic of visible light active ZnO/CuO photocatalyst for the degradation of organic pollutant. Appl. Surf. Sci. 2017, 418, 103–112. [Google Scholar] [CrossRef]
- Islam, M.; Dominguez, A.; Alvarado-Tenorio, B.; Bernal, R.; Montes, M.; Noveron, J. Sucrose-Mediated Fast Synthesis of Zinc Oxide Nanoparticles for the Photocatalytic Degradation of Organic Pollutants in Water. ACS Omega 2019, 4, 6560–6572. [Google Scholar] [CrossRef]
- Renuka, L.; Anantharaju, K.; Vidya, Y.; Nagaswarupa, H.; Prashantha, S.; Nagabhushana, H. Synthesis of Sunlight Driven ZnO/CuO Nanocomposite: Characterization, Optical, Electrochemical and Photocatalytic Studies. Mater. Today Proc. 2017, 4, 11782–11790. [Google Scholar] [CrossRef]
- Govindasamy, G.; Mydin, R.; Sreekantan, S.; Harun, N. Compositions and antimicrobial properties of binary ZnO–CuO nanocomposites encapsulated calcium and carbon from Calotropis gigantea targeted for skin pathogens. Sci. Rep. 2021, 11, 99. [Google Scholar] [CrossRef]
- Parra, M.; Haque, F. Poly (Ethylene Glycol) (PEG)-assisted shape-controlled synthesis of one-dimensional ZnO nanorods. Optik 2015, 126, 1562–1566. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, F.; Chen, J.; Sun, X.; Xian, T.; Yang, H. In Situ Construction of CNT/CuS Hybrids and Their Application in Photodegradation for Removing Organic Dyes. Nanomaterials 2020, 10, 178. [Google Scholar] [CrossRef]
- Kumar, A.; Rout, L.; Achary, L.; Mohanty, S.; Dash, P. A combustion synthesis route for magnetically separable graphene oxide-CuFe2O4-ZnO nanocomposites with enhanced solar light-mediated photocatalytic activity. New J. Chem. 2017, 41, 10568–10583. [Google Scholar] [CrossRef]
- Galani, S.; Panda, A. Enhanced Thermocatalytic Activity of Porous Yellow ZnO Nanoflakes: Defect- and Morphology-Induced Perspectives. Chem.—An Asian J. 2019, 14, 612–620. [Google Scholar] [CrossRef]
- Arias, F.; Guevara, M.; Tene, T.; Angamarca, P.; Molina, R.; Valarezo, A.; Salguero, O.; Vacacela Gomez, C.; Arias, M.; Caputi, L.S. The adsorption of methylene blue on eco-friendly reduced graphene oxide. Nanomaterials 2020, 10, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, G.; Ramalingam, S.; Rayappan, J.; Kesavan, S.; Periathambi, T.; Nesakumar, N. Green preparation of reduced graphene oxide by Bougainvillea glabra flower extract and sensing application. J. Mater. Sci. Mater. Electron. 2020, 31, 14345–14356. [Google Scholar] [CrossRef]
- Johra, F.; Lee, J.; Jung, W. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Momeni, M.; Ghayeb, Y.; Menati, M. Fabrication, characterization and photoelectrochemical properties of cuprous oxide-reduced graphene oxide photocatalysts for hydrogen generation. J. Mater. Sci. Mater. Electron. 2018, 29, 4136–4146. [Google Scholar] [CrossRef]
- Rabchinskii, M.; Dideikin, A.; Kirilenko, D.; Baidakova, M.V.; Shnitov, V.V.; Roth, F.; Konyakhin, S.V.; Besedina, N.A.; Pavlov, S.I.; Kuricyn, R.A.; et al. Facile reduction of graphene oxide suspensions and films using glass wafers. Sci. Rep. 2018, 8, 14154. [Google Scholar] [CrossRef]
- Singh, D.; Pandey, D.; Yadav, R.; Singh, D. A study of ZnO nanoparticles and ZnO-EG nanofluid. J. Exp. Nanosci. 2013, 8, 731–741. [Google Scholar] [CrossRef]
- Saravanakkumar, D.; Sivaranjani, S.; Kaviyarasu, K.; Ayeshamariam, A.; Ravikumar, B.; Pandiarajan, S.; Veeralakshmi, C.; Jayachandran, M.; Maaza, M. Synthesis and characterization of ZnO-CuO nanocomposites powder by modified perfume spray pyrolysis method and its antimicrobial investigation. J. Semicond. 2018, 39, 033001. [Google Scholar] [CrossRef]
- Lei, X.; Cao, Y.; Chen, Q.; Ao, X.; Fang, Y.; Liu, B. ZIF-8 derived hollow CuO/ZnO material for study of enhanced photocatalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 1–10. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Sweena, R.; Wu, J.; Anandan, S. Synthesis of CuO-ZnO nanophotocatalyst for visible light assisted degradation of a textile dye in aqueous solution. Chem. Eng. J. 2011, 171, 136–140. [Google Scholar] [CrossRef]
- Yu, J.; Zhuang, S.; Xu, X.; Zhu, W.; Feng, B.; Hu, J. Photogenerated electron reservoir in hetero-p-n CuO-ZnO nanocomposite device for visible-light-driven photocatalytic reduction of aqueous Cr(vi). J. Mater. Chem. A 2015, 3, 1199–1207. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, Y.; Wu, Z.; Zheng, G.; He, J.; Zhou, Y. Improved photocatalytic activity of nanocrystalline ZnO by coupling with CuO. J. Phys. Chem. Solids 2017, 106, 29–36. [Google Scholar] [CrossRef]
- Lavín, A.; Sivasamy, R.; Mosquera, E.; Morel, M. High proportion ZnO/CuO nanocomposites: Synthesis, structural and optical properties, and their photocatalytic behavior. Surf. Interfaces 2019, 17, 100367. [Google Scholar] [CrossRef]
- Park, Y.; Yang, J.; Ryu, B.; Cho, J.; Cuong, T.; Hong, C.-H. Solution-processed multidimensional ZnO/CuO heterojunction as ultraviolet sensing. Opt. Mater. Express 2015, 5, 1752. [Google Scholar] [CrossRef]
- Zou, W.; Zhang, L.; Liu, L.; Wang, X.; Sun, J.; Wu, S.; Deng, Y.; Tang, C.; Gao, F.; Dong, L. Engineering the Cu2O-reduced graphene oxide interface to enhance photocatalytic degradation of organic pollutants under visible light. Appl. Catal. B Environ. 2016, 181, 495–503. [Google Scholar] [CrossRef]
- Chen, C.; Liu, X.; Fang, Q.; Chen, X.; Liu, T.; Zhang, M. Self-assembly synthesis of CuO/ZnO hollow microspheres and their photocatalytic performance under natural sunlight. Vacuum 2020, 174, 109198. [Google Scholar] [CrossRef]
- Prabhu, Y.; Rao, V.N.; Shankar, M.; Sreedhar, B.; Pal, U. The facile hydrothermal synthesis of CuO@ZnO heterojunction nanostructures for enhanced photocatalytic hydrogen evolution. New J. Chem. 2019, 43, 6794–6805. [Google Scholar] [CrossRef]
- Tufa, L.; Oh, S.; Tran, V.; Kim, J.; Jeong, K.-J.; Park, T.; Kim, H.J.; Lee, J. Electrochemical immunosensor using nanotriplex of graphene quantum dots, Fe3O4, and Ag nanoparticles for tuberculosis. Electrochim. Acta 2018, 290, 369–377. [Google Scholar] [CrossRef]
- Xu, H.; Fang, W.; Xu, L.; Liu, F. Batch Preparation of CuO/ZnO-Loaded Nanofiber Membranes for Photocatalytic Degradation of Organic Dyes. Langmuir 2020, 36, 14189–14202. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, Y.; Qin, H.; Wu, J.; Lu, Y.; Chi, H.; Yang, F.; Zhou, B.; Yu, H.; Liu, J. Construction of rGO wrapping octahedral Ag-Cu2O heterostructure for enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2018, 227, 132–144. [Google Scholar] [CrossRef]
- Guo, F.; Huang, X.; Chen, Z.; Cao, L.; Cheng, X.; Chen, L.; Shi, W. Construction of Cu3P-ZnSnO3-g-C3N4 p-n-n heterojunction with multiple built-in electric fields for effectively boosting visible-light photocatalytic degradation of broad-spectrum antibiotics. Sep. Purif. Technol. 2021, 265, 118477. [Google Scholar] [CrossRef]
- Thatikayala, D.; Min, B. Ginkgo leaves extract-assisted synthesis of ZnO/CuO nanocrystals for efficient UV-induced photodegradation of organic dyes and antibacterial activity. J. Mater. Sci. Mater. Electron. 2021, 32, 17154–17169. [Google Scholar] [CrossRef]
- Shaker-Agjekandy, S.; Habibi-Yangjeh, A. Microwave-assisted one-pot method for preparation of ZnO/AgI nanocomposites with highly enhanced photocatalytic activity under visible-light irradiation. Desalin. Water Treat. 2016, 57, 16015–16023. [Google Scholar] [CrossRef]
- Fang, H.; Guo, Y.; Wu, T.; Liu, Y. Biomimetic synthesis of urchin-like CuO/ZnO nanocomposites with excellent photocatalytic activity. New J. Chem. 2018, 42, 12779–12786. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.; Alnashef, I. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Jenny, S.; Masaya, M.; Masato, T.; Jinlong, Z.; Yu, H.; Masakazu, A.; Detlef, W.B. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar]
- Imangaliyeva, A.; Mastai, Y.; Seilkhanova, G. In situ synthesis and catalytic properties of Cu2O nanoparticles based on clay materials and polyethylene glycol. J. Nanoparticle Res. 2019, 21, 97. [Google Scholar] [CrossRef]
- Kandula, S.; Jeevanandam, P. Synthesis of Cu2O@Ag Polyhedral Core-Shell Nanoparticles by a Thermal Decomposition Approach for Catalytic Applications. Eur. J. Inorg. Chem. 2016, 2016, 1548–1557. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, R.; Jiang, P.; Zhang, K.; Dong, Y.; Xie, W. Mechanistic Study of Catalytic Hydride Reduction of -NO2 to -NH2 Using Isotopic Solvent and Reducer: The Real Hydrogen Source. J. Phys. Chem. C 2019, 123, 15582–15588. [Google Scholar] [CrossRef]
- Emiru, T.; Ayele, D. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef]
- Majumdar, D.; Baugh, N.; Bhattacharya, S. Ultrasound assisted formation of reduced graphene oxide-copper (II) oxide nanocomposite for energy storage applications. Colloids Surf. A Physicochem. Eng. Asp. 2017, 512, 158–170. [Google Scholar] [CrossRef]
| Catalyst | Synthesis Method | Experimental Conditions | Performance | Ref. | |||
|---|---|---|---|---|---|---|---|
| Catalyst (mg) | Conc. of MB | Light | Efficiency (%) | Ratio Constant, K (min−1g−1) | |||
| ZnO/CuO | Combustion | 40 | 20 ppm | Solar | 93 in 60 min | 0.5575 | [51] |
| CuO-ZnO | Wet chemical | 5 | 5 µM | Solar | 93.8 in 20 min | 27.2 | [18] |
| ZnO–CuO | Co-precipitation | 25 | 0.001 M | Vis | 95.6 | 0.94 | [16] |
| GO–CuFe2O4–ZnO | Combustion | 10 | 40 ppm | Solar | 100 in 40 min | 9.94 | [55] |
| ZnO/CuO | Biocombustion | 40 | 10 ppm | UV | 99.2 in 60 min | 1.45 | [77] |
| MoS2-ZnO-rGO | Hydrothermal | 100 | 20 ppm | Solar | 90 in 75 min | - | [48] |
| (rGO-ZnO)/CuO | MW | 40 | 10 ppm | Vis | 90 in 105 min | 11.7 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekru, A.G.; Tufa, L.T.; Zelekew, O.A.; Gwak, J.; Lee, J.; Sabir, F.K. Microwave-Assisted Synthesis of rGO-ZnO/CuO Nanocomposites for Photocatalytic Degradation of Organic Pollutants. Crystals 2023, 13, 133. https://doi.org/10.3390/cryst13010133
Bekru AG, Tufa LT, Zelekew OA, Gwak J, Lee J, Sabir FK. Microwave-Assisted Synthesis of rGO-ZnO/CuO Nanocomposites for Photocatalytic Degradation of Organic Pollutants. Crystals. 2023; 13(1):133. https://doi.org/10.3390/cryst13010133
Chicago/Turabian StyleBekru, Aklilu Guale, Lemma Teshome Tufa, Osman Ahmed Zelekew, Juyong Gwak, Jaebeom Lee, and Fedlu Kedir Sabir. 2023. "Microwave-Assisted Synthesis of rGO-ZnO/CuO Nanocomposites for Photocatalytic Degradation of Organic Pollutants" Crystals 13, no. 1: 133. https://doi.org/10.3390/cryst13010133
APA StyleBekru, A. G., Tufa, L. T., Zelekew, O. A., Gwak, J., Lee, J., & Sabir, F. K. (2023). Microwave-Assisted Synthesis of rGO-ZnO/CuO Nanocomposites for Photocatalytic Degradation of Organic Pollutants. Crystals, 13(1), 133. https://doi.org/10.3390/cryst13010133








