Terpenoids as Natural Agents against Food-Borne Bacteria—Evaluation of Biofilm Biomass versus Viability Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Chemicals
2.3. Minimum Inhibitory and Bactericidal Concentration
Biofilm Biomass and Viability
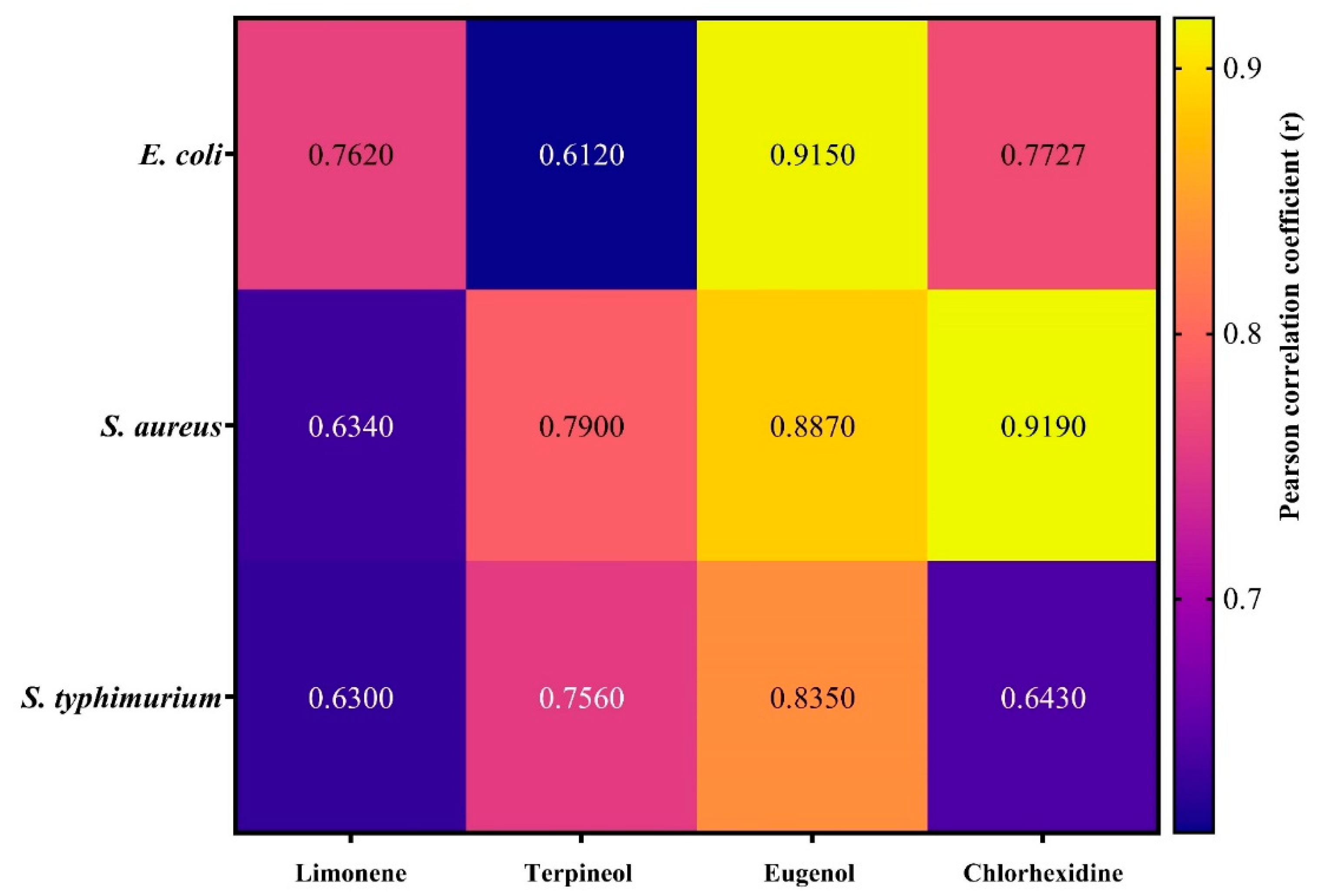
3. Results and Discussion
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schachter, H.; Kamerling, H. 4.06-Glycobiology of Caenorhabditis elegans. In Comprehensive Glycoscience; Elsevier: Oxford, UK, 2007; pp. 81–100. [Google Scholar]
- Magalhães, R.P.; Vieira, T.F.; Fernandes, H.S.; Melo, A.; Simões, M.; Sousa, S.F. The biofilms structural database. Trends Biotechnol. 2020, 38, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Abebe, G.M. The role of bacterial biofilm in antibiotic resistance and food contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.Y.L.; Scott, W.C.; Williams, E.S.; Ciarlo, M.; DeLeo, P.; Brooks, B.W. Comparative influences of dermal and inhalational routes of exposure on hazards of cleaning product ingredients among mammalian model organisms. Environ. Int. 2021, 157, 106777. [Google Scholar] [CrossRef] [PubMed]
- Dewey, H.M.; Jones, J.M.; Keating, M.R.; Budhathoki-Uprety, J. Increased use of disinfectants during the COVID-19 pandemic and its potential impacts on health and safety. ACS Chem. Health Saf. 2021, 29, 27–38. [Google Scholar] [CrossRef]
- Lobie, T.A.; Roba, A.A.; Booth, J.A.; Kristiansen, K.I.; Aseffa, A.; Skarstad, K.; Bjørås, M. Antimicrobial resistance: A challenge awaiting the post-COVID-19 era. Int. J. Infect. Dis. 2021, 111, 322–325. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Van den Poel, B.; Saegeman, V.; Schuermans, A. Increasing usage of chlorhexidine in health care settings: Blessing or curse? A narrative review of the risk of chlorhexidine resistance and the implications for infection prevention and control. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 349–362. [Google Scholar] [CrossRef]
- Nourbakhsh, F.; Nasrollahzadeh, M.S.; Tajani, A.S.; Soheili, V.; Hadizadeh, F. Bacterial biofilms and their resistance mechanisms: A brief look at treatment with natural agents. Folia Microbiol. 2022, 67, 535–554. [Google Scholar] [CrossRef]
- Saeed, K.; Pasha, I.; Chughtai, M.F.J.; Ali, Z.; Bukhari, H.; Zuhair, M. Application of essential oils in food industry: Challenges and innovation. J. Essent. Oil Res. 2022, 34, 97–110. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Nakamura, A.; Kawahara, A.; Takahashi, H.; Kuda, T.; Kimura, B. Comparison between the Antimicrobial Activity of Essential Oils and Their Components in the Vapor Phase against Food-related Bacteria. J. Oleo Sci. 2022, 71, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.S.; Vasilieva, N.Y.; Fetisova, O.Y.; Sychev, V.V.; Elsuf’ev, E.V.; Malyar, Y.N.; Issaoui, N.; Miroshnikova, A.V.; Borovkova, V.S.; Kazachenko, A.S. New reactions of betulin with sulfamic acid and ammonium sulfamate in the presence of solid catalysts. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Yamaguchi, T. Antibacterial effect of the combination of terpenoids. Arch. Microbiol. 2022, 204, 520. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: An updated review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef] [PubMed]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar] [PubMed]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Journey of limonene as an antimicrobial agent. J. Pure Appl. Microbiol. 2021, 15, 1094–1110. [Google Scholar] [CrossRef]
- Lee, S.-B.; Cha, K.-H.; Kim, S.-N.; Altantsetseg, S.; Shatar, S.; Sarangerel, O.; Nho, C.-W. The antimicrobial activity of essential oil from Dracocephalum foetidum against pathogenic microorganisms. J. Microbiol. 2007, 45, 53–57. [Google Scholar]
- Subramenium, G.A.; Vijayakumar, K.; Pandian, S.K. Limonene inhibits streptococcal biofilm formation by targeting surface-associated virulence factors. J. Med. Microbiol. 2015, 64, 879–890. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.-W.; Im, G.J.; Chung, J.-W.; Song, J.-J. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. PLoS ONE 2015, 10, e0119564. [Google Scholar] [CrossRef]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Ding, Q.; Zhuang, T.; Fu, P.; Zhou, Q.; Luo, L.; Dong, Z.; Li, H.; Tang, S. Alpha-terpineol grafted acetylated lentinan as an anti-bacterial adhesion agent. Carbohydr. Polym. 2022, 277, 118825. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, M.; Olas, B. Biological Properties and prospects for the application of eugenol—A review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.P. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Fink, R.; Potočnik, A.; Oder, M. Plant-based natural saponins for Escherichia coli surface hygiene management. LWT 2020, 122, 109018. [Google Scholar] [CrossRef]
- Babajanian, M.; Marden, M. Safer cleaning products and asthma: Tips and resources for health professionals. AAP Action 2021, 6–10. Available online: https://nyscheck.org/wp-content/uploads/2021/11/AAP_-in_Action_November_2021_Newsletter_6-10.pdf (accessed on 25 November 2022).
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, S.; Tian, Q.; Peng, W.; Tao, Y.; Bo, R.; Liu, M.; Li, J. Eugenol exposure in vitro inhibits the expressions of T3SS and TIF virulence genes in Salmonella Typhimurium and reduces its pathogenicity to chickens. Microb. Pathog. 2022, 162, 105314. [Google Scholar] [CrossRef]
- Huang, J.; Yang, L.; Zou, Y.; Luo, S.; Wang, X.; Liang, Y.; Du, Y.; Feng, R.; Wei, Q. Antibacterial activity and mechanism of three isomeric terpineols of Cinnamomum longepaniculatum leaf oil. Folia Microbiol. 2021, 66, 59–67. [Google Scholar] [CrossRef]
- Costa, M.D.S.; Rocha, J.E.; Campina, F.F.; Silva, A.R.; Da Cruz, R.P.; Pereira, R.L.; Quintans-Júnior, L.J.; De Menezes, I.R.; Adriano, A.D.S.; De Freitas, T.S. Comparative analysis of the antibacterial and drug-modulatory effect of d-limonene alone and complexed with β-cyclodextrin. Eur. J. Pharm. Sci. 2019, 128, 158–161. [Google Scholar] [CrossRef]
- Van de Vel, E.; Sampers, I.; Raes, K. A review on influencing factors on the minimum inhibitory concentration of essential oils. Crit. Rev. Food Sci. Nutr. 2019, 59, 357–378. [Google Scholar] [CrossRef]
- Kampf, G. Adaptive bacterial response to low level chlorhexidine exposure and its implications for hand hygiene. Microb. Cell 2019, 6, 307. [Google Scholar] [CrossRef] [PubMed]
- Cota, J.B.; Carvalho, A.C.; Dias, I.; Reisinho, A.; Bernardo, F.; Oliveira, M. Salmonella spp. in pet reptiles in portugal: Prevalence and chlorhexidine gluconate antimicrobial efficacy. Antibiotics 2021, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Ulhaq, Z.S.; Hendyatama, T.H.; Hameed, F.; Santosaningsih, D. Antibacterial activity of Citrus hystrix toward Salmonella spp. infection. Enferm. Infecc. Y Microbiol. Clin. 2021, 39, 283–286. [Google Scholar] [CrossRef]
- Umagiliyage, A.L.; Becerra-Mora, N.; Kohli, P.; Fisher, D.J.; Choudhary, R. Antimicrobial efficacy of liposomes containing d-limonene and its effect on the storage life of blueberries. Postharvest Biol. Technol. 2017, 128, 130–137. [Google Scholar] [CrossRef]
- Condell, O.; Power, K.A.; Händler, K.; Finn, S.; Sheridan, A.; Sergeant, K.; Renaut, J.; Burgess, C.M.; Hinton, J.C.; Nally, J.E. Comparative analysis of Salmonella susceptibility and tolerance to the biocide chlorhexidine identifies a complex cellular defense network. Front. Microbiol. 2014, 5, 373. [Google Scholar] [CrossRef]


| Limonene | Terpineol | Eugenol | Chlorhexidine | |||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| E. coli ATCC 25922 | 0.9 | 3.7 | 0.1 | 0.4 | 0.1 | 0.4 | 0.02 | 0.03 |
| S. aureus ATCC 25923 | 1.9 | 3.7 | 0.1 | 0.3 | 0.1 | 0.6 | 0.02 | 0.06 |
| S. typhimurium ATCC 14028 | 0.9 | 2.8 | 0.2 | 0.7 | 0.1 | 0.6 | 0.03 | 0.09 |
| Active Component | Concentration | Bacteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | S. aureus | S. typhimurium | |||||||||
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | ||||||
| Biofilm biomass (OD 620 nm) | Limonene | Control | 0.0454 a | 90.28 | <0.0000 * | 0.0616 a | 47.75 | <0.0000 * | 0.1221 a | 22.21 | <0.0000 * |
| 1 MIC | 0.0332 b | 0.0510 b | 0.1033 b | ||||||||
| 2 MIC | 0.0275 c | 0.0452 c | 0.0922 c | ||||||||
| 3 MIC | 0.0201 d | 0.0335 d | 0.0897 c | ||||||||
| Terpineol | Control | 0.0454 a | 69.35 | <0.0000 * | 0.0616 a | 16.66 | <0.0000 * | 0.1221 a | 29.73 | <0.0000 * | |
| 1 MIC | 0.0360 b | 0.0587 a | 0.1001 b | ||||||||
| 2 MIC | 0.0254 c | 0.0497 b | 0.0863 c | ||||||||
| 3 MIC | 0.0252 c | 0.0458 b | 0.0756 d | ||||||||
| Eugenol | Control | 0.0454 a | 190.4 | <0.0000 * | 0.0616 a | 61.3 | <0.0000 * | 0.1221 a | 81.89 | <0.0000 * | |
| 1 MIC | 0.0334 b | 0.0467 b | 0.1017 b | ||||||||
| 2 MIC | 0.0224 c | 0.0368 b | 0.0849 c | ||||||||
| 3 MIC | 0.0150 d | 0.0292 d | 0.0665 d | ||||||||
| Chlorhexidine | Control | 0.0421 a | 27.75 | <0.0000 * | 0.0649 a | 124.5 | <0.0000 * | 0.1192 a | 17.36 | <0.0000 * | |
| 1 MIC | 0.0340 b | 0.0509 b | 0.0103 b | ||||||||
| 2 MIC | 0.0225 c | 0.0400 c | 0.0949 b | ||||||||
| 3 MIC | 0.0194 c | 0.0301 d | 0.0762 c | ||||||||
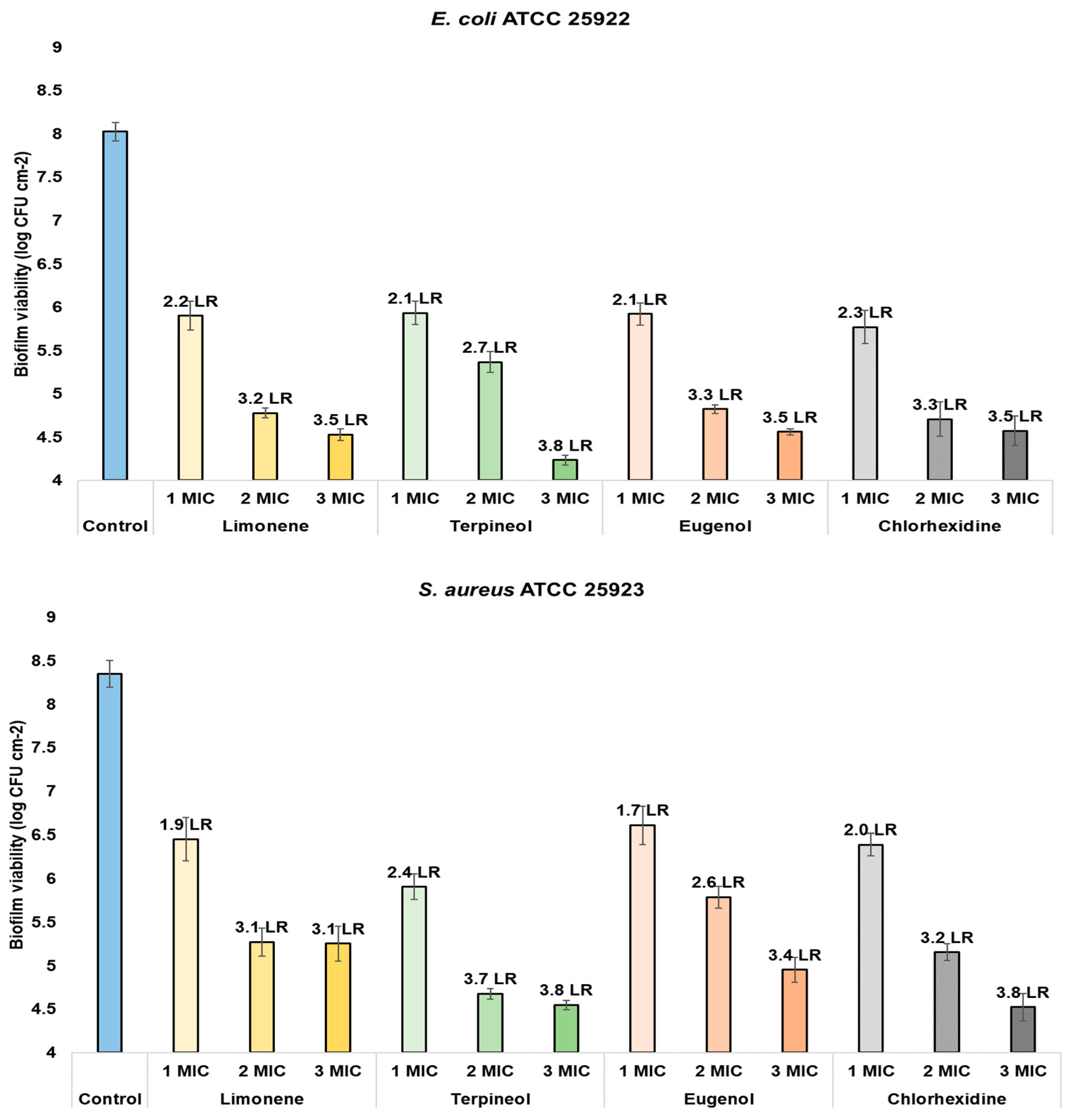
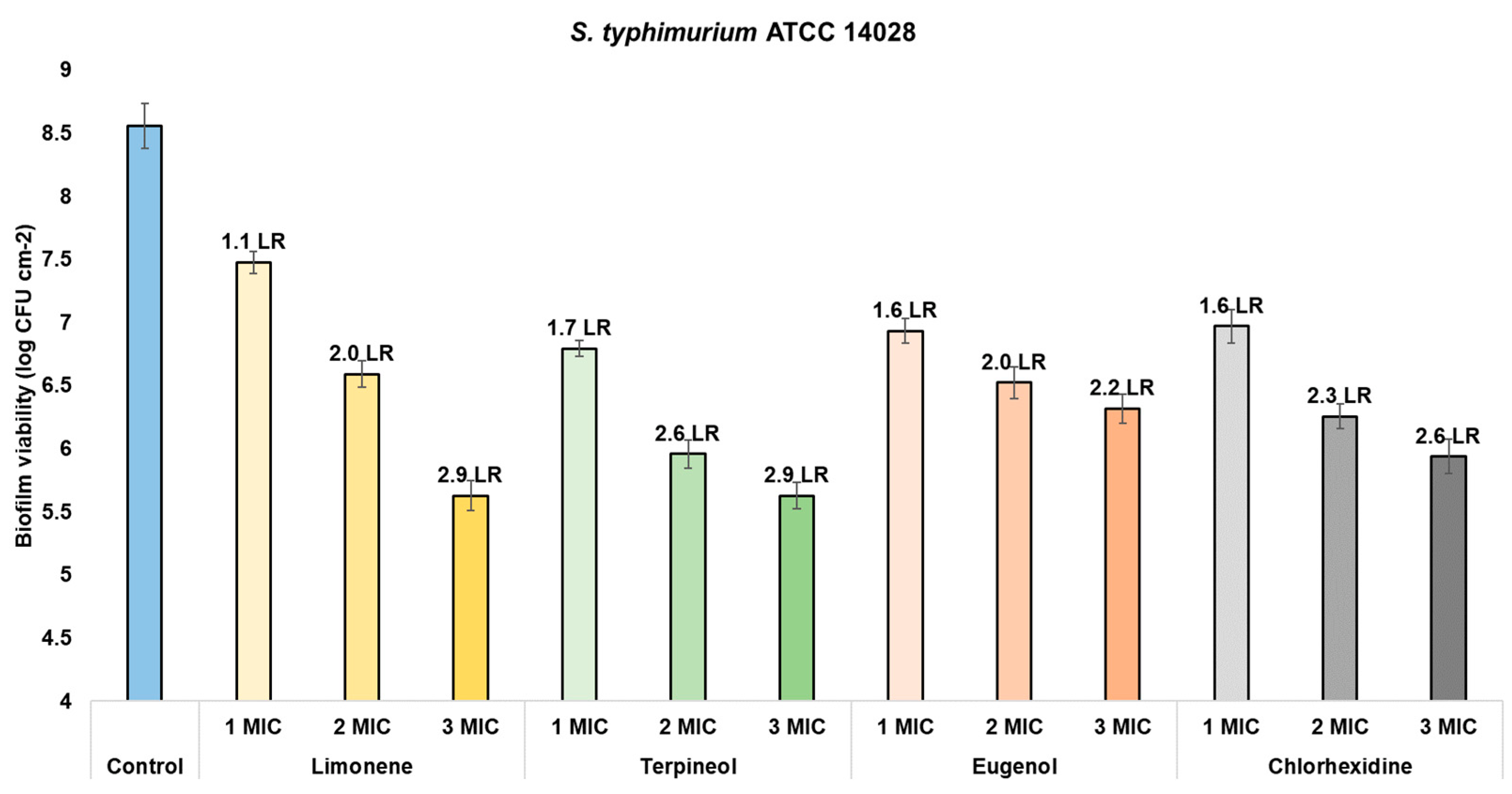
| Biofilm viability (CFU cm−2) | Limonene | Control | 8.0261 a | 1530 | <0.0000 * | 8.3507 a | 493.5 | <0.0000 * | 8.5591 a | 348.3 | <0.0000 * |
| 1 MIC | 5.8995 b | 6.4502 b | 7.4750 b | ||||||||
| 2 MIC | 4.7727 c | 5.3065 c | 6.5878 c | ||||||||
| 3 MIC | 4.5634 d | 5.2276 c | 5.8535 d | ||||||||
| Terpineol | Control | 8.0261 a | 471.6 | <0.0000 * | 8.3507 a | 366.6 | <0.0000 * | 8.5591 a | 869.6 | <0.0000 * | |
| 1 MIC | 5.9338 b | 5.9054 b | 6.7936 b | ||||||||
| 2 MIC | 5.3726 c | 4.6700 c | 5.9604 c | ||||||||
| 3 MIC | 4.3589 d | 4.5578 c | 5.6755 d | ||||||||
| Eugenol | Control | 8.0261 a | 2073 | <0.0000 * | 8.3507 a | 1,965 | <0.0000 * | 8.5591 a | 470.3 | <0.0000 * | |
| 1 MIC | 5.9214 b | 6.6092 b | 6.9335 b | ||||||||
| 2 MIC | 4.8198 c | 5.7823 c | 6.5252 c | ||||||||
| 3 MIC | 4.5893 d | 5.0638 d | 6.3395 d | ||||||||
| Chlorhexidine | Control | 8.1222 a | 109.2 | <0.0000 * | 8.2914 a | 475.1 | <0.0000 * | 8.6001 a | 163 | <0.0000 * | |
| 1 MIC | 3.3453 b | 3.8277 b | 2.6214 b | ||||||||
| 2 MIC | 3.3193 b | 3.1963 c | 2.3027 c | ||||||||
| 3 MIC | 3.2561 c | 1.9623 d | 1.5898 d | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fink, R. Terpenoids as Natural Agents against Food-Borne Bacteria—Evaluation of Biofilm Biomass versus Viability Reduction. Processes 2023, 11, 148. https://doi.org/10.3390/pr11010148
Fink R. Terpenoids as Natural Agents against Food-Borne Bacteria—Evaluation of Biofilm Biomass versus Viability Reduction. Processes. 2023; 11(1):148. https://doi.org/10.3390/pr11010148
Chicago/Turabian StyleFink, Rok. 2023. "Terpenoids as Natural Agents against Food-Borne Bacteria—Evaluation of Biofilm Biomass versus Viability Reduction" Processes 11, no. 1: 148. https://doi.org/10.3390/pr11010148
APA StyleFink, R. (2023). Terpenoids as Natural Agents against Food-Borne Bacteria—Evaluation of Biofilm Biomass versus Viability Reduction. Processes, 11(1), 148. https://doi.org/10.3390/pr11010148





