Abstract
DNA-based biomimetic enzymes have attracted extensive attention due to their unique structure and stability compared to natural enzymes. Meanwhile, the specific sequences of DNA itself also have a catalytic effect. Herein, we first designed three guanine-rich DNA sequences numbered c−Myc3c, PG4TC, and TTT to construct g−quadruplex/hemin DNAzymes. Then, the g−quadruplex/hemin DNAzymes with the best activity were selected by a comprehensive examination of activity, degradation rate, and affinity. Subsequently, the stability and reusability of UiO66−DNAzymes were investigated using UiO66 as the carrier to immobilize DNAzymes. The results showed that UiO66−DNAzymes had excellent reusability and stability. Finally, UiO66−DNAzymes were successfully used for glucose detection by cascading with glucose oxidase (GOx) with a detection limit of 0.62 μM. The constructed glucose sensor had a good specificity, which is of great significance for developing a novel, accurate, fast, and economical glucose detection sensor.
1. Introduction
Natural enzymes are efficient biocatalysts with mild reaction conditions and high selectivity specificity. However, the stability and activity of natural enzymes are difficult to reach the level of practical application [1,2]. Therefore, it is urgent to study biomimetic enzymes with good stability and low cost to meet the needs of practical production and application. Natural enzymes tend to be susceptible to inactivation in cascade reactions, while biomimetic enzymes have become ideal catalysts to replace natural enzymes [3,4,5]. Particularly, deoxyribozymes (DNAzymes) are single-stranded DNA molecules with enzymatic catalytic activity and structure recognition ability obtained by in vitro screening technology [6]. DNA molecules provide a rich reaction microenvironment due to their unique helical structure, hairpin structure, tetrad structure, and various hair non-canonical structures [7,8,9,10]. DNAzymes have numerous advantages compared to natural enzymes, such as easy access, strong hydrolytic stability, resistance to extreme environments, etc. Thereupon, DNA-based mimetic enzymes have attracted extensive interest [11]. Nevertheless, the catalytic activity of early developed DNAzymes was not satisfactory, so it is crucial to develop methods to improve the catalytic activity.
G−quadruplex/hemin DNAzymes are also known as peroxide-mimicking DNAzymes [12,13]. They are composed of a guanine-rich DNA sequence folded into a quadruplex structure and have peroxidase catalytic activity in the presence of hemin [14]. Different DNA sequences affect how g−quadruplexes bind to hemin, thereby affecting the activity of DNAzymes [15,16]. Therefore, the design of specific DNA sequences is one of the directions to optimize the activity of DNAzymes. Moreover, immobilized DNAzymes are another means to improve the activity of DNAzymes. Enzyme immobilization is one of the most attractive methods to improve enzyme performance due to the inherent fragility and difficult recovery of natural enzymes [17]. Metal-organic frameworks (MOFs) have the advantages of flexible pore structure, abundant coordination sites, and diverse functional groups [18,19]. The structural characteristics of MOFs make them the preferred materials for enzyme immobilization, which can be used in biosensors and other fields. Therefore, MOFs are an ideal choice to immobilize DNAzymes. Currently, there are many studies about embedding DNAzymes on MOFs to improve the activity of DNAzymes [20,21,22,23], but all of them were used in electrocatalysis and have few biological applications.
Peroxidase can oxidize chromogenic substrates such as ABTS and TMB in the presence of H2O2 [24]. Based on this, researchers have developed various biosensors for detection applications, including fluorescent g−quadruplex DNAzyme sensors, colorimetric g−quadruplex DNAzyme sensors, electrochemical g−quadruplex sensors, etc. [25]. However, there was no report on g−quadruplex sensors for glucose detection to our knowledge. Glucose, as an important intermediate involved in human metabolism, is closely related to various human diseases [26]. Therefore, the importance of developing an accurate, fast, facile, and economical sensor to detect glucose is self-evident.
Herein, we first designed three DNA sequences and screened out the g−quadruplex/hemin DNAzymes system with high activity through a series of optimization. Then, the DNAzymes were immobilized on UiO66 to improve their stability and catalytic ability. Subsequently, UiO66−DNAzymes were cascaded with GOx to construct an efficient, accurate, and cost-effective biosensor platform for glucose detection. This work constructed a cascade catalytic platform between DNA-mimetic enzymes and natural enzymes, which provided an inspiring general strategy for glucose detection.
2. Results and Discussion
2.1. Design and Characterization of G−Quadruplex/Hemin DNAzymes
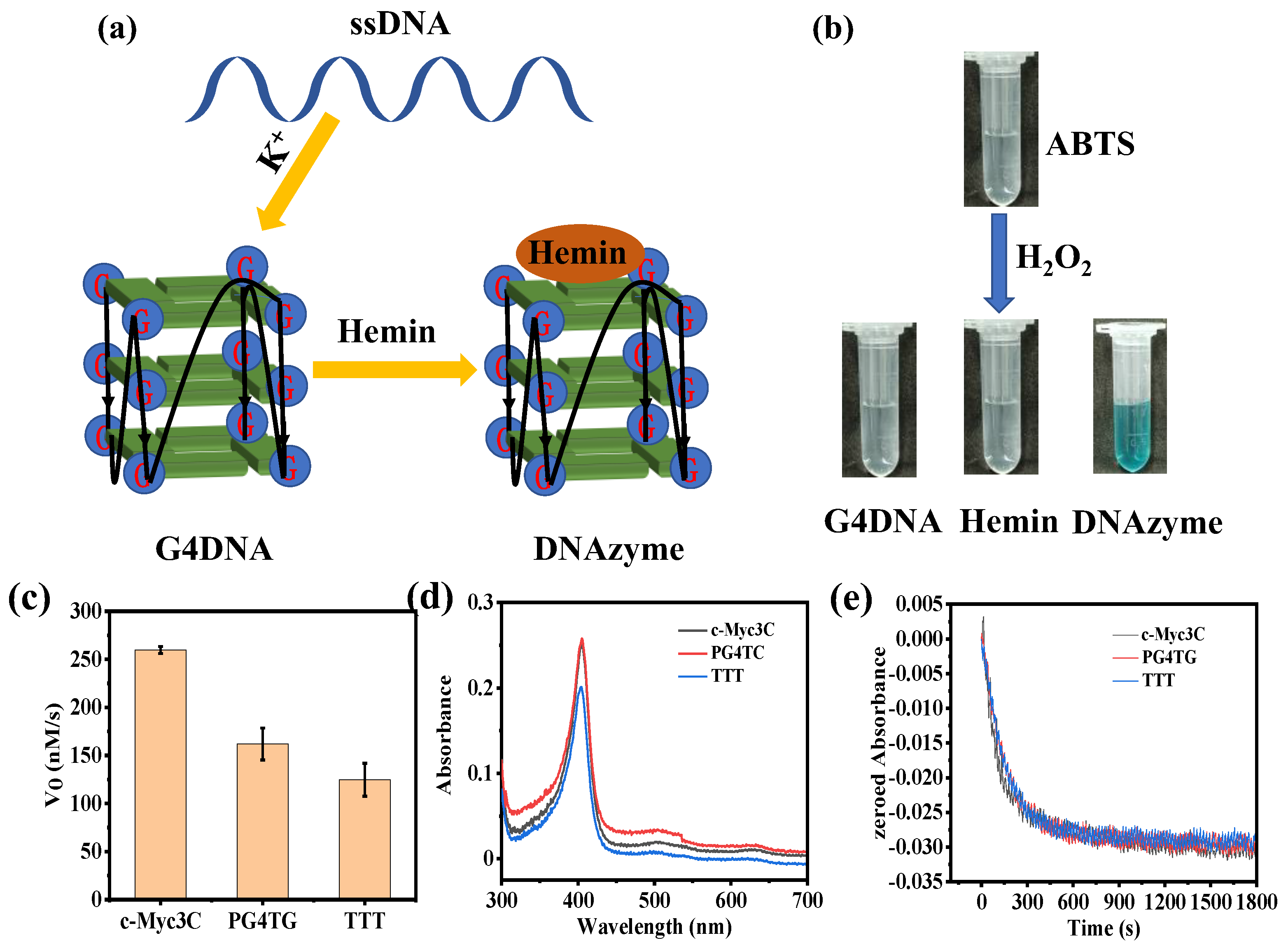
The preparation process of g−quadruplex/hemin DNAzymes is shown in Figure 1a. In the presence of K+, ssDNA molecules fold into a parallel g−quadruplex structure. After adding hemin, the g−quadruplex will form peroxidase-activated DNAzymes with hemin. Peroxidase enzyme can oxidize ABTS to generate chromogenic ABTS+ in the presence of H2O2. To verify the peroxidase activity of DNAzymes, ABTS and H2O2 were added to DNAzyme solution, g−quadruplex DNA solution, or hemin solution, respectively, and then the color change of the solution was observed. As shown in Figure 1b, the DNAzyme solutions appeared blue in the presence of H2O2, but g−quadruplex DNA solutions or hemin solutions were colorless. This result proved that DNAzymes did have peroxidase activity, while g−quadruplex DNA or hemin had no peroxidase activity. At the same time, it also verified the successful formation of g−quadruplex/hemin DNAzymes.
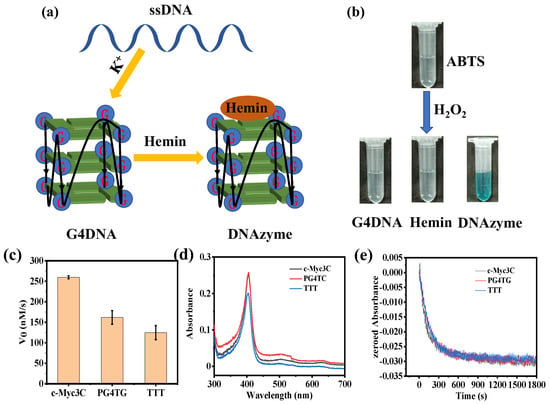
Figure 1.
(a) The schematic diagram of the formation of g−quadruplex/hemin DNAzymes. (b) DNAzymes oxidized ABTS to generate ABTS+ in the presence of H2O2. (c) The initial speed of the three DNA sequences catalyzed the ABTS reaction. (d) Full-wavelength scanning of three DNA sequences and (e) change in the absorption spectra of H2O2.
Then, three DNA sequences were designed to optimize the activity of DNAzymes (Table S1). By studying the initial reaction rate and enzyme kinetics of the three sequences, the g−quadruplex/hemin DNAzymes with higher activity were screened out. As shown in Figure 1c, the initial reaction rate of c−Myc3c was 259.72 nM/s, which was much higher than that of PG4TG and TTT. The enzymatic kinetic parameters of the three DNA sequences were further studied (Figure S1). The V0 and H2O2 concentration curves were fitted by using the Michaelis–Menten model, and the specific enzyme kinetic parameters are summarized in Table 1. According to the data in Table 1, the Km of the c−Myc3c sequence was the smallest among the three sequences, indicating that the c−Myc3c had the highest affinity for the substrate. Meanwhile, the Vmax and kcat of the c−Myc3c sequence were much higher than the other two sequences, showing that the c−Myc3c had the most excellent activity among the three sequences.

Table 1.
The kinetic parameters of the three DNA sequences.
The stability of the enzyme is significant for practical applications, so the initial degradation rate of DNAzymes synthesized from three DNA sequences in the presence of 0.5 mM H2O2 was investigated. To obtain the specific degradation rate, the extinction coefficient of DNAzymes should be acquired first. DNAzymes have a characteristic absorption peak at 404 nm, while hemin also has an absorption peak at the same wavelength. To avoid interference with DNAzymes by hemin, hemin should be fully saturated with the g-quadruplex sequences. Therefore, the DNAzyme was constructed with a hemin concentration of 2 μM and a DNA concentration of 5 μM. As shown in Figure 1d, the calculated extinction coefficients of c−Myc3c, PG4TC, and TTT were 1.265 × 105 M−1cm−1, 1.005 × 105 M−1cm−1, and 1.270 × 105 M−1cm−1, respectively. According to Figure 1e, the DNAzymes of the three sequences started to degrade in the presence of 0.5 mM H2O2. The slowest degradation rate was TTT among the three sequences, with a degradation rate of 1.030 nM s−1. The initial degradation rates of c-Myc3c and PG4TC were 1.212 nM s−1 and 1.318 nM s−1, respectively. The specific data were listed in Table 2.

Table 2.
The degradation and dissociation constant of the three DNA sequences.
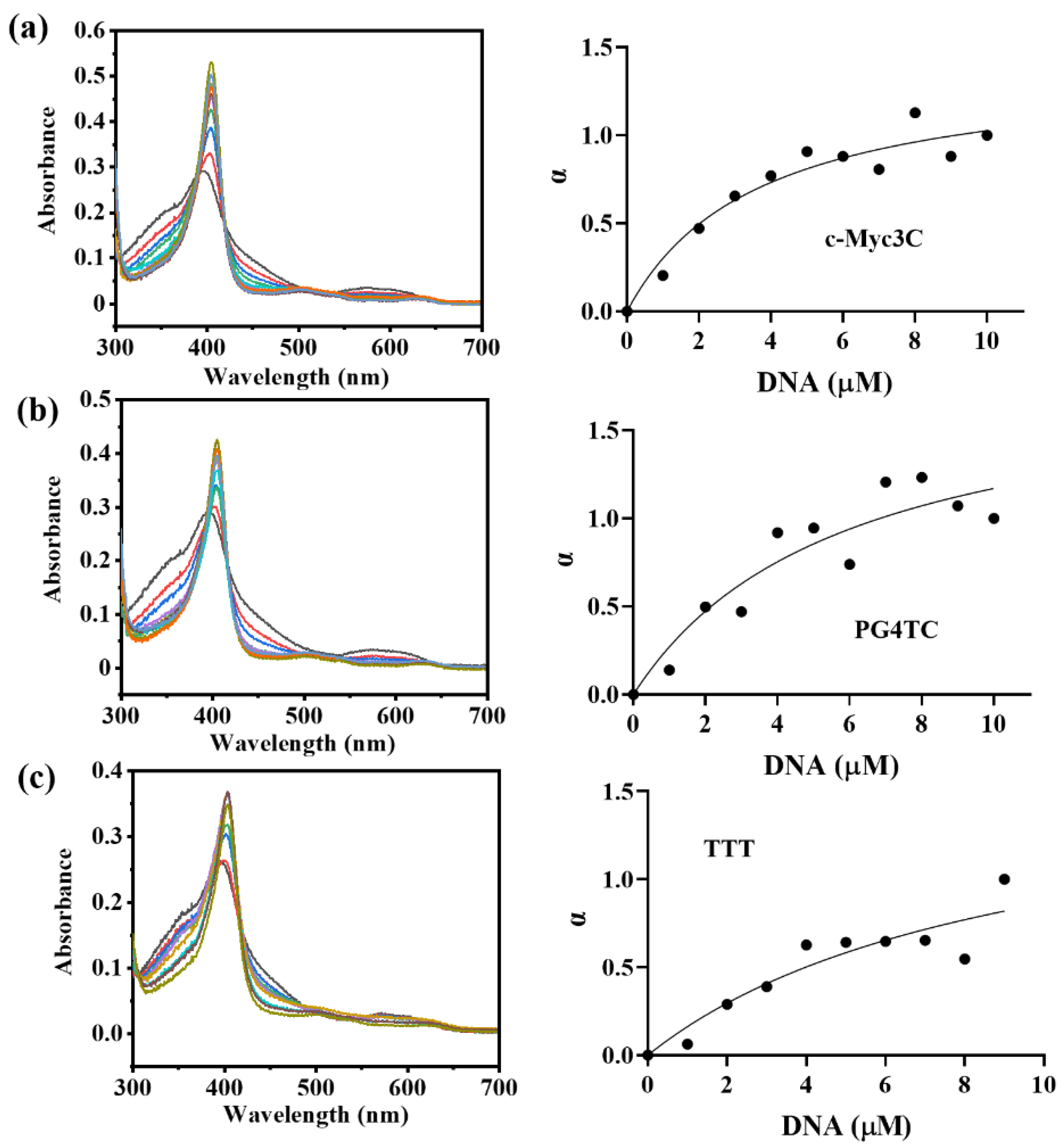
To assess the affinity magnitude of the three g−quadruplex sequences to hemin, the dissociation constant Kd of the DNAzymes was determined. The absorbance change of DNAzymes at 404 nm was recorded by titrating DNA solutions with different concentrations (0–10 μM) into 5 μM hemin, and Kd was reckoned by fitting a curve of α versus DNA concentration. Figure 2 showed the changes in the titration spectra of three g−quadruplex sequences, and the specific Kd statistics were shown in Table 2. The affinity of the three g−quadruplex sequences to hemin was c−Myc3c, PG4TC, and TTT in descending order. Combined with the previous activity experiments, the affinity between the g−quadruplex sequences and hemin was positively correlated with the formation of DNAzymes. Therefore, considering the catalytic activity, stability, and affinity, the c−Myc3c sequence was finally selected for subsequent immobilization studies.
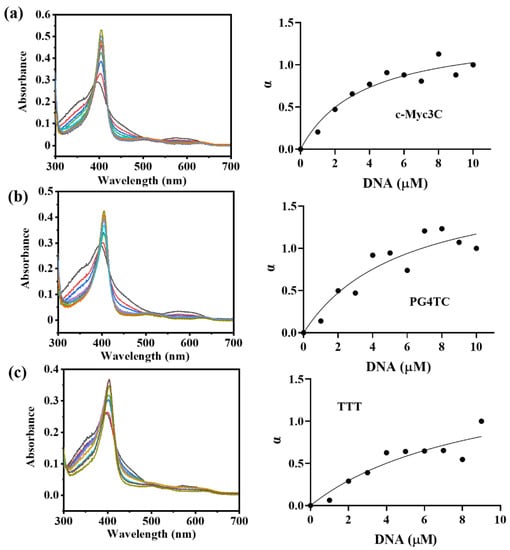
Figure 2.
The UV absorption titration diagram of three DNA sequences with 5 μM hemin (in the presence of K+). (a–c) c−Myc3c, PG4TC and TTT.
2.2. UiO66−DNAzymes Immobilization
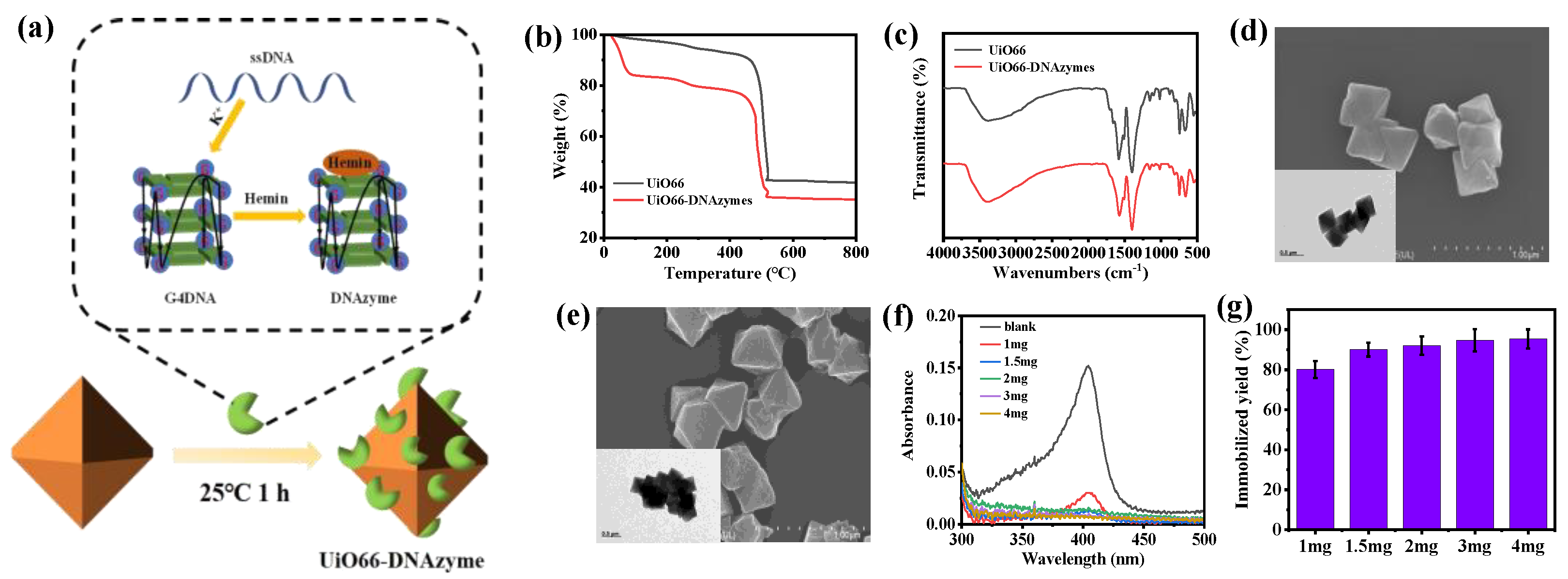
Subsequently, the DNAzymes with a c−Myc3c sequence were immobilized on UiO66 (Figure 3a) and the properties of the immobilized DNAzymes were studied. To demonstrate the successful immobilization of UiO66−DNAzymes, TG was used to characterize UiO66 and UiO66−DNAzymes (Figure 3b). The UiO66−DNAzymes showed a slight weight loss below 100 °C, which was not seen in the UiO66 curve. When heated to 800 °C, UiO66 retained 43% of its initial weight, which was slightly higher than UiO66−DNAzymes. The breakdown of DNAzymes was responsible for the excess loss in UiO66−DNAzymes, indicating that DNAzymes were successfully immobilized on UiO66. FTIR was further performed to investigate the relationship between immobilized UiO66−DNAzymes. As illustrated in Figure 3c, the peak of UiO66−DNAzymes changed slightly but not noticeably, which could be attributed to the small amount of immobilized DNAzymes. To better observe the morphology, particle size, and dispersion of UiO66 and UiO66−DNAzymes, the UiO66 and UiO66−DNAzymes were characterized by SEM and TEM. As shown in Figure 3d, the morphology of UiO66 was an ortho-octahedron with about 500 nm. Likewise, the morphology, dispersibility, and particle size of UiO66−DNAzymes were unchanged (Figure 3e), indicating that DNAzymes had no effect on UiO66. Subsequently, the optimal immobilized ratio was attained by varying the amount of UiO66 (0–4 mg). As shown in Figure 3f, the absorbance of the supernatant at 404 nm decreased significantly with the addition of UiO66, indicating that most of the DNAzymes were immobilized on UiO66. The immobilization yield was up to 92% after adding 2 mg of UiO66. However, the immobilization rate of the DNAzymes increased slowly with the further amount of UiO66 (Figure 3g). Considering the economy, 2 mg UiO66 was finally selected for immobilization.
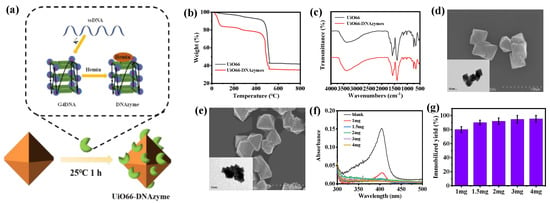
Figure 3.
(a) Scheme of UiO66−DNAzymes; (b) TG and (c) FTIR of UiO66 and UiO66−DNAzymes; SEM and TEM images of (d) UiO66 and (e) UiO66−DNAzymes; (f,g) optimization of immobilization ratio UiO66−DNAzymes.
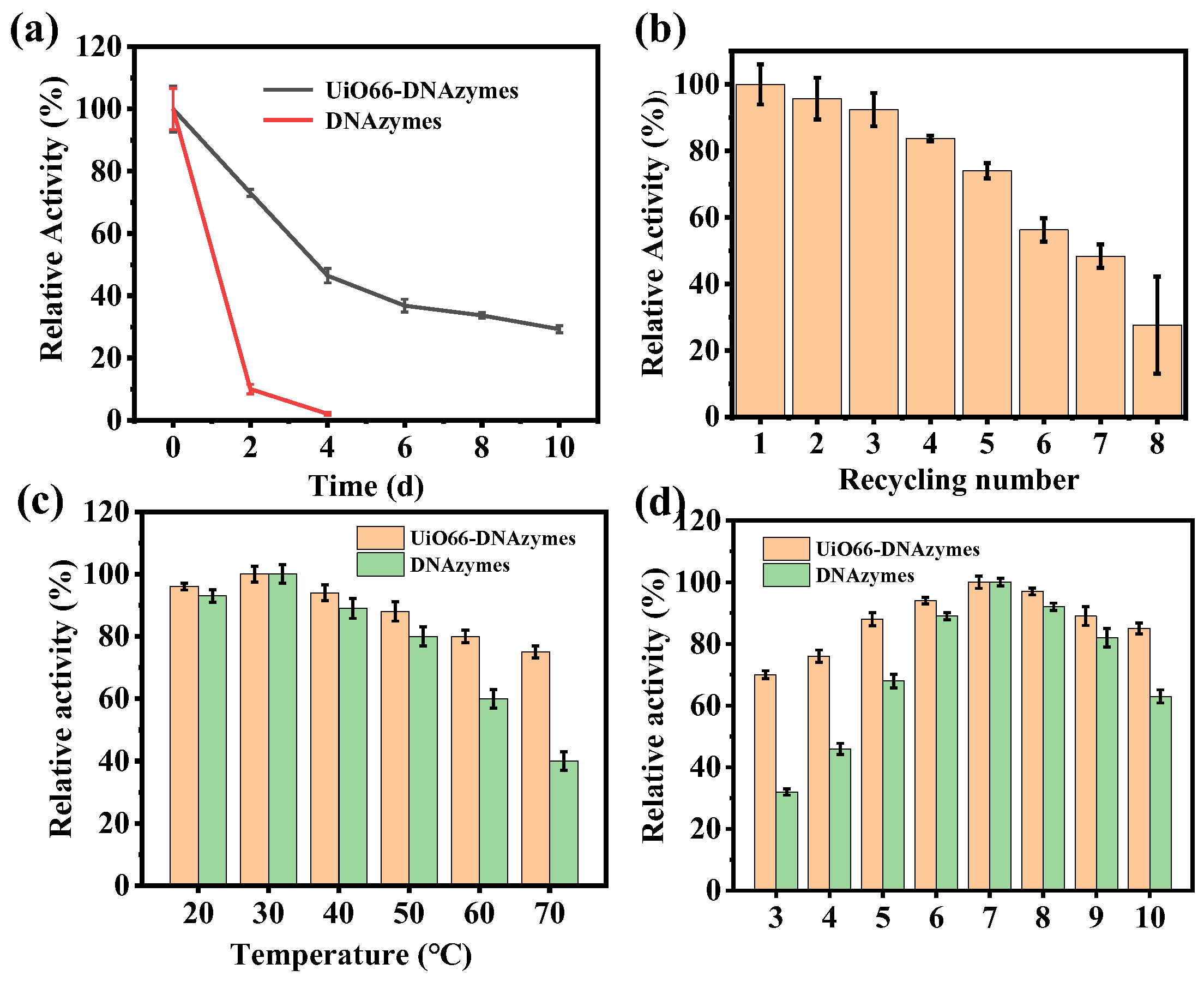
Subsequently, the storage stability and reusability of the immobilized DNAzymes were investigated. As shown in Figure 4a, the free DNAzymes were inactivated after 4 days of storage, while the UiO66−DNAzymes retained 46.4% activity at this time. Remarkably, UiO66−DNAzymes still maintained 29.25% activity after 10 days. From Figure 4b, the UiO66−DNAzymes preserved approximately 50% activity after repeated use 7 times. Furthermore, UiO66−DNAzymes still retained high activity in different temperatures and pH (Figure 4c,d), displaying the robust stability of the UiO66−DNAzymes. These results all indicated that UiO66 had a protective effect on the DNAzymes structure, preventing DNAzyme decomposition.
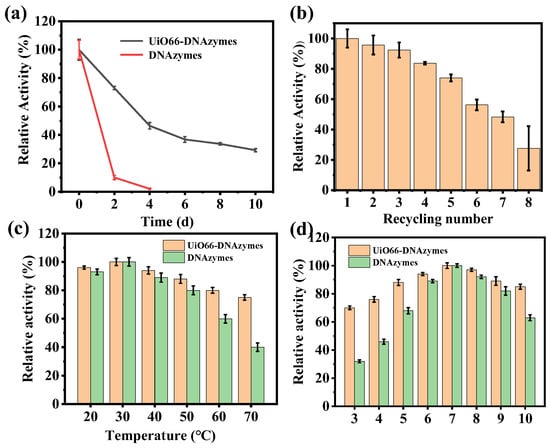
Figure 4.
(a) The storage stability of DNAzymes and UiO66−DNAzymes; (b) reusability of UiO66−DNAzymes; (c) temperature and (d) pH stability of DNAzymes and UiO66−DNAzymes.
Then, the enzymatic kinetics of UiO66−DNAzymes were studied, and the specific enzyme kinetic parameters were shown in Table S2. The Km of UiO66−DNAzymes was higher than that of free DNAzymes, indicating that immobilization reduced the affinity of DNAzymes to a substrate. DNAzymes were adsorbed on the UiO66 surface, making them more difficult to contact the substrate with greater resistance. At the same time, the Vmax of immobilized DNAzymes was smaller than that of free DNAzymes, which also proved the above conclusion.
2.3. Glucose Detection
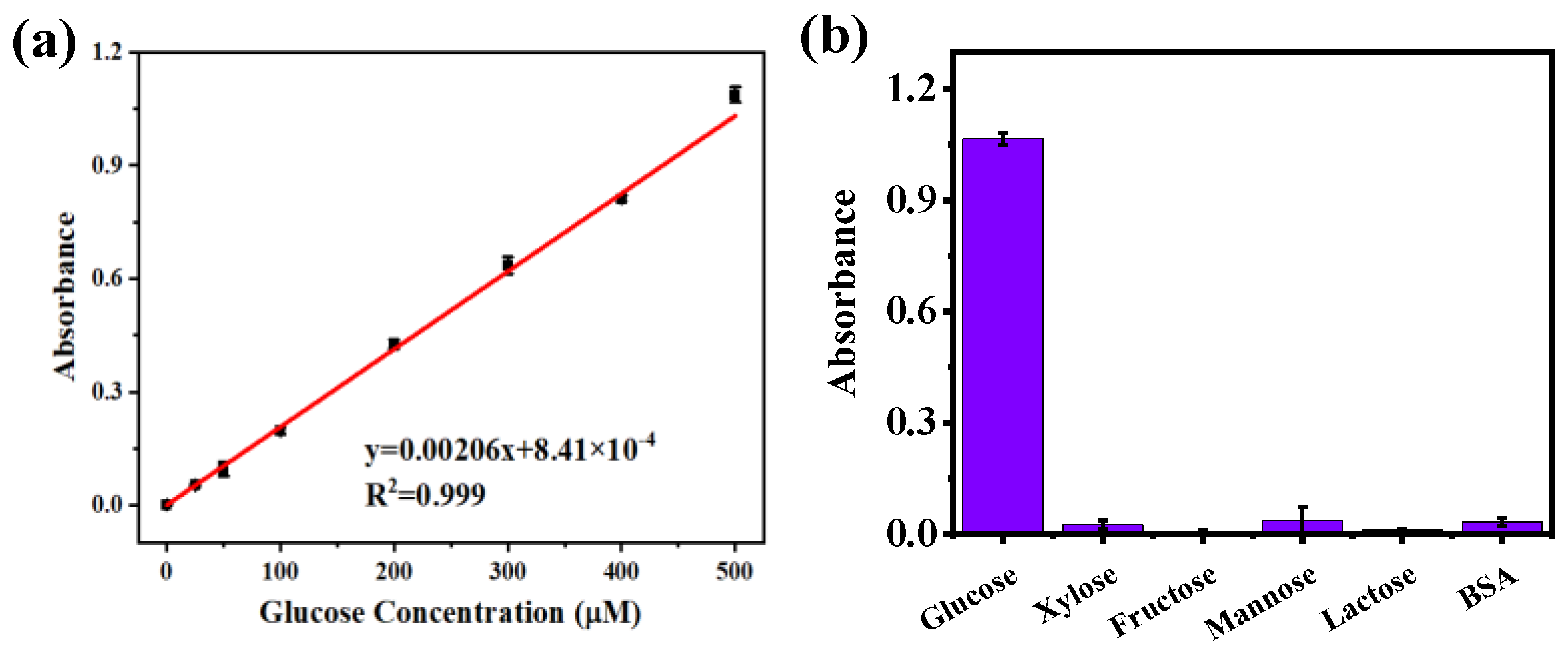
With the increase in glucose-related diseases, the development of methods to detect glucose has been a research hotspot. Therefore, an efficient, accurate, and economical sensor for glucose detection was constructed by cascading UiO66−DNAzymes and GOx. The response of the glucose sensor was investigated in HEPES buffer (pH = 7.5) using different glucose concentrations (0–500 μM). As shown in Figure 5a, the detection limit of the constructed sensor was 0.62 μM. Then, to verify the selection specificity of the sensor, 500 μM glucose was replaced with equal concentrations of other sugars (xylose, fructose, mannose, lactose) and reacted with BSA. The selection specificity of the sensor was assessed by the absorbance change of the supernatant at 420 nm. It could be seen that the absorbance of other interfering substances was extremely low (Figure 5b), showing that the constructed glucose sensor had excellent specificity.
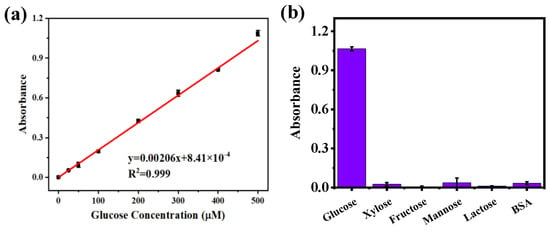
Figure 5.
(a) Glucose sensor based on UiO66−DNAzymes and GOx cascaded to detect glucose (0–500 μM). (b) The study of UiO66−DNAzymes specificity.
3. Experimental
3.1. Materials
Zirconium (IV) tetrachloride (ZrCl4, 99%), terephthalic acid (H2BDC, 98%), N, N-dimethylformamide (DMF, ≥99.9%), and glucose were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Hemin (AR), dimethyl sulfoxide (DMSO), GOx, and H2O2 were purchased from Shanghai yuanye Bio-Technology Co., Ltd. Hydrochloric acid (HCl, 36.0–38.0 wt%) was obtained from Damao (Tianjin, China).
3.2. Characterization
Transmission electron microscopy (TEM, Hitachi H-800) and scanning electron microscopy (SEM, JSM-5600LV) were used to observe the morphologies and structures of UiO66 and UiO66−DNAzymes. Fourier transform infrared spectroscopy (FTIR, Nicolet model 205 spectrometer) was performed to characterize the functional groups of UiO66 and UiO66−DNAzymes. Thermogravimetric (TG) analysis was undertaken on a STA7300 Thermal gravimetric analyzer (Hitachi HTIACHI) heating from 25 °C to 800 °C in air at a rate of 10 °C/min.
3.3. Preparation Methods
3.3.1. Preparation of G−Quadruplex/Hemin DNAzymes
The three DNA sequences were designed according to the literature [15,16,27] and are shown in Table S1. DNA stock solution was prepared in a HEPES buffer (50 mM, pH 7.5) and stored at 4 °C. The diluted DNA solution was added to a HEPES buffer containing 100 mM KCl and the g−quadruplex structure was obtained after a 5 min reaction at 95 °C. Then, DNA and hemin were dissolved in DMSO at a molar concentration of 1:2, and the g−quadruplex/hemin DNAzymes were produced after incubation for 1 h at room temperature.
3.3.2. Preparation of UiO66
UiO66 was prepared by the conventional hydrothermal method [18]: ZrCl4 (104 mg) and H2BDC (72.5 mg) were dissolved in 100 mL DMF. Then, a little hydrochloric acid was added to ultrasonicate for 10 min. The solution was placed in a Teflon-lined autoclave, followed by a hydrothermal reaction at 130 °C for 10 h. The products were centrifuged and washed three times with ethanol and water to remove the unreacted substrate.
3.3.3. Preparation of UiO66−DNAzymes
The above obtained UiO66 (2 mg) was added to the prepared DNAzyme solution and immobilized at room temperature for 1 h at 800 rpm. The precipitate was collected by centrifugation and washed three times with HEPES buffer to remove the non-immobilized DNAzymes. The amount of UiO66 was changed (0–4 mg) to optimize the immobilization ratio.
3.4. Enzymatic Properties Determination
3.4.1. Enzyme Activity
Enzyme activity was calculated by measuring the ability of the g−quadruplex/hemin DNAzymes to oxidize ABTS. A total of 0.5 mM H2O2 was added to the prepared DNAzyme solution to oxidize ABTS (0.5 mM), and then the absorbance change of the solution was measured at 420 nm. The molar absorption coefficients were 36,000 M−1 cm−1 for ABTS+ [14]. The standard deviations were calculated from three parallel experiments.
The enzyme kinetic constant was determined by changing the H2O2 concentration (0–5 mM). The enzyme kinetic curve of V0 and H2O2 was nonlinearly fitted to obtain the Michaelis constant Km and the maximum velocity Vmax. kcat was calculated using the formula kcat = Vmax/[catalyst concentration].
3.4.2. DNAzymes Degradation Rate
H2O2 (0.5 mM) was added to the prepared DNAzyme solution, and the change of absorbance was observed at 404 nm within 30 min. The initial degradation rate Vd was calculated by Equation (1).
where complex is DNAzymes, dt is the change of time, dA is the change of absorbance at 404 nm, b is the optical path, and ε is the extinction coefficient of the DNAzyme at 404 nm.
3.4.3. DNAzymes Dissociation Constant (Kd)
DNAzymes were prepared by titrating different concentrations of DNA sequences (0–10 μM) to 5 μM hemin solution, and the change of absorbance between 300–700 nm was recorded by UV-vis. The fraction of hemin (α) was calculated by Equation (2). Kd was obtained by fitting a curve of α versus DNA concentration.
where Af is the absorbance of hemin alone at 404 nm, Ab is the absorbance of DNAzymes saturated with hemin at 404 nm, and A is the absorbance when DNA was added.
3.5. Enzyme Stability
Storage stability: free and immobilized DNAzymes were stored at 4 °C for 10 days, and the changes in enzyme activity were recorded at 2, 4, 6, 8, and 10 days, respectively. Maximum enzyme activity was set to 100%. The three parallel experiments were performed.
Reusability: the reacted UiO66−DNAzymes were centrifuged to collect the precipitate, washed three times with a HEPES buffer, and then the substrate ABTS and H2O2 were added to react again. The enzyme activity was taken to be 100% the first time.
Temperature stability: free and immobilized DNAzymes were incubated at a different temperature (20–70 °C) for 1 h, and other conditions remained unchanged. The enzyme activity was measured according to method 3.4.1. The maximum enzyme activity was set to 100%. The three parallel experiments were performed.
pH stability: free and immobilized DNAzymes were incubated in different pH (3–10) for 1 h, and other conditions remained unchanged. The enzyme activity was measured according to method 3.4.1. The maximum enzyme activity was set to 100%. The three parallel experiments were performed.
3.6. Glucose Detection Application
The total reaction system was 1 mL. The prepared UiO66−DNAzymes were added to a HEPES buffer containing 100 μg/mL GOx, 1.5 mM ABTS, and 0–500 μM glucose for 30 min. Then, the solution was centrifuged and the absorbance of the supernatant at 420 nm was measured by a UV-vis spectrophotometer. The three parallel experiments were performed.
Selection specificity: the total reaction system was 1 mL. The prepared UiO66−DNAzymes were added to a HEPES buffer containing 100 μg/mL GOx, 1.5 mM ABTS, and 500 μM of other sugars (xylose, fructose, mannose, lactose) for 30 min. Then, the solution was centrifuged and the absorbance of the supernatant at 420 nm was measured by a UV-vis spectrophotometer.
4. Conclusions
In summary, DNAzymes were constructed with hemin based on three guanine-rich DNA sequences, c−Myc3c, PG4TC, and TTT. Comprehensively examining the activity, degradation rate, and affinity of the three sequences, the results showed that the peroxidase activity and affinity with the substrate of the DNAzymes by the c−Myc3c sequence were much higher than those of the other two sequences. Then, the DNAzymes numbered c−Myc3c were immobilized on UiO66, and the stability and reusability of UiO66−DNAzymes were improved. Finally, an efficient, accurate, and economical sensor for glucose detection was successfully constructed by cascading UiO66−DNAzymes and GOx. Using different glucose concentrations (0–500 μM), the response of the glucose sensor in a HEPES buffer (pH = 7.5) was studied. The detection limit of the constructed sensor was 0.62 μM, and the glucose sensor also had excellent specificity. Our study explored a new direction for DNAzyme catalysis and offered a facile and versatile biosensor platform for glucose detection. Considering that the base sequences affect the catalytic activity of enzymes, exploring the design of specific DNA sequences seems limitless.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13010081/s1, Figure S1. Michaelis-Menten analysis of three sequences; Table S1. DNA sequences used in the paper; Table S2 The kinetic parameters of the UiO66-DNAzymes.
Author Contributions
Y.Z.: experimental design and planning, performing the experiments, writing the initial draft and revised manuscript; J.L.: performing the experiments, drawing of the diagrams; H.L.: ideas, oversight and leadership responsibility for the research activity planning and execution, revision of the initial draft, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The National Natural Science Foundation of China [22078014]; the Hebei Key Research and Development Project [20372508D].
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors acknowledged financial support from the National Natural Science Foundation of China (22078014) and the Hebei Key Research and Development Project (20372508D).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cao, P.; Liu, H.; Wu, D.; Wang, X. Immobilization of Laccase on Phase-Change Microcapsules as Self-Thermoregulatory Enzyme Carrier for Biocatalytic Enhancement. Chem. Eng. J. 2021, 405, 126695. [Google Scholar] [CrossRef]
- Ghannadi, S.; Abdizadeh, H.; Miroliaei, M.; Saboury, A.A. Immobilization of Alcohol Dehydrogenase on Titania Nanoparticles to Enhance Enzyme Stability and Remove Substrate Inhibition in the Reaction of Formaldehyde to Methanol. Ind. Eng. Chem. Res. 2019, 58, 9844–9854. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, S.; Yuan, Q.; Liang, H. Zr-Based Acid-Stable Nucleotide Coordination Polymers: An Excellent Platform for Acidophilic Enzymes Immobilization. J. Inorg. Biochem. 2021, 216, 111338. [Google Scholar] [CrossRef] [PubMed]
- Jiao, M.; Li, Z.; Li, X.; Zhang, Z.; Yuan, Q.; Vriesekoop, F.; Liang, H.; Liu, J. Solving the H2O2 by-Product Problem Using a Catalase-Mimicking Nanozyme Cascade to Enhance Glycolic Acid Oxidase. Chem. Eng. J. 2020, 388, 124249. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, F.; Yuan, Q.; Liu, J.; Li, Y.; Liang, H. Robust Magnetic Laccase-Mimicking Nanozyme for Oxidizing O-Phenylenediamine and Removing Phenolic Pollutants. J. Environ. Sci. 2020, 88, 103–111. [Google Scholar] [CrossRef]
- Silverman, S.K. In Vitro Selection, Characterization, and Application of Deoxyribozymes That Cleave RNA. Nucleic Acids Res. 2005, 33, 6151–6163. [Google Scholar] [CrossRef]
- Amirbekyan, K.; Duchemin, N.; Benedetti, E.; Joseph, R.; Colon, A.; Markarian, S.A.; Bethge, L.; Vonhoff, S.; Klußmann, S.; Cossy, J.; et al. Design, Synthesis, and Binding Affinity Evaluation of Hoechst 33258 Derivatives for the Development of Sequence-Specific DNA-Based Asymmetric Catalysts. ACS Catal. 2016, 6, 3096–3105. [Google Scholar] [CrossRef]
- Rioz-Martínez, A.; Roelfes, G. DNA-Based Hybrid Catalysis. Curr. Opin. Chem. Biol. 2015, 25, 80–87. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Jia, G.; Liu, Y.; Lu, S.; Li, C. Enantioselective Friedel–Crafts Reactions in Water Catalyzed by a Human Telomeric G−Quadruplex DNA Metalloenzyme. Chem. Commun. 2012, 48, 6232–6234. [Google Scholar] [CrossRef]
- Park, S.; Okamura, I.; Sakashita, S.; Yum, J.H.; Acharya, C.; Gao, L.; Sugiyama, H. Development of DNA Metalloenzymes Using a Rational Design Approach and Application in the Asymmetric Diels–Alder Reaction. ACS Catal. 2015, 5, 4708–4712. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, L.; Wang, Y.; Li, W.; Zhang, J.; Gu, J.; Fu, Y. Guanine-Rich DNA-Based Peroxidase Mimetics for Colorimetric Assays of Alkaline Phosphatase. Biosens. Bioelectron. 2016, 77, 549–556. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, M.; Hao, J.; Jia, G.; Monchaud, D.; Li, C. The Noncovalent Dimerization of a G−Quadruplex/Hemin Dnazyme Improves Its Biocatalytic Properties. Chem. Sci. 2020, 11, 8846–8853. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, J.; Cheng, M.; Monchaud, D.; Zhou, J.; Ju, H. A Thermophilic Tetramolecular G−Quadruplex/Hemin Dnazyme. Angew. Chem. Int. Ed. 2017, 56, 16636–16640. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Cheng, M.; Guo, Y.; Šponer, J.; Monchaud, D.; Mergny, J.-L.; Ju, H.; Zhou, J. How Proximal Nucleobases Regulate the Catalytic Activity of G−Quadruplex/Hemin Dnazymes. ACS Catal. 2018, 8, 11352–11361. [Google Scholar] [CrossRef]
- Chang, T.; Gong, H.; Ding, P.; Liu, X.; Li, W.; Bing, T.; Cao, Z.; Shangguan, D. Activity Enhancement of G−Quadruplex/Hemin Dnazyme by Flanking D(Ccc). Chem. Eur. J. 2016, 22, 4015–4021. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; Cheng, M.; Mergny, J.-L.; Lin, Q.; Zhou, J.; Ju, H. Highly Active G−Quadruplex/Hemin Dnazyme for Sensitive Colorimetric Determination of Lead(Ii). Mikrochim. Acta 2019, 186, 786. [Google Scholar] [CrossRef] [PubMed]
- Ashkan, Z.; Hemmati, R.; Homaei, A.; Dinari, A.; Jamlidoost, M.; Tashakor, A. Immobilization of Enzymes on Nanoinorganic Support Materials: An Update. Int. J. Biol. Macromol. 2021, 168, 708–721. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, J.; Li, D.; Zhang, S.; Gu, B.; Qiu, Q.; Sun, Y.; Zhang, Y.; Cai, Z.; Jiang, Z. Synergy of Electron Transfer and Electron Utilization Via Metal–Organic Frameworks as an Electron Buffer Tank for Nicotinamide Regeneration. ACS Catal. 2020, 10, 2894–2905. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.-L.; Xiong, J.; Zong, M.-H.; Lou, W.-Y. Metal-Organic Frameworks as Novel Matrices for Efficient Enzyme Immobilization: An Update Review. Coordin. Chem. Rev. 2020, 406, 213149. [Google Scholar] [CrossRef]
- Ma, J.; Bai, W.; Zheng, J. A Novel Self-Cleaning Electrochemical Biosensor Integrating Copper Porphyrin-Derived Metal-Organic Framework Nanofilms, G−Quadruplex, and DNA Nanomotors for Achieving Cyclic Detection of Lead Ions. Biosens. Bioelectron. 2022, 197, 113801. [Google Scholar] [CrossRef]
- Shi, X.; Xie, Y.; Chen, L.; Lu, J.; Zhang, L.; Sun, D. Combining Quasi-Zif-67 Hybrid Nanozyme and G−Quadruplex/Hemin Dnazyme for Highly Sensitive Electrochemical Sensing. Bioelectrochemistry 2023, 149, 108278. [Google Scholar] [CrossRef]
- Wu, S.-H.; Tang, Y.; Chen, L.; Ma, X.-G.; Tian, S.-M.; Sun, J.-J. Amplified Electrochemical Hydrogen Peroxide Reduction Based on Hemin/G−Quadruplex Dnazyme as Electrocatalyst at Aold Particles Modified Heated Copper Disk Electrode. Biosens. Bioelectron. 2015, 73, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Lo, W.-S.; Man, T.; Williams, B.P.; Li, D.; Chen, S.-Y.; Pei, H.; Li, L.; Tsung, C.-K. Stabilizing Dnazymes through Encapsulation in a Metal–Organic Framework. Chem. Eur. J. 2020, 26, 12931–12935. [Google Scholar] [CrossRef] [PubMed]
- Oloketuyi, S.; Annovi, G.; de Marco, A. Peroxidase Zymograms Obtained by Agarose Native Gel Electrophoresis Have Unmet Resolution and Completeness. Int. J. Biol. Macromol. 2020, 156, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Man, Y.; Li, A.; Jin, X.; Liu, X.; Pan, L. Dnazyme-Based Biosensor for Detection of Lead Ion: A Review. Microchem. J. 2017, 131, 145–153. [Google Scholar] [CrossRef]
- Liu, S.; Su, W.; Ding, X. A Review on Microfluidic Paper-Based Analytical Devices for Glucose Detection. Sensors 2016, 16, 2086. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, W.; Gao, T.; Ding, P.; Pei, R. One Terminal Guanosine Flip of Intramolecular Parallel G−Quadruplex: Catalytic Enhancement of G−Quadruplex/Hemin Dnazymes. Chemistry 2020, 26, 8631–8638. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).