Nano-Microbial Remediation of Polluted Soil: A Brief Insight
Abstract
:1. Introduction
2. An Evaluation of Nanobioremediation-Based Pollutant Reduction with a Focus on Microbe-Mediated Remediation
Nanobioremediation of Heavy Metals
3. Soil Nanoremediation
4. Microorganism-Assisted Nanoremediation
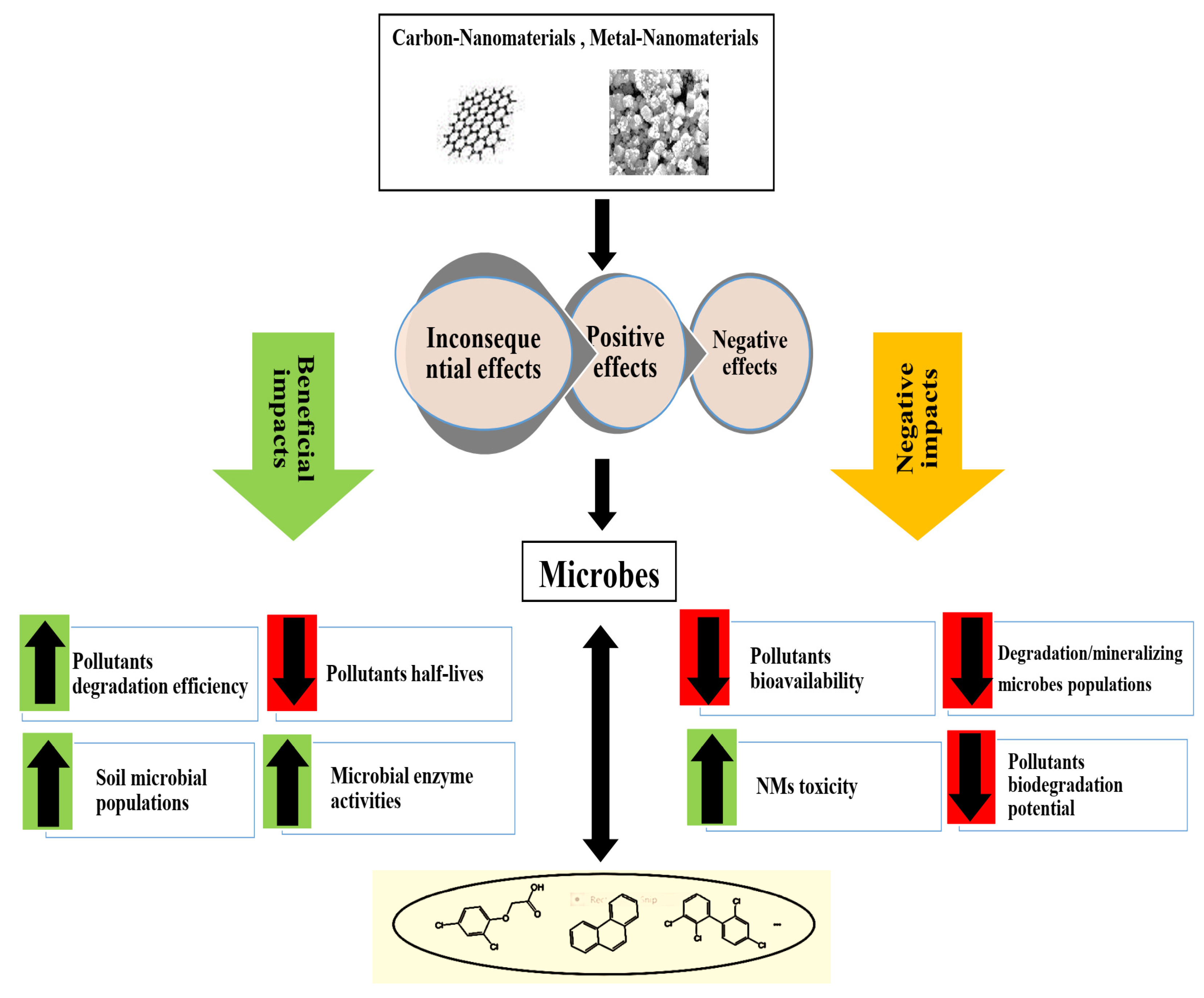
5. Utilization of Nanomaterials for Micro-Remediation of Polluted Soils
6. Advancements in Agricultural Techniques by Using Nanotechnology
7. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, H.; Qin, Y.; Niu, Z.; Wang, L.; Ge, S. Summer Maize Mapping by Compositing Time Series Sentinel-1A Imagery Based on Crop Growth Cycles. J. Indian Soc. Remote Sens. 2021, 49, 2863–2874. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Liu, L.; Li, T.; Dou, Y.; Qiao, J.; Wang, Y.; An, S.; Chang, S.X. Nitrogen fertilization weakens the linkage between soil carbon and microbial diversity: A global meta-analysis. Glob. Chang. Biol. 2022, 28, 6446–6461. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, T.; Pokharel, P.; Liu, L.; Qiao, J.; Wang, Y.; An, S.; Chang, S.X. Global effects on soil respiration and its temperature sensitivity depend on nitrogen addition rate. Soil Biol. Biochem. 2022, 174, 108814. [Google Scholar] [CrossRef]
- Khan, M.I.; Cheema, S.A.; Anum, S.; Niazi, N.K.; Azam, M.; Bashir, S.; Ashraf, I.; Qadri, R. Phytoremediation of agricultural pollutants. In Phytoremediation; Springer: Cham, Switzerland, 2020; pp. 27–81. [Google Scholar]
- Ghodszad, L.; Reyhanitabar, A.; Maghsoodi, M.R.; Lajayer, B.A.; Chang, S.X. Biochar affects the fate of phosphorus in soil and water: A critical review. Chemosphere 2021, 283, 131176. [Google Scholar] [CrossRef] [PubMed]
- Karimi, H.; Mahdavi, S.; Asgari Lajayer, B.; Moghiseh, E.; Rajput, V.D.; Minkina, T.; Astatkie, T. Insights on the bioremediation technologies for pesticide-contaminated soils. Environ. Geochem. Health 2022, 44, 1329–1354. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.R.R.; Pomarolli, L.C.; da Veiga, M.A.M.S. From classic methodologies to application of nanomaterials for soil remediation: An integrated view of methods for decontamination of toxic metal (oid) s. Environ. Sci. Pollut. Res. 2020, 27, 10205–10227. [Google Scholar] [CrossRef]
- Dai, J.; Feng, H.; Shi, K.; Ma, X.; Yan, Y.; Ye, L.; Xia, Y. Electrochemical degradation of antibiotic enoxacin using a novel PbO2 electrode with a graphene nanoplatelets inter-layer: Characteristics, efficiency and mechanism. Chemosphere 2022, 307, 135833. [Google Scholar] [CrossRef]
- Keshavarzi, A.; Kumar, V. Spatial distribution and potential ecological risk assessment of heavy metals in agricultural soils of Northeastern Iran. Geol. Ecol. Landsc. 2020, 4, 87–103. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.; Dong, B.; Xing, M.; Sun, X.; Xi, B.; Dai, W.; He, C.; Luo, Y.; Huang, Y. Electric field applications enhance the electron transfer capacity of dissolved organic matter in sludge compost. Environ. Technol. 2022, 1–11. [Google Scholar] [CrossRef]
- Butu, M.; Stef, R.; Corneanu, M.; Butnariu, M. Mycoremediation: A Sustainable Approach for Pesticide Pollution Abatement. In Bioremediation and Biotechnology; Springer: Cham, Switzerland, 2020; Volume 2, pp. 73–96. [Google Scholar]
- Shahi Khalaf Ansar, B.; Kavusi, E.; Dehghanian, Z.; Pandey, J.; Asgari Lajayer, B.; Price, G.W.; Astatkie, T. Removal of organic and inorganic contaminants from the air, soil, and water by algae. Environ. Sci. Pollut. Res. 2022, 1–29. [Google Scholar] [CrossRef]
- Kavusi, E.; Ansar, B.S.K.; Ebrahimi, S.; Sharma, R.; Ghoreishi, S.S.; Nobaharan, K.; Abdoli, S.; Dehghanian, Z.; Lajayer, B.A.; Senapathi, V. Critical review on phytoremediation of polyfluoroalkyl substances from environmental matrices: Need for global concern. Environ. Res. 2022, 217, 114844. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, L.; Zhai, L.; Zhang, H.; Wang, S.; Zou, J.; Shen, Z.; Lian, C.; Chen, Y. Analysis of the long-term effectiveness of biochar immobilization remediation on heavy metal contaminated soil and the potential environmental factors weakening the remediation effect: A review. Ecotoxicol. Environ. Saf. 2021, 207, 111261. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, X.; Zhao, H.; Liu, F.; Wang, L.; Zhao, X.; Gao, P.; Li, X.; Ji, P. Possibility of using modified fly ash and organic fertilizers for remediation of heavy-metal-contaminated soils. J. Clean. Prod. 2021, 284, 124713. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H.; Zhao, Y.; Menzembere, E.R.G.Y. Remediation of vanadium-contaminated soils by the combination of natural clay mineral and humic acid. J. Clean. Prod. 2021, 279, 123874. [Google Scholar] [CrossRef]
- Gu, W.; Li, X.; Li, Q.; Hou, Y.; Zheng, M.; Li, Y. Combined remediation of polychlorinated naphthalene-contaminated soil under multiple scenarios: An integrated method of genetic engineering and environmental remediation technology. J. Hazard. Mater. 2021, 405, 124139. [Google Scholar] [CrossRef]
- Delangiz, N.; Aliyar, S.; Pashapoor, N.; Nobaharan, K.; Lajayer, B.A.; Rodríguez-Couto, S. Can polymer-degrading microorganisms solve the bottleneck of plastics’ environmental challenges? Chemosphere 2022, 294, 133709. [Google Scholar] [CrossRef]
- Delangiz, N.; Varjovi, M.B.; Lajayer, B.A.; Ghorbanpour, M. Beneficial microorganisms in the remediation of heavy metals. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 417–423. [Google Scholar]
- Atigh, Z.B.Q.; Sardari, P.; Moghiseh, E.; Lajayer, B.A.; Hursthouse, A.S. Purified montmorillonite as a nano-adsorbent of potentially toxic elements from environment: An overview. Nanotechnol. Environ. Eng. 2021, 6, 12. [Google Scholar] [CrossRef]
- Yousefi, S.R.; Alshamsi, H.A.; Amiri, O.; Salavati-Niasari, M. Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J. Mol. Liq. 2021, 337, 116405. [Google Scholar] [CrossRef]
- Mahdi, M.A.; Yousefi, S.R.; Jasim, L.S.; Salavati-Niasari, M. Green synthesis of DyBa2Fe3O7. 988/DyFeO3 nanocomposites using almond extract with dual eco-friendly applications: Photocatalytic and antibacterial activities. Int. J. Hydrogen Energy 2022, 47, 14319–14330. [Google Scholar] [CrossRef]
- Maghsoodi, M.R.; Lajayer, B.A.; Hatami, M.; Mirjalili, M.H. Challenges and opportunities of nanotechnology in plant-soil mediated systems: Beneficial role, phytotoxicity, and phytoextraction. Adv. Phytonanotechnol. 2019, 2019, 379–404. [Google Scholar]
- Khalkhal, K.; Asgari Lajayer, B.; Ghorbanpour, M. An overview on the effect of soil physicochemical properties on the immobilization of biogenic nanoparticles. In Biogenic Nano-Particles and their Use in Agro-Ecosystems; Springer: Singapore, 2020; pp. 133–160. [Google Scholar]
- Tratnyek, P.G.; Johnson, R.L. Nanotechnologies for environmental cleanup. Nano Today 2006, 1, 44–48. [Google Scholar] [CrossRef]
- Patil, S.S.; Shedbalkar, U.U.; Truskewycz, A.; Chopade, B.A.; Ball, A.S. Nanoparticles for environmental clean-up: A review of potential risks and emerging solutions. Environ. Technol. Innov. 2016, 5, 10–21. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Upadhyay, S.K.; Kumari, A.; Ranjan, A.; Mandzhieva, S.; Sushkova, S.; Singh, R.K.; Verma, K.K. Nanotechnology in the Restoration of Polluted Soil. Nanomaterials 2022, 12, 769. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, A.; Yadav, K.K.; Yadav, S.; Gupta, N.; Singh, J.K.; Katiyar, R.; Kumar, V. Nano-phytoremediation of pollutants from contaminated soil environment: Current scenario and future prospects. In Phytoremediation; Springer: Cham, Switzerland, 2018; pp. 383–401. [Google Scholar]
- Romeh, A.A.; Saber, R.A.I. Green nano-phytoremediation and solubility improving agents for the remediation of chlorfenapyr contaminated soil and water. J. Environ. Manag. 2020, 260, 110104. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Behera, M.; Kumar, S. Nano-bioremediation: An innovative remediation technology for treatment and management of contaminated sites. In Bioremediation of Industrial Waste for Environmental Safety; Springer: Singapore, 2020; pp. 165–182. [Google Scholar]
- Tran, T.D.; Dao, N.T.; Sasaki, R.; Tu, M.B.; Dang, G.H.M.; Nguyen, H.G.; Dang, H.M.; Vo, C.H.; Inigaki, Y.; van Nguyen, N. Accelerated remediation of organochlorine pesticide-contaminated soils with phyto-Fenton approach: A field study. Environ. Geochem. Health 2020, 42, 3597–3608. [Google Scholar] [CrossRef] [PubMed]
- Gamallo, M.; Fernández, L.; Feijoo, G.; Moreira, M. Nano-based technologies for environmental soil remediation. In Nanomaterials for Sustainable Energy and Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 307–331. [Google Scholar]
- Liao, Y.; Yang, J. Remediation of vanadium contaminated soil by nano-hydroxyapatite. J. Soils Sediments 2020, 20, 1534–1544. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, K.; Cheng, J. Different nanomaterials for soil remediation affect avoidance response and toxicity response in earthworm (Eisenia fetida). Bull. Environ. Contam. Toxicol. 2020, 104, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Huang, S.; Huang, G.; Liu, Y.; Yang, G.; Lin, C.; Xiao, G.; Wang, Y.; Liu, M. Remediation of organic arsenic contaminants with heterogeneous Fenton process mediated by SiO2-coated nano zero-valent iron. Environ. Sci. Pollut. Res. 2020, 27, 12017–12029. [Google Scholar] [CrossRef]
- Ahmad, A.; Ghufran, R.; Al-Hosni, T.K. Bioavailability of zinc oxide nano particle with fly ash soil for the remediation of metals by Parthenium hysterophorus. J. Environ. Health Sci. Eng. 2019, 17, 1195–1203. [Google Scholar] [CrossRef]
- Sundararaghavan, A.; Mukherjee, A.; Suraishkumar, G.K. Investigating the potential use of an oleaginous bacterium, Rhodococcus opacus PD630, for nano-TiO2 remediation. Environ. Sci. Pollut. Res. 2020, 27, 27394–27406. [Google Scholar] [CrossRef]
- Sarkar, A.; Sengupta, S.; Sen, S. Nanoparticles for soil remediation. In Nanoscience and Biotechnology for Environmental Applications; Springer: Cham, Switzerland, 2019; pp. 249–262. [Google Scholar]
- Wang, Y.; Liu, Y.; Zhan, W.; Zheng, K.; Lian, M.; Zhang, C.; Ruan, X.; Li, T. Long-term stabilization of Cd in agricultural soil using mercapto-functionalized nano-silica (MPTS/nano-silica): A three-year field study. Ecotoxicol. Environ. Saf. 2020, 197, 110600. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Ghosh Sachan, S. Nanobioremediation of heavy metals: Perspectives and challenges. J. Basic Microbiol. 2022, 62, 428–443. [Google Scholar]
- Abdi, O.; Kazemi, M. A review study of biosorption of heavy metals and comparison between different biosorbents. J. Mater Env. Sci. 2015, 6, 1386–1399. [Google Scholar]
- Ayangbenro, A.S.; Babalola, O.O. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Abebe, B.; Murthy, H.A.; Amare, E. Summary on adsorption and photocatalysis for pollutant remediation: Mini review. J. Encapsulation Adsorpt. Sci. 2018, 8, 225–255. [Google Scholar] [CrossRef] [Green Version]
- Desiante, W.L.; Minas, N.S.; Fenner, K. Micropollutant biotransformation and bioaccumulation in natural stream biofilms. Water Res. 2021, 193, 116846. [Google Scholar] [CrossRef]
- Cao, X.; Alabresm, A.; Chen, Y.P.; Decho, A.W.; Lead, J. Improved metal remediation using a combined bacterial and nanoscience approach. Sci. Total Environ. 2020, 704, 135378. [Google Scholar] [CrossRef]
- Gil-Díaz, M.; Diez-Pascual, S.; González, A.; Alonso, J.; Rodríguez-Valdés, E.; Gallego, J.; Lobo, M.C. A nanoremediation strategy for the recovery of an As-polluted soil. Chemosphere 2016, 149, 137–145. [Google Scholar] [CrossRef]
- Baragaño, D.; Forján, R.; Welte, L.; Gallego, J.L.R. Nanoremediation of As and metals polluted soils by means of graphene oxide nanoparticles. Sci. Rep. 2020, 10, 1896. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.E.; Abou Ali, S.A.; Elweshahy, S.M. Microwave functionalization of titanium oxide nanoparticles with chitosan nanolayer for instantaneous microwave sorption of Cu (II) and Cd (II) from water. Int. J. Biol. Macromol. 2018, 111, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.; Zhu, N.; Shang, R.; Shi, C.; Cui, J.; Sohoo, I.; Wu, P.; Cao, Y. Biorecovery of palladium as nanoparticles by Enterococcus faecalis and its catalysis for chromate reduction. Chem. Eng. J. 2016, 288, 246–254. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Abdou, A.E.; Mohamed, S.M.; Osman, M.M. Engineered staphylococcus aureus via immobilization on magnetic Fe3O4-phthalate nanoparticles for biosorption of divalent ions from aqueous solutions. J. Environ. Chem. Eng. 2016, 4, 3810–3824. [Google Scholar] [CrossRef]
- Akhtar, N.; Khan, S.; Rehman, S.U.; Rehman, Z.U.; Khatoon, A.; Rha, E.S.; Jamil, M. Synergistic effects of zinc oxide nanoparticles and bacteria reduce heavy metals toxicity in rice (Oryza sativa L.) plant. Toxics 2021, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajput, V.D.; Minkina, T.; Kimber, R.L.; Singh, V.K.; Shende, S.; Behal, A.; Sushkova, S.; Mandzhieva, S.; Lloyd, J.R. Insights into the Biosynthesis of Nanoparticles by the Genus Shewanella. Appl. Environ. Microbiol. 2021, 87, e01390-21. [Google Scholar] [CrossRef]
- Capeness, M.J.; Echavarri-Bravo, V.; Horsfall, L.E. Production of biogenic nanoparticles for the reduction of 4-Nitrophenol and oxidative laccase-like reactions. Front. Microbiol. 2019, 10, 997. [Google Scholar] [CrossRef]
- Alabresm, A.; Chen, Y.P.; Decho, A.W.; Lead, J. A novel method for the synergistic remediation of oil-water mixtures using nanoparticles and oil-degrading bacteria. Sci. Total Environ. 2018, 630, 1292–1297. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [Green Version]
- Laslo, V.; Pinzaru, S.C.; Zaguła, G.; Kluz, M.; Vicas, S.I.; Cavalu, S. Synergic effect of selenium nanoparticles and lactic acid bacteria in reduction cadmium toxicity. J. Mol. Struct. 2022, 1247, 131325. [Google Scholar] [CrossRef]
- Dong, G.; Wang, Y.; Gong, L.; Wang, M.; Wang, H.; He, N.; Zheng, Y.; Li, Q. Formation of soluble Cr (III) end-products and nanoparticles during Cr (VI) reduction by Bacillus cereus strain XMCr-6. Biochem. Eng. J. 2013, 70, 166–172. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef] [PubMed]
- Ahkami, A.H.; White III, R.A.; Handakumbura, P.P.; Jansson, C. Rhizosphere engineering: Enhancing sustainable plant ecosystem productivity. Rhizosphere 2017, 3, 233–243. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Ahmad, M.; Srivastava, A.K.; Abhilash, P.C.; Sharma, B. Optimization of eco-friendly novel amendments for sustainable utilization of Fly ash based on growth performance, hormones, antioxidant, and heavy metal translocation in chickpea (Cicer arietinum L.) plant. Chemosphere 2021, 267, 129216. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; Imran, M. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 2019, 248, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B. Dynamic probabilistic modeling of environmental emissions of engineered nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Usman, K.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci. Rep. 2019, 9, 5658. [Google Scholar] [CrossRef] [Green Version]
- Kisku, G.C.; Kumar, V.; Sahu, P.; Kumar, P.; Kumar, N. Characterization of coal fly ash and use of plants growing in ash pond for phytoremediation of metals from contaminated agricultural land. Int. J. Phytoremed. 2018, 20, 330–337. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O.O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, G.K.; Bhat, B.A.; Mushtaq, M.; Tariq, L.; Rai, P.K.; Basu, U.; Dar, A.A.; Islam, S.T.; Dar, T.U.; Bhat, J.A. Insights into decontamination of soils by phytoremediation: A detailed account on heavy metal toxicity and mitigation strategies. Physiol. Plant. 2021, 173, 287–304. [Google Scholar] [CrossRef]
- Yu, G.; Wang, X.; Liu, J.; Jiang, P.; You, S.; Ding, N.; Guo, Q.; Lin, F. Applications of nanomaterials for heavy metal removal from water and soil: A review. Sustainability 2021, 13, 713. [Google Scholar] [CrossRef]
- Mazarji, M.; Bayero, M.T.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Tereshchenko, A.; Timofeeva, A.; Bauer, T.; Burachevskaya, M.; Kızılkaya, R. Realizing united nations sustainable development goals for greener remediation of heavy metals-contaminated soils by biochar: Emerging trends and future directions. Sustainability 2021, 13, 13825. [Google Scholar] [CrossRef]
- Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for environmental remediation: Materials and applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [Green Version]
- Machado, S.; Pinto, S.; Grosso, J.; Nouws, H.; Albergaria, J.T.; Delerue-Matos, C. Green production of zero-valent iron nanoparticles using tree leaf extracts. Sci. Total Environ. 2013, 445, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in situ remediation: A review of the benefits and potential risks. Environ. Health Perspect. 2009, 117, 1813–1831. [Google Scholar] [CrossRef] [Green Version]
- Gillham, R.W.; O’Hannesin, S.F. Enhanced degradation of halogenated aliphatics by zero-valent iron. Groundwater 1994, 32, 958–967. [Google Scholar] [CrossRef]
- Roehl, K.E.; Meggyes, T.; Simon, F.; Stewart, D. Long-Term Performance of Permeable Reactive Barriers; Gulf Professional Publishing: Houston, TX, USA, 2005. [Google Scholar]
- Shih, Y.-h.; Tai, Y.-t. Reaction of decabrominated diphenyl ether by zerovalent iron nanoparticles. Chemosphere 2010, 78, 1200–1206. [Google Scholar] [CrossRef]
- Elliott, D.W.; Lien, H.-L.; Zhang, W.-X. Degradation of lindane by zero-valent iron nanoparticles. J. Environ. Eng. 2009, 135, 317–324. [Google Scholar] [CrossRef]
- Machado, S.; Pacheco, J.; Nouws, H.; Albergaria, J.T.; Delerue-Matos, C. Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci. Total Environ. 2015, 533, 76–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.-n.; Zhang, X.; Chen, Z.-l. Removal of chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water Res. 2011, 45, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Reyes, J.; Peralta-Videa, J.; Gardea-Torresdey, J. Supported and unsupported nanomaterials for water and soil remediation: Are they a useful solution for worldwide pollution? J. Hazard. Mater. 2014, 280, 487–503. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Ang, H.-M.; Tadé, M.O. Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem. Eng. J. 2013, 226, 336–347. [Google Scholar] [CrossRef]
- Zhang, W. Nanoscale Iron Particles for Environmental Remediation: An Overview. J. Nanoparticle Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Qiu, X.; Fang, Z.; Liang, B.; Gu, F.; Xu, Z. Degradation of decabromodiphenyl ether by nano zero-valent iron immobilized in mesoporous silica microspheres. J. Hazard. Mater. 2011, 193, 70–81. [Google Scholar] [CrossRef]
- Xie, Y.; Cheng, W.; Tsang, P.E.; Fang, Z. Remediation and phytotoxicity of decabromodiphenyl ether contaminated soil by zero valent iron nanoparticles immobilized in mesoporous silica microspheres. J. Environ. Manag. 2016, 166, 478–483. [Google Scholar] [CrossRef]
- Su, H.; Fang, Z.; Tsang, P.E.; Zheng, L.; Cheng, W.; Fang, J.; Zhao, D. Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles. J. Hazard. Mater. 2016, 318, 533–540. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, Y.; Xiong, Z.; Kaback, D.; Zhao, D. Immobilization of mercury in field soil and sediment using carboxymethyl cellulose stabilized iron sulfide nanoparticles. Nanotechnology 2012, 23, 294007. [Google Scholar] [CrossRef]
- Olson, M.R.; Blotevogel, J.; Borch, T.; Petersen, M.; Royer, R.; Sale, T. Long-term potential of in situ chemical reduction for treatment of polychlorinated biphenyls in soils. Chemosphere 2014, 114, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Peng, R.; Li, X.; Ren, J.; Liu, T.; Wang, X. Effect of titanium dioxide nanoparticles on copper toxicity to Daphnia magna in water: Role of organic matter. Water Res. 2016, 105, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Mahanty, S.; Chatterjee, S.; Ghosh, S.; Tudu, P.; Gaine, T.; Bakshi, M.; Das, S.; Das, P.; Bhattacharyya, S.; Bandyopadhyay, S. Synergistic approach towards the sustainable management of heavy metals in wastewater using mycosynthesized iron oxide nanoparticles: Biofabrication, adsorptive dynamics and chemometric modeling study. J. Water Process Eng. 2020, 37, 101426. [Google Scholar] [CrossRef]
- Govarthanan, M.; Jeon, C.-H.; Jeon, Y.-H.; Kwon, J.-H.; Bae, H.; Kim, W. Non-toxic nano approach for wastewater treatment using Chlorella vulgaris exopolysaccharides immobilized in iron-magnetic nanoparticles. Int. J. Biol. Macromol. 2020, 162, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.; Shahid, M.; Ahmed, T.; Niazi, M.B.K.; Hussain, S.; Song, F.; Manzoor, I. Use of biogenic copper nanoparticles synthesized from a native Escherichia sp. as photocatalysts for azo dye degradation and treatment of textile effluents. Environ. Pollut. 2020, 257, 113514. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, H.; Lu, Y.; Hou, K.; Wang, Y.; Ning, Q.; Li, L.; Wang, B.; Zhang, L.; Zeng, G. Toxicity of sulfide-modified nanoscale zero-valent iron to Escherichia coli in aqueous solutions. Chemosphere 2019, 220, 523–530. [Google Scholar] [CrossRef]
- Abuhatab, S.; El-Qanni, A.; Al-Qalaq, H.; Hmoudah, M.; Al-Zerei, W. Effective adsorptive removal of Zn2+, Cu2+, and Cr3+ heavy metals from aqueous solutions using silica-based embedded with NiO and MgO nanoparticles. J. Environ. Manag. 2020, 268, 110713. [Google Scholar] [CrossRef]
- Sarioglu, O.F.; San Keskin, N.O.; Celebioglu, A.; Tekinay, T.; Uyar, T. Bacteria encapsulated electrospun nanofibrous webs for remediation of methylene blue dye in water. Colloids Surf. B Biointerfaces 2017, 152, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Chen, S.; Fan, J.; Shang, T.; Huang, D.; Li, G. Novel mesoporous organosilica nanoparticles with ferrocene group for efficient removal of contaminants from wastewater. J. Colloid Interface Sci. 2019, 554, 565–571. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Oyekunle, J.A.; Durosinmi, L.M.; Oluwafemi, O.S.; Olayanju, D.S.; Akinola, A.S.; Obisesan, O.R.; Akinyele, O.F.; Ajayeoba, T.A. Potential of cobalt and cobalt oxide nanoparticles as nanocatalyst towards dyes degradation in wastewater. Nano-Struct. Nano-Objects 2020, 21, 100405. [Google Scholar] [CrossRef]
- San Keskin, N.O.; Celebioglu, A.; Sarioglu, O.F.; Uyar, T.; Tekinay, T. Encapsulation of living bacteria in electrospun cyclodextrin ultrathin fibers for bioremediation of heavy metals and reactive dye from wastewater. Colloids Surf. B Biointerfaces 2018, 161, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debnath, B.; Majumdar, M.; Bhowmik, M.; Bhowmik, K.L.; Debnath, A.; Roy, D.N. The effective adsorption of tetracycline onto zirconia nanoparticles synthesized by novel microbial green technology. J. Environ. Manag. 2020, 261, 110235. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Nguyen, L.N.; Hou, J.; Hai, F.I.; Chen, V. Direct immobilization of laccase on titania nanoparticles from crude enzyme extracts of P. ostreatus culture for micro-pollutant degradation. Sep. Purif. Technol. 2017, 178, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, M.A.; Jabbari, V.; Imam, M.A.; Castro, E.; Kim, H.; Curry, M.L.; Valles-Rosales, D.J.; Noveron, J.C. Nanoscale nickel metal organic framework decorated over graphene oxide and carbon nanotubes for water remediation. Sci. Total Environ. 2020, 698, 134214. [Google Scholar] [CrossRef]
- Mohanraj, R.; Gnanamangai, B.M.; Poornima, S.; Oviyaa, V.; Ramesh, K.; Vijayalakshmi, G.; Nithya, M.; Karthik, N.; Ponmurugan, P.; Robinson, J.P. Decolourisation efficiency of immobilized silica nanoparticles synthesized by actinomycetes. Mater. Today Proc. 2020, 48, 129–135. [Google Scholar] [CrossRef]
- Cornu, J.-Y.; Huguenot, D.; Jézéquel, K.; Lollier, M.; Lebeau, T. Bioremediation of copper-contaminated soils by bacteria. World J. Microbiol. Biotechnol. 2017, 33, 26. [Google Scholar] [CrossRef]
- Wu, G.; Kang, H.; Zhang, X.; Shao, H.; Chu, L.; Ruan, C. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities. J. Hazard. Mater. 2010, 174, 1–8. [Google Scholar] [CrossRef]
- Huang, D.-L.; Zeng, G.-M.; Feng, C.-L.; Hu, S.; Jiang, X.-Y.; Tang, L.; Su, F.-F.; Zhang, Y.; Zeng, W.; Liu, H.-L. Degradation of lead-contaminated lignocellulosic waste by Phanerochaete chrysosporium and the reduction of lead toxicity. Environ. Sci. Technol. 2008, 42, 4946–4951. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Lai, C.; Zhao, M.H.; Wei, Z.; Li, N.J.; Huang, C.; Xie, G.X. Adsorption of Pb (II) by iron oxide nanoparticles immobilized Phanerochaete chrysosporium: Equilibrium, kinetic, thermodynamic and mechanisms analysis. Chem. Eng. J. 2012, 203, 423–431. [Google Scholar] [CrossRef]
- Polti, M.A.; García, R.O.; Amoroso, M.J.; Abate, C.M. Bioremediation of chromium (VI) contaminated soil by Streptomyces sp. MC1. J. Basic Microbiol. 2009, 49, 285–292. [Google Scholar] [CrossRef]
- He, Y.K.; Sun, J.G.; Feng, X.Z.; Czakó, M.; Márton, L. Differential mercury volatilization by tobacco organs expressing a modified bacterial merA gene. Cell Res. 2001, 11, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Pan, L.P.; Li, H.H. Isolation, identification and characterization of soil microbes which degrade phenolic allelochemicals. J. Appl. Microbiol. 2010, 108, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, D.; Shim, H.; Canada, K.; Barbieri, P.; Wood, T.K. Aerobic degradation of tetrachloroethylene by toluene-o-xylene monooxygenase of Pseudomonas stutzeri OX1. Nat. Biotechnol. 2000, 18, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Acosta-Martinez, V.; Cox, S.B.; Green, M.J.; Li, S.; Cañas-Carrell, J.E. An evaluation of the impact of multiwalled carbon nanotubes on soil microbial community structure and functioning. J. Hazard. Mater. 2013, 261, 188–197. [Google Scholar] [CrossRef]
- Fang, G.; Si, Y.; Tian, C.; Zhang, G.; Zhou, D. Degradation of 2, 4-D in soils by Fe3O4 nanoparticles combined with stimulating indigenous microbes. Environ. Sci. Pollut. Res. 2012, 19, 784–793. [Google Scholar] [CrossRef]
- Tilston, E.L.; Collins, C.D.; Mitchell, G.R.; Princivalle, J.; Shaw, L.J. Nanoscale zerovalent iron alters soil bacterial community structure and inhibits chloroaromatic biodegradation potential in Aroclor 1242-contaminated soil. Environ. Pollut. 2013, 173, 38–46. [Google Scholar] [CrossRef]
- Singh, J.; Lee, B.-K. Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): A possible mechanism for the removal of Cd from the contaminated soil. J. Environ. Manag. 2016, 170, 88–96. [Google Scholar] [CrossRef]
- Oyelami, A.O.; Semple, K.T. The impact of carbon nanomaterials on the development of phenanthrene catabolism in soil. Environ. Sci. Process. Impacts 2015, 17, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Li, Y.; Zhou, Z.; Feng, C. Bioavailability of adsorbed phenanthrene by black carbon and multi-walled carbon nanotubes to Agrobacterium. Chemosphere 2010, 78, 1329–1336. [Google Scholar] [CrossRef]
- Zhou, W.; Shan, J.; Jiang, B.; Wang, L.; Feng, J.; Guo, H.; Ji, R. Inhibitory effects of carbon nanotubes on the degradation of 14C-2, 4-dichlorophenol in soil. Chemosphere 2013, 90, 527–534. [Google Scholar] [CrossRef]
- Bawa, R.; Bawa, S.; Maebius, S.B.; Flynn, T.; Wei, C. Protecting new ideas and inventions in nanomedicine with patents. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanoparticle Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- World Health Organization. State of the Art on the Initiatives and Activities Relevant to Risk Assessment and Risk Management of Nanotechnologies in the Food and Agriculture Sectors; FAO/WHO Technical Paper; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Parisi, C.; Vigani, M.; Rodríguez-Cerezo, E. Agricultural nanotechnologies: What are the current possibilities? Nano Today 2015, 10, 124–127. [Google Scholar] [CrossRef]
- Aragay, G.; Pino, F.; Merkoçi, A. Nanomaterials for sensing and destroying pesticides. Chem. Rev. 2012, 112, 5317–5338. [Google Scholar] [CrossRef]
- bin Hussein, M.Z.; Zainal, Z.; Yahaya, A.H.; Foo, D.W.V. Controlled release of a plant growth regulator, α-naphthaleneacetate from the lamella of Zn–Al-layered double hydroxide nanocomposite. J. Control. Release 2002, 82, 417–427. [Google Scholar] [CrossRef]
- Kottegoda, N.; Munaweera, I.; Madusanka, N.; Karunaratne, V. A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Curr. Sci. 2011, 101, 73–78. [Google Scholar]
- Guo, J. Synchrotron radiation, soft-X-ray spectroscopy and nanomaterials. Int. J. Nanotechnol. 2004, 1, 193–225. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, R.; Xiao, Q.; Wang, Y.; Zhang, J. Effects of slow/controlled-release fertilizer cemented and coated by nano-materials on biology. II. Eff. Slow/Control. -Release Fertil. Cem. Coat. Nano-Mater. Plants. Nanosci. 2006, 11, 18–26. [Google Scholar]
- El Salmawi, K.M. Application of polyvinyl alcohol (PVA)/carboxymethyl cellulose (CMC) hydrogel produced by conventional crosslinking or by freezing and thawing. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 44, 619–624. [Google Scholar] [CrossRef]
- Sekhon, B.S. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Jatav, G.K.; Mukhopadhyay, R.; De, N. Characterization of swelling behaviour of nanoclay composite. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 1560–1563. [Google Scholar]
- Mahfoudhi, N.; Boufi, S. Poly (acrylic acid-co-acrylamide)/cellulose nanofibrils nanocomposite hydrogels: Effects of CNFs content on the hydrogel properties. Cellulose 2016, 23, 3691–3701. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Z.; Chen, J.F.; Liu, F.; Liu, A.Q.; Wang, Q.; Sun, H.Y.; Wen, L.X. Study of UV-shielding properties of novel porous hollow silica nanoparticle carriers for avermectin. Pest Manag. Sci. Former. Pestic. Sci. 2007, 63, 241–246. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Zhao, R.; Torley, P.; Halley, P.J. Emerging biodegradable materials: Starch-and protein-based bio-nanocomposites. J. Mater. Sci. 2008, 43, 3058–3071. [Google Scholar] [CrossRef]
- Aragay, G.; Pons, J.; Ros, J.; Merkoci, A. Aminopyrazole-based ligand induces gold nanoparticle formation and remains available for heavy metal ions sensing. A simple “mix and detect” approach. Langmuir 2010, 26, 10165–10170. [Google Scholar] [CrossRef]

| Nanoparticles | Contaminant Remediated | Factors of Performance and Removal Efficiency | References |
|---|---|---|---|
| Iron oxide nanoparticles with a polyvinyl pyrrolidone coating | Cd, Pb | The use of nanoparticles was combined with a bioremediation process driven by Halomonas sp. Halomonas sp. was inoculated for 48 h at 180 rpm and 28 °C in the Cd and Pb removal system. After 24 h, 100% removal was detected, whereas it took 48 h for Cd. | [46] |
| Industrial suspension of zero-valent iron (nZVI) at two dosages (1% and 10%) | As | The pH of the nZVI suspension was adjusted to 12.2 ± 0.1. Polyacrylic acid was utilized as a stabilizer to prevent the accumulation of nZVI in the suspension. The maximum amount of As immobilized in brownfield soil was 10% of nZVI. | [47] |
| Graphene oxide nanoparticles (nGOx) and nZVI | Metals such as Cd, Pb, Zn, Cu, and As were found in As- and metal-contaminated soil. | The application of nZVI and nGOx to contaminated soils had a significant influence on the availability of As and metals. nGOx immobilized Cu, Pb, and Cd while mobilizing As and P. In the case of nZVI, it successfully immobilized As and Pb (but not Cd) while increasing Cu’s availability. This study discovered that both NPs may work as techniques for immobilization and stabilization, which can then be used for phytoremediation. | [48] |
| Titanium oxide nanoparticles bonded to a chitosan nanolayer (NTiO2–NCh) | Cd and Cu | During the experiment, the pH was adjusted at 7.0. The elimination was aided by a microwave-enforced sorption technique that lasted 60–70 s. Cu and Cd were eliminated at a rate of 88.01% and 70.67%, respectively, when NTiO2–NCh was used. | [49] |
| Palladium (Pd), Pd NPs | Cr | Pd NPs were investigated as a bionanocatalyst. Pd NPs were shown to decrease Cr6+ completely in 12 h. To decrease 5.0 mol of Cr6+, 6.3 mg of Pd NPs was utilized. | [50] |
| Magnetic iron oxide nanoparticles (Fe3O4 NPs) were treated with Staphylococcus aureus and had their surfaces encapsulated in phthalic acid (n-Fe3O4–Phth–S. aureus) | Cu, Ni, Pb | The remediation efficiency of n-Fe3O4–Phth–S. aureus was reported to be 83.0–89.5% for Cu2+, 99.4–100% for Pb2+, and 92.6–7.5% for Ni2+. The researchers also discovered that n-Fe3O4–Phth–S. aureus was an effective biosorbent for removing pollutants. | [51] |
| ZnO NPs | Cu, Cd, Cr, and Pb | The maximum removal of Cr, Cu, and Pb by ZnO-NPs at 5 mg L−1 with Bacillus cereus and Lysinibacillus macroides was 60%, 70%, and 85%, respectively. The ideal pH for effective removal was 8.0. The elimination was reduced in the case of bacteria-mediated remediation, which was determined to be 83% and 70% with B. cereus, and 60% and 65% for L. macroides. | [52] |
| Nanotechnology Administered | Modification | Affiliated Microorganisms | Deletion or Adsorption Efficiency | Advantage/Technique | Distinct Attribute | References |
|---|---|---|---|---|---|---|
| NiO and MgO nanoparticles | SiO2 embedding | - | Maximal absorption at a rate of 41.4, 13.8, and 7.2 (ions/nm2) for Cr3+, Cu2+, and Zn2+, respectively | Physical, spontaneous, and endothermic absorption of Cu2+ and Cr3+, but chemical and exothermic Zn2+ uptake | Renewal, reusability, and proven sustainability | [96] |
| Electrospun nanofibrous webs | Bacterial encapsulation | Pseudomonas aeruginosa | 55–70% deletion of methylene blue at various concentrations. | Biological deletion of dye | Potent bacterial cells or genetic engineering can be rather promising. | [97] |
| Mesoporous organosilica nanoparticles (MONs) | Ferrocene amalgamation | - | Application of MONs increased the removal rates of dyes by 50% and metals by 25% | Ferrocene facilitated the non-covalent interaction and provided a larger surface area and conjugation | Advanced organic–inorganic hybrid nanomaterial | [98] |
| Co and CoO nanoparticles | Microwave and reductive chemical heating | - | Respectively, cobalt and cobalt oxide nanoparticles destroyed murexide dye by 43.6 and 39.4%. | Irradiation and greater surface area | Eco-friendly, easy to build, fast, and highly efficient photocatalytic degradation | [99] |
| Electrospun cyclodextrin fibers | Bacterial encapsulation | Lysinibacillus sp. | Reduction efficacy: Cr(VI) = 58 ± 1.4%; reaction black 5 = 82 ± 0.8%; Ni(II) = 70 ± 0.2% | Bacterial bioremediation | Cyclodextrin contributes to bacterial growth by providing an additional carbon source | [100] |
| Zirconia (ZrO2) nanoparticles | Synthesis based on a microbial acellular culture supernatant | Pseudomonas aeruginosa | Tetracycline accumulation up to a concentration of 526.32 mg/g | Chemisorption and potent electrostatic reaction amongst zwitterions | Synthesis of green nanoparticles and steady bioremediation | [101] |
| Enzyme-immobilized nanoparticles | Laccase immobilization | P. ostreatus | Breakdown of 90% bisphenol-A and 10% carbamazepine | Immobilized laccase-mediated oxidation | Reusable and cost-effective enzyme | [102] |
| Graphene oxide (GO) and carbon nanotubes | Nanosized Ni metal–organic framework | - | Methylene blue accumulation up to 222 mg/g | Mixed nanocomposites consist of hydrophobic and/or π-π interactions, a large surface area, pores between MOFs, and diverse morphological characteristics. | The nanocomposite’s interaction was far better. | [103] |
| Silica (SiO2) nanoparticles | Synthesized from actinomycetes | Actinomycetes | Approx. 80% clearing of industrial wastewater | Photocatalytic deterioration | Cost-effective and stable | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliyari Rad, S.; Nobaharan, K.; Pashapoor, N.; Pandey, J.; Dehghanian, Z.; Senapathi, V.; Minkina, T.; Ren, W.; Rajput, V.D.; Asgari Lajayer, B. Nano-Microbial Remediation of Polluted Soil: A Brief Insight. Sustainability 2023, 15, 876. https://doi.org/10.3390/su15010876
Aliyari Rad S, Nobaharan K, Pashapoor N, Pandey J, Dehghanian Z, Senapathi V, Minkina T, Ren W, Rajput VD, Asgari Lajayer B. Nano-Microbial Remediation of Polluted Soil: A Brief Insight. Sustainability. 2023; 15(1):876. https://doi.org/10.3390/su15010876
Chicago/Turabian StyleAliyari Rad, Shiva, Khatereh Nobaharan, Neda Pashapoor, Janhvi Pandey, Zahra Dehghanian, Venkatramanan Senapathi, Tatiana Minkina, Wenjie Ren, Vishnu D. Rajput, and Behnam Asgari Lajayer. 2023. "Nano-Microbial Remediation of Polluted Soil: A Brief Insight" Sustainability 15, no. 1: 876. https://doi.org/10.3390/su15010876
APA StyleAliyari Rad, S., Nobaharan, K., Pashapoor, N., Pandey, J., Dehghanian, Z., Senapathi, V., Minkina, T., Ren, W., Rajput, V. D., & Asgari Lajayer, B. (2023). Nano-Microbial Remediation of Polluted Soil: A Brief Insight. Sustainability, 15(1), 876. https://doi.org/10.3390/su15010876










