Efficient Detection of Nerve Agents through Carbon Nitride Quantum Dots: A DFT Approach
Abstract
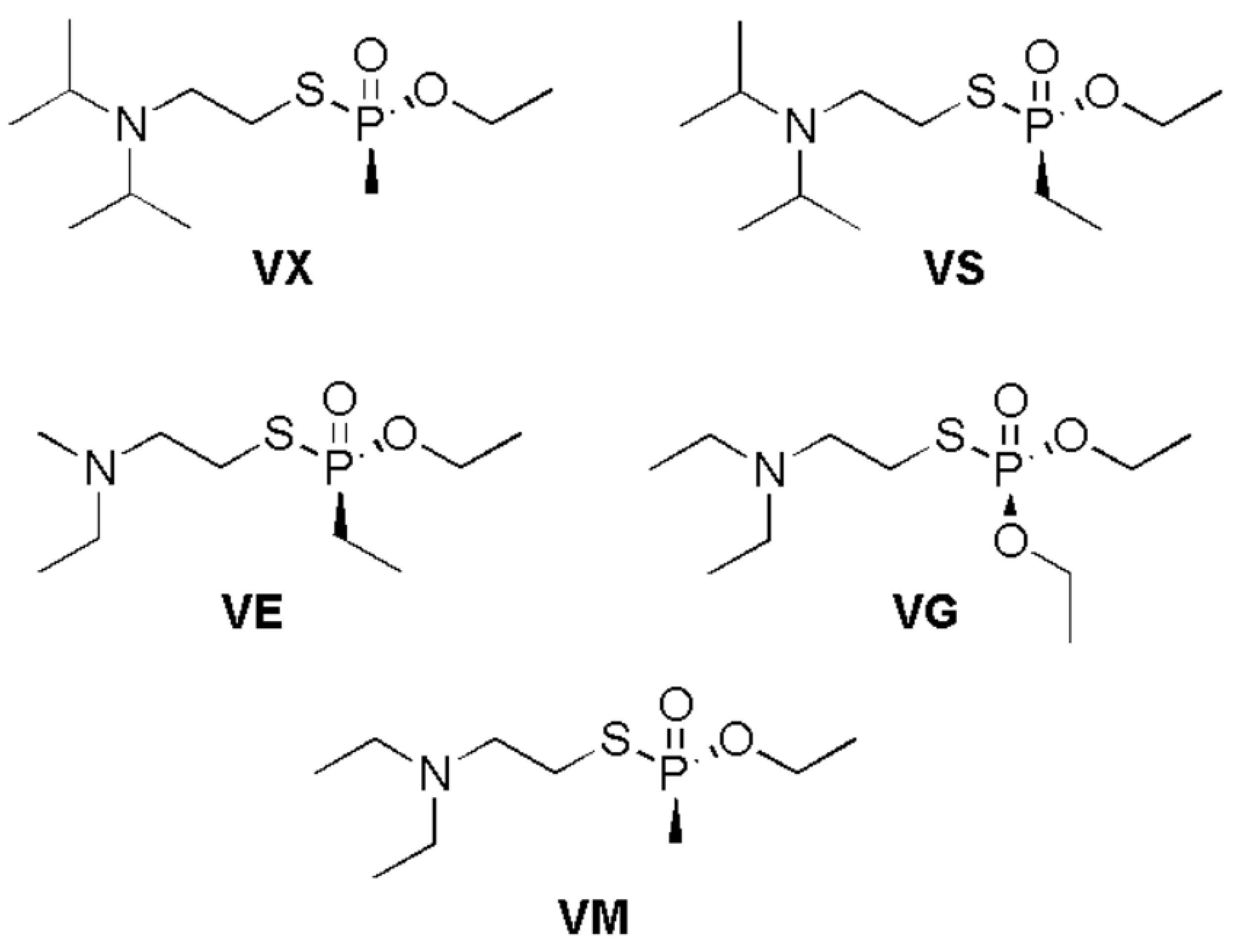
:1. Introduction
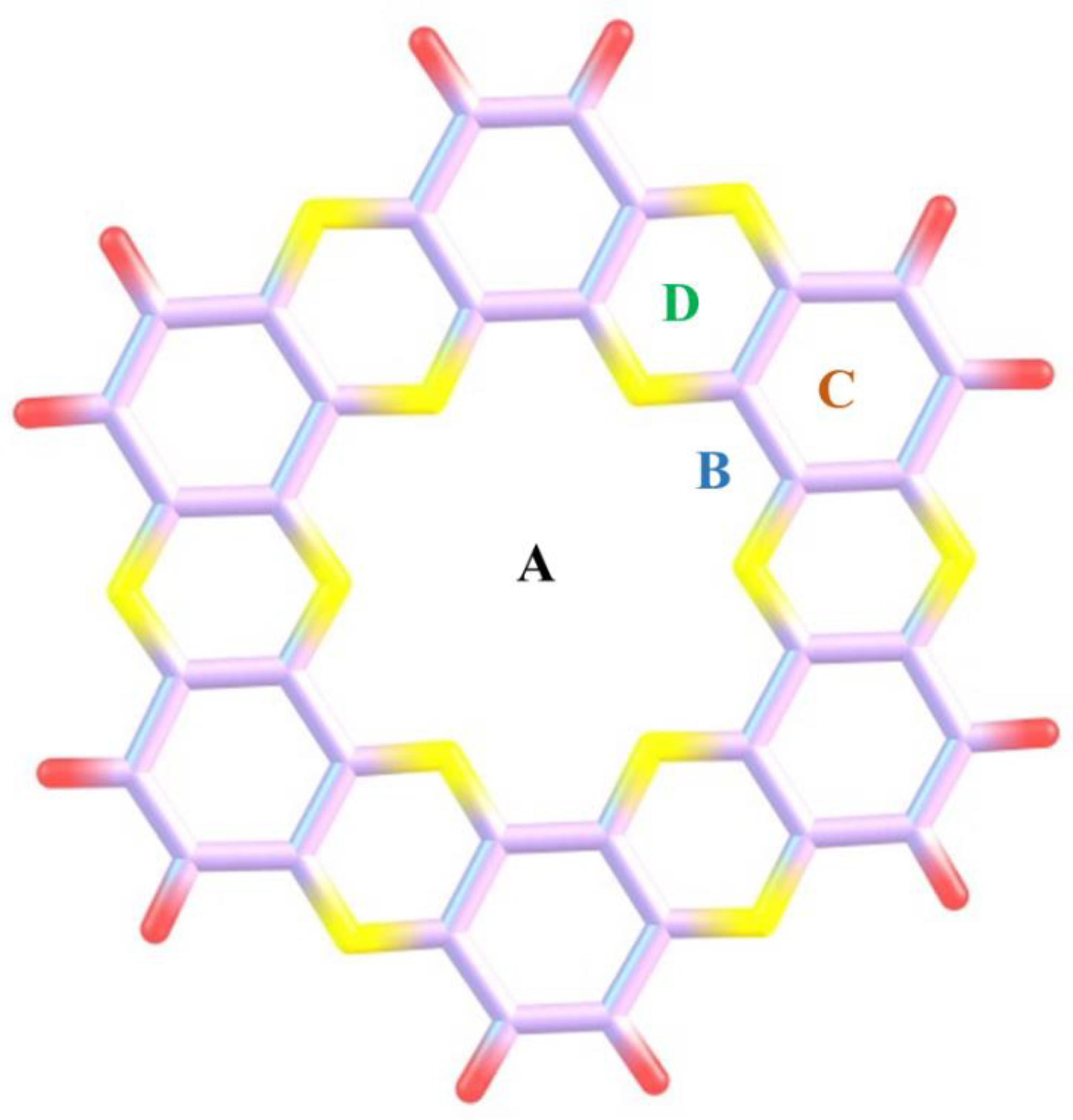
2. Computational Methodology
3. Results and Discussion
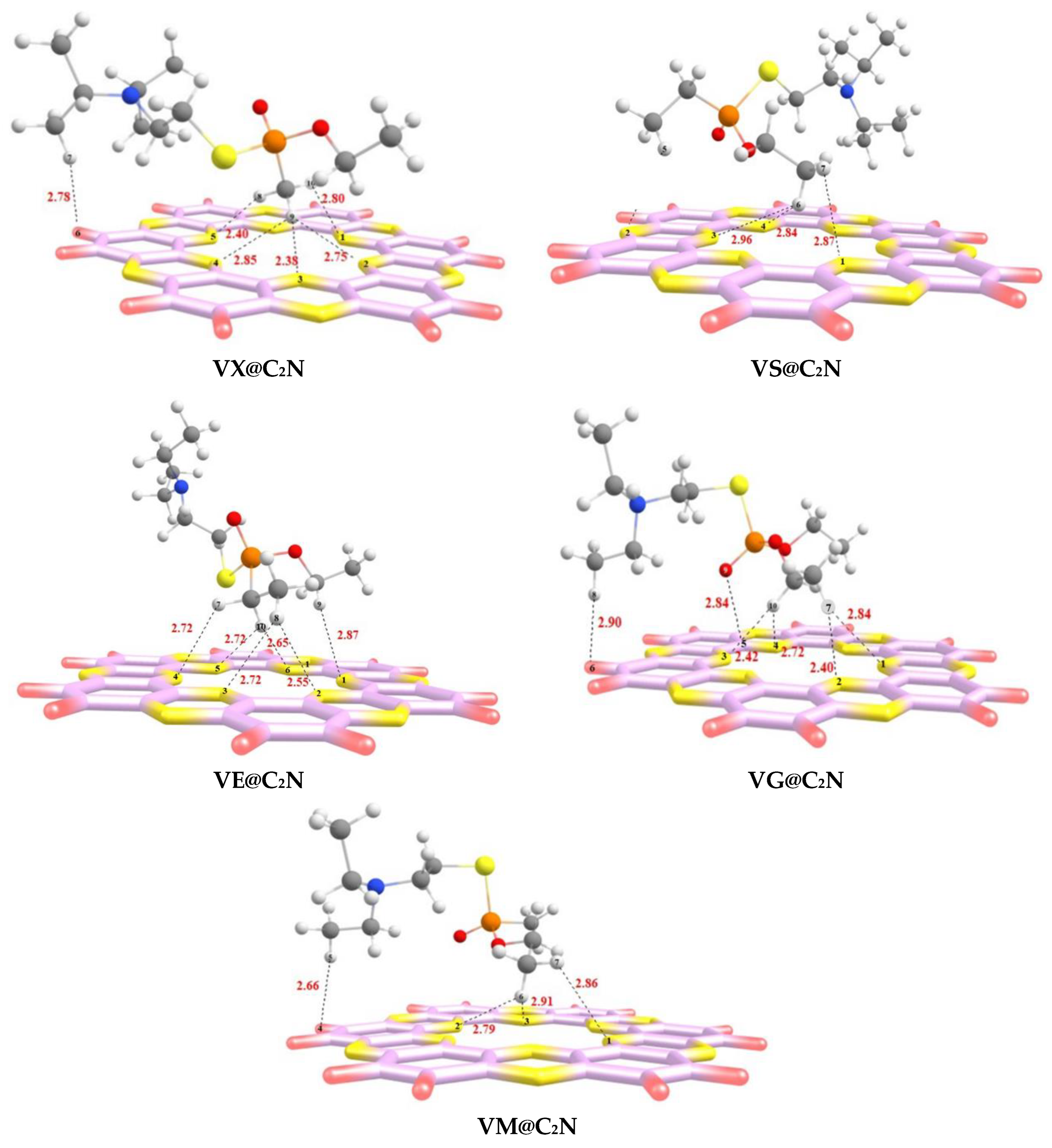
3.1. Geometric Optimization and Adsorption Energies
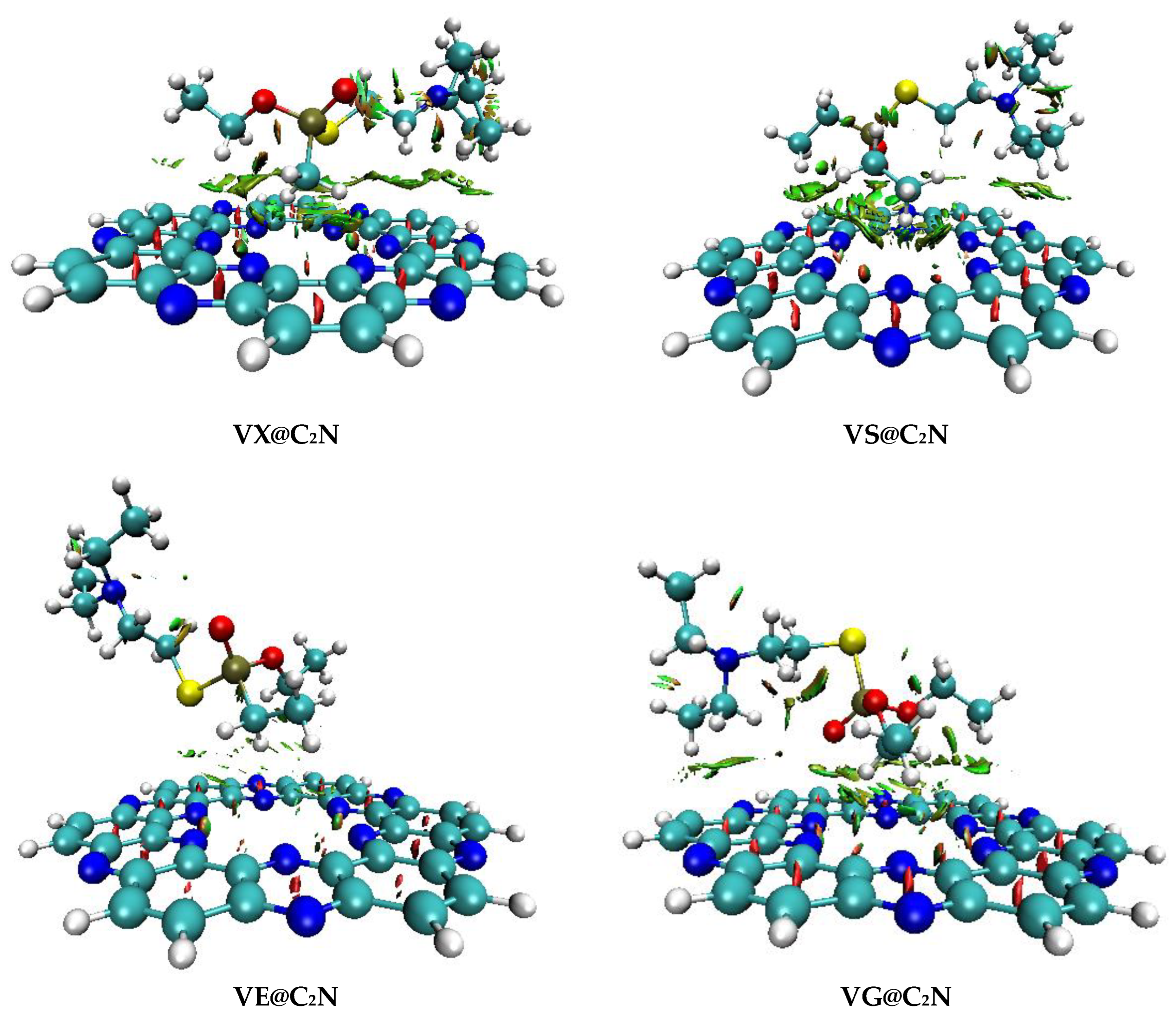
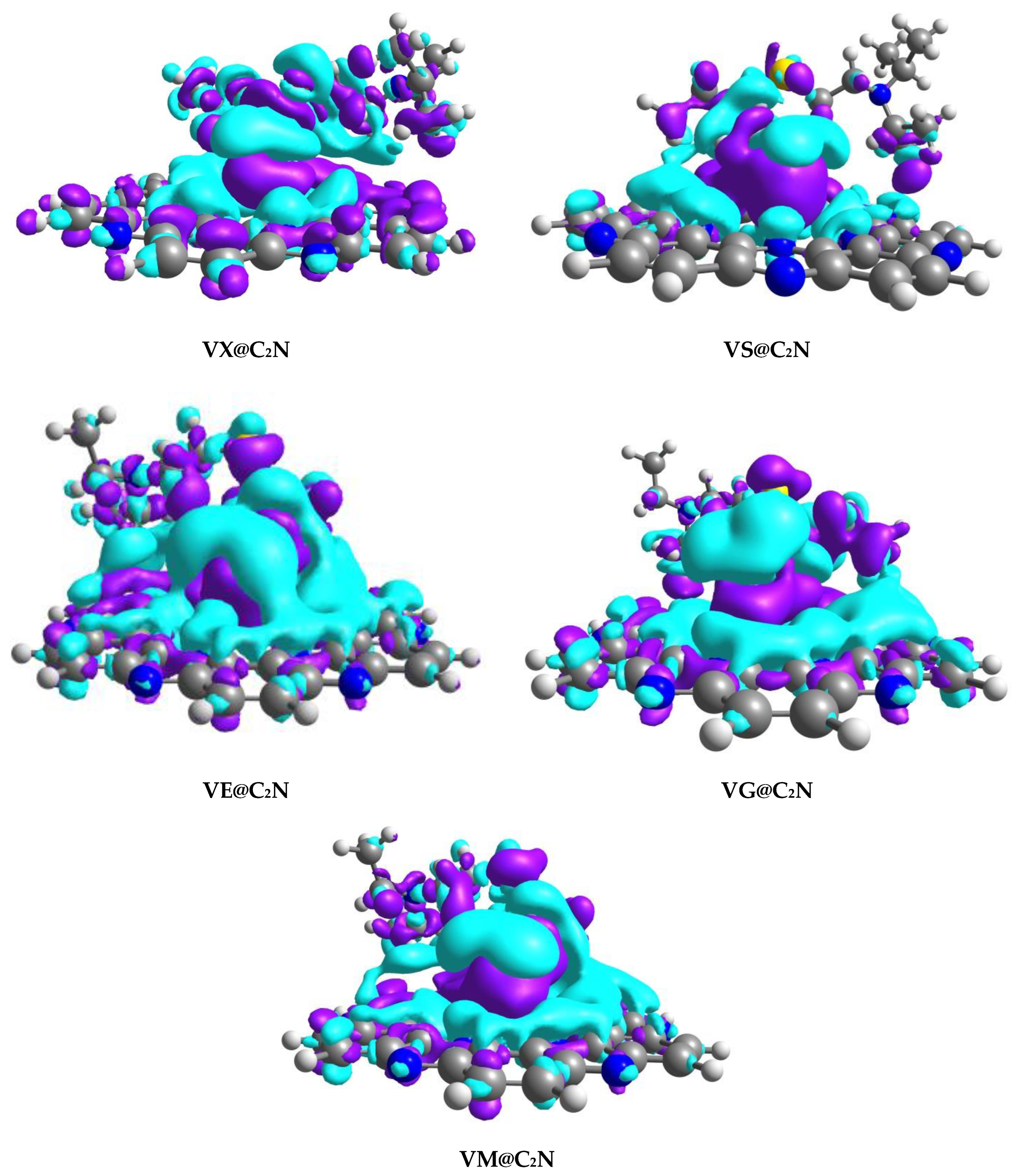
3.2. Non-Covalent Interactions (NCI) Analysis
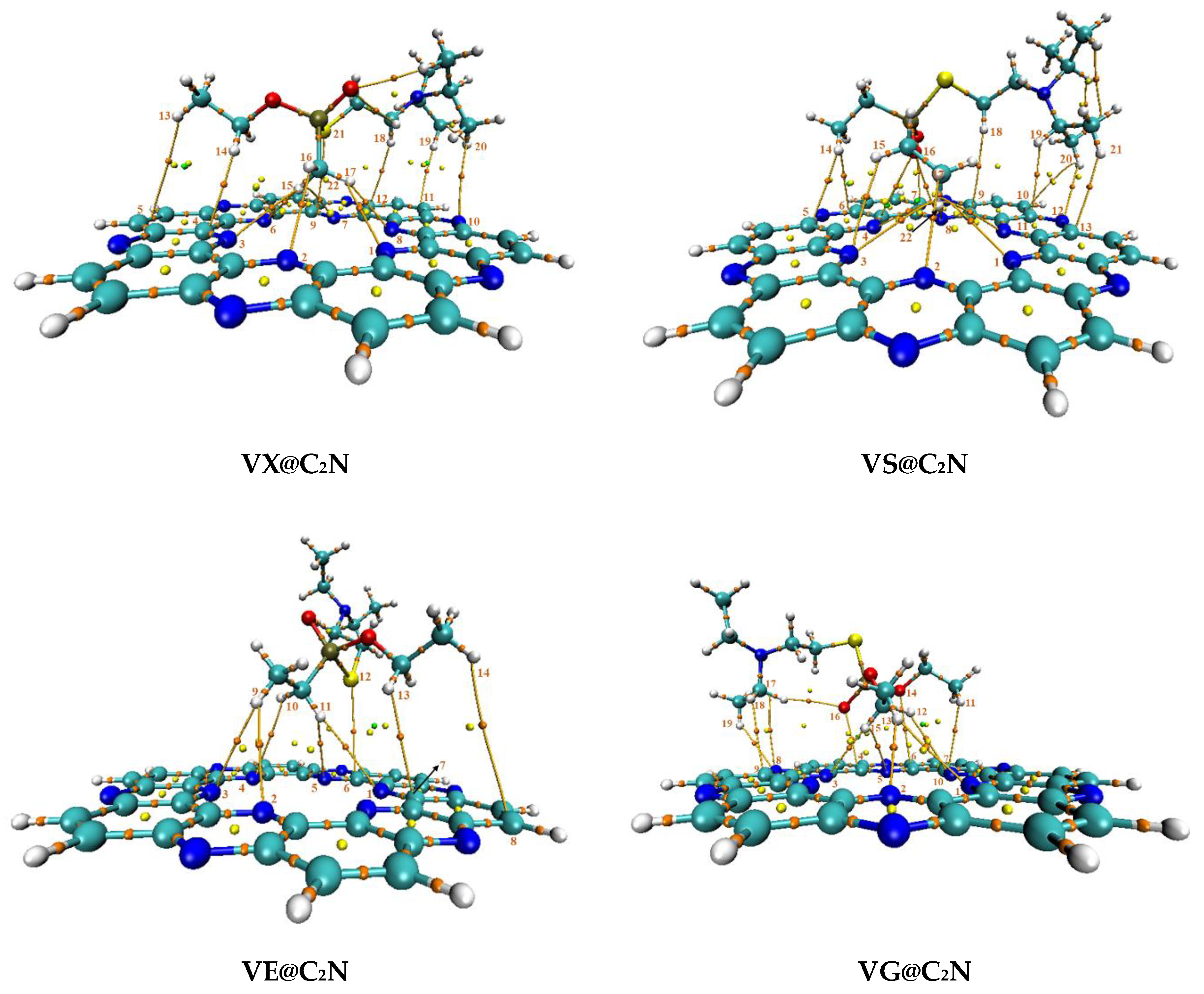
3.3. Quantum Theory of Atoms in Molecules (QTAIM) Analysis
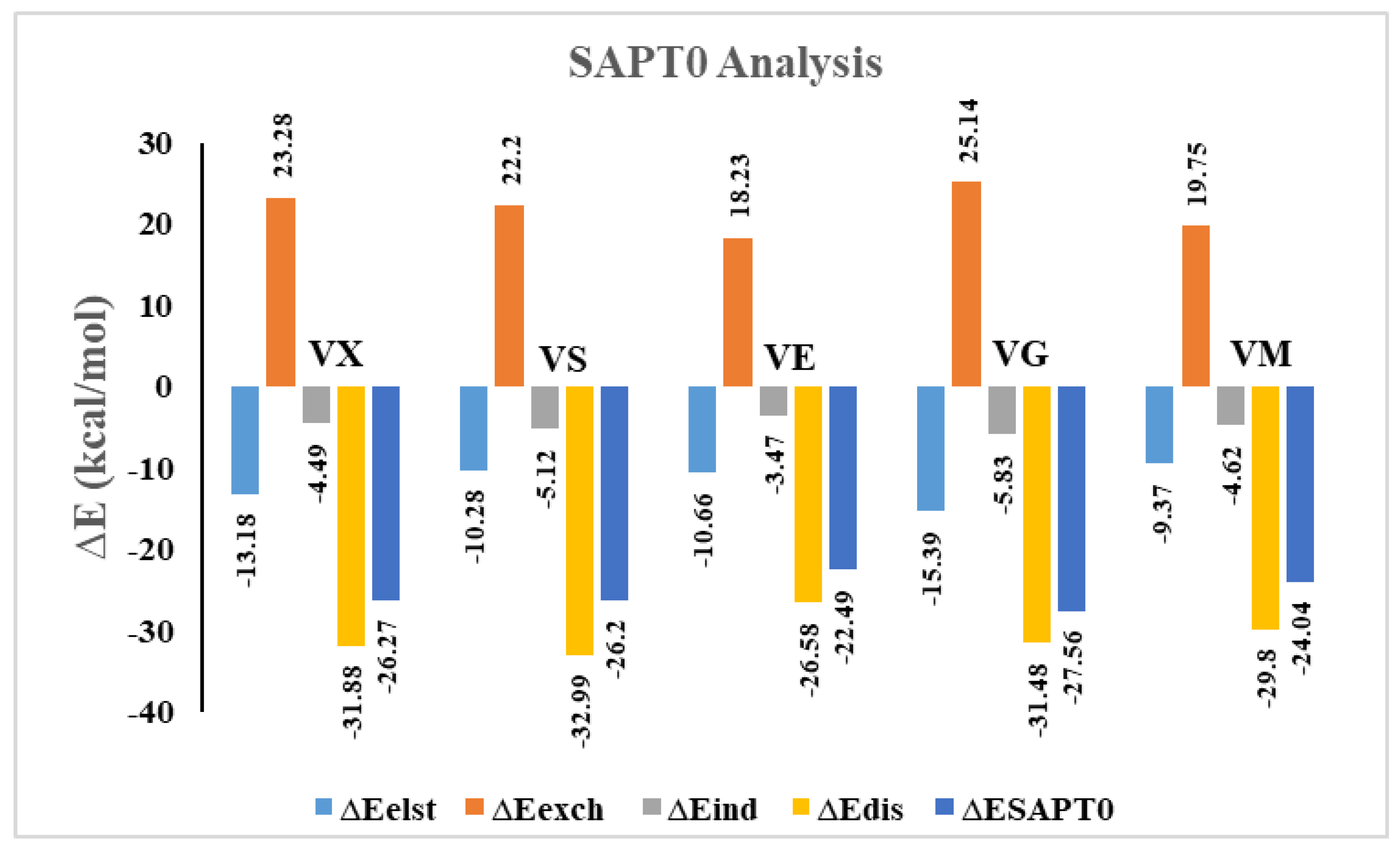
3.4. SAPT0 Analysis
4. Electronic Properties
4.1. Natural Bond Orbital (NBO) Analysis
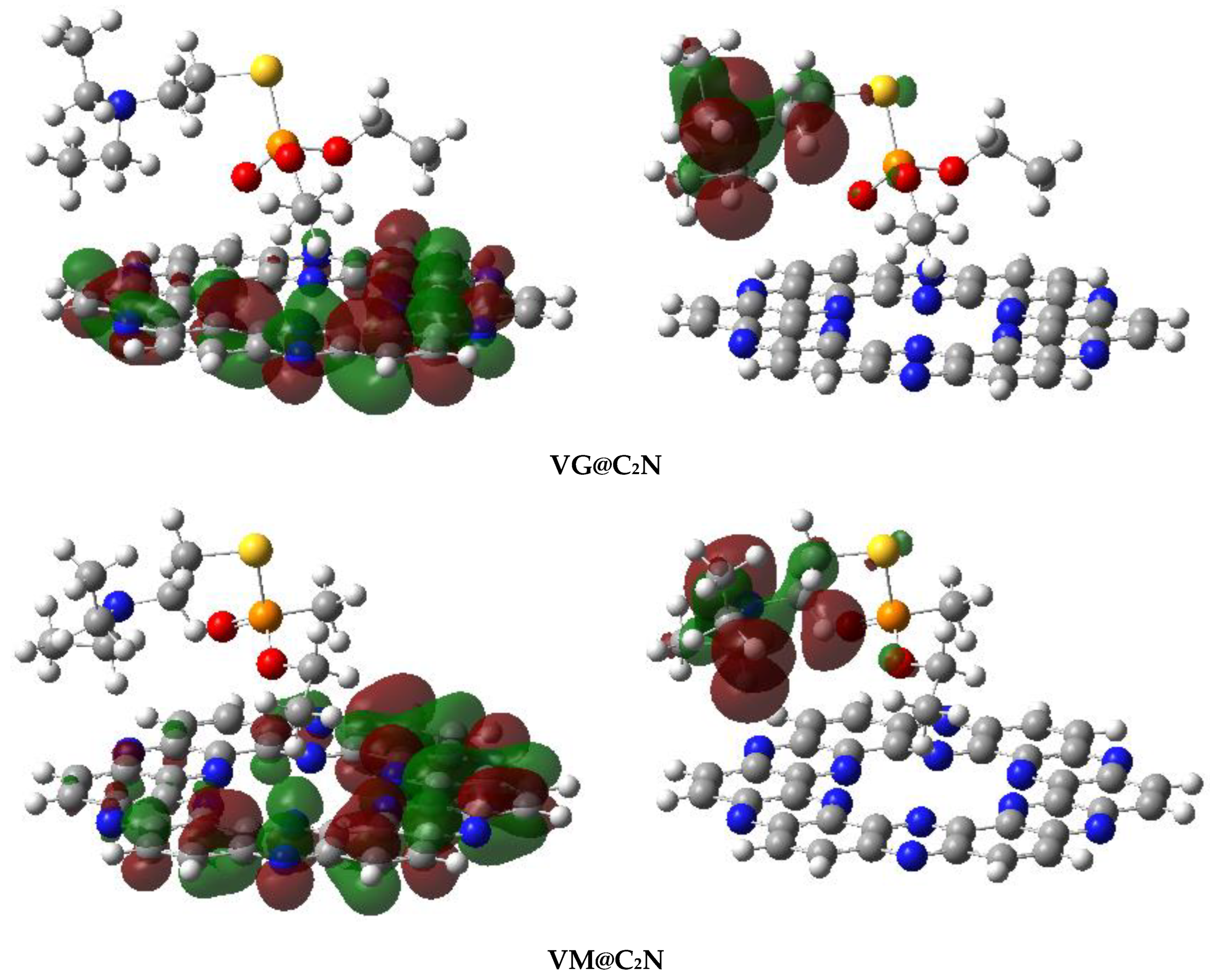
4.2. Electron Density Differences (EDD)
4.3. Frontier Molecular Orbital (FMO) Analysis
5. Recovery Time
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, W.; Sui, S.; Li, J.; Zong, L.; Li, D.; Xiao, Y.; Wang, L.; Zhu, H.; Shen, Y.; Jiang, Z. A fluorescent probe bearing two reactive groups discriminates between fluoride-containing G series and sulfur-containing V series nerve agents. Analyst 2020, 145, 5425–5429. [Google Scholar]
- Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Destruction and detection of chemical warfare agents. Chem. Rev. 2011, 111, 5345–5403. [Google Scholar] [CrossRef]
- Snyder, D.T.; Demond, P.S.; Szalwinski, L.J.; Dhummakupt, E.S.; McBride, E.M.; Cooks, R.G.; Glaros, T.; Mach, P.M. Two-dimensional MS/MS scans on a linear ion trap mass analyzer: Identification of V-series chemical warfare agents. Int. J. Mass Spectrom. 2019, 444, 116171. [Google Scholar] [CrossRef]
- Dennison, G.H.; Johnston, M.R. Mechanistic insights into the luminescent sensing of organophosphorus chemical warfare agents and simulants using trivalent lanthanide complexes. Chem. A Eur. J. 2015, 21, 6328–6338. [Google Scholar] [CrossRef]
- Marrs, T.C.; Rice, P.; Vale, J.A. The role of oximes in the treatment of nerve agent poisoning in civilian casualties. Toxicol. Rev. 2006, 25, 297–323. [Google Scholar] [CrossRef]
- Shamai Yamin, T.; Prihed, H.; Madmon, M.; Blanca, M.; Weissberg, A. Structural elucidation of V-type nerve agents by liquid chromatography/electrospray ionization mass spectrometry. J. Mass Spectrom. 2020, 55, e4617. [Google Scholar] [CrossRef]
- Rosman, Y.; Eisenkraft, A.; Milk, N.; Shiyovich, A.; Ophir, N.; Shrot, S.; Kreiss, Y.; Kassirer, M. Lessons learned from the Syrian sarin attack: Evaluation of a clinical syndrome through social media. Ann. Intern. Med. 2014, 160, 644–648. [Google Scholar] [CrossRef]
- Sulaiman, I.S.C.; Chieng, B.W.; Pojol, F.E.; Ong, K.K.; Rashid, J.I.A.; Yunus, W.M.Z.W.; Kasim, N.A.M.; Halim, N.A.; Noor, S.A.M.; Knight, V.F. A review on analysis methods for nerve agent hydrolysis products. Forensic Toxicol. 2020, 38, 297–313. [Google Scholar] [CrossRef]
- Tanimoto, T.; Oshima, Y.; Yuji, K.; Ozaki, A.; Kami, M. Organophosphate poisoning and terrorism. Malays. J. Med. Sci. 2017, 24, 111–112. [Google Scholar] [CrossRef]
- Stojiljković, M.P. Nerve agents–a clear and present danger to mankind. Scr. Med. 2019, 50, 109–111. [Google Scholar] [CrossRef]
- Salem, H.; Ternay Jr, A.L.; Smart, J.K. Brief history and use of chemical warfare agents in warfare and terrorism. In Chemical Warfare Agents; CRC Press: Boca Raton, FL, USA, 2019; pp. 3–15. [Google Scholar]
- Huo, D.; Yang, L.; Hou, C. Optical detection of dimethyl methyl-phosphonate with monosulfonate tetraphenyl porphyrin-plant-esterase complex. Sens. Lett. 2009, 7, 72–78. [Google Scholar] [CrossRef]
- Rowell, F.; Seviour, J.; Lim, A.Y.; Elumbaring-Salazar, C.G.; Loke, J.; Ma, J. Detection of nitro-organic and peroxide explosives in latent fingermarks by DART-and SALDI-TOF-mass spectrometry. Forensic Sci. Int. 2012, 221, 84–91. [Google Scholar] [CrossRef]
- Seto, Y.; Kanamori-Kataoka, M.; Komano, A.; Nagoya, T.; Sasano, R.; Matsuo, S. Gas chromatography-mass spectrometry with spiral large-volume injection for determination of fluoridated phosphonates produced by fluoride-mediated regeneration of nerve agent adduct in human serum. J. Chromatogr. A 2019, 1583, 108–116. [Google Scholar] [CrossRef]
- Dennison, G.H.; Bochet, C.G.; Curty, C.; Ducry, J.; Nielsen, D.J.; Sambrook, M.R.; Zaugg, A.; Johnston, M.R. Supramolecular agent–simulant correlations for the luminescence based detection of V-series chemical warfare agents with trivalent lanthanide complexes. Eur. J. Inorg. Chem. 2016, 2016, 1348–1358. [Google Scholar] [CrossRef]
- Song, Y.; Cooks, R.G. Reactive desorption electrospray ionization for selective detection of the hydrolysis products of phosphonate esters. J. Mass Spectrom. 2007, 42, 1086–1092. [Google Scholar] [CrossRef]
- Cinti, S.; Minotti, C.; Moscone, D.; Palleschi, G.; Arduini, F. Fully integrated ready-to-use paper-based electrochemical biosensor to detect nerve agents. Biosens. Bioelectron. 2017, 93, 46–51. [Google Scholar] [CrossRef]
- Takahashi, F.; Kazui, Y.; Miyaguchi, H.; Ohmori, T.; Tanaka, R.; Jin, J. Simple colorimetric screening of the nerve agent VX using gold nanoparticles and a hand-powered extraction device. Sens. Actuators B Chem. 2021, 327, 128902. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Y. Electrochemical stripping analysis of organophosphate pesticides and nerve agents. Electrochem. Commun. 2005, 7, 339–343. [Google Scholar]
- Privett, B.J.; Shin, J.H.; Schoenfisch, M.H. Electrochemical sensors. Anal. Chem. 2008, 80, 4499–4517. [Google Scholar] [CrossRef] [Green Version]
- Bobbitt, N.S.; Mendonca, M.L.; Howarth, A.J.; Islamoglu, T.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Metal–organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents. Chem. Soc. Rev. 2017, 46, 3357–3385. [Google Scholar] [CrossRef]
- Stassen, I.; Burtch, N.; Talin, A.; Falcaro, P.; Allendorf, M.; Ameloot, R. An updated roadmap for the integration of metal–organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 2017, 46, 3185–3241. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, B.; Liu, G.; Zhang, D. Electrochemical Sensors Based on Covalent Organic Frameworks: A Critical Review. Front. Chem. 2020, 8, 1082. [Google Scholar] [CrossRef]
- Alkhalifah, M.A.; Yar, M.; Bayach, I.; Sheikh, N.S.; Ayub, K. Covalent Organic Framework (C6N6) as a Drug Delivery Platform for Fluorouracil to Treat Cancerous Cells: A DFT Study. Materials 2022, 15, 7425. [Google Scholar] [CrossRef]
- Yar, M.; Hashmi, M.A.; Khan, A.; Ayub, K. Carbon nitride 2-D surface as a highly selective electrochemical sensor for V-series nerve agents. J. Mol. Liq. 2020, 311, 113357. [Google Scholar] [CrossRef]
- Islamoglu, T.; Chen, Z.; Wasson, M.C.; Buru, C.T.; Kirlikovali, K.O.; Afrin, U.; Mian, M.R.; Farha, O.K. Metal–organic frameworks against toxic chemicals. Chem. Rev. 2020, 120, 8130–8160. [Google Scholar] [CrossRef]
- Chen, L.; Wu, D.; Yoon, J. Recent advances in the development of chromophore-based chemosensors for nerve agents and phosgene. ACS Sens. 2018, 3, 27–43. [Google Scholar] [CrossRef] [Green Version]
- Royo, S.; Martínez-Máñez, R.; Sancenón, F.; Costero, A.M.; Parra, M.; Gil, S. Chromogenic and fluorogenic reagents for chemical warfare nerve agents’ detection. Chem. Commun. 2007, 46, 4839–4847. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Huo, D.; Zhao, Y.; Ma, N.; Hou, J.; Liu, M.; Shen, C.; Hou, C. A non-enzymatic electro-chemical sensor for organophosphorus nerve agents mimics and pesticides detection. Sens. Actuators B Chem. 2017, 252, 1118–1124. [Google Scholar]
- Medeiros, P.V.; Gueorguiev, G.; Stafström, S. Bonding, charge rearrangement and interface dipoles of benzene, graphene, and PAH molecules on Au (1 1 1) and Cu (1 1 1). Carbon 2015, 81, 620–628. [Google Scholar] [CrossRef]
- Kakanakova-Georgieva, A.; Giannazzo, F.; Nicotra, G.; Cora, I.; Gueorguiev, G.K.; Persson, P.O.; Pécz, B. Material proposal for 2D indium oxide. Appl. Surf. Sci. 2021, 548, 149275. [Google Scholar] [CrossRef]
- Mahmood, J.; Lee, E.K.; Jung, M.; Shin, D.; Jeon, I.-Y.; Jung, S.-M.; Choi, H.-J.; Seo, J.-M.; Bae, S.-Y.; Sohn, S.-D. Nitrogenated holey two-dimensional structures. Nat. Commun. 2015, 6, 6486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panigrahi, P.; Desai, M.; Talari, M.K.; Bae, H.; Lee, H.; Ahuja, R.; Hussain, T. Selective decoration of nitrogenated holey graphene (C2N) with titanium clusters for enhanced hydrogen storage application. Int. J. Hydrogen Energy 2021, 46, 7371–7380. [Google Scholar] [CrossRef]
- Tian, Z.; Fechler, N.; Oschatz, M.; Heil, T.; Schmidt, J.; Yuan, S.; Antonietti, M. C2NxO1−x framework carbons with defined microporosity and Co-doped functional pores. J. Mater. Chem. A 2018, 6, 19013–19019. [Google Scholar] [CrossRef]
- Ahsan, F.; Yar, M.; Gulzar, A.; Ayub, K. Therapeutic potential of C2N as targeted drug delivery system for fluorouracil and nitrosourea to treat cancer: A theoretical study. J. Nanostruct. Chem. 2022, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Ju, W.; Wang, J.; Yao, H.; Zhang, L.; Wang, J.; Li, Z. Molten salt synthesis of water-dispersible polymeric carbon nitride nanoseaweeds and their application as luminescent probes. Carbon 2016, 102, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Shinde, S.; Sami, A.; Lee, J.-H. Sulfur mediated graphitic carbon nitride/S-Se-graphene as a metal-free hybrid photocatalyst for pollutant degradation and water splitting. Carbon 2016, 96, 929–936. [Google Scholar] [CrossRef]
- Zhang, Y.; Antonietti, M. Photocurrent generation by polymeric carbon nitride solids: An initial step towards a novel photovoltaic system. Chem. Asian J. 2010, 5, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Mathew, S.C.; Möhlmann, L.; Antonietti, M.; Wang, X.; Blechert, S. Aerobic oxidative coupling of amines by carbon nitride photocatalysis with visible light. Angew. Chem. 2011, 123, 683–686. [Google Scholar] [CrossRef]
- Shah, A.B.; Sarfaraz, S.; Yar, M.; Sheikh, N.S.; Hammud, H.H.; Ayub, K. Remarkable Single Atom Catalyst of Transition Metal (Fe, Co & Ni) Doped on C2N Surface for Hydrogen Dissociation Reaction. Nanomaterials 2023, 13, 29. [Google Scholar]
- Qin, G.; Cui, Q.; Yun, B.; Sun, L.; Du, A.; Sun, Q. High capacity and reversible hydrogen storage on two dimensional C2N monolayer membrane. Int. J. Hydrogen Energy 2018, 43, 9895–9901. [Google Scholar] [CrossRef]
- Yar, M.; Shah, A.B.; Hashmi, M.A.; Ayub, K. Selective detection and removal of picric acid by C2N surface from a mixture of nitro-explosives. New J. Chem. 2020, 44, 18646–18655. [Google Scholar] [CrossRef]
- Yar, M.; Hashmi, M.A.; Ayub, K. Nitrogenated holey graphene (C2N) surface as highly selective electrochemical sensor for ammonia. J. Mol. Liq. 2019, 296, 111929. [Google Scholar] [CrossRef]
- Panigrahi, P.; Sajjad, M.; Singh, D.; Hussain, T.; Andreas Larsson, J.; Ahuja, R.; Singh, N. Two-dimensional Nitrogenated Holey Graphene (C2N) monolayer based glucose sensor for diabetes mellitus. Appl. Surf. Sci. 2022, 573, 151579. [Google Scholar] [CrossRef]
- Sajjad, M.; Hussain, T.; Singh, N.; Larsson, J.A. Superior Anchoring of Sodium Polysulfides to the Polar C2N 2D Material: A Potential Electrode Enhancer in Sodium–Sulfur Batteries. Langmuir 2020, 36, 13104–13111. [Google Scholar] [CrossRef]
- Yong, Y.; Cui, H.; Zhou, Q.; Su, X.; Kuang, Y.; Li, X. C2N monolayer as NH3 and NO sensors: A DFT study. Appl. Surf. Sci. 2019, 487, 488–495. [Google Scholar] [CrossRef]
- Yar, M.; Ahsan, F.; Gulzar, A.; Ayub, K. Adsorption and sensor applications of C2N surface for G-series and mustard series chemical warfare agents. Microporous Mesoporous Mater. 2021, 317, 110984. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Yar, M.; Khan, A.A.; Ahmad, R.; Ayub, K. DFT investigation of adsorption of nitro-explosives over C2N surface: Highly selective towards trinitro benzene. J. Mol. Liq. 2022, 352, 118652. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Deshpande, M.D.; Hussain, T.; Ahuja, R. Investigating CO2 storage properties of C2N monolayer functionalized with small metal clusters. J. CO2 Util. 2020, 35, 1–13. [Google Scholar] [CrossRef]
- Yar, M.; Ayub, K. Expanding the horizons of covalent organic frameworks to electrochemical sensors; A case study of CTF-FUM. Microporous Mesoporous Mater. 2020, 300, 110146. [Google Scholar] [CrossRef]
- Burns, L.A.; Mayagoitia, Á.V.; Sumpter, B.G.; Sherrill, C.D. Density-functional approaches to noncovalent interactions: A comparison of dispersion corrections (DFT-D), exchange-hole dipole moment (XDM) theory, and specialized functionals. J. Chem. Phys. 2011, 134, 084107. [Google Scholar] [CrossRef]
- Sajid, H.; Mahmood, T.; Ayub, K. High sensitivity of polypyrrole sensor for uric acid over urea, acetamide and sulfonamide: A density functional theory study. Synth. Met. 2018, 235, 49–60. [Google Scholar] [CrossRef]
- Blanco, F.; Alkorta, I.; Rozas, I.; Solimannejad, M.; Elguero, J. A theoretical study of the interactions of NF3 with neutral ambidentate electron donor and acceptor molecules. Phys. Chem. Chem. Phys. 2011, 13, 674–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munsif, S.; Ayub, K. Permeability and storage ability of inorganic X12Y12 fullerenes for lithium atom and ion. Chem. Phys. Lett. 2018, 698, 51–59. [Google Scholar] [CrossRef]
- Wasim, F.; Mahmood, T.; Ayub, K. An accurate cost effective DFT approach to study the sensing behaviour of polypyrrole towards nitrate ions in gas and aqueous phases. Phys. Chem. Chem. Phys. 2016, 18, 19236–19247. [Google Scholar] [CrossRef]
- Yar, M.; Hashmi, M.A.; Ayub, K. The C2N surface as a highly selective sensor for the detection of nitrogen iodide from a mixture of NX3 (X = Cl, Br, I) explosives. RSC Adv. 2020, 10, 31997–32010. [Google Scholar] [CrossRef]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of Density Functionals by Combining the Method of Constraint Satisfaction with Parametrization for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Shah, A.-u.-H.A.; Bilal, S.; Ayub, K. DFT study of polyaniline NH3, CO2, and CO gas sensors: Comparison with recent experimental data. J. Phys. Chem. C 2013, 117, 23701–23711. [Google Scholar] [CrossRef]
- Tirado-Rives, J.; Jorgensen, W.L. Performance of B3LYP density functional methods for a large set of organic molecules. J. Chem. Theory Comput. 2008, 4, 297–306. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Kondratowicz, M.; Lis, T.; Chmielewski, P.J.; Cybińska, J.; Zafra, J.L.; Casado, J.; Vives, T.; Crassous, J.; Favereau, L. Lemniscular [16] cycloparaphenylene: A radially conjugated figure-eight aromatic molecule. J. Am. Chem. Soc. 2019, 141, 7421–7427. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Yar, M.; Ayub, K. Covalent triazine framework (CTF-0) surface as a smart sensing material for the detection of CWAs and industrial pollutants. Mater. Sci. Semicond. Process. 2022, 139, 106334. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Yar, M.; Ans, M.; Gilani, M.A.; Ludwig, R.; Hashmi, M.A.; Hussain, M.; Muhammad, S.; Ayub, K. Computational investigation of a covalent triazine framework (CTF-0) as an efficient electrochemical sensor. RSC Adv. 2022, 12, 3909–3923. [Google Scholar] [CrossRef]
- Sajid, H.; Ayub, K.; Mahmood, T. A comprehensive DFT study on the sensing abilities of cyclic oligothiophenes (n CTs). New J. Chem. 2019, 43, 14120–14133. [Google Scholar] [CrossRef]
- Hussain, R.; Saeed, M.; Mehboob, M.Y.; Khan, S.U.; Khan, M.U.; Adnan, M.; Ahmed, M.; Iqbal, J.; Ayub, K. Density functional theory study of palladium cluster adsorption on a graphene support. RSC Adv. 2020, 10, 20595–20607. [Google Scholar] [CrossRef]
- Jadoon, T.; Mahmood, T.; Ayub, K. Silver-graphene quantum dots based electrochemical sensor for trinitrotoluene and p-nitrophenol. J. Mol. Liq. 2020, 306, 112878. [Google Scholar] [CrossRef]
- Khan, S.; Sajid, H.; Ayub, K.; Mahmood, T. Adsorption behaviour of chronic blistering agents on graphdiyne; excellent correlation among SAPT, reduced density gradient (RDG) and QTAIM analyses. J. Mol. Liq. 2020, 316, 113860. [Google Scholar] [CrossRef]
- Khan, S.; Sajid, H.; Ayub, K.; Mahmood, T. High sensitivity of graphdiyne nanoflake toward detection of phosgene, thiophosgene and phosogenoxime; a first-principles study. J. Mol. Graph. Model. 2020, 100, 107658. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Ahmad, R.; Ahmad, I. Density functional theory study of emerging pollutants removal from water by covalent triazine based framework. J. Mol. Liq. 2020, 309, 113008. [Google Scholar] [CrossRef]
- Farooqi, B.A.; Yar, M.; Ashraf, A.; Farooq, U.; Ayub, K. Graphene-polyaniline composite as superior electrochemical sensor for detection of cyano explosives. Eur. Polym. J. 2020, 138, 109981. [Google Scholar] [CrossRef]
- Dennison, G.H.; Sambrook, M.R.; Johnston, M.R. VX and VG chemical warfare agents bidentate complexation with lanthanide ions. Chem. Commun. 2013, 50, 195–197. [Google Scholar] [CrossRef]
- Mushtaq, M.; Khan, S.; Tit, N. Magnetization effect of Mn-embedded in C2N on hydrogen adsorption and gas-sensing properties: Ab-initio analysis. Appl. Surf. Sci. 2021, 537, 147970. [Google Scholar] [CrossRef]
- Zhou, X.; Han, J. Design and simulation of C2N based solar cell by SCAPS-1D software. Mater. Res. Express 2020, 7, 126303. [Google Scholar] [CrossRef]
- Sajid, H.; Mahmood, T.; Ayub, K. An accurate comparative theoretical study of the interaction of furan, pyrrole, and thiophene with various gaseous analytes. J. Mol. Model. 2017, 23, 295. [Google Scholar] [CrossRef] [PubMed]
- Sajid, H.; Ullah, F.; Ayub, K.; Mahmood, T. Cyclic versus straight chain oligofuran as sensor: A detailed DFT study. J. Mol. Graph. Model. 2020, 97, 107569. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. Chemical bonds without bonding electron density—Does the difference electron-density analysis suffice for a description of the chemical bond? Angew. Chem. Int. Ed. Engl. 1984, 23, 627–628. [Google Scholar] [CrossRef]
- Yuan, K.; Liu, C.; Han, J.; Yu, G.; Wang, J.; Duan, H.; Wang, Z.; Jian, X. Phthalazinone structure-based covalent triazine frameworks and their gas adsorption and separation properties. RSC Adv. 2016, 6, 12009–12020. [Google Scholar] [CrossRef]
- Mata, I.; Alkorta, I.; Espinosa, E.; Molins, E. Relationships between interaction energy, intermolecular distance and electron density properties in hydrogen bonded complexes under external electric fields. Chem. Phys. Lett. 2011, 507, 185–189. [Google Scholar] [CrossRef]
- Pastorczak, E.; Corminboeuf, C. Perspective: Found in translation: Quantum chemical tools for grasping non-covalent interactions. J. Chem. Phys. 2017, 146, 120901. [Google Scholar] [CrossRef] [Green Version]
- Varadwaj, A.; Marques, H.M.; Varadwaj, P.R. Is the fluorine in molecules dispersive? Is molecular electrostatic potential a valid property to explore fluorine-centered non-covalent interactions? Molecules 2019, 24, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rad, A.S.; Ayub, K. O3 and SO2 sensing concept on extended surface of B12N12 nanocages modified by Nickel decoration: A comprehensive DFT study. Solid State Sci. 2017, 69, 22–30. [Google Scholar] [CrossRef]
- Arshad, M.N.; Faidallah, H.M.; Asiri, A.M.; Kosar, N.; Mahmood, T. Structural, spectroscopic and nonlinear optical properties of sulfonamide derivatives; experimental and theoretical study. J. Mol. Struct. 2020, 1202, 127393. [Google Scholar] [CrossRef]
- Peng, S.; Cho, K.; Qi, P.; Dai, H. Ab initio study of CNT NO2 gas sensor. Chem. Phys. Lett. 2004, 387, 271–276. [Google Scholar] [CrossRef]
- Bai, L.; Zhou, Z. Computational study of B-or N-doped single-walled carbon nanotubes as NH3 and NO2 sensors. Carbon 2007, 45, 2105–2110. [Google Scholar] [CrossRef]
- Hosseinian, A.; Asadi, Z.; Edjlali, L.; Bekhradnia, A.; Vessally, E. NO2 sensing properties of a borazine doped nanographene: A DFT study. Comput. Theor. Chem. 2017, 1106, 36–42. [Google Scholar] [CrossRef]
- Khan, S.; Yar, M.; Kosar, N.; Ayub, K.; Arshad, M.; Zahid, M.N.; Mahmood, T. First-principles study for exploring the adsorption behavior of G-series nerve agents on graphdyine surface. Comput. Theor. Chem. 2020, 1191, 113043. [Google Scholar] [CrossRef]
- Ma, D.; Ju, W.; Li, T.; Zhang, X.; He, C.; Ma, B.; Lu, Z.; Yang, Z. The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 2016, 383, 98–105. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, J.; Li, X.; He, C.; Lu, Z.; Lu, Z.; Yang, Z.; Wang, Y. C3N monolayers as promising candidates for NO2 sensors. Sens. Actuators B Chem. 2018, 266, 664–673. [Google Scholar] [CrossRef]
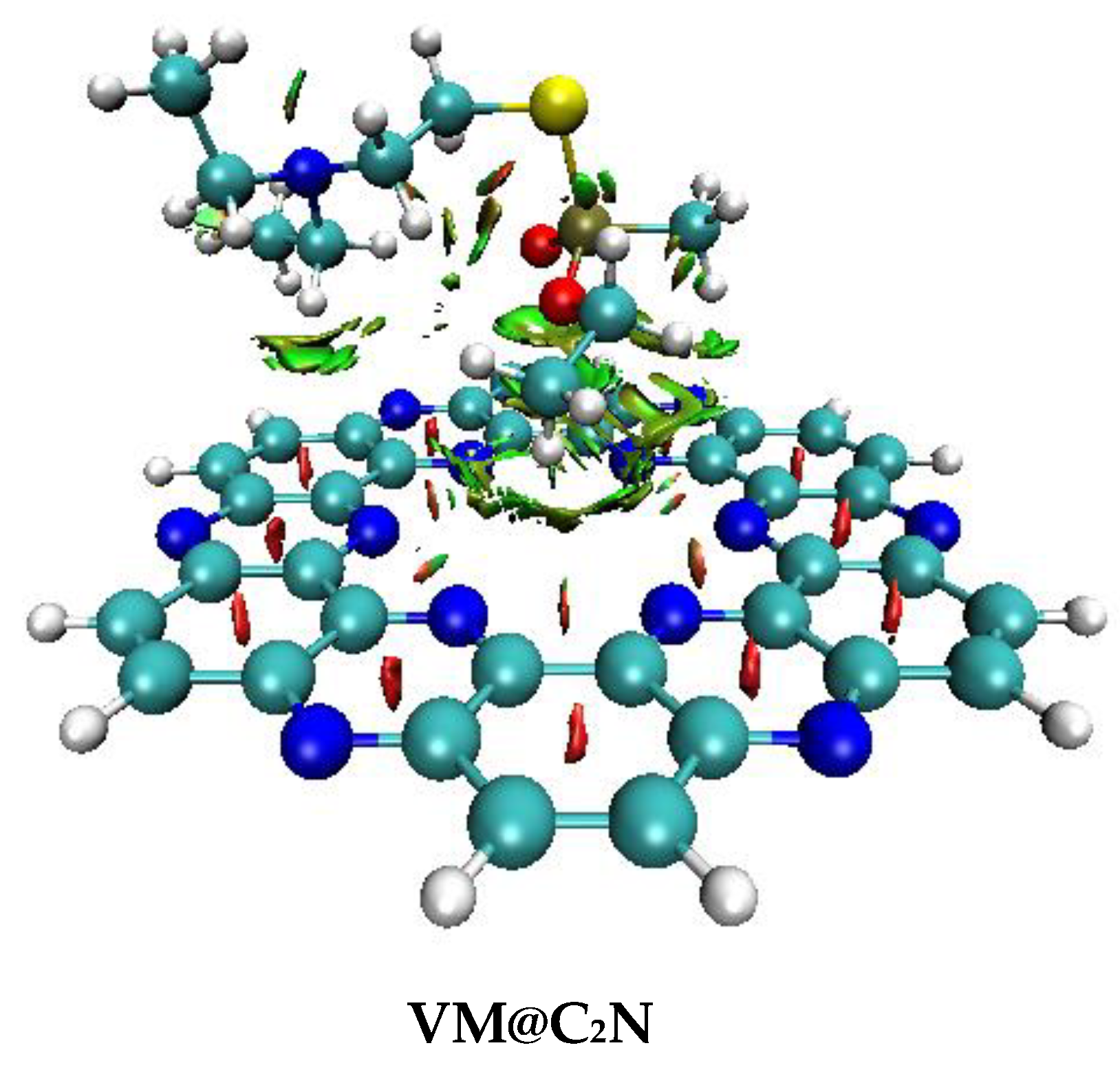
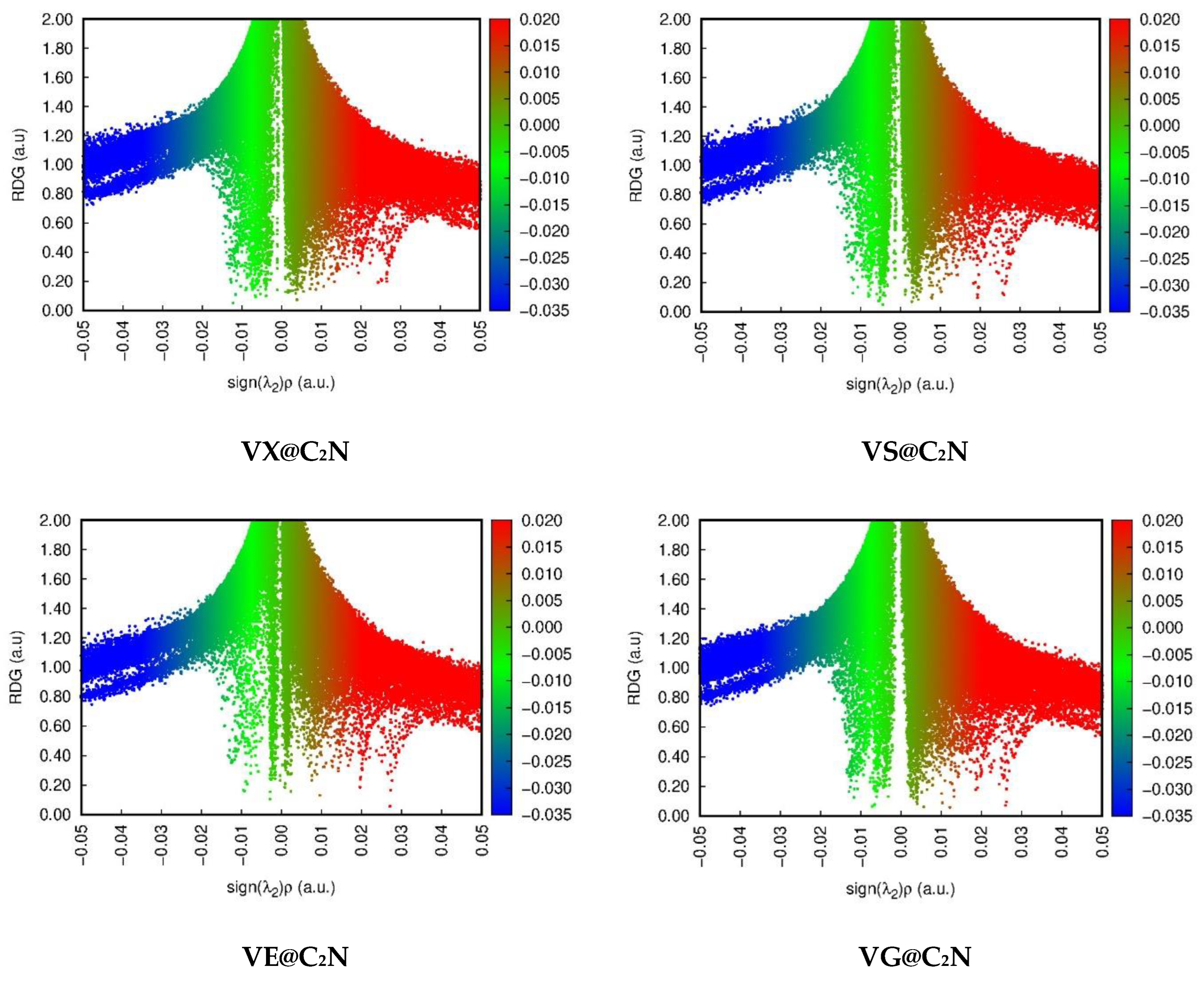
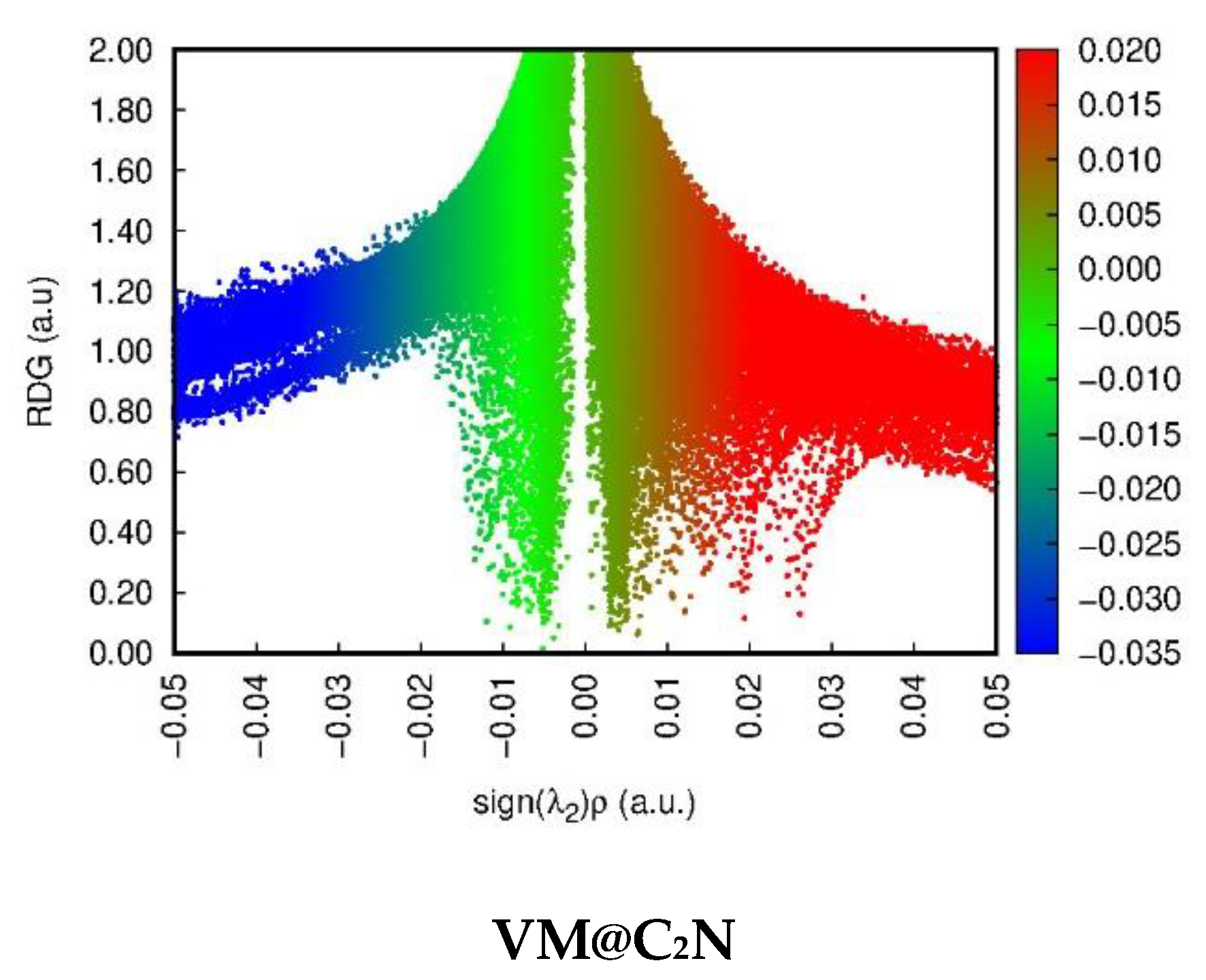
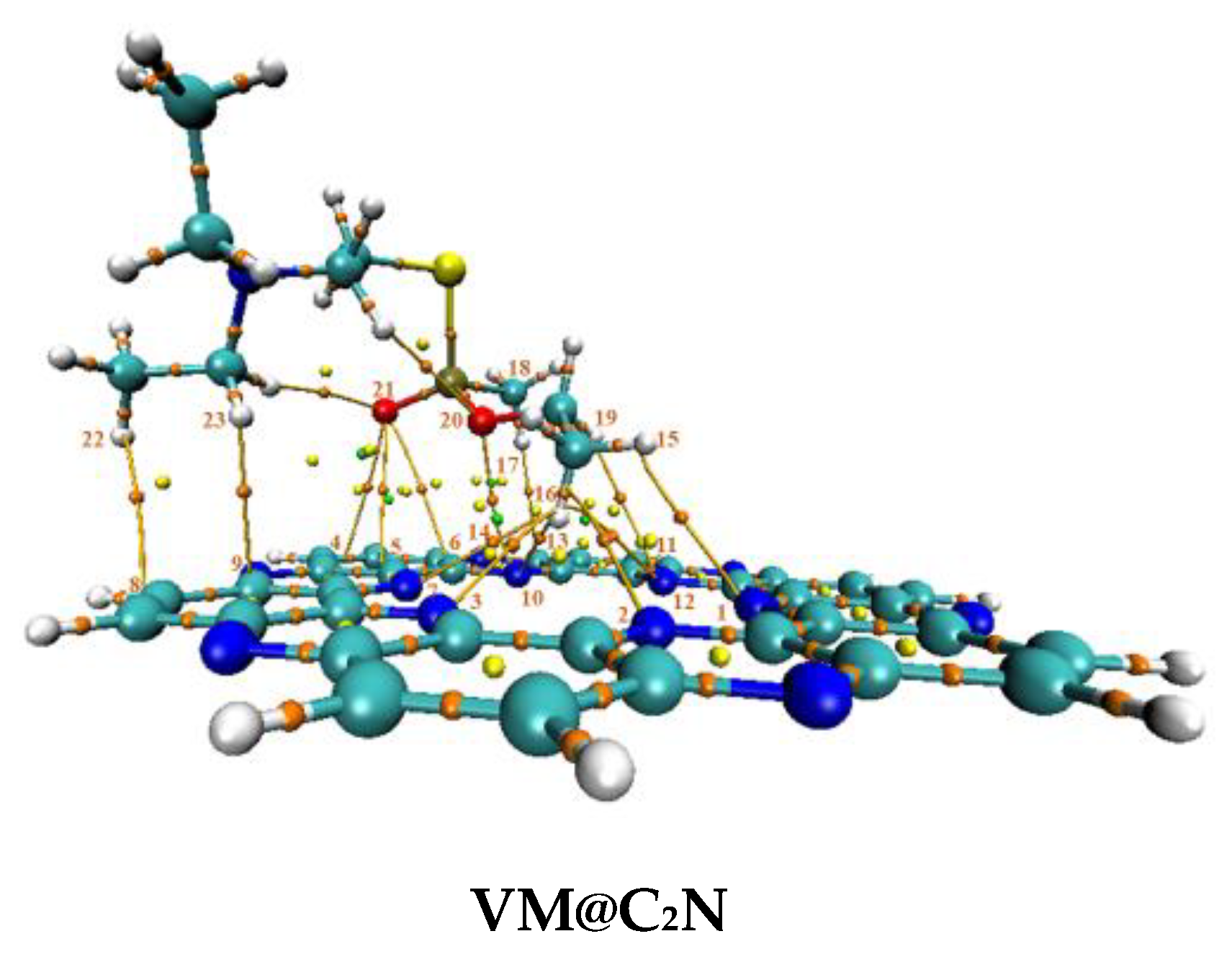

| Nitro@C2N | |||
|---|---|---|---|
| Complex | Intermolecular Bond | Bond Length (Å) | Eint (kcal/mol) |
| VX@C2N | H10--N1 | 2.80 | −17.64 |
| H9--N2 | 2.75 | ||
| H9--N3 | 2.38 | ||
| H9--N4 | 2.85 | ||
| H8--N5 | 2.40 | ||
| H7--H6 | 2.78 | ||
| VS@C2N | H7--N1 | 2.87 | −12.93 |
| H6--N3 | 2.96 | ||
| H6--N4 | 2.84 | ||
| H5--N2 | 2.99 | ||
| VE@C2N | H9--N1 | 2.87 | −13.97 |
| H8--N2 | 2.55 | ||
| H8--N3 | 2.72 | ||
| H10--N5 | 2.72 | ||
| H10--N6 | 2.65 | ||
| H7--N4 | 2.72 | ||
| VG@C2N | H7--N1 | 2.84 | −17.81 |
| H7--N2 | 2.40 | ||
| H10--N3 | 2.42 | ||
| H10--N4 | 2.72 | ||
| H8--H6 | 2.90 | ||
| O9--C5 | 2.84 | ||
| VM@C2N | H7--N1 | 2.86 | −17.69 |
| H6--N2 | 2.79 | ||
| H6--N3 | 2.91 | ||
| H5--H4 | 2.66 | ||
| Analyte---C2N | ρ (a.u) | ∇2ρ (a.u) | G (r) (a.u) | V(r) (a.u) | H(r) (a.u) | −V/G | Eint (kcal/mol) |
|---|---|---|---|---|---|---|---|
| VX@C2N | |||||||
| H19---C11 | 0.007 | 0.022 | 0.004 | −0.0035 | 0.0010 | 0.87 | −1.10 |
| H17---N1 | 0.012 | 0.037 | 0.008 | −0.0077 | 0.0007 | 0.96 | −2.41 |
| H20---N10 | 0.007 | 0.022 | 0.005 | −0.0037 | 0.0010 | 0.74 | −1.16 |
| H16---N2 | 0.006 | 0.020 | 0.004 | −0.0031 | 0.0009 | 0.77 | −0.97 |
| H15---N6 | 0.006 | 0.022 | 0.004 | −0.0035 | 0.0010 | 0.87 | −1.10 |
| H15---N3 | 0.011 | 0.036 | 0.008 | −0.0073 | 0.0009 | 0.91 | −2.29 |
| H17---N8 | 0.006 | 0.021 | 0.004 | −0.0032 | 0.0010 | 0.80 | −1.00 |
| H14---C4 | 0.004 | 0.011 | 0.002 | −0.0017 | 0.0006 | 0.85 | −0.53 |
| H13---C5 | 0.005 | 0.017 | 0.004 | −0.0021 | 0.0008 | 0.52 | −0.66 |
| S21---C22 | 0.008 | 0.023 | 0.005 | −0.0037 | 0.0010 | 0.74 | −1.16 |
| H18---C12 | 0.005 | 0.015 | 0.003 | −0.0023 | 0.0008 | 0.77 | −0.72 |
| VS@C2N | |||||||
| H20---C10 | 0.003 | 0.012 | 0.002 | −0.0018 | 0.0006 | 0.90 | −0.56 |
| H22---N1 | 0.004 | 0.014 | 0.003 | −0.0019 | 0.0007 | 0.63 | −0.59 |
| H22---N3 | 0.005 | 0.019 | 0.004 | −0.0029 | 0.0009 | 0.72 | −0.91 |
| H20---N12 | 0.005 | 0.018 | 0.004 | −0.0029 | 0.0008 | 0.72 | −0.91 |
| H22---N4 | 0.005 | 0.019 | 0.004 | −0.0028 | 0.0009 | 0.70 | −0.88 |
| H22---N8 | 0.006 | 0.021 | 0.004 | −0.0032 | 0.0010 | 0.80 | −1.00 |
| H22---N11 | 0.005 | 0.019 | 0.004 | −0.0029 | 0.0009 | 0.72 | −0.91 |
| H19---C10 | 0.004 | 0.014 | 0.003 | −0.0020 | 0.0008 | 0.67 | −0.62 |
| H18---C9 | 0.004 | 0.013 | 0.003 | −0.0018 | 0.0007 | 0.60 | −0.56 |
| O16---N8 | 0.008 | 0.026 | 0.006 | −0.0049 | 0.0009 | 0.82 | −1.54 |
| H14---C5 | 0.005 | 0.017 | 0.003 | −0.0027 | 0.0008 | 0.90 | −0.85 |
| H14---C6 | 0.005 | 0.017 | 0.003 | −0.0027 | 0.0008 | 0.90 | −0.85 |
| H21---C13 | 0.003 | 0.012 | 0.002 | −0.0018 | 0.0006 | 0.90 | −0.56 |
| O16---N4 | 0.009 | 0.032 | 0.007 | −0.0067 | 0.0007 | 0.96 | −2.10 |
| O16---C7 | 0.008 | 0.029 | 0.006 | −0.0055 | 0.0009 | 0.92 | −1.72 |
| VE@C2N | |||||||
| H9---N3 | 0.002 | 0.008 | 0.002 | −0.0011 | 0.0005 | 0.55 | −0.34 |
| H9---N2 | 0.002 | 0.008 | 0.002 | −0.0010 | 0.0005 | 0.50 | −0.31 |
| S12---C6 | 0.003 | 0.009 | 0.002 | −0.0011 | 0.0005 | 0.55 | −0.34 |
| H10---N4 | 0.002 | 0.008 | 0.002 | −0.0011 | 0.0005 | 0.55 | −0.34 |
| H11---N1 | 0.003 | 0.010 | 0.002 | −0.0014 | 0.0006 | 0.70 | −0.44 |
| H11---N5 | 0.003 | 0.010 | 0.002 | −0.0014 | 0.0006 | 0.70 | −0.44 |
| VG@C2N | |||||||
| H12---N10 | 0.007 | 0.025 | 0.005 | −0.0040 | 0.0010 | 0.80 | −1.25 |
| O14---C6 | 0.004 | 0.014 | 0.003 | −0.0023 | 0.0007 | 0.77 | −0.72 |
| O16---C4 | 0.007 | 0.025 | 0.005 | −0.0040 | 0.0011 | 0.80 | −1.25 |
| H13---N1 | 0.011 | 0.033 | 0.008 | −0.0070 | 0.0007 | 0.87 | −2.19 |
| H15---N3 | 0.011 | 0.036 | 0.008 | −0.0073 | 0.0008 | 0.91 | −2.29 |
| H15---N5 | 0.005 | 0.018 | 0.004 | −0.0030 | 0.0008 | 0.75 | −0.94 |
| H13---N2 | 0.006 | 0.020 | 0.004 | −0.0034 | 0.0008 | 0.85 | −1.07 |
| H11---C7 | 0.006 | 0.019 | 0.004 | −0.0029 | 0.0010 | 0.72 | −0.91 |
| H18---C9 | 0.004 | 0.014 | 0.003 | −0.0021 | 0.0007 | 0.70 | −0.66 |
| H17---N8 | 0.006 | 0.020 | 0.004 | −0.0035 | 0.0008 | 0.87 | −1.10 |
| H19---N8 | 0.004 | 0.014 | 0.003 | −0.0023 | 0.0006 | 0.77 | −0.72 |
| VM@C2N | |||||||
| O21---C6 | 0.005 | 0.018 | 0.004 | −0.0031 | 0.0008 | 0.77 | −0.97 |
| H16---N12 | 0.005 | 0.017 | 0.003 | −0.0025 | 0.0008 | 0.83 | −0.78 |
| O21---C4 | 0.005 | 0.017 | 0.004 | −0.0029 | 0.0007 | 0.72 | −0.91 |
| O21---C5 | 0.005 | 0.018 | 0.004 | −0.0031 | 0.0007 | 0.77 | −0.97 |
| O20---N10 | 0.009 | 0.029 | 0.007 | −0.0060 | 0.0006 | 0.86 | −1.88 |
| H17---C13 | 0.007 | 0.023 | 0.005 | −0.0037 | 0.0010 | 0.74 | −1.16 |
| H19---C11 | 0.004 | 0.014 | 0.003 | −0.0020 | 0.0008 | 0.67 | −0.62 |
| H16---N2 | 0.006 | 0.019 | 0.004 | −0.0029 | 0.0010 | 0.72 | −0.91 |
| H16---N3 | 0.006 | 0.020 | 0.004 | −0.0031 | 0.0010 | 0.77 | −0.97 |
| H16---N7 | 0.005 | 0.016 | 0.003 | −0.0024 | 0.0008 | 0.80 | −0.75 |
| H16---N10 | 0.004 | 0.014 | 0.003 | −0.0020 | 0.0007 | 0.67 | −0.63 |
| H15---N1 | 0.005 | 0.018 | 0.004 | −0.0029 | 0.0008 | 0.72 | −0.91 |
| H23---N9 | 0.004 | 0.015 | 0.003 | −0.0024 | 0.0007 | 0.80 | −0.75 |
| Complexes | ∆Eelst | % | ∆Eexch | ∆Eind | % | ∆Edis | % | ∆ESAPT0 |
|---|---|---|---|---|---|---|---|---|
| VX@C2N | −13.18 | 26.60 | 23.28 | −4.49 | 9.06 | −31.88 | 64.34 | −26.27 |
| VS@C2N | −10.28 | 21.24 | 22.20 | −5.12 | 10.59 | −32.99 | 68.17 | −26.20 |
| VE@C2N | −10.66 | 26.18 | 18.23 | −3.47 | 8.52 | −26.58 | 65.29 | −22.49 |
| VG@C2N | −15.39 | 29.20 | 25.14 | −5.83 | 11.06 | −31.48 | 59.73 | −27.56 |
| VM@C2N | −9.37 | 21.39 | 19.75 | −4.62 | 10.55 | −29.80 | 68.05 | −24.04 |
| Complexes | HOMO eV | LUMO eV | EH-L | NBO (e−) |
|---|---|---|---|---|
| VX@C2N | −5.02 | −2.89 | 2.13 | −0.023 |
| VS@C2N | −5.37 | −2.74 | 2.63 | −0.020 |
| VE@C2N | −5.35 | −2.88 | 2.47 | −0.012 |
| VG@C2N | −5.56 | −2.75 | 2.81 | −0.013 |
| VM@C2N | −5.57 | −2.78 | 2.80 | −0.002 |
| C2N | −6.40 | −2.69 | 3.71 | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Faiyz, Y.S.S.; Sarfaraz, S.; Yar, M.; Munsif, S.; Khan, A.A.; Amin, B.; Sheikh, N.S.; Ayub, K. Efficient Detection of Nerve Agents through Carbon Nitride Quantum Dots: A DFT Approach. Nanomaterials 2023, 13, 251. https://doi.org/10.3390/nano13020251
Al-Faiyz YSS, Sarfaraz S, Yar M, Munsif S, Khan AA, Amin B, Sheikh NS, Ayub K. Efficient Detection of Nerve Agents through Carbon Nitride Quantum Dots: A DFT Approach. Nanomaterials. 2023; 13(2):251. https://doi.org/10.3390/nano13020251
Chicago/Turabian StyleAl-Faiyz, Yasair S. S., Sehrish Sarfaraz, Muhammad Yar, Sajida Munsif, Adnan Ali Khan, Bin Amin, Nadeem S. Sheikh, and Khurshid Ayub. 2023. "Efficient Detection of Nerve Agents through Carbon Nitride Quantum Dots: A DFT Approach" Nanomaterials 13, no. 2: 251. https://doi.org/10.3390/nano13020251
APA StyleAl-Faiyz, Y. S. S., Sarfaraz, S., Yar, M., Munsif, S., Khan, A. A., Amin, B., Sheikh, N. S., & Ayub, K. (2023). Efficient Detection of Nerve Agents through Carbon Nitride Quantum Dots: A DFT Approach. Nanomaterials, 13(2), 251. https://doi.org/10.3390/nano13020251







