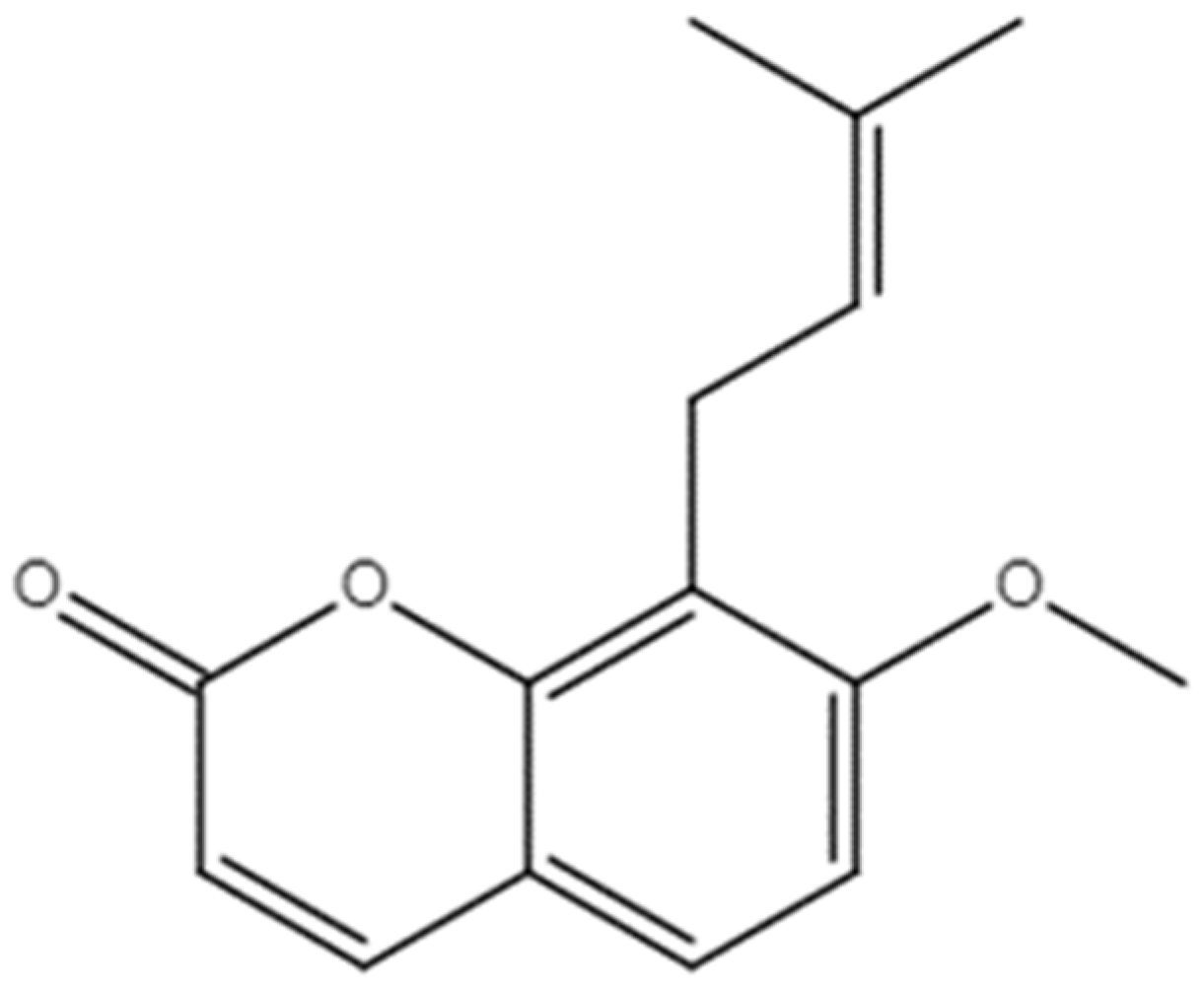
UPLC-Q-TOF/MS-Based Metabolomics Approach Reveals Osthole Intervention in Breast Cancer 4T1 Cells
Abstract
1. Introduction
2. Results
2.1. Effect of OST on Breast Cancer 4T1 Cell Activity
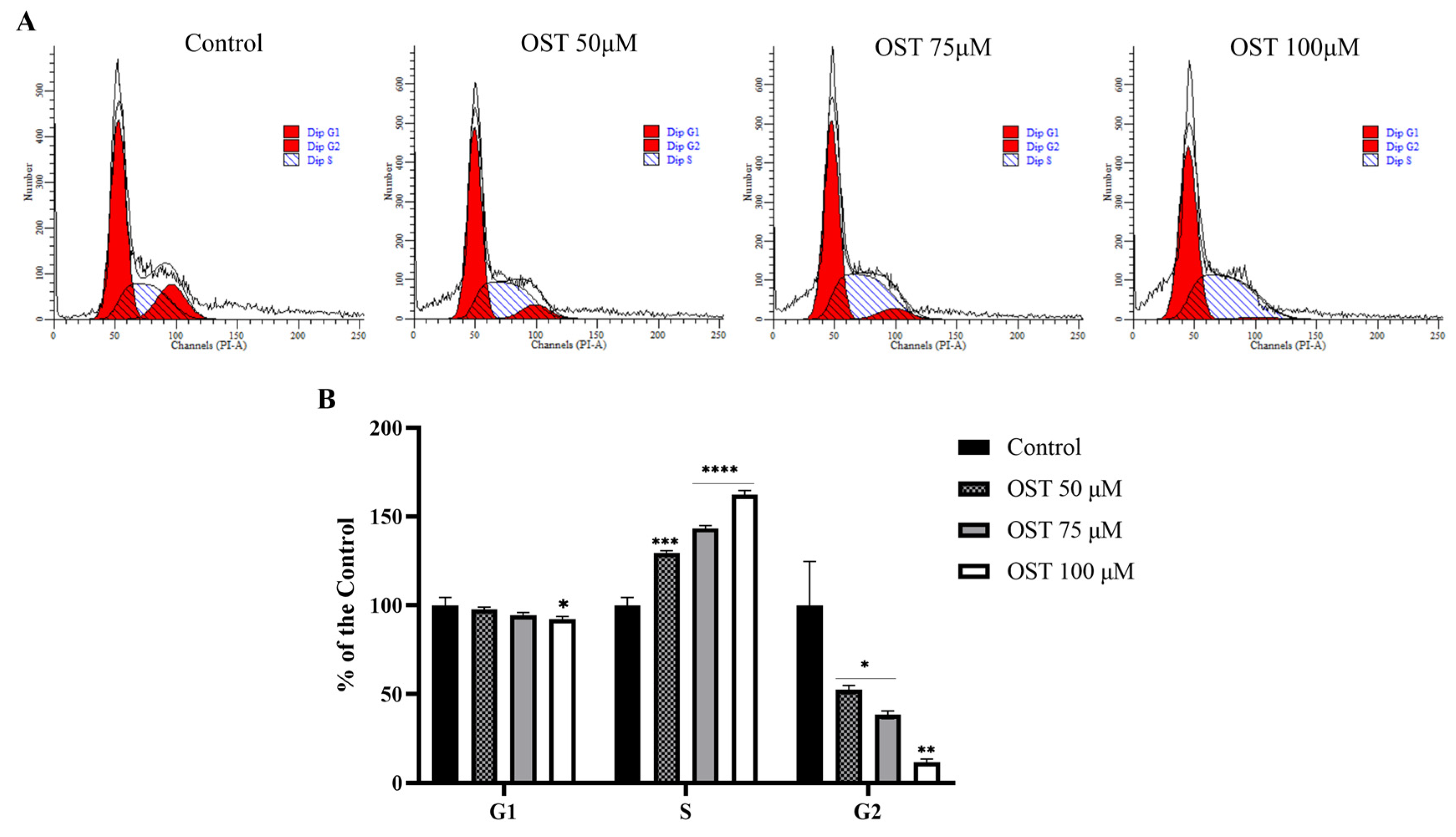
2.2. Effect of OST on Cell Cycle of Breast Cancer 4T1 Cells
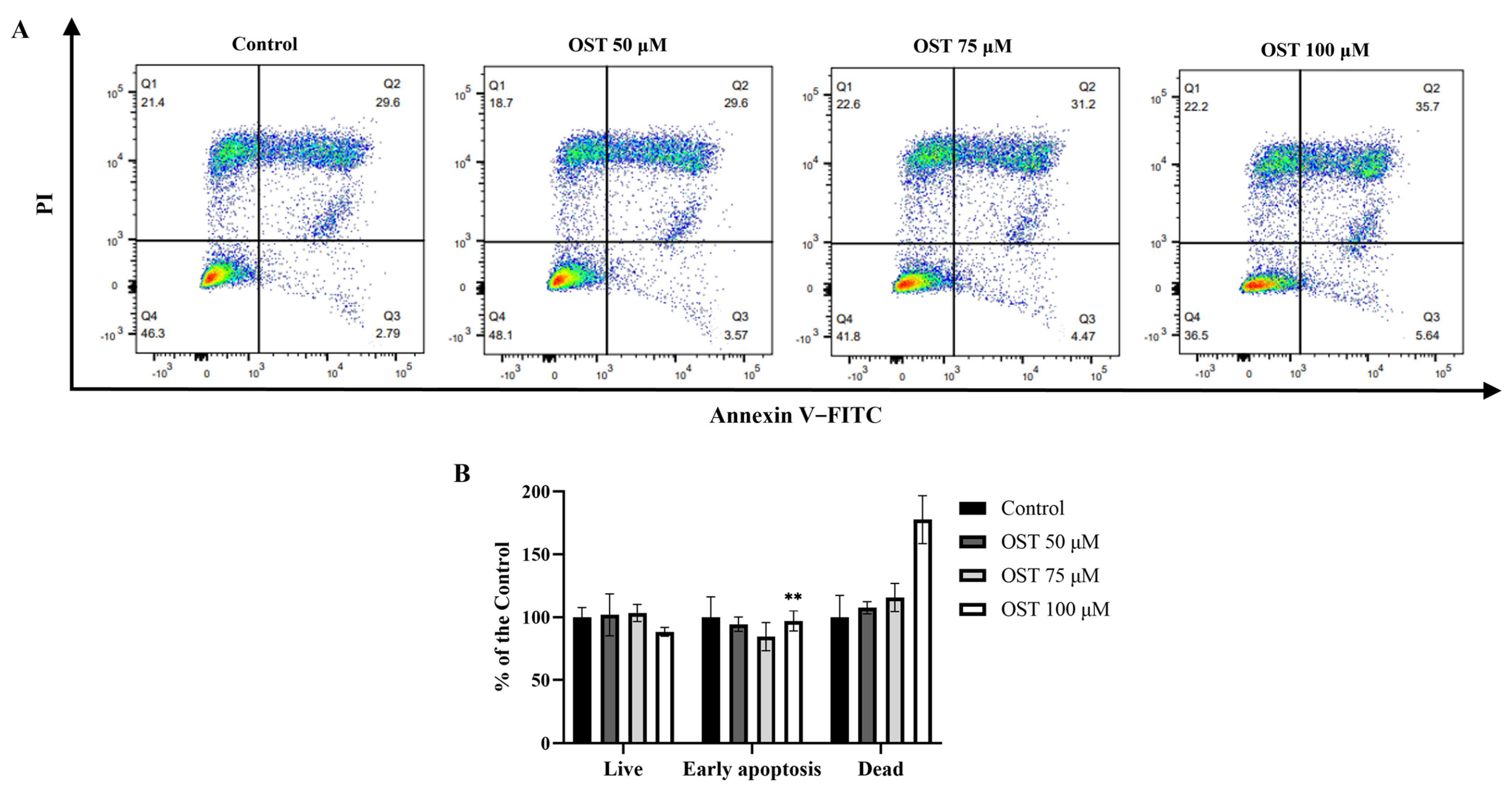
2.3. Effect of OST on Apoptosis of Breast Cancer 4T1 Cells
2.4. Metabolomics Analysis
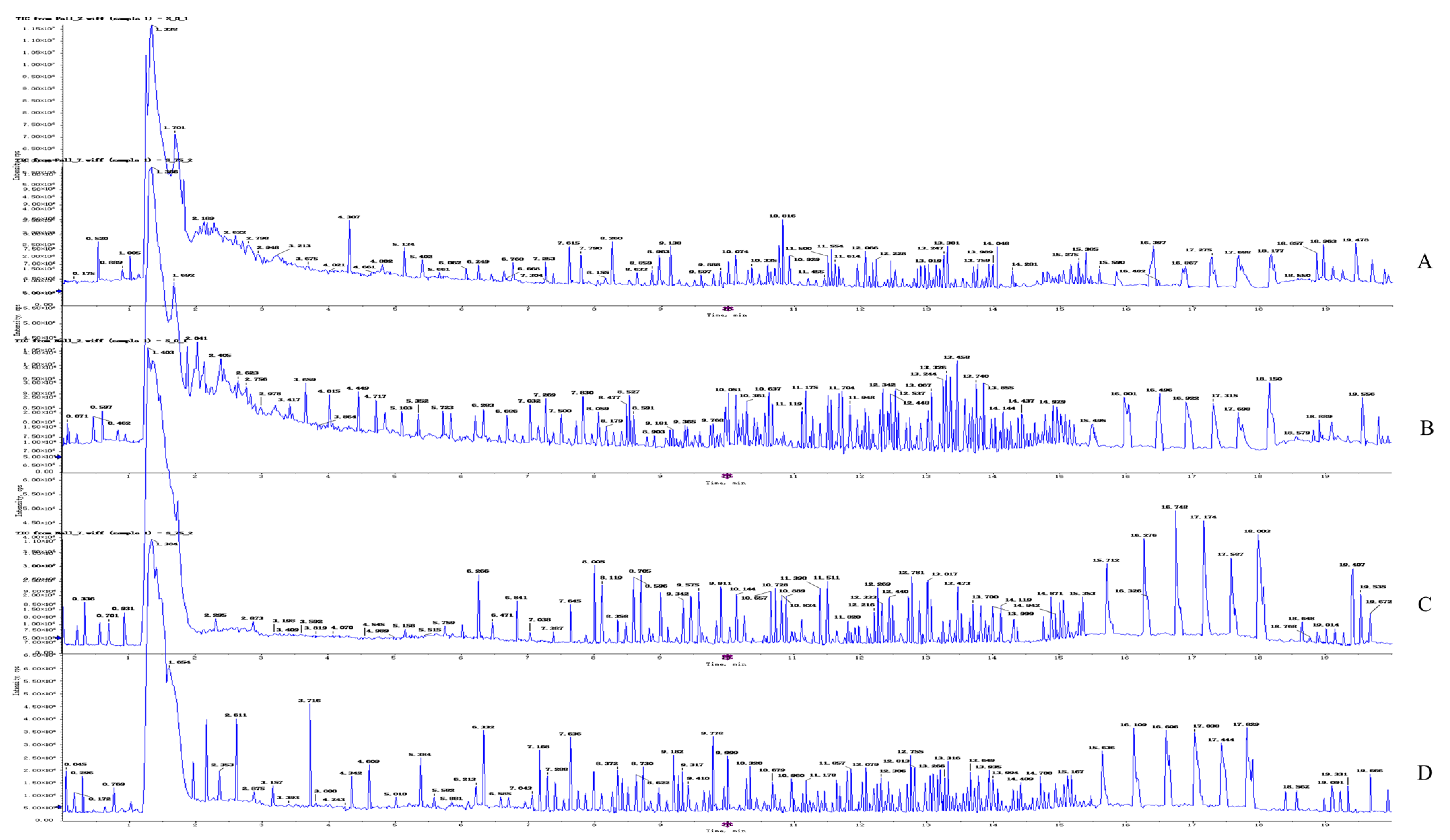
2.4.1. Quality Control Sample Detection and System Stability Analysis
2.4.2. Metabolite Profiling of 4T1 Cells after OST Treatment
2.5. Multivariate Statistical Analysis and Screening to Identify Potential Marker Metabolites
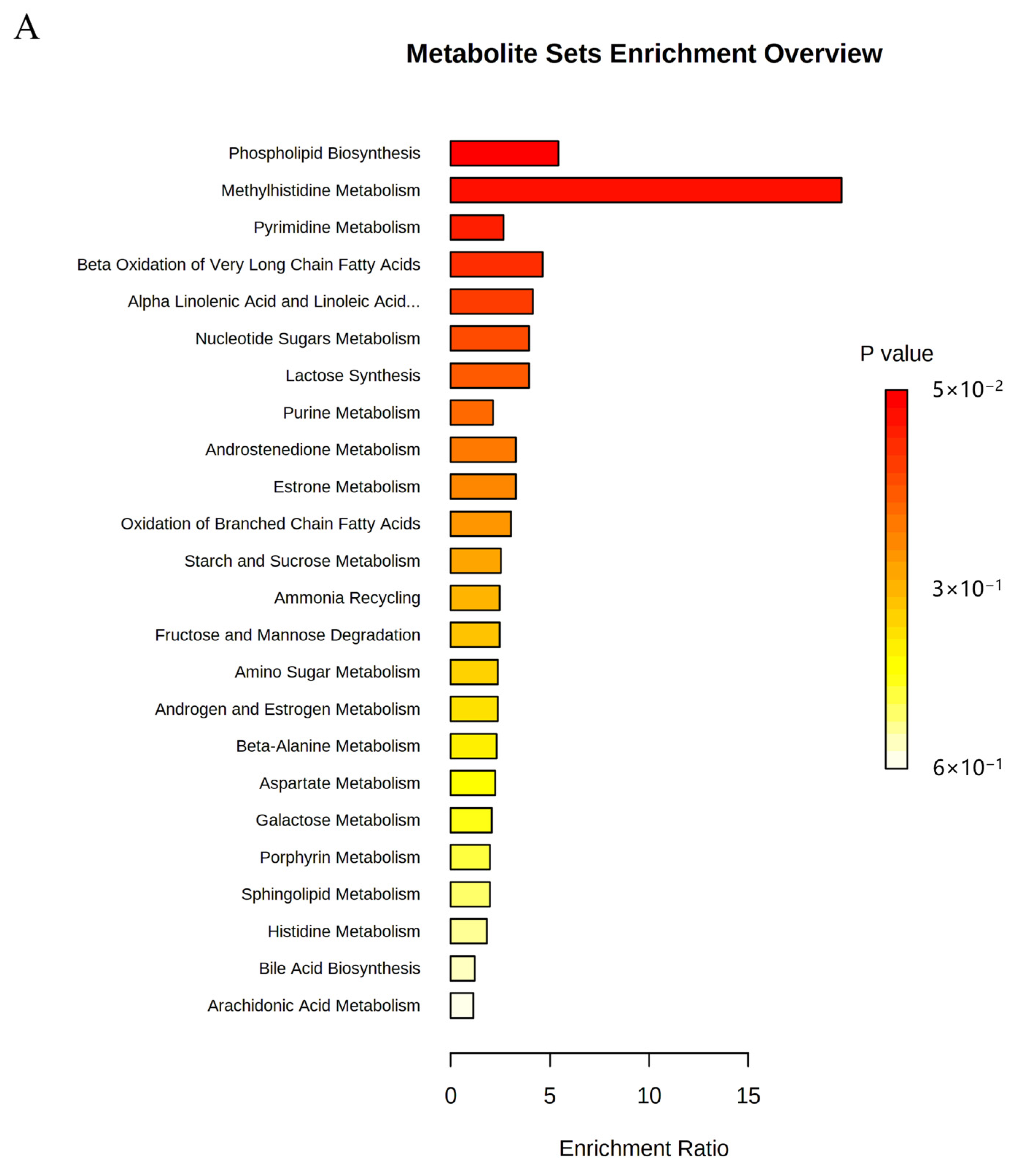
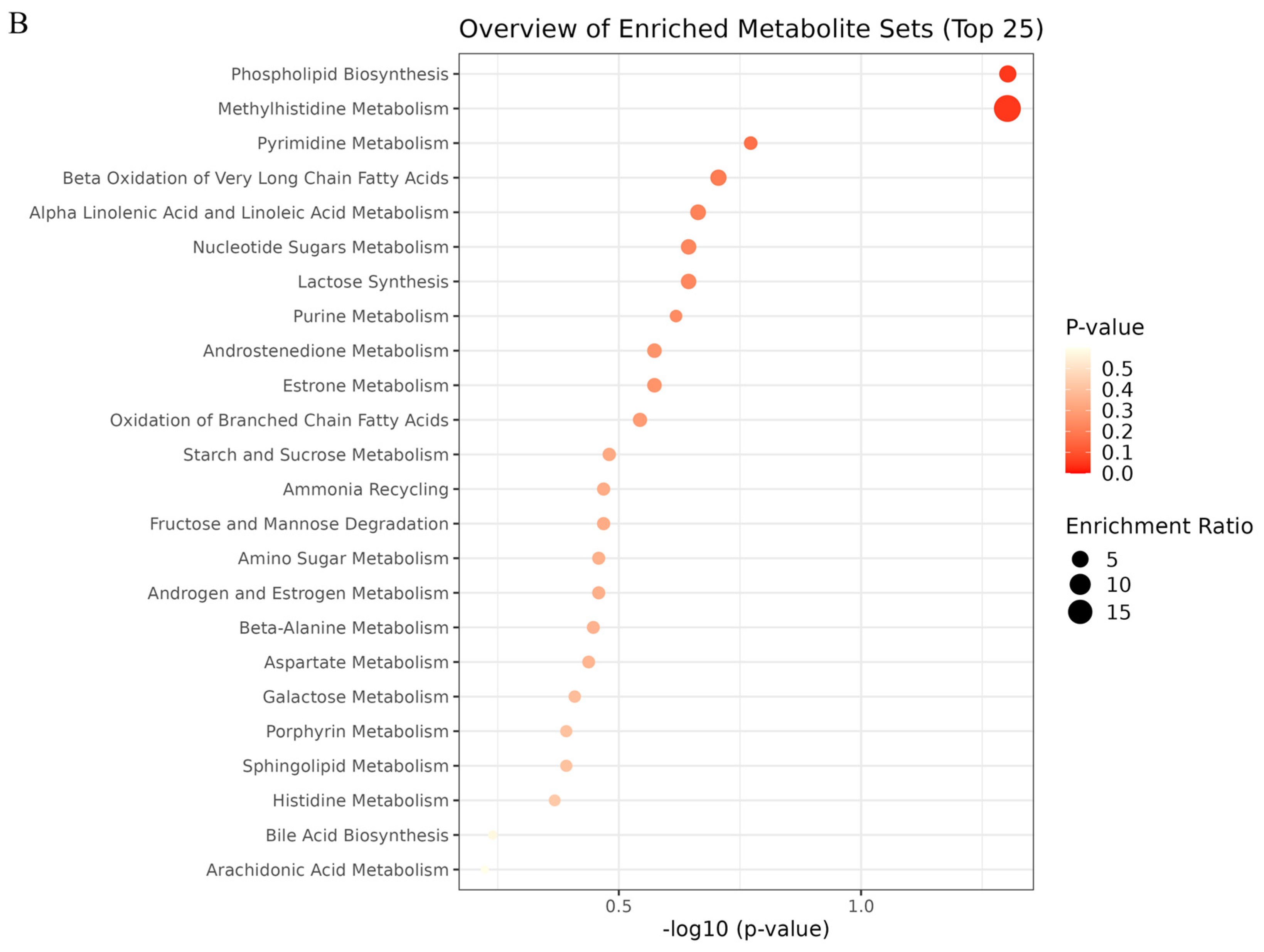
2.6. Metabolic Pathway Analysis
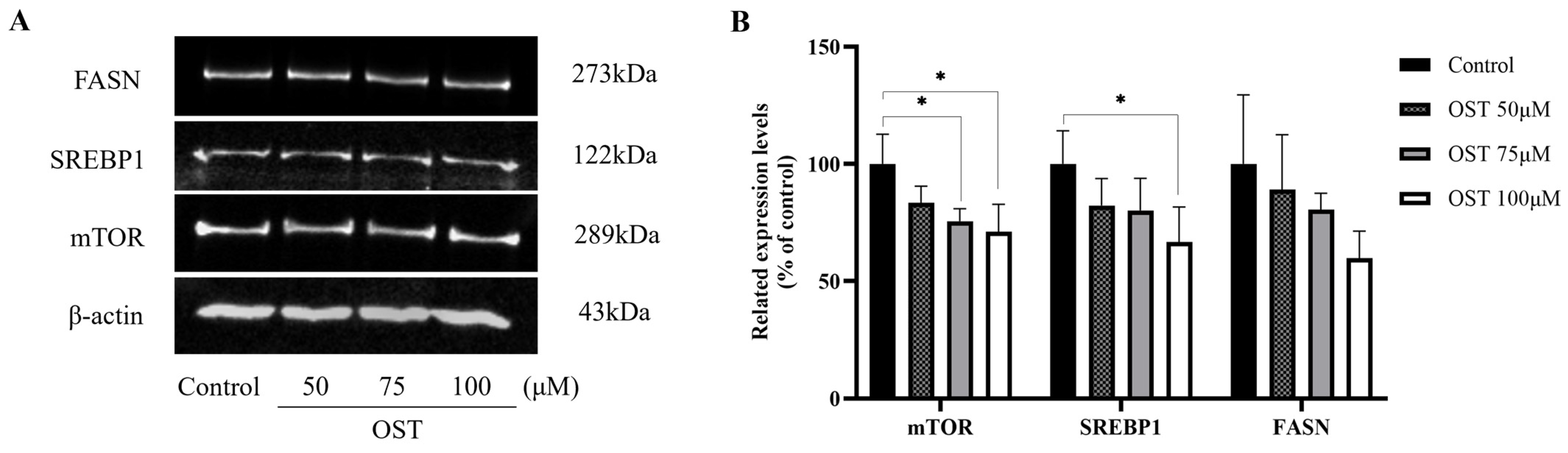
2.7. Effect of OST on mTOR/SREBP1/FASN Pathway Proteins in Breast Cancer 4T1 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Cell Cycle Assay
4.5. Cell Apoptosis Assay
4.6. Sample Preparation for Metabolomics
4.7. UPLC-Q-TOF/MS Conditions
4.8. Metabolomics Data Analysis
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Deng, Y.; Zhou, L.; Tian, T.; Yang, S.; Wu, Y.; Zheng, Y.; Zhai, Z.; Hao, Q.; Song, D.; et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: Results from the Global Burden of Disease Study 2017. J. Hematol. Oncol. 2019, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, H.; Song, X.; Yang, Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin. Cancer Biol. 2020, 60, 14–27. [Google Scholar] [CrossRef]
- Park, W.; Park, S.; Song, G.; Lim, W. Inhibitory Effects of Osthole on Human Breast Cancer Cell Progression via Induction of Cell Cycle Arrest, Mitochondrial Dysfunction, and ER Stress. Nutrients 2019, 11, 2777. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, F.; Liang, Y.; Yang, Q. Breast cancer brain metastasis: Insight into molecular mechanisms and therapeutic strategies. Br. J. Cancer 2021, 125, 1056–1067. [Google Scholar] [CrossRef]
- Ataseven, B.; Frindte, J.; Harter, P.; Gebers, G.; Vogt, C.; Traut, A.; Breit, E.; Bluni, V.; Reinisch, M.; Heitz, F.; et al. Perception of side effects associated with anticancer treatment in women with breast or ovarian cancer (KEM-GO-1): A prospective trial. Support. Care Cancer 2020, 28, 3605–3615. [Google Scholar] [CrossRef]
- Zhang, Y. The root cause of drug resistance in HER2-positive breast cancer and the therapeutic approaches to overcoming the resistance. Pharmacol. Ther. 2021, 218, 107677. [Google Scholar] [CrossRef]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.B.; Im, S.A.; Wang, Y.; Salgado, R.; Mani, A.; et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): A phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020, 21, 1283–1295. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Q.; Yu, L.; Zhu, J.; Cao, Y.; Gao, X. The signaling pathways and targets of traditional Chinese medicine and natural medicine in triple-negative breast cancer. J. Ethnopharmacol. 2021, 264, 113249. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Lin, J.; Jiang, J. Osthole inhibited cell proliferation and induced cell apoptosis through decreasing CPEB2 expression via up-regulating miR-424 in endometrial carcinoma. J. Recept. Signal. Transduct. Res. 2020, 40, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Tao, S.Y.; Lu, C.; Li, J.J.; Li, X.M.; Jiang, Q.; Yan, B. Osthole: A Traditional Chinese Medicine for Ocular Anti-Angiogenic Therapy. Ophthalmic Res. 2020, 63, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Shokoohinia, Y.; Jafari, F.; Mohammadi, Z.; Bazvandi, L.; Hosseinzadeh, L.; Chow, N.; Bhattacharyya, P.; Farzaei, M.H.; Farooqi, A.A.; Nabavi, S.M.; et al. Potential Anticancer Properties of Osthol: A Comprehensive Mechanistic Review. Nutrients 2018, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Mohammadinejad, R.; Samarghandian, S.; Yaribeygi, H.; Johnston, T.P.; Sahebkar, A. Anti-Tumor Effects of Osthole on Different Malignant Tissues: A Review of Molecular Mechanisms. Anti-Cancer Agents Med. Chem. 2020, 20, 918–931. [Google Scholar] [CrossRef]
- Raggi, C.; Taddei, M.L.; Rae, C.; Braconi, C.; Marra, F. Metabolic reprogramming in cholangiocarcinoma. J. Hepatol. 2022, 77, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.V.; Tilija Pun, N.; Park, P.H. Autophagy activation and SREBP-1 induction contribute to fatty acid metabolic reprogramming by leptin in breast cancer cells. Mol. Oncol. 2021, 15, 657–678. [Google Scholar] [CrossRef]
- Wright, H.J.; Hou, J.; Xu, B.; Cortez, M.; Potma, E.O.; Tromberg, B.J.; Razorenova, O.V. CDCP1 drives triple-negative breast cancer metastasis through reduction of lipid-droplet abundance and stimulation of fatty acid oxidation. Proc. Natl. Acad. Sci. USA 2017, 114, E6556–E6565. [Google Scholar] [CrossRef]
- Tsachaki, M.; Strauss, P.; Dunkel, A.; Navrátilová, H.; Mladenovic, N.; Odermatt, A. Impact of 17β-HSD12, the 3-ketoacyl-CoA reductase of long-chain fatty acid synthesis, on breast cancer cell proliferation and migration. Cell. Mol. Life Sci. 2020, 77, 1153–1175. [Google Scholar] [CrossRef]
- Huang, L.H.; Chung, H.Y.; Su, H.M. Docosahexaenoic acid reduces sterol regulatory element binding protein-1 and fatty acid synthase expression and inhibits cell proliferation by inhibiting pAkt signaling in a human breast cancer MCF-7 cell line. BMC Cancer 2017, 17, 890. [Google Scholar] [CrossRef]
- Xu, S.; Chen, T.; Dong, L.; Li, T.; Xue, H.; Gao, B.; Ding, X.; Wang, H.; Li, H. Fatty acid synthase promotes breast cancer metastasis by mediating changes in fatty acid metabolism. Oncol. Lett. 2021, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jee, W.; An, E.J.; Ko, H.M.; Jung, J.H.; Na, Y.C.; Jang, H.J. Timosaponin A3 Inhibits Palmitate and Stearate through Suppression of SREBP-1 in Pancreatic Cancer. Pharmaceutics 2022, 14, 945. [Google Scholar] [CrossRef] [PubMed]
- Murugan, A.K. mTOR: Role in cancer, metastasis and drug resistance. Semin. Cancer Biol. 2019, 59, 92–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qi, Q.; Wu, N.; Wang, Y.; Feng, Q.; Jin, R.; Jiang, L. Aspirin promotes RSL3-induced ferroptosis by suppressing mTOR/SREBP-1/SCD1-mediated lipogenesis in PIK3CA-mutatnt colorectal cancer. Redox Biol. 2022, 55, 102426. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, Q.; Zhang, A.; Liu, Y.; Li, Z.; Tang, H.; Cao, D.; Zhang, D. Revealing the Hypoglycemic Effects and Mechanism of GABA-Rich Germinated Adzuki Beans on T2DM Mice by Untargeted Serum Metabolomics. Front. Nutr. 2021, 8, 791191. [Google Scholar] [CrossRef]
- Duan, X.; Pan, L.; Bao, Q.; Peng, D. UPLC-Q-TOF-MS Study of the Mechanism of THSWD for Breast Cancer Treatment. Front. Pharmacol. 2020, 10, 1625. [Google Scholar] [CrossRef]
- Kozar, N.; Kruusmaa, K.; Bitenc, M.; Argamasilla, R.; Adsuar, A.; Takač, I.; Arko, D. Identification of Novel Diagnostic Biomarkers in Breast Cancer Using Targeted Metabolomic Profiling. Clin. Breast Cancer 2021, 21, e204–e211. [Google Scholar] [CrossRef]
- Ma, S.; Wang, F.; Zhang, C.; Wang, X.; Wang, X.; Yu, Z. Cell metabolomics to study the function mechanism of Cyperus rotundus L. on triple-negative breast cancer cells. BMC Complement. Med. Ther. 2020, 20, 262. [Google Scholar] [CrossRef]
- Butler, L.M.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020, 159, 245–293. [Google Scholar] [CrossRef]
- Lin, V.C.; Chou, C.H.; Lin, Y.C.; Lin, J.N.; Yu, C.C.; Tang, C.H.; Lin, H.Y.; Way, T.D. Osthole suppresses fatty acid synthase expression in HER2-overexpressing breast cancer cells through modulating Akt/mTOR pathway. J. Agric. Food Chem. 2010, 58, 4786–4793. [Google Scholar] [CrossRef]
- Sunami, Y.; Rebelo, A.; Kleeff, J. Lipid Metabolism and Lipid Droplets in Pancreatic Cancer and Stellate Cells. Cancers 2017, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aljarbou, A.N.; Aldebasi, Y.H.; Faisal, S.M.; Khan, M.A. Resveratrol suppresses the proliferation of breast cancer cells by inhibiting fatty acid synthase signaling pathway. Cancer Epidemiol. 2014, 38, 765–772. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Lu, X.; Zheng, S.; Li, F.; Xiong, Z. Metabonomic study on the anti-osteoporosis effect of Rhizoma Drynariae and its action mechanism using ultra-performance liquid chromatography-tandem mass spectrometry. J. Ethnopharmacol. 2012, 139, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Catalá, A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids 2009, 157, 1–11. [Google Scholar] [CrossRef]
- Gao, D.; Wang, Y.; Xie, W.; Yang, T.; Jiang, Y.; Guo, Y.; Guan, J.; Liu, H. Metabolomics study on the antitumor effect of marine natural compound flexibilide in HCT-116 colon cancer cell line. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1014, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, F.B. The biochemistry and physiology of nucleotides. J. Nutr. 1994, 124, 124S–127S. [Google Scholar] [CrossRef]
- Lovászi, M.; Németh, Z.H.; Gause, W.C.; Beesley, J.; Pacher, P.; Haskó, G. Inosine monophosphate and inosine differentially regulate endotoxemia and bacterial sepsis. FASEB J. 2021, 35, e21935. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Löffler, M.; Carrey, E.A.; Zameitat, E. Orotate (orotic acid): An essential and versatile molecule. Nucleosides Nucleotides Nucleic Acids 2016, 35, 566–577. [Google Scholar] [CrossRef]


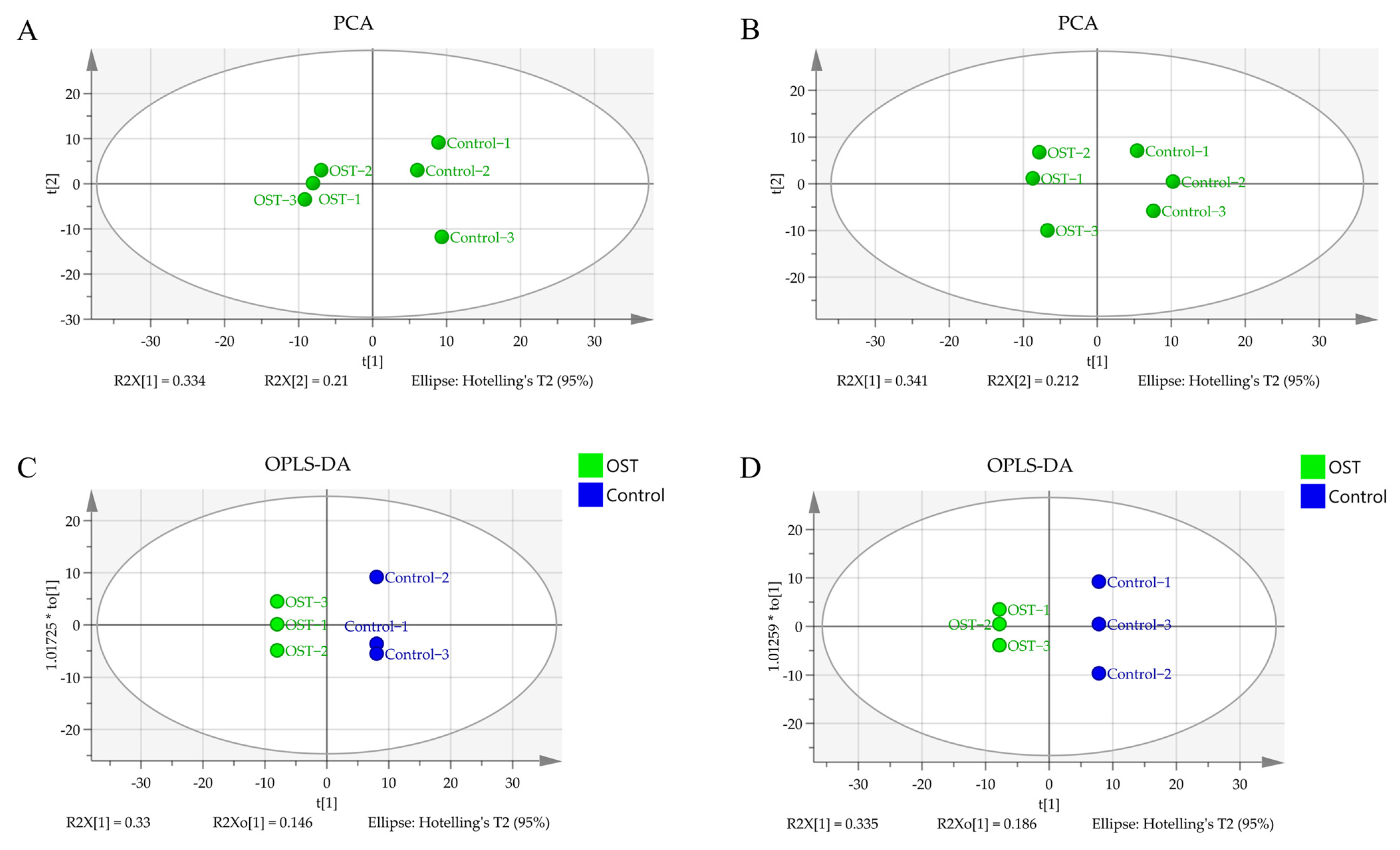
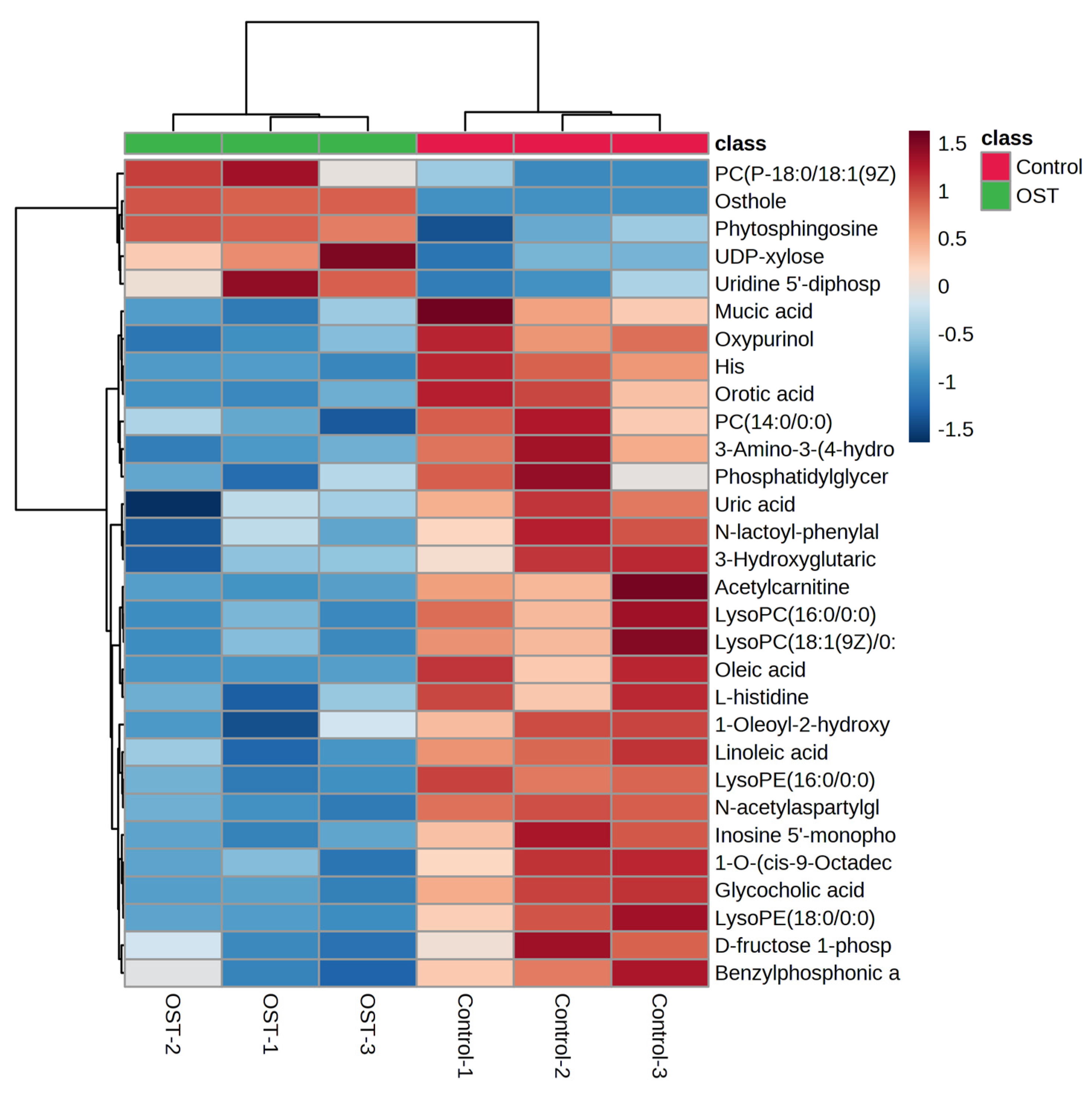
| NO | M/Z | Compound Name | Chemical Formula | p-Value | Trend |
|---|---|---|---|---|---|
| 1 | 245.1170 | Osthole | C15H16O3 | 0.0001 | ↑ |
| 2 | 521.3430 | Linoleic acid | C18H32O2 | 0.0048 | ↓ |
| 3 | 466.3285 | Glycocholic acid | C26H43NO6 | 0.0061 | ↓ |
| 4 | 468.3065 | PC (14:0/0:0) | C22H46NO7P | 0.0151 | ↓ |
| 5 | 496.3388 | LysoPC (16:0/0:0) | C24H50NO7P | 0.0151 | ↓ |
| 6 | 318.2995 | Phytosphingosine | C18H39NO3 | 0.0195 | ─ |
| 7 | 550.3854 | 1-O-(cis-9-octadecenyl)-2-O-acetyl-sn-glycero-3-phosphocholine | C28H57NO7P+ | 0.0203 | ↓ |
| 8 | 522.3544 | LysoPC (18:1(9Z)/0:0) | C26H52NO7P | 0.0246 | ↓ |
| 9 | 521.3430 | Oleic acid | C18H34O2 | 0.0254 | ↓ |
| 10 | 283.0186 | D-fructose-1-phosphate | C6H13O9P | 0.0361 | ─ |
| 11 | 204.1222 | L-acetylcarnitine | C9H18NO4 | 0.0407 | ↓ |
| 12 | 772.6059 | PC (P-18:0/18:1(9Z)) | C44H86NO7P | 0.0445 | ↑ |
| 13 | 452.2755 | LysoPE (16:0/0:0) | C21H44NO7P | 0.0005 | ↓ |
| 14 | 303.2319 | N-acetylaspartylglutamate (NAAG) | C11H16N2O8 | 0.0011 | ↓ |
| 15 | 151.0257 | Oxypurinol | C5H4N4O2 | 0.0017 | ─ |
| 16 | 154.0617 | His | C6H9N3O2 | 0.0049 | ─ |
| 17 | 306.0765 | Glutathione | C10H17N3O6S | 0.0071 | ─ |
| 18 | 195.0508 | Galactonic acid | C6H12O7 | 0.0083 | ─ |
| 19 | 180.0660 | 3-amino-3-(4-hydroxyphenyl) propionic acid | C9H11NO3 | 0.0092 | ↓ |
| 20 | 154.0617 | L-histidine | C6H9N3O2 | 0.0098 | ─ |
| 21 | 155.0097 | Orotic acid | C5H4N2O4 | 0.0156 | ↓ |
| 22 | 347.1826 | Inosine 5′-monophosphate (IMP) | C10H13N4O8P | 0.0173 | ─ |
| 23 | 236.0922 | N-lactoyl-phenylalanine | C12H15NO4 | 0.0210 | ─ |
| 24 | 147.0295 | 3-hydroxyglutaric acid | C5H8O5 | 0.0236 | ↓ |
| 25 | 509.2864 | 1-oleoyl-2-hydroxy-sn-glycero-3-PG (sodium salt) | C24H47NaO9P | 0.0254 | ─ |
| 26 | 480.3070 | LysoPE (18:0/0:0) | C23H48NO7P | 0.0298 | ─ |
| 27 | 535.0338 | UDP-xylose | C14H22N2O16P2 | 0.0302 | ─ |
| 28 | 171.0260 | Benzylphosphonic acid | C7H9O3P | 0.0315 | ↓ |
| 29 | 209.0300 | Mucic acid | C6H10O8 | 0.0372 | ↓ |
| 30 | 402.9918 | Uridine 5′-diphosphate | C9H14N2O12P2 | 0.0390 | ─ |
| 31 | 771.5151 | Phosphatidylglyceride18:2–18:2 | C42H75O10P | 0.0459 | ↓ |
| 32 | 167.0204 | Uric acid | C5H4N4O3 | 0.0491 | ─ |
| NO | Pathway Name | Total | Expected | Hits | Raw p |
|---|---|---|---|---|---|
| 1 | Phospholipid Biosynthesis | 29 | 0.368 | 2 | 0.0498 |
| 2 | Methyl Histidine Metabolism | 4 | 0.051 | 1 | 0.0499 |
| 3 | Pyrimidine Metabolism | 59 | 0.749 | 2 | 0.169 |
| 4 | Beta Oxidation of Very Long Chain Fatty Acids | 17 | 0.216 | 1 | 0.197 |
| 5 | Alpha Linolenic Acid and Linoleic Acid Metabolism | 19 | 0.241 | 1 | 0.217 |
| 6 | Nucleotide Sugar Metabolism | 20 | 0.254 | 1 | 0.227 |
| 7 | Lactose Synthesis | 20 | 0.254 | 1 | 0.227 |
| 8 | Purine Metabolism | 74 | 0.939 | 2 | 0.241 |
| 9 | Androstenedione Metabolism | 24 | 0.305 | 1 | 0.267 |
| 10 | Estrone Metabolism | 24 | 0.305 | 1 | 0.267 |
| 11 | Oxidation of Branched Chain Fatty Acids | 26 | 0.330 | 1 | 0.286 |
| 12 | Starch and Sucrose Metabolism | 31 | 0.394 | 1 | 0.331 |
| 13 | Ammonia Recycling | 32 | 0.406 | 1 | 0.340 |
| 14 | Fructose and Mannose Degradation | 32 | 0.406 | 1 | 0.340 |
| 15 | Amino Sugar Metabolism | 33 | 0.419 | 1 | 0.348 |
| 16 | Androgen and Estrogen Metabolism | 33 | 0.419 | 1 | 0.348 |
| 17 | Beta-Alanine Metabolism | 34 | 0.432 | 1 | 0.357 |
| 18 | Aspartate Metabolism | 35 | 0.444 | 1 | 0.365 |
| 19 | Galactose Metabolism | 38 | 0.482 | 1 | 0.390 |
| 20 | Porphyrin Metabolism | 40 | 0.508 | 1 | 0.406 |
| 21 | Sphingolipid Metabolism | 40 | 0.508 | 1 | 0.406 |
| 22 | Histidine Metabolism | 43 | 0.546 | 1 | 0.429 |
| 23 | Bile Acid Biosynthesis | 65 | 0.825 | 1 | 0.576 |
| 24 | Arachidonic Acid Metabolism | 69 | 0.876 | 1 | 0.598 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, C.; Wu, E.; Han, L.; Deng, X.; Shi, Z. UPLC-Q-TOF/MS-Based Metabolomics Approach Reveals Osthole Intervention in Breast Cancer 4T1 Cells. Int. J. Mol. Sci. 2023, 24, 1168. https://doi.org/10.3390/ijms24021168
Li X, Zhang C, Wu E, Han L, Deng X, Shi Z. UPLC-Q-TOF/MS-Based Metabolomics Approach Reveals Osthole Intervention in Breast Cancer 4T1 Cells. International Journal of Molecular Sciences. 2023; 24(2):1168. https://doi.org/10.3390/ijms24021168
Chicago/Turabian StyleLi, Xiuyun, Chenglun Zhang, Enhui Wu, Liang Han, Xiangliang Deng, and Zhongfeng Shi. 2023. "UPLC-Q-TOF/MS-Based Metabolomics Approach Reveals Osthole Intervention in Breast Cancer 4T1 Cells" International Journal of Molecular Sciences 24, no. 2: 1168. https://doi.org/10.3390/ijms24021168
APA StyleLi, X., Zhang, C., Wu, E., Han, L., Deng, X., & Shi, Z. (2023). UPLC-Q-TOF/MS-Based Metabolomics Approach Reveals Osthole Intervention in Breast Cancer 4T1 Cells. International Journal of Molecular Sciences, 24(2), 1168. https://doi.org/10.3390/ijms24021168







